Introduction
Papillary thyroid carcinoma (PTC) is the most common
type of thyroid tumor and despite developments in imaging
techniques, the incidence rate of PTC has increased over the last
decade (1). Locoregional lymph node
(LN) metastasis is typically one of the first steps in the
progression of PTC to distant metastasis from the thyroid (2,3).
According to a previous report from the National Comprehensive
Cancer Network, the LN metastasis rate in PTC is 50–80% (4).
The most effective therapeutic strategies for
well-differentiated thyroid carcinoma are thyroid surgery and
iodine-131 (131I) radiotherapy (5). 131I radiotherapy is not only
appropriate for primary tumors but also for LN and distant
metastasis (6). Kim et al
(7) reported that for lesions with
high serum thyroglobulin (Tg), a negative cervical sonography and
an 18F-fluorodeoxyglucose (FDG) positron emission
tomography (PET) scan exhibit limited therapeutic effect with
131I therapy. Positron emission tomography and computed
tomography (PET/CT) has been applied for the evaluation and
monitoring of PTC and nodal metastasis, particularly for patients
with elevated serum Tg levels but produced negative findings with
an iodine-131 (131I) whole-body scan (WBS) (8). Previously, small LNs have been detected
with PET/CT, which are easily misdiagnosed by 131I-WBS
due to a low resolution and the presence of remnant thyroid tissue
(9). To the best of our knowledge,
the association between metastatic LNs (mLNs) and the effect of
131I therapy has not yet been reported. The aim of the
present retrospective study was to evaluate the clinical efficiency
of 18F-FDG PET/CT in predicting the therapeutic response
of mLNs in patients with PTC following 131I therapy.
Patients and methods
Patients
The present retrospective study was approved by
Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiaotong
University School of Medicine and the requirement for informed
consent was waived. Between January 2012 and August 2017, 106
patients at the Nuclear Medicine Department of Xinhua Hospital
Affiliated with The Shanghai Jiaotong University School of Medicine
(Shanghai, China) were assessed based on the following criteria: i)
All patients underwent a near-total or a total thyroidectomy; ii)
patients received a PET/CT examination prior to their first and
following their second or later 131I radioiodine
ablation treatment; iii) patients with LN metastasis were
identified by fine-needle aspiration biopsy (FNAB), postoperative
pathology and other clinical methods, including neck
ultrasonography, CT, 131I-WBS, and serum Tg and
thyroglobulin-antibody levels. Patients were excluded if they had a
history or coexistence of other metastasis, including lung or bone
metastasis. In addition, patients were excluded if the LNs were not
confirmed by pathology, biopsy or other clinical findings. In
total, 74 patients were excluded, including 16 patients with bone
metastasis, 35 patients with lung metastasis and 23 patients who
did not possess appropriate evidence of LN metastasis. Therefore,
18 female and 14 male patients were enrolled in the present
study.
mLNs
The mLNs involved in the present study were included
according to the following criteria: i) The mLN was diagnosed by
postoperative pathology or FNAB; ii) the LN had a positive
131I accumulation on the 131I WBS; iii)
ultrasonographic features of malignant thyroid nodules included
hypoechoic lesions, a longitudinal/transverse ratio <2, a
blurred or spicular margin, microlobular contour, single or
multiple microcalcifications, both internal and peripheral flow,
and extrathyroidal extension or an interrupted and discontinuous
echogenicity of a capsule (10); iv)
a mLN on CT manifestations exhibited an unenhanced center and rim
enhancement, with or without fine sand calcification and mottle
calcification, or a LN with a spherical shape and minimal axial
diameter (MAD) >10 mm (11) and
an increase in size on follow-up CT; and v) a LN of spherical
shape, peak standardized uptake value (SULpeak) >2.5 with a
history of stimulated Tg level >10 ng/ml during thyroid hormone
withdrawal. A total of 50 nodes were identified as mLNs.
18F-FDG PET/CT
PET/CT imaging was performed using a Biograph 64
PET/CT scanner (Siemens AG, Munich, Germany). Prior to 18F-FDG
PET/CT, all patients were instructed not to eat for ≥6 h to
maintain their serum glucose levels at <11.1 mmol/l prior to
injection. Image acquisition started ~1 h (mean, 56±2 min; range,
50–60 min) following intravenous injection of FDG (3.7
megabecquerel/kg). The lowest possible milliampere setting on the
scanner was used to acquire the CT scans for attenuation
correction. The whole-body CT imaging was performed from the skull
to the upper part of the thigh with 120 kV, 0.5 sec rotation, 3 mm
slice thickness and 0.8 mm intervals. The PET scans were obtained
immediately following the whole-body CT scan, including 5–7 bed
positions (1.5 min acquisition time per bed position) over the same
range as the CT scan. Prior to PET/CT imaging, the serum
thyrotropin (TSH) levels of patients were maintained at 30
mU/l.
131I therapy and
post-therapy 131I whole-body scintigraphic imaging
Following radioiodine ablation of the residual
normal thyroid tissue, 19 patients received a second dose of
therapy and five also received an additional third dose of therapy.
The TSH level was measured prior to 131I administration
and a minimum TSH of 30 mU/l was required. 131I-NaI was
orally administered, with activities ranging between 3.7 and 7.4
gigabecquerel (100–200 mCi). The 131I WBS was conducted
3–4 days following 131I administration, using a
dual-head large-field γ camera with high energy collimators. An
additional single-photon emission CT/CT (SPECT/CT) scan was
performed with extensive cervical iodine accumulation on the WBS
scan in four patients (4/32).
Imaging analysis
The acquired images were analyzed with Siemens True
D (Syngo TrueD VE13A). The tracer uptake was expressed as a
standardized uptake value, which was calculated according to the
following formula: Measured activity concentration (Bq/ml) × body
weight (g)/injected activity (Bq). To achieve a larger region of
interest, the SULpeak that corrected for lean body mass was
selected instead of the widely used single-pixel maximum
standardized uptake value. The maximum standardized uptake has also
been demonstrated to be subjected to upward bias in low-count
studies compared with the SULpeak (12). All 18F-FDG PET/CT images and
131I-WBS images were visually interpreted by two
experienced nuclear physicians, and a final consensus was reached
for all patients.
Statistical analysis
All quantitative data are expressed as the mean ±
standard deviation. A χ2 test and t-test were used to
compare the SULpeak, minimum diameter, maximum diameter, LN zone,
shape and density, punctate calcification, and the longitudinal and
transverse ratios of the mLNs in the effective treatment group and
ineffective treatment group. To assess the predictive role of
SULpeak for the therapeutic effect, receiver operating
characteristic (ROC) analysis was performed in both the effective
treatment group and ineffective treatment group. The cut-off value
was selected as 5.85, above which the best compromise between
sensitivity and specificity could be achieved. Multivariate
analysis of categorical variables was conducted using the survival
analysis model. All statistical analysis was performed using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Patients
Among the 32 enrolled patients, the mean ± standard
deviation age was 40.6±10.6 years (range, 24–58 years). According
to the 7th Edition of The TNM staging system from The American
Joint Committee on Cancer (13), 18
patients had stage I disease, 6 patients had stage II disease, 3
patients had stage III disease and 5 patients had stage IV disease.
During surgery, 13 patients were identified to have extrathyroidal
extension and 18 patients had residual thyroid tissue on the
initial 131I WBS. In the present study, clinical serum
outcomes of Tg were obtained under a TSH stimulated state <1
week prior to or following the PET/CT examination. Follow-up was
performed ≥9 months following examination, with a mean follow-up
time of 17.87±6.80 months (range, 9–36 months).
mLNs
A total of 50 lesions were diagnosed as mLNs in 32
patients, with 1–4 lesions identified per patient. A total of ten
lesions were diagnosed by histopathological findings; eight were
diagnosed by positive 131I accumulation on the
131I-WBS; ten were diagnosed by neck ultrasonography and
18F-FDG PET with six lesions exhibiting a blurred
margin, multiple microcalcifications, internal flow and peripheral
flow, and four lesions exhibiting single microcalcification and
extrathyroidal extension; and twelve lesions were diagnosed by CT
and 18F-FDG PET with seven lesions exhibiting a MAD
>10 mm, and four lesions demonstrating an increase in size
following ≥9 months. The characteristics of the mLNs are presented
in Table I.
 | Table I.Characteristics of metastatic lymph
nodes. |
Table I.
Characteristics of metastatic lymph
nodes.
|
Characteristics | Mean (standard
deviation), range | n (50) |
|---|
| Minimum axial
diameter, mm | 7.29 (2.42),
3.00–14.00 |
|
| Maximum axial
diameter, mm | 9.77 (2.66),
4.70–16.00 |
|
| Peak standardized
uptake value | 6.80 (3.06),
2.60–16.60 |
|
| Lymph node zone
(41) |
| I |
| 0 |
| II |
| 14 |
|
III |
| 9 |
| IV |
| 9 |
| V |
| 2 |
| VI |
| 15 |
|
VII |
| 1 |
| Spherical shape
with solid density |
| 34 |
| Spherical shape
with soft density |
| 16 |
| Longitudinal and
transverse ratio (<2) |
|
Yes |
| 38 |
| No |
| 12 |
| Punctate
calcification |
|
Yes |
| 19 |
| No |
| 31 |
The metabolic response was determined separately for
changes in the SULpeak based on the PET Response Criteria in Solid
Tumors 1.0 (14). A complete
response (CR) was defined as a complete resolution of abnormal
18F-FDG uptake within measurable target lesions, such
that the uptake was equal to or less than the mean healthy-liver
activity on the follow-up examination. A partial response (PR) was
defined as a decrease of >30% in any baseline PET parameter.
Progressive disease (PD) was defined as an increase in any baseline
PET parameter by >30% between the baseline and follow-up, or by
the appearance of a new 18F-FDG-avid lesion. Stable
disease (SD) was defined as non-PD, non-PR and non-CR. All mLNs
were divided into the following two groups: An effective treatment
group (group A) and an ineffective treatment group (group B). The
lesions in group A achieved either CR or PR, while the lesions in
the group B achieved either SD or PD.
The standard uptake value of lean body mass (SUL) of
the 29 mLNs in group B (mean, 7.85±3.20; range, 3.00–16.60) was
significantly higher compared with that of the 21 mLNs in group A
(mean, 5.36±2.19; range, 2.60–12.80; P=0.027). A cut-off value of
5.85 was used to distinguish the ineffective treatment lesions from
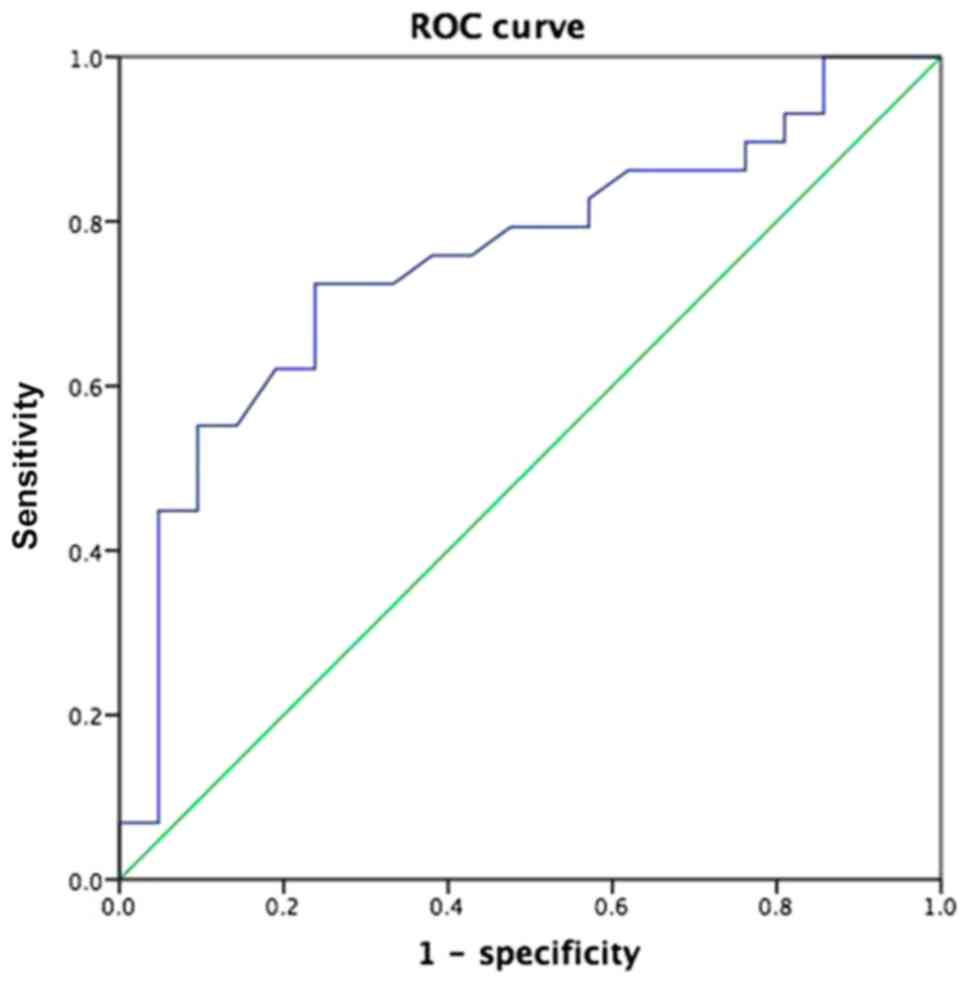
the mLNs receiving radioactive ablation based on the ROC curve
analysis, with an area under the ROC curve (AUC) of 0.755. The
accuracy of predicting the therapeutic effect of group A and group
B was 76.19 and 72.4%, respectively (Fig. 1). Furthermore, a significant
difference was identified in the shape and density between the two
groups (P=0.044; Table II).
However, the minimum diameter, maximum diameter, LN zone, punctate
calcification, and longitudinal and transverse ratios (<2)
demonstrated no significant difference between the two groups
(P>0.05; Table II).
 | Table II.Clinical characteristics of
metastatic lymph nodes in group A and group B prior to therapy. |
Table II.
Clinical characteristics of
metastatic lymph nodes in group A and group B prior to therapy.
|
Characteristics | Group A | Group B | P-value |
|---|
| Number | 21 | 29 | 0.422 |
| Minimum diameter,
mm |
|
| 0.338 |
| Mean
(SD) | 7.02 (2.16) | 7.48 (2.61) |
|
|
Range | 4.00–12.00 | 3.00–14.00 |
|
| Maximum diameter,
mm |
|
| 0.457 |
| Mean
(SD) | 9.67 (2.40) | 9.84 (2.86) |
|
|
Range | 4.70–14.00 | 5.00–16.00 |
|
| Peak standardized
uptake value |
|
| 0.027 |
| Mean
(SD) | 5.36 (2.19) | 7.85 (3.20) |
|
|
Range | 2.60–12.80 | 3.00–16.6 |
|
| Lymph node zone, n
(41) |
|
| 0.416 |
| VI | 5 | 10 |
|
|
Non-VI | 16 | 19 |
|
| Shape and density,
n |
|
| 0.044 |
|
Spherical shape with solid
density | 10 | 19 |
|
|
Spherical shape with soft
density | 8 | 5 |
|
| Punctate
calcification, n |
|
| 0.242 |
|
Yes | 6 | 13 |
|
| No | 15 | 16 |
|
| Longitudinal and
transverse ratios (<2), n |
|
| 0.314 |
|
Yes | 14 | 23 |
|
| No | 7 | 6 |
|
The effective treatment of mLNs following
131I radiotherapy was independently associated with
SULpeak and extrathyroidal extension. The sex, stage, size and
location of LN, Tg level, spherical shape with solid density, LN
zone, punctate calcification, longitudinal and transverse ratios
(<2), and residual thyriod tissue prior to therapy were not
identified as risk factors for 131I therapy (Table III).
 | Table III.Multivariate analysis of prognostic
factors for patients with papillary thyroid cancer. |
Table III.
Multivariate analysis of prognostic
factors for patients with papillary thyroid cancer.
|
Characteristics | Odds ratio (95%
confidence interval) | P-value |
|---|
| Standardized uptake
value | 1.194
(1.061–1.345) | 0.003 |
| Extrathyroidal
extension | 0.436
(0.206–0.923) | 0.030 |
| Age | – | 0.268 |
| Thyroglobulin | – | 0.635 |
| Sex | – | 0.785 |
| Spherical shape
with solid density | – | 0.874 |
| Minimal axial
diameter | – | 0.300 |
| Maximum axial
diameter | – | 0.510 |
| In the central
area | – | 0.105 |
| Punctate
calcification | – | 0.609 |
| Longitudinal and
transverse ratios (<2) | – | 0.677 |
| Residual thyroid
tissue | – | 0.274 |
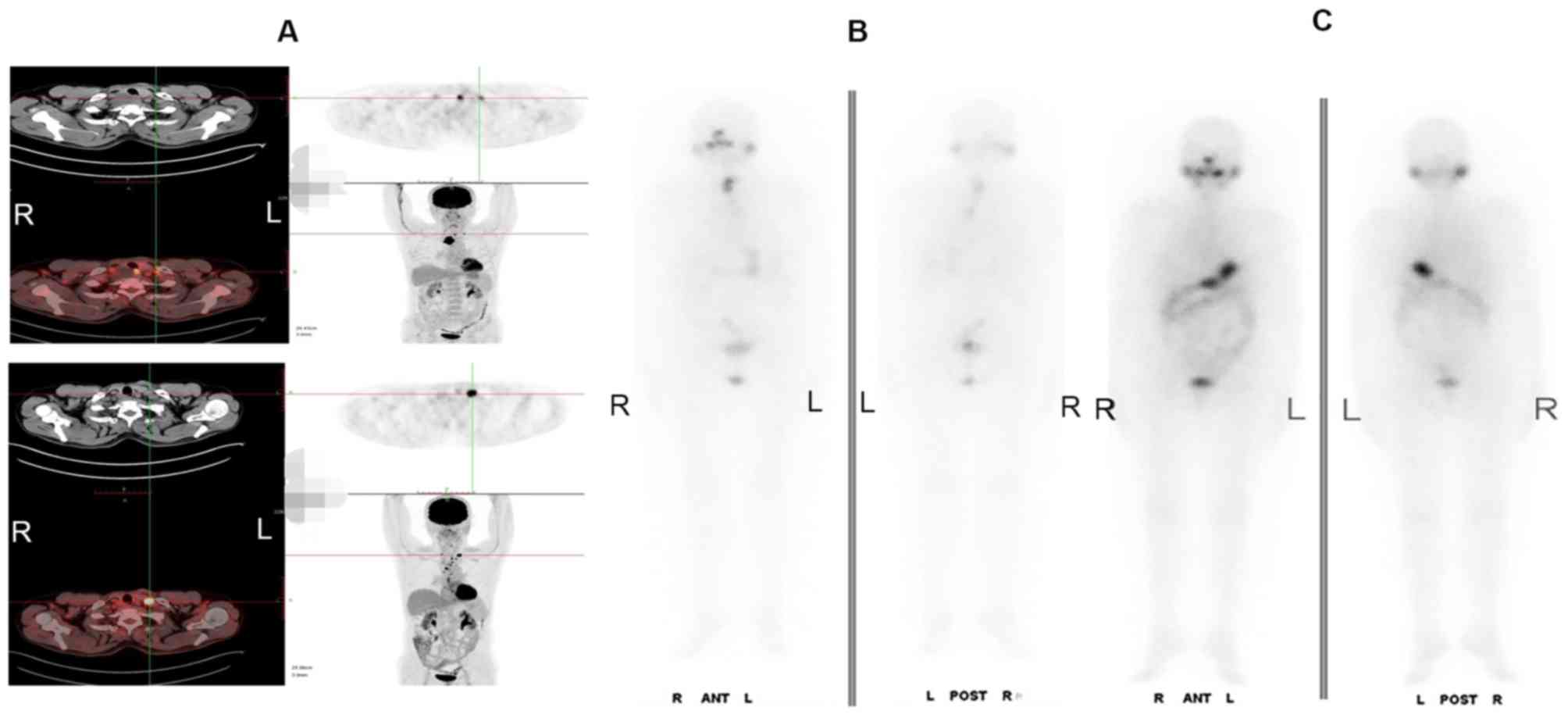
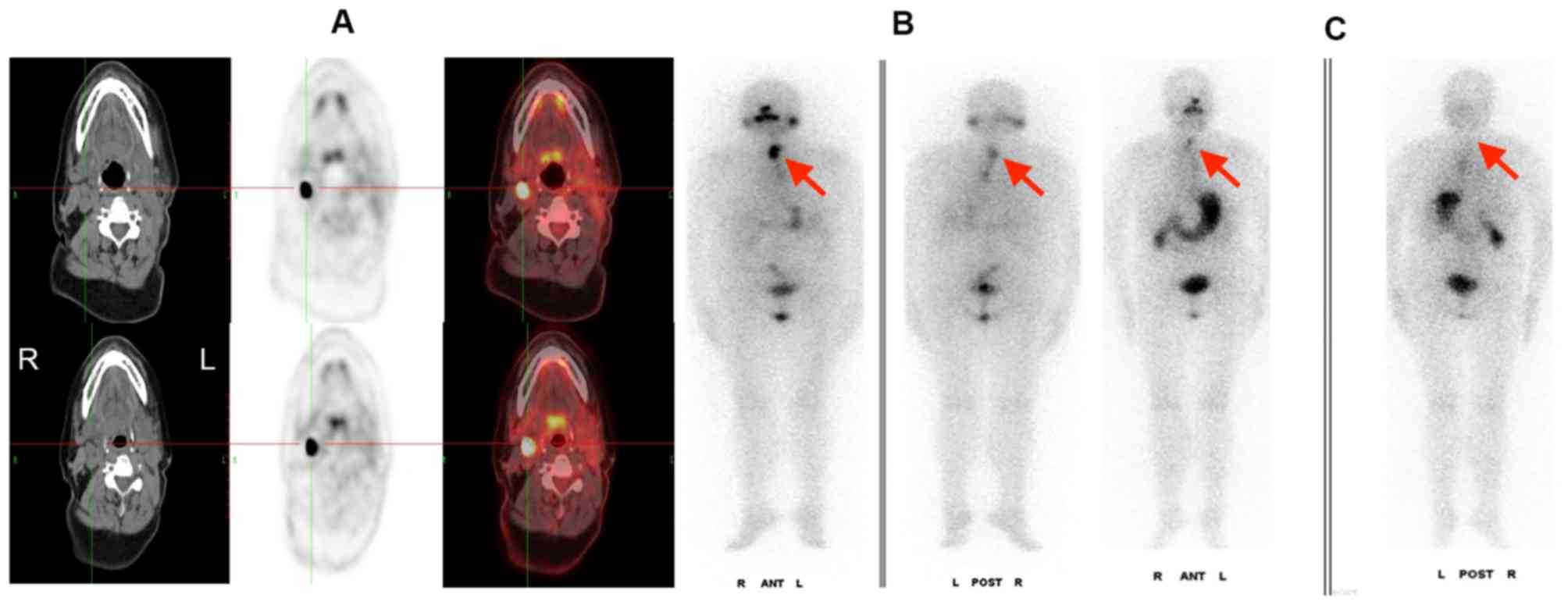
In this study, seven mLNs were found positive on
both 131I WBS and PET/CT image. During follow-up, it was
identified that three LNs progressed into PD following
radiotherapy, while the uptake of 131I WBS decreased
(Fig. 3) and four nodes in the SD
group demonstrated no significant changes in 18F-FDG PET/CT and a
decreased uptake on the 131I-WBS (Fig. 4).
Discussion
LN metastasis is common in patients with PTC
(15). A previous study based on a
Surveillance, Epidemiology, and End Results database with 14 years
of follow-up demonstrated that an mLN can serve as a specific
independent predictor for PTC, and the total survival rate of
patients with mLNs was lower compared with patients without mLNs
(hazard ratio, 12.597) (16).
18F-FDG PET/CT is useful for detecting the sites of
metastatic disease and recurrence, which appear on radio-iodine
images (17). In the present study,
all 18F-FDG PET/CT acquisitions were performed under a
TSH stimulated state and FDG uptake was positive in all patients
with PTC with mLNs, while 131I accumulation was
identified in eight lesions from 6 patients on the first WBS. In
addition, 42 mLNs demonstrated a minimum diameter ≤1 cm and 15
patients with a total of 27 LNs exhibited residual thyroid tissue
and exhibited star emission in the thyroid bed area on
131I WBS, which may explain why the majority of the LNs
did not demonstrate positive 131I on the initial WBS.
Therefore, the present study attempted to use PET/CT imaging to
predict the therapeutic effect of mLNs receiving 131I
radiotherapy.
At present, the most widely used imaging
technologies for diagnosis of thyroid cacinoma with mLNs include
ultrasonography, magnetic resonance imaging (MRI), CT, SPECT and
PET/CT (18–22). Pathology is the gold standard for
diagnosing LN metastasis of thyroid carcinoma (23). However, certain mLNs are located deep
in the neck, which presents difficulties for diagnosis. Imaging
examination serves an important role in the diagnosis and
therapeutic evaluation of thyroid cancer LN metastasis. Mulla and
Schulte (24) reported that
ultrasonography in the diagnosis of cervical LN metastases exhibits
a sensitivity of 63% and specificity of 93%. However,
ultrasonography possesses limitations for the diagnosis of central
compartment lymph nodal metastases, as certain LNs are located in
deep regions, the trachea and surrounding structures (25). 131I SPECT/CT has been used
to evaluate patients with differentiated thyroid cancer (DTC);
however, residual thyroid tissue following thyroidectomy affects
the uptake of 131I and imaging of mLNs (26). Compared with CT, which provides
low-resolution images for soft tissue, MRI exhibits distinct
advantages for the diagnosis of metastatic thyroid carcinoma,
including high-resolution determination of soft tissue, no
radiation exposure and a multi-sequence, multi-parameter imaging
capability (27). For detection of
cervical nodal metastasis in patients with DTC, MRI demonstrates a
high sensitivity, accuracy, specificity and positive predictive
value (19); however, MRI exhibits a
low specificity and negative predictive value for the detection of
lateral neck lymph nodal metastasis (28). PET/CT is useful in localizing
metastasis that does not accumulate iodine in patients with
elevated Tg levels (29) and has
been demonstrated to have a specificity ≤90% (30). In the present study, mLNs were
diagnosed by pathology, ultrasonography, CT, SPECT, PET/CT and
clinical follow up. However, MRI was not analyzed in this study due
to the limited number of patients examined.
The present study identified that the SULpeak of
group A was significantly lower compared with that of group B prior
to therapy. FDG is highly concentrated in cells with high
glycolytic rates, including poorly differentiated, proliferating
thyroid cancer cells (31). The
combination of a low iodine concentration and a high FDG
concentration has been considered to be an indicator of poorly
differentiated or dedifferentiated thyroid cancer cells (32), which typically indicates a poor
prognosis. Therefore, a low uptake of 18F-FDG in LNs
indicates low tumor activity and a good prognosis for patients with
PTC with mLNs. Sun et al (33) demonstrated that tumor/normal tissue
(T/NT) of cervical LN metastases in 18F-FDG dual-head
coincidence imaging is associated with the efficacy of radioiodine
therapy; a high T/NT indicates a poor therapeutic effect. Based on
the present results, the cut-off value of mLNs, involved in SUL
with or without 131I accumulation, was >5.85 on the
PET/CT image, which demonstrated improved sensitivity and
specificity for predicting a poor prognosis following
131I ablation.
The present results also demonstrated that a high
SULpeak of mLNs and extrathyroidal extension following thyroid
surgery were significant poor prognostic factors for patients
receiving therapy. A number of previous studies have demonstrated
that the 131I WBS is negative for dedifferentiated PTC
lesions that lack the ability to take up iodine and these
dedifferentiated PTC lesions are 18F-FDG-avid (32,34,35). The
present study identified that certain mLNs demonstrated positive
results in both imaging examinations; however, the therapeutic
effect of these mLNs has rarely been reported. In the present
study, among the eight positive mLNs revealed by
131I-WBS, two maintained SD, one achieved CR and the
other five progressed to PD. A possible reason for this poor
prognosis may be that the LN metastases had a lack of oxygen or
were poorly differentiated, which reduced the effectiveness of
131I and led to a poorer curative effect. Of the 42
negative 131I WBS, ten LNs achieved CR, 11 reached a PR,
16 remained in SD and five developed into PD. Only eight LNs
possessed a minimum diameter ≥10 mm and 27 LNs displayed a star
emission image in the thyroid bed area on the 131I WBS,
which may have led to the majority of the LNs not being
131I-positive on the initial WBS. During subsequent
therapy, 131I accumulation was observed in two mLNs,
which were negative on the first 131I WBS and had
SULpeaks, of 5.5 and 5.3, respectively on the PET/CT prior to
therapy. Finally, both LNs achieved CR (Fig. 2).
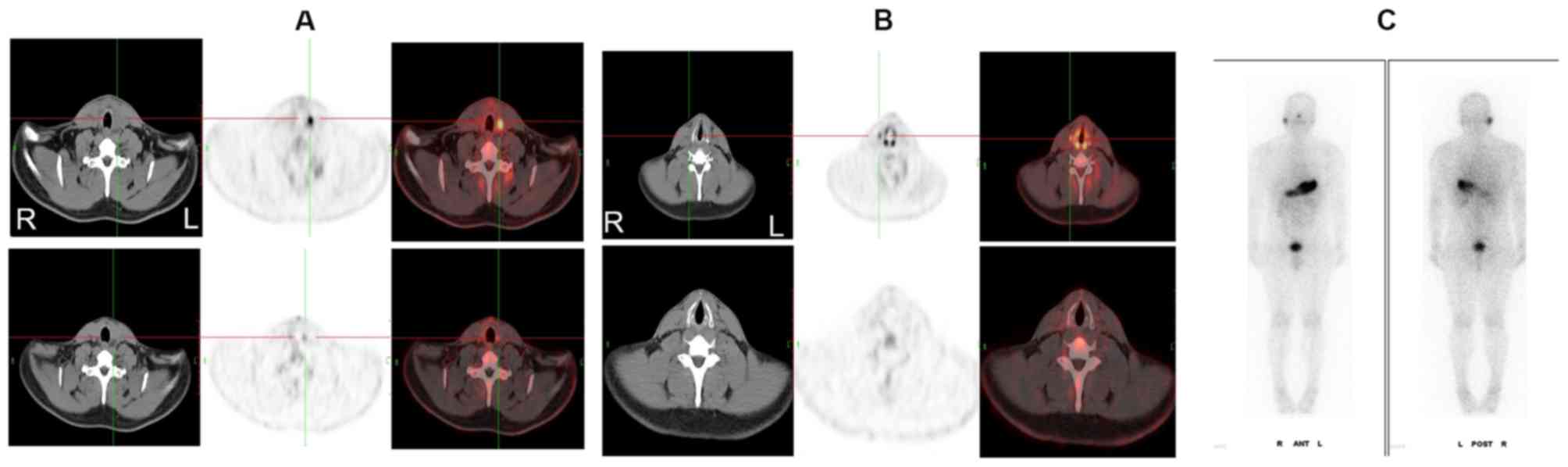 | Figure 2.A 24-year-old male patient with
papillary thyroid carcinoma with mLNs. The LNs detected by CT
exhibited a spherical shape and a homogeneous density, which
revealed hypermetabolic LNs in the (A) left side of the neck and in
the (B) right side of the neck. The standardized uptake values of
the LNs on the PET/CT images before (upper images) and after (lower
images) 131I therapy were 5.7, 3.5, and 3.0, 1.1,
respectively. (C) 131I whole-body scan image revealed no
131I accumulation in the two mLNs. The lesions achieved
a partial response (LN in the left neck) and complete response (LN
in the right neck), respectively. mLN, metastatic lymph node; LN,
lymph node; PET, positron emission tomography; CT, computed
tomography; 131I, iodine-131. |
A number of previous studies have confirmed that
macroscopic LN metastasis is a predictor that is specifically
associated with PTC persistence or recurrence (36–40). Wu
et al (41) investigated
prognostic factors of 131I treatment for cervical LN
metastases of PTC and suggested that the therapeutic effectiveness
was associated with the size of the mLNs. However, the present
results revealed that patients with a high SULpeak and a history of
extrathyroidal extension exhibited a poorer therapeutic effect
following 131I ablation. mLNs with a SULpeak >5.85
could be considered as poor prognostic factors for therapy with or
without a positive 131I result. Furthermore, seven mLNs
from 4 patients with PTC were both positive on 131I WBS
and PET/CT, which demonstrated no remission following
131I therapy. These lesions may be associated with more
aggressive characteristics and 131I therapy appears to
have little to no effect on the viability of such lesions (42).
The present study had a number of limitations.
Firstly, the number of subjects was small as only 50 mLNs were
involved from 32 patients with PTC, which may lead to missing
131I on SPECT imaging due to a low resolution. In
addition, the pathological characteristics of the primary tumor and
LNs were not completely analyzed due to the limited number of
samples. Furthermore, the present study was retrospective in nature
and excluded certain imaging methods that could additionally be
performed, including ultrasound, CT and MRI. Therefore, future
studies should aim to compare the predictive value of various
imaging examinations for the prognosis of thyroid carcinoma with LN
metastases.
In conclusion, a high SULpeak and spherical shape
with a solid density of mLNs were identified as risk predictors for
the clinical outcome of patients with PTC treated with radioiodine
therapy, regardless of whether 131I dose has accumulated
on those nodes. When the SULpeak was >5.85, a poor therapeutic
effect was revealed for mLNs. Therefore, patients with a high
SULpeak of LNs and extrathyroidal extension may be considered to
exhibit a poor prognosis. By introducing associated factors of mLNs
on the predictive value of PET/CET, these may exhibit a useful role
in the management of PTC. Further studies should focus on the
detection of LNs with two positive images to identify the
association between mLNs and the clinical outcome following
131I therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL contributed to the study design and wrote the
manuscript. JZ collected and analyzed the clinical data of
patients. HW was responsible for the statistical analysis of the
data and revising for important intellectual content. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The approvals of all patients were waived due to the
retrospective nature of this study. The research protocol was
approved by Ethics Committee of Xinhua Hospital Affiliated to
Shanghai Jiaotong University School of Medicine (Shaghai,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
FDG
|
fluorodeoxyglucose
|
|
mLN
|
metastatic LN
|
|
PTC
|
papillary thyroid carcinoma
|
|
WBS
|
whole-body scan
|
|
SULpeak
|
peak standardized uptake value
|
|
PET/CT
|
positron emission tomography/computed
tomography
|
|
Tg
|
thyroglobulin
|
|
FNAB
|
fine-needle aspiration biopsy
|
|
LN
|
lymph node
|
|
MAD
|
minimal axial diameter
|
|
TSH
|
serum thyrotropin
|
|
SD
|
stable disease
|
|
ROC
|
receiver operating characteristic
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
PD
|
progressive disease
|
|
AUC
|
area under the curve
|
|
131I
|
iodine-131
|
References
|
1
|
Kitahara CM and Sosa JA: The changing
incidence of thyroid cancer. Nat Rev Endocrinol. 12:646–653. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schneider DF, Chen H and Sippel RS: Impact
of lymph node ratio on survival in papillary thyroid cancer. Ann
Surg Oncol. 20:1906–1911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee YM, Lo CY, Lam KY, Wan KY and Tam PK:
Well-differentiated thyroid carcinoma in Hong Kong Chinese patients
under 21 years of age: A 35-year experience. J Am Coll Surg.
194:711–716. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mazzaferri EL and Massoll N: Management of
papillary and follicular (differentiated) thyroid cancer: New
paradigms using recombinant human thyrotropin. Endocr Relat Cancer.
9:227–247. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giannoula E, Iakovou I and Verburg FA:
Long term quality of life in differentiated thyroid cancer patients
after thyroidectomy and high doses of 131I with or
without suppressive treatment. Hell J Nucl Med. 21:69–73.
2018.PubMed/NCBI
|
|
6
|
Ronga G, Toteda M, D'Apollo R, De
Cristofaro F, Filesi M, Acqualagna G, Argirò R, Ciancamerla M,
Ugolini F and Montesano T: Lymph node metastases from
differentiated thyroid carcinoma: Does radioiodine still play a
role? Clin Ter. 163:377–381. 2012.PubMed/NCBI
|
|
7
|
Kim WG, Ryu JS, Kim EY, Lee JH, Baek JH,
Yoon JH, Hong SJ, Kim ES, Kim TY, Kim WB and Shong YK: Empiric
high-dose 131-iodine therapy lacks efficacy for treated papillary
thyroid cancer patients with detectable serum thyroglobulin, but
negative cervical sonography and 18F-fluorodeoxyglucose positron
emission tomography scan. J Clin Endocrinol Metab. 95:1169–1173.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bertagna F, Albano D, Bosio G, Piccardo A,
Dib B and Giubbini R: 18F-FDG-PET/CT in patients affected by
differentiated thyroid carcinoma with positive thyroglobulin level
and negative 131I whole body scan. It's value confirmed by a
bicentric experience. Curr Radiopharm. 9:228–234. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu YH, Shen CT, Xue YL, Qiu ZL and Luo QY:
Iodine-131 SPET/CT and 18F-FDG PET/CT for the identification and
localization of mediastinal lymph node metastases from
differentiated thyroid carcinoma. Hell J Nucl Med. 16:199–203.
2013.PubMed/NCBI
|
|
10
|
Wang QC, Cheng W, Wen X, Li JB, Jing H and
Nie CL: Shorter distance between the nodule and capsule has greater
risk of cervical lymph node metastasis in papillary thyroid
carcinoma. Asian Pac J Cancer Prev. 15:855–860. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van den Brekel MW, Stel HV, Castelijns JA,
Nauta JJ, van der Waal I, Valk J, Meyer CJ and Snow GB: Cervical
lymph node metastasis: Assessment of radiologic criteria.
Radiology. 177:379–384. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lodge MA, Chaudhry MA and Wahl RL: Noise
considerations for PET quantification using maximum and peak
standardized uptake value. J Nucl Med. 53:1041–1047. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Turk A, Asa SL, Baloch ZW, Faquin WC,
Fellegara G, Ghossein RA, Giordano TJ, LiVolsi VA, Lloyd R, Mete O,
et al: Interobserver variability in the histopathologic assessment
of extrathyroidal extension of well differentiated thyroid
carcinoma supports the new american joint committee on cancer
eighth edition criteria for tumor staging. Thyroid. Apr
27–2019.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wahl RL, Jacene H, Kasamon Y and Lodge MA:
From RECIST to PERCIST: Evolving considerations for PET response
criteria in solid tumors. J Nucl Med. 50 (Suppl 1):122S–150S. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shaha AR: Prognostic factors in papillary
thyroid carcinoma and implications of large nodal metastasis.
Surgery. 135:237–239. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Podnos YD, Smith D, Wagman LD and
Ellenhorn JD: The implication of lymph node metastasis on survival
in patients with well-differentiated thyroid cancer. Am Surg.
71:731–734. 2005.PubMed/NCBI
|
|
17
|
Shammas A, Degirmenci B, Mountz JM, McCook
BM, Branstetter B, Bencherif B, Joyce JM, Carty SE, Kuffner HA and
Avril N: 18F-FDG PET/CT in patients with suspected recurrent or
metastatic well-differentiated thyroid cancer. J Nucl Med.
48:221–226. 2007.PubMed/NCBI
|
|
18
|
Zhao H and Li H: Meta-analysis of
ultrasound for cervical lymph nodes in papillary thyroid cancer:
Diagnosis of central and lateral compartment nodal metastases. Eur
J Radiol. 112:14–21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Renkonen S, Lindén R, Bäck L, Silén R,
Mäenpää H, Tapiovaara L and Aro K: Accuracy of preoperative MRI to
assess lateral neck metastases in papillary thyroid carcinoma. Eur
Arch Otorhinolaryngol. 274:3977–3983. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hempel JM, Kloeckner R, Krick S, Pinto Dos
Santos D, Schadmand-Fischer S, Boeßert P, Bisdas S, Weber MM,
Fottner C, Musholt TJ, et al: Impact of combined FDG-PET/CT and MRI
on the detection of local recurrence and nodal metastases in
thyroid cancer. Cancer Imaging. 16:372016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lou K, Gu Y, Hu Y, Wang S and Shi H:
Technetium-99m-pertechnetate whole-body SPET/CT scan in
thyroidectomized differentiated thyroid cancer patients is a useful
imaging modality in detecting remnant thyroid tissue, nodal and
distant metastases before 131I therapy. A study of 416
patients. Hell J Nucl Med. 21:121–124. 2018.PubMed/NCBI
|
|
22
|
Lee Y, Kim JH, Baek JH, Jung SL, Park SW,
Kim J, Yun TJ, Ha EJ, Lee KE, Kwon SY, et al: Value of CT added to
ultrasonography for the diagnosis of lymph node metastasis in
patients with thyroid cancer. Head Neck. 40:2137–2148. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garau LM, Rubello D, Morganti R, Boni G,
Volterrani D, Colletti PM and Manca G: Sentinel lymph node biopsy
in small papillary thyroid cancer: A meta-analysis. Clin Nucl Med.
44:107–118. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mulla M and Schulte KM: The accuracy of
ultrasonography in the preoperative diagnosis of cervical lymph
node (LN) metastasis in patients with papillary thyroid carcinoma:
A meta-analysis. Eur J Radiol. 81:1965.author reply 1966. 2012.
|
|
25
|
Na DK, Choi YJ, Choi SH, Kook SH and Park
HJ: Evaluation of cervical lymph node metastasis in thyroid cancer
patients using real-time CT-navigated ultrasonography: Preliminary
study. Ultrasonography. 34:39–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He Y, Pan MZ, Huang JM, Xie P, Zhang F and
Wei LG: Iodine-131: An effective method for treating lymph node
metastases of differentiated thyroid cancer. Med Sci Monit.
22:4924–4928. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hoang JK, Vanka J, Ludwig BJ and
Glastonbury CM: Evaluation of cervical lymph nodes in head and neck
cancer with CT and MRI: Tips, traps, and a systematic approach. AJR
Am J Roentgenol. 200:W17–W25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mihailovic J, Prvulovic M, Ivkovic M,
Markoski B and Martinov D: MRI versus ¹3¹I whole-body
scintigraphy for the detection of lymph node recurrences in
differentiated thyroid carcinoma. AJR Am J Roentgenol.
195:1197–1203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Freudenberg LS, Antoch G, Frilling A,
Jentzen W, Rosenbaum SJ, Kühl H, Bockisch A and Görges R: Combined
metabolic and morphologic imaging in thyroid carcinoma patients
with elevated serum thyroglobulin and negative cervical
ultrasonography: Role of 124I-PET/CT and FDG-PET. Eur J Nucl Med
Mol Imaging. 35:950–957. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morita S, Mizoguchi K, Suzuki M and Iizuka
K: The accuracy of (18)[F]-fluoro-2-deoxy-D-glucose-positron
emission tomography/computed tomography, ultrasonography and
enhanced computed tomography alone in the preoperative diagnosis of
cervical lymph node metastasis in patients with papillary thyroid
carcinoma. World J Surg. 34:2564–2569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang W, Larson SM, Tuttle RM, Kalaigian H,
Kolbert K, Sonenberg M and Robbins RJ; Resistance of
[18f]-fluorodeoxyglucose-avid metastatic thyroid cancer lesions to
treatment with high-dose radioactive iodine, : Thyroid.
11:1169–1175. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feine U, Lietzenmayer R, Hanke JP, Held J,
Wöhrle H and Müller-Schauenburg W: Fluorine-18-FDG and
iodine-131-iodide uptake in thyroid cancer. J Nucl Med.
37:1468–1472. 1996.PubMed/NCBI
|
|
33
|
Sun YG, Feng HJ, Liu JH, Hu R and Ouyang
W: Value of (18)F-FDG dual head coincidence imaging in predicting
the efficacy of radioiodine therapy for papillary thyroid carcinoma
with cervical lymph node metastasis. Nan Fang Yi Ke Da Xue Xue Bao.
31:1571–1574. 2011.(In Chinese). PubMed/NCBI
|
|
34
|
Weigel RJ and McDougall IR: The role of
radioactive iodine in the treatment of well-differentiated thyroid
cancer. Surg Oncol Clin N Am. 15:625–638. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang W, Macapinlac H, Larson SM, Yeh SD,
Akhurst T, Finn RD, Rosai J and Robbins RJ:
[18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography
localizes residual thyroid cancer in patients with negative
diagnostic (131)I whole body scans and elevated serum thyroglobulin
levels. J Clin Endocrinol Metab. 84:2291–2302. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mazzaferri EL and Jhiang SM: Long-term
impact of initial surgical and medical therapy on papillary and
follicular thyroid cancer. Am J Med. 97:418–428. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bardet S, Ciappuccini R, Quak E, Rame JP,
Blanchard D, de Raucourt D, Babin E, Michels JJ, Vaur D and Heutte
N: Prognostic value of microscopic lymph node involvement in
patients with papillary thyroid cancer. J Clin Endocrinol Metab.
100:132–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ito Y, Miyauchi A, Jikuzono T, Higashiyama
T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Ichihara K and
Kuma K: Risk factors contributing to a poor prognosis of papillary
thyroid carcinoma: Validity of UICC/AJCC TNM classification and
stage grouping. World J Surg. 31:838–848. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
de Meer SG, Dauwan M, de Keizer B, Valk
GD, Borel Rinkes IH and Vriens MR: Not the number but the location
of lymph nodes matters for recurrence rate and disease-free
survival in patients with differentiated thyroid cancer. World J
Surg. 36:1262–1267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
de Castro TP, Waissmann W, Simões TC, de
Mello RC and Carvalho DP: Predictors for papillary thyroid cancer
persistence and recurrence: A retrospective analysis with a 10-year
follow-up cohort study. Clin Endocrinol (Oxf). 85:466–474. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu Q, Zhang YM, Sun S, Li JJ, Wu J, Li X,
Zhu S, Wei W and Sun SR: Clinical and sonographic assessment of
cervical lymph node metastasis in papillary thyroid carcinoma. J
Huazhong Univ Sci Technolog Med Sci. 36:823–827. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Diab Y: Sentinel lymph nodes mapping in
cervical cancer a comprehensive review. Int J Gynecol Cancer.
27:154–158. 2017. View Article : Google Scholar : PubMed/NCBI
|