Introduction
Primary liver cancer is one of the most common
causes for cancer-associated mortality worldwide (1). Due to its nonspecific symptoms, the
diagnosis of liver cancer is often made at an intermediate-advanced
stage, rendering patients unsuitable for curative treatments
(2). Transcatheter arterial
embolization/chemoembolization (TAE/TACE) provides a modest
survival benefit for patients with unresectable liver cancer
(3), which limits hepatic arterial
blood supply to liver cancer and exerts antitumor effects by
inducing tumor ischemic hypoxia and necrosis (4). However, the results of TAE/TACE remain
unsatisfactory, with local recurrence rates as high as 30–60%
(4). Among the factors interfering
with the effectiveness of TAE/TACE, a neoangiogenic reaction
following treatment is frequently observed (5). In addition to inducing complete
necrosis in small-sized liver cancer, TAE/TACE inevitably results
in an acute hypoxic insult in the majority of liver cancer lesions
(5). It is apparent that
hypoxia-induced angiogenesis contributes to tumor regrowth
(5–7). Of note, the stimulation of vascular
endothelial growth factor (VEGF) following treatment has emerged as
a negative factor affecting treatment efficacy and patient survival
rates (8–12). Bevacizumab, an antibody targeting
VEGF, can attenuate the increase in VEGF post-TACE and reduce
neo-vessel formation detected using angiography (13,14).
However, the optimal embolic endpoint of TACE, which is correlated
with the extent of hypoxia, has not been clarified. It has been
reported that the embolization of tumor blood supply to a
sub-stasis endpoint during TACE improves survival rates compared
with embolization to a stasis endpoint (15), suggesting that the extent or patterns
of embolization-induced hypoxia affect treatment outcomes. This
prompted the hypothesis that the pattern of tumor hypoxia induction
influences tumor progression.
In the present study, it was shown that intermittent
hypoxia alleviated the acute hypoxia-induced increase of VEGF, and
consequently decreased the pro-angiogenic potential of liver
cancer; these findings suggest the potential use of a novel
embolism strategy using TACE to induce intermittent hypoxia.
Materials and methods
Reagents and antibodies
Monoclonal anti-hypoxia-inducible factor (HIF)-1α
antibody (cat. no. ab51608; Abcam), anti-VEGF antibody (cat. no.
ab51745; Abcam), anti-β-actin antibody (cat. no. ab8224; Abcam),
anti-P38 mitogen-activated protein kinase (MAPK) antibody (cat. no.
ab31828; Abcam), anti-phosphorylated (p-)P38MAPK antibody (cat. no.
ab4822; Abcam), monoclonal anti-Akt antibody (cat. no. ab8805;
Abcam), anti-p-Akt antibody (cat. no. ab38449; Abcam), anti-NF-κB
antibody (cat. no. ab16502; Abcam), anti-p-NF-κB antibody (cat. no.
3033S; Cell Signaling Technology), anti-Src antibody (cat. no.
184Q20; Thermo Fisher Scientific, Inc.), anti-p-Src antibody (cat.
no. 44-660G; Thermo Fisher Scientific, Inc.), anti-extracellular
signal-regulated kinase (ERK)1/2 antibody (cat. no. 13-6200; Thermo
Fisher Scientific, Inc.) and anti-p-ERK1/2 antibody (cat. no.
44-680G; Thermo Fisher Scientific, Inc.) were used for western
blotting. N-acetyl-cysteine (NAC), ammonium
pyrrolidinedithiocarbamate (PDTC) and the reactive oxygen species
(ROS) assay were purchased from Beyotime Institute of
Biotechnology.
Cell culture and treatment
The HepG2 (ATCC) and Huh7 (Japanese Cancer Research
Resources Bank) liver cancer cell lines and the EA.hy926 human
umbilical vein cell line (ATCC) were maintained in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin-streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) in a 5% CO2 incubator at 37°C. The
HepG2 cell line is derived from hepatoblastoma. The cell lines were
authenticated using short tandem repeat validation analysis. For
acute hypoxia, the liver cancer cells were cultured under 1% oxygen
for 48 h. For intermittent hypoxia exposure, the cells were
subjected to three cycles of hypoxia-reoxygenation comprising
culture under 1% oxygen for 24 h followed by 21% oxygen for 24 h,
and finally under 1% oxygen for 48 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract the total cellular RNA, which
was then reverse-transcribed into cDNA using a RevertAid First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) in
accordance with the manufacturer's instructions. The primers used
were as follows: VEGF, 5′-CCAACTTCTGGGCTGTTCTC-3′ (sense) and
5′-CCCCTCTCCTCTTCCTTCTC-3′ (antisense); platelet-derived growth
factor (PDGF), 5′-GATGATCTCCAACGCCTGCT-3′ (sense) and
5′-TCTTCCACGAGCCAAGCTCT-3′ (antisense); β-actin,
5′-CATGTACGTTGCTATCCAGGC-3′ (sense) and 5′-CTCCTTAATGTCACGCACGAT-3′
(antisense). FastStart Universal Probe Master (Roche Diagnostics)
was used to amplify the resultant cDNA The thermocycling conditions
were as follows: 50°C for 2 min; 95°C for 10 min; 40 cycles of 95°C
for 15 sec and 60°C for 30 sec. The relative expression of each
target gene was quantified using the 2-∆∆Cq method (16), with normalization using the level of
β-actin.
Western blotting
The cells were lysed using RIPA buffer containing
PMSF and phosphatase inhibitor (Beyotime Institute of
Biotechnology) on ice and protein concentration was measured using
a BCA protein assay (Pierce; Thermo Fisher Scientific, Inc.). The
proteins (20 µg/well) were separated via 10% SDS-PAGE, following
which the protein samples were transferred onto a PVDF membrane
(EMD Millipore), which was blocked with 5% low fat milk for 1 h and
then incubated with the primary antibodies (1:1,000) at 4°C
overnight. The following day, the membrane was incubated with
secondary antibodies (1:1,000; cat. no. A0208 and A0216, Beyotime
Institute of Biotechnology) for 1 h at room temperature and
developed using ECL (Pierce; Thermo Fisher Scientific, Inc.). The
target protein level was normalized to that of β-actin, and that of
the phosphorylated target protein was normalized to that of the
corresponding total protein.
Proliferation analysis
The WST-1 cell proliferation assay kit (Roche
Diagnostics) and 5-ethynyl-2′-deoxyuridine (EdU) assay (RiboBio
Co., Ltd.) were used to measure the proliferation of endothelial
cells. The endothelial cells (2×103 cells/well) were incubated with
conditioned medium (CM) derived from the acute hypoxia or
intermittent hypoxia-exposed HepG2 cells, in 96-well plates for 1–3
days. At different time points (24, 48 and 72 h), WST-1 (10 µl) was
added, followed by measuring the absorbance at 450 nm using a
microplate reader (Thermo Fisher Scientific, Inc.). For the EDU
assays, the endothelial cells were incubated with EdU working
solution (50 µM) for 2 h at 37°C, and then stained with Hoechst
solution. Images were captured in six randomly selected fields and
analyzed with an Olympus fluorescence microscope (Olympus
Corporation). The experiments were performed in triplicate.
In vitro tube formation assay
The endothelial cells (4×104 cells/well) were
incubated with the CM derived from acute hypoxia or intermittent
hypoxia-exposed HepG2 cells at 37°C for 4 h in 24-well plates
coated using Matrigel matrix (BD Biosciences). ImageJ software
(National Institutes of Health) was used to quantify the number of
tubes in three randomly selected fields that were observed with an
Olympus fluorescence microscope (magnification, ×100).
In vivo tumor angiogenesis
The protocols for animal experiments were reviewed
and approved by the Ethical Committee on Animal Experiments of
Animal Care Committee of Fudan University (Fudan, China). Male
BALB/c nu/nu mice at 4–6 weeks of age weighing 18–20 g were
obtained from SLAC Laboratory Animal Co., Ltd. Mice were housed in
animal rooms with a 10-h light/14-h dark cycle and at a constant
temperature (22–27°C). Animals had free access to standard rodent
chow and water. Following exposure to acute or intermittent
hypoxia, liver cancer cells (2×107) mixed with endothelial cells
(5×106) were injected subcutaneously into the flanks of the mice
(each group, n=3) for the evaluation of tumor angiogenesis. The
immunohistologic staining of endothelial cells was subsequently
performed. Briefly, subcutaneous tumors were fixed in 10%
formaldehyde solution for 10 h at room temperature, embedded in
paraffin and cut into 5 µm thick slices. Some slices were stained
with hematoxylin and eosin (H&E; Beyotime Institute of
Biotechnology) according to a standard procedure (17). For VEGF and CD31 detection, tissue
slices were deparaffinized in xylene for 20 min at room
temperature, rehydrated in gradient ethanol (100, 95, 90 and 80%)
and treated with 0.3% H2O2 for 15 min at room temperature to block
endogenous peroxidase activity. To retrieve the antigen, slices
were heated in 10 mM citrate buffer (pH 6.0) in a microwave oven at
95°C three times for 5 min. Slices were then blocked with 20% goat
serum (Beyotime Institute of Biotechnology) for 30 min at room
temperature, and were incubated with VEGF antibody (1:100; cat. no.
ab51745; Abcam) or CD31 antibody (1:50; cat. no. ab28364; Abcam) at
4°C overnight and with the secondary antibody (1:50; cat. no.
A0208, Beyotime Institute of Biotechnology) at room temperature for
30 min. Slices were treated with 3,3′-diaminobenzidine
tetrahydrochloride (DAB; Beyotime Institute of Biotechnology),
counterstained with hematoxylin and visualized under an Olympus
IX73 inverted microscope. The cellular brown staining was
considered as positive reaction.
ROS measurement
The generation of ROS was quantified using
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA; Beyotime
Institute of Biotechnology). Briefly, following acute hypoxia or
intermittent hypoxia exposure, the liver cancer cells were
incubated with serum-free media containing 10 µM DCFH-DA
fluorescent probe 30 min for at 37°C in the dark. The cells were
then washed with serum-free DMEM three times to completely remove
residual DCFH-DA, and fluorescence of the DCFH-DA-loaded cells was
measured using a fluorescence plate reader.
The liver cancer cells were pre-treated with NAC (10
mmol/l, a ROS scavenger) for 30 min at 37°C to reduce intracellular
ROS sources, and exposed to hypoxia for 24 h. Images were captured
with an Olympus fluorescence microscope at ×200 magnification.
Statistical analysis
SPSS 13.0 software (SPSS, Inc.) was used for data
analysis. All values are expressed as the means ± standard
deviation. Student's unpaired t-test and one-way analysis of
variance were used to compare between two samples and among three
groups, respectively. P<0.05 was considered to indicate a
statistically significant difference.
Results
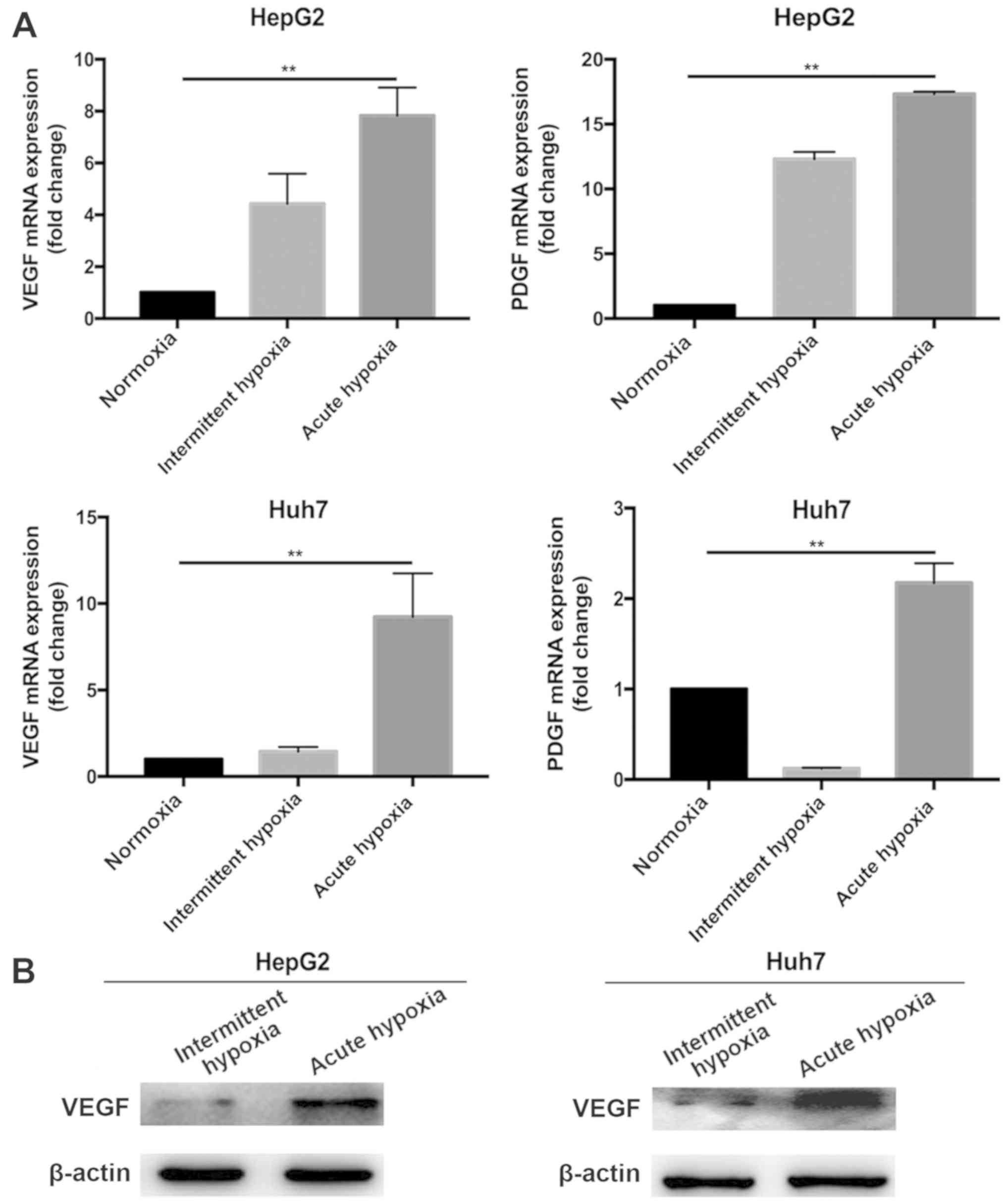
Expression of VEGF is attenuated in
liver cancer cells under intermittent hypoxia
The liver cancer cells were cultured under normoxia,
or under acute hypoxia or intermittent hypoxia conditions. As shown
using RT-qPCR analysis (Fig. 1A),
the expression of VEGF in the acute and intermittent
hypoxia-exposed liver cancer cells was significantly higher than
that in the cells under normoxic conditions. However, the magnitude
of this increase in the liver cancer cells exposed to intermittent
hypoxia was significantly lower than that in the cells exposed to
acute hypoxia. This was confirmed using western blotting (Fig. 1B). These results suggest that the
expression of pro-angiogenic factor VEGF in liver cancer cells can
be modulated by the patterns of hypoxia. In subsequent experiments,
the biological effects of different hypoxic patterns (intermittent
hypoxia vs. intermittent hypoxia) were compared. Although the mRNA
expression of PDGF was also significantly increased in the acute
hypoxia-exposed liver cancer cells, the effects of intermittent
hypoxia on the expression of PDGF were inconsistent with those of
normoxia in the two liver cancer cell lines (Fig. 1A). Therefore, the role of VEGF was
investigated in the subsequent experiments.
Effects of CM from intermittent
hypoxia- or acute hypoxia-exposed liver cancer cells on endothelial
cell in vitro and in vivo
Subsequently, the effects of CM from acute or
intermittent hypoxia-exposed liver cancer cells on angiogenesis
were investigated.
In vitro cell proliferation (Fig. 2A and B) and tube formation (Fig. 2C) in endothelial cells cultured in CM
from the intermittent hypoxia-exposed liver cancer cells were
significantly reduced compared with those cultured in CM from acute
hypoxia-exposed liver cancer cells. In parallel, activation of the
Src, p38MAPK, AKT and ERK1/2 pathways was attenuated in the
endothelial cells cultured in CM from intermittent hypoxia-exposed
liver cancer cells (Fig. 2D).
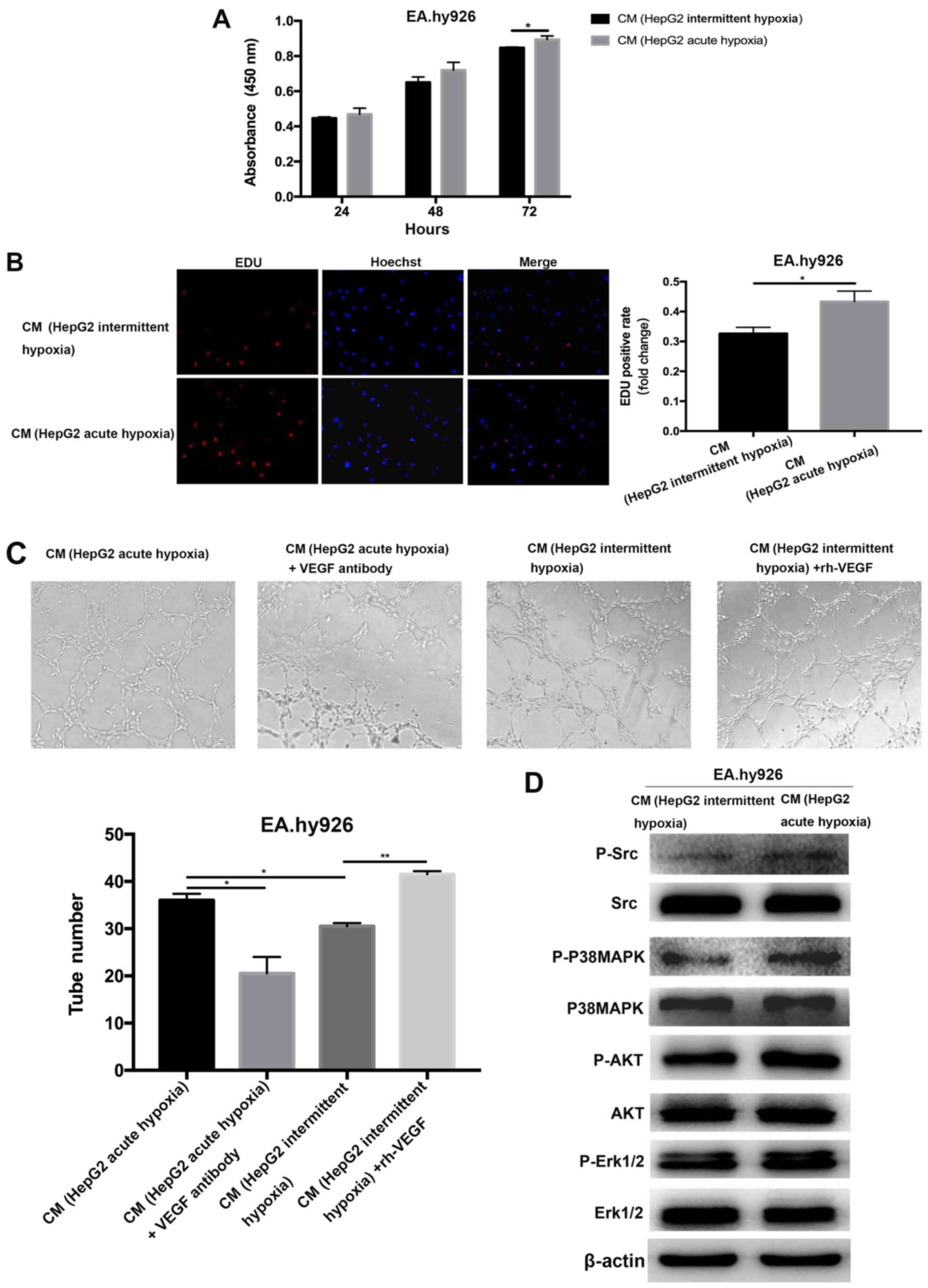 | Figure 2.Effects of CM from intermittent or
acute hypoxia-exposed HepG2 cells on endothelial cells. CM from
acute hypoxia-exposed HepG2 markedly promoted the proliferation of
endothelial cells, as detected using (A) WST-1 and (B) EdU assay
(magnification, ×200). (C) In vitro tube formation from endothelial
cells cultured in CM from acute or intermittent hypoxia-exposed
HepG2 with or without VEGF neutralizing antibody or rhVEGF
(magnification, ×100). (D) Activation of Src, p38MAPK, AKT and
ERK1/2 pathways in endothelial cells cultured with CM from acute or
intermittent hypoxia-exposed HepG2 cells was evaluated using
western blotting. *P<0.05, **P<0.01. CM, conditioned media;
VEGF, vascular endothelial growth factor; rhVEGF, recombinant human
VEGF; MAPK, mitogen-activated protein kinase; ERK, extracellular
signal-regulated kinase; p-, phosphorylated; EdU,
5-ethynyl-2′-deoxyuridine. |
Experiments were then performed to verify whether
the pro-angiogenic effect of CM from acute hypoxia-treated liver
cancer cells was due to VEGF. Therefore, the VEGF in the CM from
hypoxia-exposed liver cancer cells was specifically neutralized.
The removal of VEGF in the CM from acute hypoxia-exposed HepG2
cells using anti-VEGF neutralizing antibody resulted in a decrease
in tube formation in endothelial cells (Fig. 2C). By contrast, the angiogenic
response of tube formation in endothelial cells was increased when
the endothelial cells were cultured in CM from intermittent
hypoxia-exposed liver cancer cells supplemented with recombinant
VEGF (Fig. 2C). These data confirmed
that the pro-angiogenic effects of acute hypoxia-exposed liver
cancer cells on in vitro angiogenesis were mediated through
VEGF.
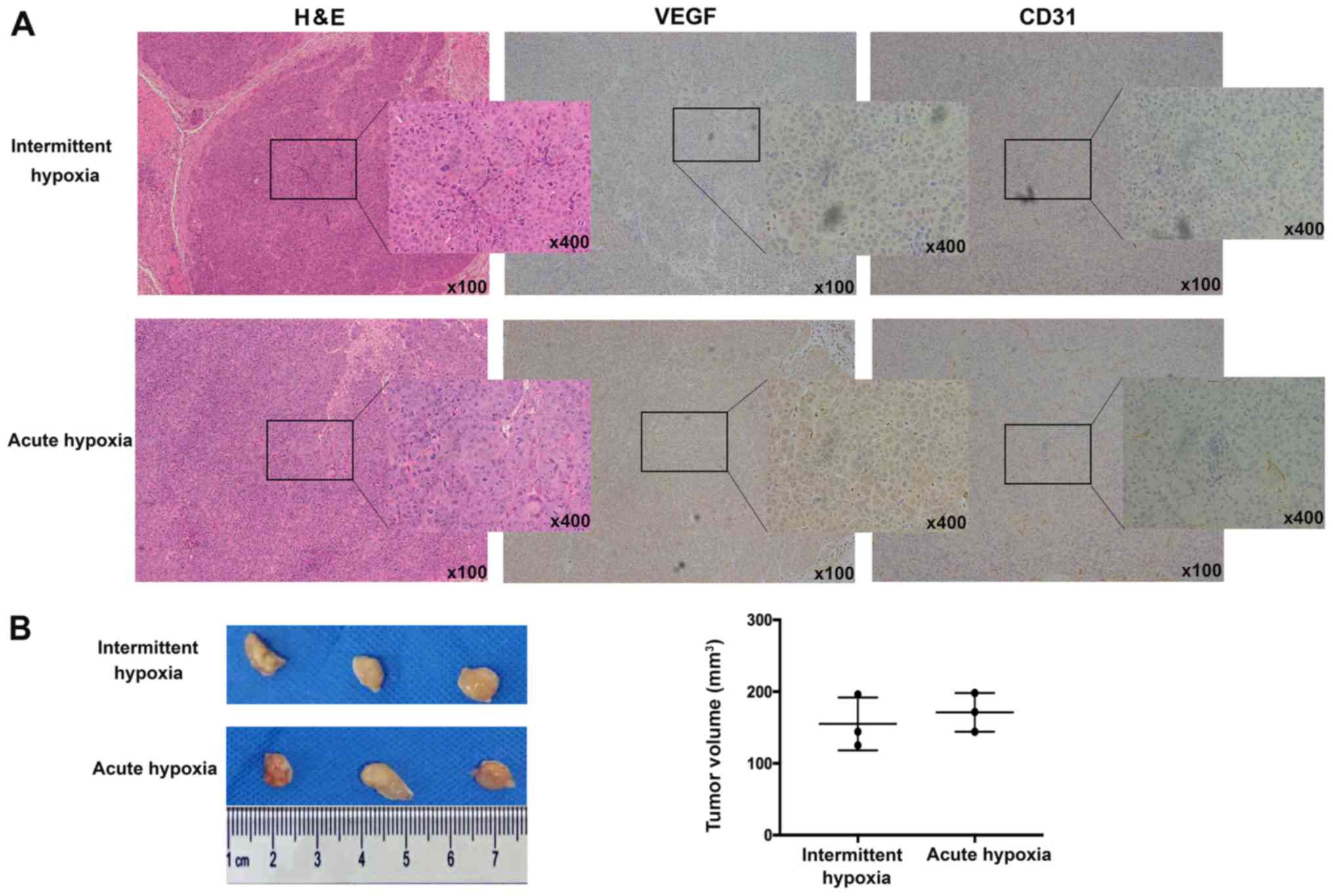
To investigate the effects of hypoxia-exposed liver
cancer cells on in vivo angiogenesis, acute or intermittent
hypoxia-exposed liver cancer cells mixed with endothelial cells
were injected subcutaneously into nude mice and tumor
neovascularization was examined. As visualized by VEGF and CD31
staining, tumors arising from intermittent hypoxia-exposed liver
cancer cells exhibited lower neovascularization than those from
acute hypoxia-exposed liver cancer cells (Fig. 3A), although tumor size did not differ
significantly (Fig. 3B).
ROS/HIF-1α pathway is responsible for
the increased expression of VEGF in liver cancer under acute
hypoxia
The ROS levels were assessed using DCFH-DA, as shown
in Fig. 4A and B. The levels of
intracellular ROS were significantly decreased in the intermittent
hypoxia-exposed liver cancer cells compared with those in the acute
hypoxia-exposed cells. Similarly, the expression of HIF-1α was
inhibited under intermittent hypoxia (Fig. 4C). To determine whether ROS was
involved in the regulation of HIF-1α, the acute hypoxia-exposed
liver cancer cells were pre-cultured with NAC, a ROS scavenger
(Fig. 4B), and the increases in
HIF-1α and VEGF were significantly downregulated (Fig. 4D). These data indicated that acute
hypoxia, with the sequential triggering of ROS accumulation and
HIF-1α pathway activation, may be involved in the increased
expression of VEGF in liver cancer under acute hypoxia, and
intermittent hypoxia may downregulate intracellular ROS levels and
attenuate the expression of VEGF.
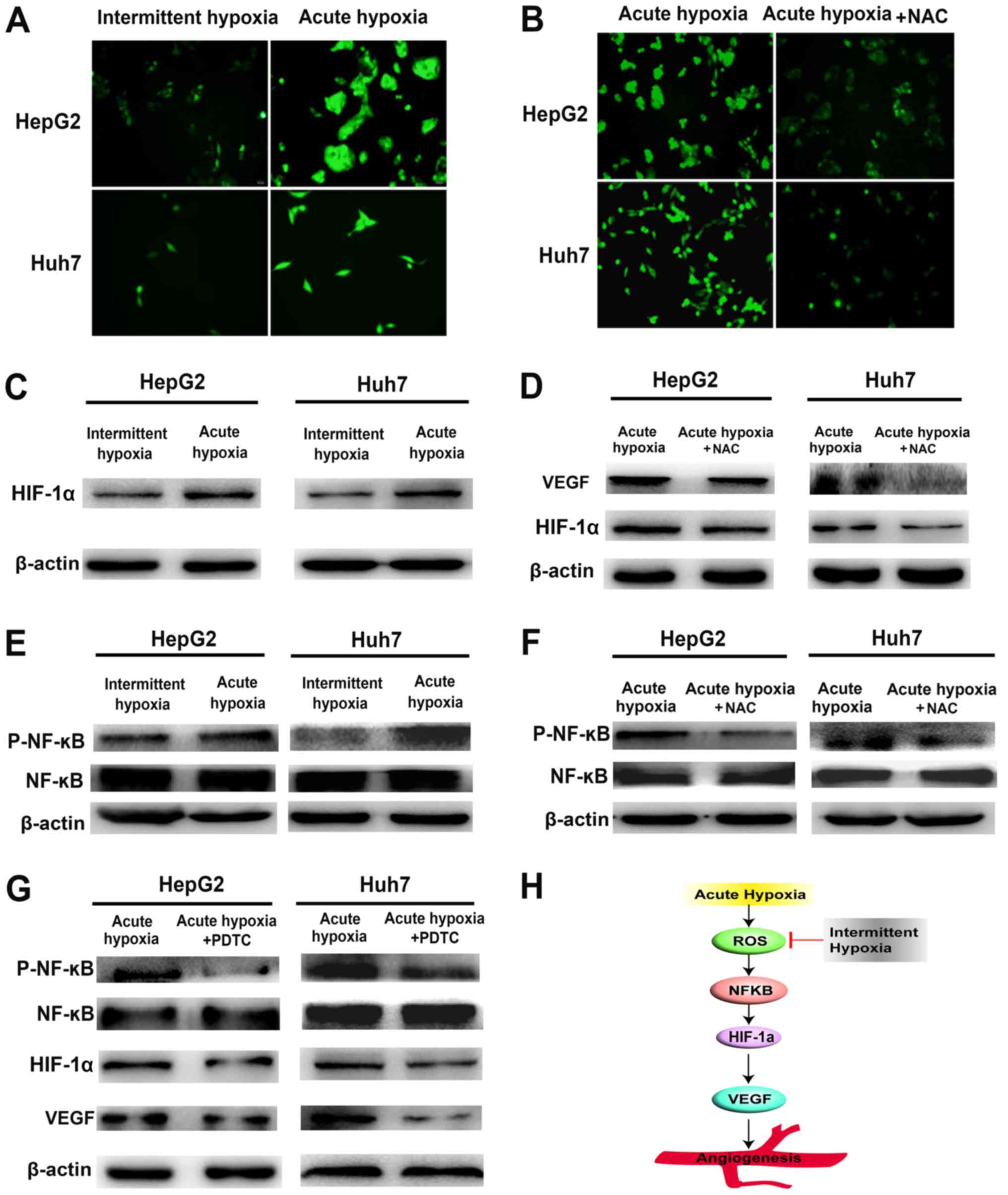 | Figure 4.NF-kB-mediated activation of the
ROS/HIF-1α pathway is involved in the expression of VEGF in liver
cancer cells under hypoxia. (A) Levels of intracellular ROS in
acute and intermittent hypoxia-exposed liver cancer cells were
measured using 2′,7′-dichlorodihydrofluorescein diacetate
(magnification, ×200). (B) When acute hypoxia-exposed liver cancer
cells were pre-cultured with NAC (a ROS scavenger), the level of
intracellular ROS was significantly decreased (magnification,
×200). (C) Expression of HIF-1α in acute and intermittent
hypoxia-exposed Huh7 and HepG2 cells. (D) In parallel with the ROS
decrease, protein expression levels of HIF-1α and VEGF were
downregulated. (E) activity of NF-κB in acute and intermittent
hypoxia-exposed liver cancer was measured using western blotting.
(F) When acute hypoxia-treated liver cancer cells were pre-treated
with NAC (a ROS scavenger), the phosphorylation of NF-κB was
significantly decreased. (G) When acute hypoxia-treated liver
cancer cells were pre-treated with PDTC (an NF-κB inhibitor), the
phosphorylation of NF-κB, and the expression of HIF-1α and VEGF
were significantly lower. (H) Diagram illustrating the effect of
intermittent hypoxia on alleviating the increase in VEGF and
decreasing the pro-angiogenic potential of liver cancer cells.
VEGF, vascular endothelial growth factor; ROS, reactive oxygen
species; HIF-1α hypoxia-inducible factor1α; p-, phosphorylated.
NAC, N-acetyl-cysteine. |
NF-kB mediates the regulation of
HIF-1α by ROS in acute hypoxia-exposed liver cancer cells
Previous studies have shown that ROS are involved in
activating NF-κB pathways, enhancing the expression of HIF-1α
(18–22). To assess whether the upregulation of
HIF-1α induced by ROS was mediated through the NF-κB pathway, the
activity of NF-κB was measured under hypoxic conditions. As
presented in Fig. 4E, the
phosphorylation of NF-κB was significantly decreased in the
intermittent hypoxia-exposed liver cancer cells compared with that
in the cells exposed to acute hypoxia. Upon pre-treated with NAC to
scavenge ROS, the phosphorylation of NF-κB in acute hypoxia-exposed
liver cancer was significantly attenuated (Fig. 4F). The use of NF-κB inhibitor PDTC
significantly lowered the phosphorylation of NF-κB, and the
expression of HIF-1α and that of its downstream target VEGF in
acute hypoxia-exposed liver cancer cells (Fig. 4G). These data indicate that
activation of the ROS/NF-κB/HIF-1α pathway may be responsible for
the increased expression of VEGF in liver cancer cells under acute
hypoxia, and intermittent hypoxia may decrease ROS/NF-κB/HIF-1α
activity to downregulate the expression of VEGF.
Discussion
In the present study, it was demonstrated that
intermittent hypoxia attenuated the expression of VEGF in liver
cancer cells compared with that in acute hypoxia. Secondly, it was
shown that the pro-angiogenic effect of intermittent
hypoxia-exposed liver cancer cells on endothelial cells was
significantly reduced compared with that of acute hypoxia-exposed
liver cancer cells. It is anticipated that these findings are of
potential value in establishing a novel embolism strategy using
TACE to improve treatment efficacy.
Due to its insidious onset, the majority of patients
with liver cancer are diagnosed at the intermediate-advanced stage
(23). TAE/TACE, which embolizes the
tumor blood supply to cause tumor necrosis by inducing ischemia,
provides a modest survival benefit to patients (3). Several studies have suggested that TACE
causes hypoxia and stimulates angiogenesis via pro-angiogenic
factors, including basic fibroblast growth factor (b-FGF) and VEGF
(9,24). Elevated levels of VEGF following
TACE, which represent an unfavorable factor, indicate poor patient
prognosis (8,9). Therefore, inhibiting the increase of
VEGF following TACE represents a potential therapeutic strategy to
prevent the rebound of blood vessel formation, and this may offer
promise in improving treatment outcomes. In the present study, it
was hypothesized that the pattern of hypoxia can modulate the
expression of VEGF in hypoxia-exposed liver cancer cells. The
results demonstrate that intermittent hypoxia can alleviate the
acute hypoxia-induced increase of VEGF, and decrease the
pro-angiogenic potential of liver cancer cells; these findings may
be of value in establishing a novel embolism strategy using
TACE.
VEGF is the most potent angiogenic factor
stimulating angiogenesis (25,26).
Acute hypoxia caused by TACE induces abnormal angiogenesis via an
increase in VEGF levels. In the present study, it was shown that
intermittent hypoxia attenuated the levels of VEGF in liver cancer
cells compared with those in acute hypoxia. It has been reported
that hypoxia (1% hypoxia, 24 h), in contrast to normoxia, impairs
the angiogenic response of endothelial cells to VEGF stimulation
(27). This suggests that, although
different studies have used their own subtypes of hypoxia exposure,
hypoxic exposure patterns can alter the neo-angiogenic process. The
findings of the present study suggest that intermittent hypoxia can
reduce the production of VEGF in liver cancer cells compared with
that following acute hypoxia, and this may inform the design of an
optimal embolism strategy using TACE. Embolization of the tumor
feeding artery to a sub-stasis (intermittent hypoxia-like
condition) may be better than occlusion of an arterial vessel to
complete stasis (severe acute hypoxia). Therefore, specific
embolism patterns using TACE therapy to induce intermittent hypoxia
may be beneficial in liver cancer treatment.
Previous reports have shown that the increased
activities of HIF-1α and NF-κB are mainly mediated by ROS in cells
under hypoxia, and the transcription of HIF-1α is enhanced
following NF-κB activation (18,21). In
the present study, it was shown that the expression of VEGF was
significantly increased in liver cancer cells under acute hypoxia,
which was mediated via the activation of ROS/NF-κB/HIF-1α
signaling, whereas intermittent hypoxia decreased ROS/NF-κB/HIF-1α
activity and downregulated the expression of VEGF. This suggests
that the expression of VEGF in liver cancer cells under hypoxia is
modulated by ROS/NF-κB/HIF-1α signaling, although this does not
exclude the possibility that ROS increase the activity of HIF-1α
through the stability of HIF-1α via inactivation of proline
hydroxylase, as previously described (28).
Taken together, the present study showed that in
vitro and in vivo angiogenesis was attenuated when
endothelial cells were cultured in CM from intermittent
hypoxia-exposed liver cancer cells or co-inoculated with
intermittent hypoxia-exposed liver cancer cells in mice,
respectively. This was correlated with reduced levels of VEGF in
intermittent hypoxia-exposed liver cancer cells.
The present study has a number of limitations.
First, although it was found that VEGF was markedly upregulated in
liver cancer cells exposed to acute hypoxia, this does not exclude
the possibility that other pro-angiogenic factors, including PDGF
and b-FGF, secreted from acute hypoxia-exposed liver cancer cells
promote neo-angiogenesis. Second, the effects of hypoxia on the
biological characteristics of liver cancer cells themselves were
not examined. It has been reported that intermittent hypoxia
promotes more malignant biological behaviors of breast cancer cells
(29,30). Therefore, it is possible that liver
cancer cells present more malignant behaviors under intermittent
hypoxia. Third, an ideal TACE animal model is required to validate
the effect of the proposed novel embolism pattern, inducing
intermittent hypoxia, on increasing the efficacy of liver cancer
treatment.
In conclusion, as presented in Fig. 4H, the present study demonstrated that
intermittent hypoxia alleviates the increase in VEGF levels and
reduces the consequent pro-angiogenic potential of liver cancer
cells induced by acute hypoxia, suggesting a novel treatment
strategy.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural
Science Foundation of China (grant no. 81272723).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RXC, JFC and ZGR designed the study and analyzed the
data. GD, XHL, HHL and DMG performed the experiments. GD, RXC, JFC
and ZGR wrote and revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The protocols for animal experiments were reviewed
and approved by the Ethical Committee on Animal Experiments of
Animal Care Committee of Fudan University (Fudan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chua CW and Choo SP: Targeted therapy in
hepatocellular carcinoma. Int J Hepatol. 2011:1–11. 2011.
View Article : Google Scholar
|
|
2
|
Zhang ZM, Guo JX, Zhang ZC, Jiang N, Zhang
ZY and Pan LJ: Therapeutic options for intermediate-advanced
hepatocellular carcinoma. World J Gastroenterol. 17:1685–1689.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang YH, Wu JC, Chen SC, Chen CH, Chiang
JH, Huo TI, Lee PC, Chang FY and Lee SD: Survival benefit of
transcatheter arterial chemoembolization in patients with
hepatocellular carcinoma larger than 10 cm in diameter. Aliment
Pharmacol Ther. 23:129–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guan YS, He Q and Wang MQ: Transcatheter
arterial chemoembolization: History for more than 30 years. ISRN
Gastroenterol. 2012:4806502012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pleguezuelo M, Marelli L, Misseri M,
Germani G, Calvaruso V, Xiruochakis E, Manousou P and Burroughs AK:
TACE versus TAE as therapy for hepatocellular carcinoma. Expert Rev
Anticancer Ther. 8:1623–1641. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y and Lai Y: A combination of
anti-angiogenesis with endostatin and transcatheter arterial
chemoembolization (TACE) enhances antitumour effects in a rabbit
VX2 liver tumor. Radiol Soc North Am 2010 Sci Assembly Meeting.
2010.
|
|
7
|
Hanks BA, Suhocki PV, DeLong DM, Doan PL,
Liu E, Tsai AL, Burke CT, Bernard SA, O'Neil BH and Morse MA: The
efficacy and tolerability of transarterial chemo-embolization
(TACE) compared with transarterial embolization (TAE) for patients
with unresectable hepatocellular carcinoma (HCC). J Clin Oncol.
26:45952008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sergio A, Cristofori C, Cardin R, Pivetta
G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A and
Farinati F: Transcatheter arterial chemoembolization (TACE) in
hepatocellular carcinoma (HCC): The role of angiogenesis and
invasiveness. Am J Gastroenterol. 103:914–921. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chao Y, Wu CY, Kuo CY, Wang JP, Luo JC,
Kao CH, Lee RC, Lee WP and Li CP: Cytokines are associated with
postembolization fever and survival in hepatocellular carcinoma
patients receiving transcatheter arterial chemoembolization.
Hepatol Int. 7:883–892. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jia ZZ, Jiang GM and Feng YL: Serum
HIF-1alpha and VEGF levels pre- and post-TACE in patients with
primary liver cancer. Chin Med Sci J. 26:158–162. 2010. View Article : Google Scholar
|
|
11
|
Liu K, Min XL, Peng J, Yang K, Yang L and
Zhang XM: The changes of HIF-1α and VEGF expression after TACE in
patients with hepatocellular carcinoma. J Clin Med Res. 8:297–302.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jia ZZ, Jiang GM and Feng YL: Serum
HIF-1alpha and VEGF levels pre- and post-TACE in patients with
primary liver cancer. Chin Med Sci J. 26:158–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Britten CD, Gomes AS, Wainberg ZA,
Elashoff D, Amado R, Xin Y, Busuttil RW, Slamon DJ and Finn RS:
Transarterial chemoembolization plus or minus intravenous
bevacizumab in the treatment of hepatocellular cancer: A pilot
study. BMC Cancer. 12:162012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pinter M, Ulbrich G, Sieghart W,
Kölblinger C, Reiberger T, Li S, Ferlitsch A, Müller C, Lammer J
and Peck-Radosavljevic M: Hepatocellular carcinoma: A phase II
randomized controlled double-blind trial of transarterial
chemoembolization in combination with biweekly intravenous
administration of bevacizumab or a placebo. Radiology. 277:903–912.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin B, Wang D, Lewandowski RJ, Riaz A, Ryu
RK, Sato KT, Larson AC, Salem R and Omary RA: Chemoembolization
endpoints: Effect on survival among patients with hepatocellular
carcinoma. AJR Am J Roentgenol. 196:919–928. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu D, Liao Y, Zhu SH, Chen QC, Xie DM,
Liao JJ, Feng X, Jiang MH and He W: Bone-derived Nestin-positive
mesenchymal stem cells improve cardiac function via recruiting
cardiac endothelial cells after myocardial infarction. Stem Cell
Res Ther. 10:1272019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu M, Ning X, Li R, Yang Z, Yang X, Sun S
and Qian Q: Signalling pathways involved in hypoxia-induced renal
fibrosis. J Cell Mol Med. 21:1248–1259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rius J, Guma M, Schachtrup C, Akassoglou
K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG and Karin M:
NF-kappaB links innate immunity to the hypoxic response through
transcriptional regulation of HIF-1alpha. Nature. 453:807–811.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang Q, Zhan L, Cao H, Li J, Lyu Y, Guo
X, Zhang J, Ji L, Ren T, An J, et al: Increased mitochondrial
fission promotes autophagy and hepatocellular carcinoma cell
survival through the ROS-modulated coordinated regulation of the
NFKB and TP53 pathways. Autophagy. 12:999–1014. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bonello S, Zähringer C, BelAiba RS,
Djordjevic T, Hess J, Michiels C, Kietzmann T and Görlach A:
Reactive oxygen species activate the HIF-1alpha promoter via a
functional NFkappaB site. Arterioscler Thromb Vasc Biol.
27:755–761. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Diebold I, Djordjevic T, Hess J and
Görlach A: Rac-1 promotes pulmonary artery smooth muscle cell
proliferation by upregulation of plasminogen activator inhibitor-1:
Role of NFkappaB-dependent hypoxia-inducible factor-1alpha
transcription. Thromb Haemost. 100:1021–1028. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
El-Halawany MS, Ismail HM, Zeeneldin AA,
Elfiky A, Tantawy M, Kobaisi MH, Hamed I and Abdel Wahab AH:
Investigating the pretreatment miRNA expression patterns of
advanced hepatocellular carcinoma patients in association with
response to TACE treatment. Biomed Res Int. 2015:6497502015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Petrillo M, Patella F, Pesapane F, Suter
MB, Ierardi AM, Angileri SA, Floridi C, de Filippo M and
Carrafiello G: Hypoxia and tumor angiogenesis in the era of
hepatocellular carcinoma transarterial loco-regional treatments.
Future Oncol. 14:2957–2967. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ahluwalia A, Jones MK, Matysiak-Budnik T
and Tarnawski AS: VEGF and colon cancer growth beyond angiogenesis:
Does VEGF directly mediate colon cancer growth via a non-angiogenic
mechanism? Curr Pharm Des. 20:1041–1044. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Petersen W, Pufe T, Stärke C, Fuchs T,
Kopf S, Neumann W, Zantop T, Paletta J, Raschke M and Becker R: The
effect of locally applied vascular endothelial growth factor on
meniscus healing: Gross and histological findings. Arch Orthop
Trauma Surg. 127:235–240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Olszewska-Pazdrak B, Hein TW, Olszewska P
and Carney DH: Chronic hypoxia attenuates VEGF signaling and
angiogenic responses by downregulation of KDR in human endothelial
cells. Am J Physiol Cell Physiol. 296:C1162–C1170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee G, Won HS, Lee YM, Choi JW, Oh TI,
Jang JH, Choi DK, Lim BO, Kim YJ, Park JW, et al: Oxidative
dimerization of PHD2 is responsible for its inactivation and
contributes to metabolic reprogramming via HIF-1α activation. Sci
Rep. 6:189282016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen A, Sceneay J, Gödde N, Kinwel T, Ham
S, Thompson EW, Humbert PO and Möller A: Intermittent hypoxia
induces a metastatic phenotype in breast cancer. Oncogene.
37:4214–4225. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Louie E, Nik S, Chen JS, Schmidt M, Song
B, Pacson C, Chen XF, Park S, Ju J and Chen EI: Identification of a
stem-like cell population by exposing metastatic breast cancer cell
lines to repetitive cycles of hypoxia and reoxygenation. Breast
Cancer Res. 12:R942010. View
Article : Google Scholar : PubMed/NCBI
|