Introduction
Originating in various tissues, neuroendocrine
neoplasm (NEN) comprises a family of tumor types that exhibit
neuroendocrine differentiation characteristics, including
neurosecretory granules, synaptic-like vesicles and production of
different peptides (1,2). Despite the shared neuroendocrine
phenotypes, NENs are represented by an extremely heterogeneous
entity of varied histopathological features (1). Therefore, NENs are divided into three
main categories according to the 2010 World Health Organization
classification system: Well-differentiated neuroendocrine tumor
(NET; Grade 1 and Grade 2, Ki-67 ≤20% and/or mitotic count ≤20 per
10 high-power fields), poorly differentiated neuroendocrine
carcinoma (NEC; Grade 3, Ki-67 >20% and/or mitotic count>20
per 10 high-power fields) and mixed adenoendocrine carcinoma
(MANEC) (3).
Different pathological types vary greatly with
regards to biological behavior and prognosis. Generally, NET tends
to be biologically indolent and has a favorable prognosis, whereas
NEC is an aggressive tumor type associated with poor patient
survival rates (1). However, at
present, the characteristics of MANEC remain poorly elucidated due
to the histological complexity of this tumor type. MANEC refers to
a composite tumor characterized by coexisting glandular and
neuroendocrine elements, with each accounting for >30% of the
lesion. Although MANEC is not frequently encountered, the
coexistence of two distinct histological components in the same
tumor provokes interest from clinicians and researchers.
Additionally, controversies exist regarding the pathogenesis of
biphasic morphology and the therapeutic protocols for this
particular subtype (4).
The extrahepatic biliary tract (EHBT) arises from
outside the liver and extends to the ampulla. It has a complex
anatomical position, as it is surrounded by diverse structures,
including the pancreas, duodenum, portal vein, hepatic artery and
autonomic nerve fibers (5).
Cholangiocarcinoma (CCA) is the most common type of tumor in the
EHBT, which accounts for >80% of cases (6). NEN at this location is rare, even more
so MANEC. However, cases of biliary NENs have been increasingly
described in the medical literature, presumably due to a true
increase in incidence or advances in diagnostic tools (4,7,8).
The data suggests that NEN in the EHBT has a
tendency to mimic conventional CCA in terms of biological
heterogeneity, presenting substantial challenges for clinical
management and prognostic stratification (8). Several attempts have been made to
clarify the clinical settings of this unusual disease. However,
prior studies only focused on a single pathological type (mainly
NET or NEC) (8–10). Additionally, currently, the
prognostic factors of NEN in the EHBT have not been investigated
(11).
In the present report, a MANEC in the distal common
bile duct (CBD) in a 64-year-old Chinese female patient was
described. Additionally, previous cases of NENs in the EHBT were
collected from the medical literature and reviewed to provide
centralized clinical data and to identify factors affecting the
survival outcome of patients with NEN.
Case report
A 64-year-old Chinese woman presented to a local
hospital (the first people's hospital of Fuyang, Hangzhou, China)
in July 2015 with complaints of abdominal pain and obstructive
jaundice. The patient had no fever, nausea or vomiting. The patient
had undergone cholecystectomy for gallstones 2 years earlier. There
was no history of trauma, bronchospasm, peptic ulcers or cancer.
Imaging examinations suggested a malignant structure at the distal
CBD, with significant upstream tract dilation. Although surgery was
offered, the patient refused and three biliary stents were placed
instead. Later, the patient's discomfort gradually subsided. The
patient was discharged after a total of 10 days in the local
hospital. However, the patient complained again of abdominal pain 5
months later (in December 2015) and was referred to the First
Affiliated Hospital, School of Medicine, Zhejiang University
(Hangzhou, China).
On admission (December 2015), the patient had no
fever, jaundice, diarrhea, hypotension or flushing. Physical
examination revealed slight upper abdominal tenderness without
rebound tenderness or guarding. Laboratory work-ups highlighted
elevated levels of serum carcinoembryonic antigen (31.3 ng/ml,
normal 0–5 ng/ml) and carbohydrate antigen 19-9 (40.4 U/ml, normal
0–35 U/ml). Liver function tests were within normal limits. The
chest X-ray was negative. An abdominal computed tomography scan
revealed that the distal CBD had thickened and moderately enhanced
duct walls. Magnetic resonance cholangiopancreatography indicated
that the common hepatic duct, proximal and middle CBD, and main
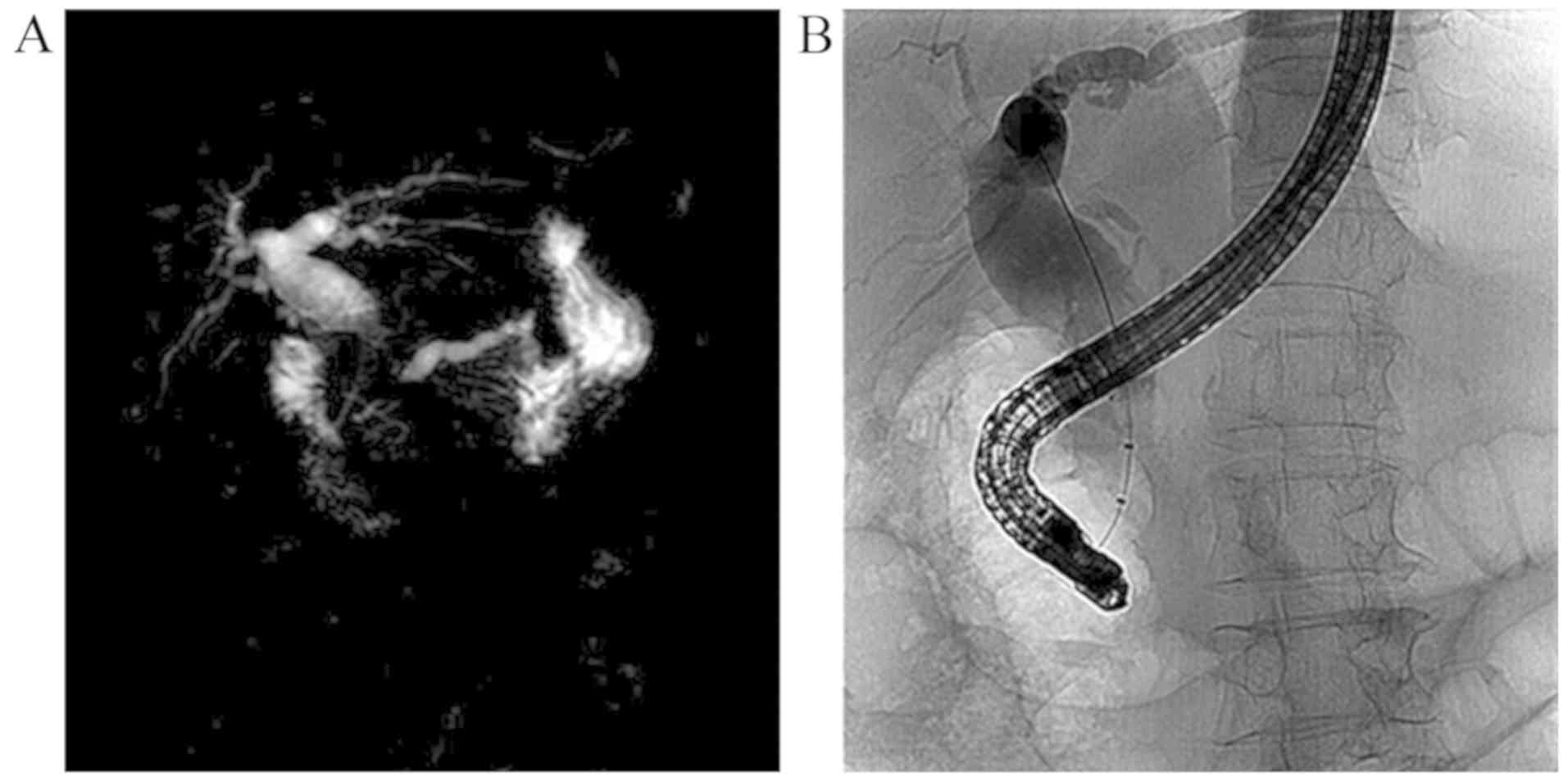
pancreatic duct were markedly dilated (Fig. 1A). Endoscopic retrograde
cholangiopancreatography (ERCP) indicated severe stenosis at the
distal CBD (Fig. 1B), and the
biliary stents were retrieved. Biliary duct brush cytology at the
time of ERCP revealed a small cluster of atypical cells. The
patient was tentatively diagnosed with distal CCA, and a standard
Whipple pancreaticoduodenectomy was scheduled. Intraoperatively,
the patient was found to have enlarged lymph nodes in the hepatic
hilum, which were removed. This procedure was considered curative
since the intraoperative frozen section revealed that the resected
margins were free of atypical cells. Macroscopically, a grayish,
solid tumor surrounded, infiltrated and extended along the distal
CBD wall. Subsequent to being resected and flattened, the tumor
measured 4.5×3.0 cm in size.
The 4 µm-thick surgical specimens were fixed with
10% neutral formaldehyde solution at room temperature, paraffin
embedded, and were then subject to detailed histopathological
analysis combined with immunohistochemical (IHC) staining. The
immunostaining was performed according to the standard protocol of
the Department of Pathology, First Affiliated Hospital, School of
Medicine, Zhejiang University (Hangzhou, China). Briefly, the
specimens were cut into 4-µm thick sections, deparaffinized, and
rehydrated and 1.5% hydrogen peroxide in methanol was used for the
blockage of endogenous peroxidase at room temperature. Then, the
sections were washed with distilled water, and immersed in the
heated EGTA solution (pH 9.0) for 20 min. After cooling down, the
sections were washed with phosphate-buffered saline (PBS; pH
7.2–7.6, three times). Subsequently, The tissue sections were
incubated with a panel of 12 primary antibodies overnight at 4°C,
including caudal type homeobox 2 (CDX2; ZA-0520 EP25; 1:100
dilution), mucin (MUC) 1 (ZA-0656 EP85; 1:100 dilution), MUC2A
(MRQ-18; 1:200 dilution), cytokeratin 19 (CK19; K19.2; 1:200
dilution), MUC5AC (ZA-0664; 1:100 dilution), mammaglobin (MMG;
ZM-0388; 1:80 dilution), cluster of differentiation 56 (CD56;
123C3.D5; 1:80 dilution), synaptophysin (ZA-0506; 1:200 dilution),
chromogranin-A (LK2H101-PHE5; 1:100 dilution), Ki-67 (ZM-0167;
1:1,000 dilution), thyroid transcription factor-1 (TTF-1; ZM-0270;
1:200 dilution) and gross cystic disease fluid protein-15
(GCDFP-15; 23A3; 1:100 dilution). CD56, chromogranin-A, CK19 and
GCDFP-15 antibodies were obtained from Shanghai Long Island Biotec.
Co., Ltd. (Shanghai, China), and the remaining antibodies were
obtained from OriGene Technologies, Inc. (Rockville, MD, USA). The
sections were subsequently washed with PBS (three times), and
anti-mice/rabbit enzyme-labeled secondary antibodies [PV-8000
(IB000086), 1:200 dilution; provided by the Zhong Shan Golden
Bridge Biological Technology Inc., Beijing, China; EnVision
detection system] were then applied at room temperature for 15 min.
The slides were rinsed in PBS again (three times), and treated with
diaminobenzidine (DAB; 1:50) for 5 min, rinsed in distilled water,
and finally counterstained with hematoxylin according to a standard
protocol. Leica DM2500 optical microscopes were used and Ki-67
scoring was performed as described by Adsay (12).
The results revealed a collision tumor composed of
poorly differentiated adenocarcinoma and NEC (Fig. 2). The adenocarcinoma component was
poorly differentiated, exhibited an intestinal phenotype, and
accounted for more than 30% of the tumor. Mucin pool and signet
ring-like cells were observed. Tumor cells were stained for CDX2,
MUC2A, CK19 and focally MUC5AC. The NEC component accounted for
~60% of the tumor, and was characterized by small tumor cells with
scant cytoplasm, hyperchromatic nuclei and inconspicuous nucleoli.
These tumor cells were arranged in a nesting pattern and they
strongly expressed CD56, synaptophysin and chromogranin-A, with a
Ki-67 labeling index >50%. MUC1, GCDFP-15, TTF-1 and MMG were
negative. The angiolymphatic invasion were predominantly NEC. In
view of these findings, the tumor was definitively diagnosed as a
MANEC in the distal CBD.
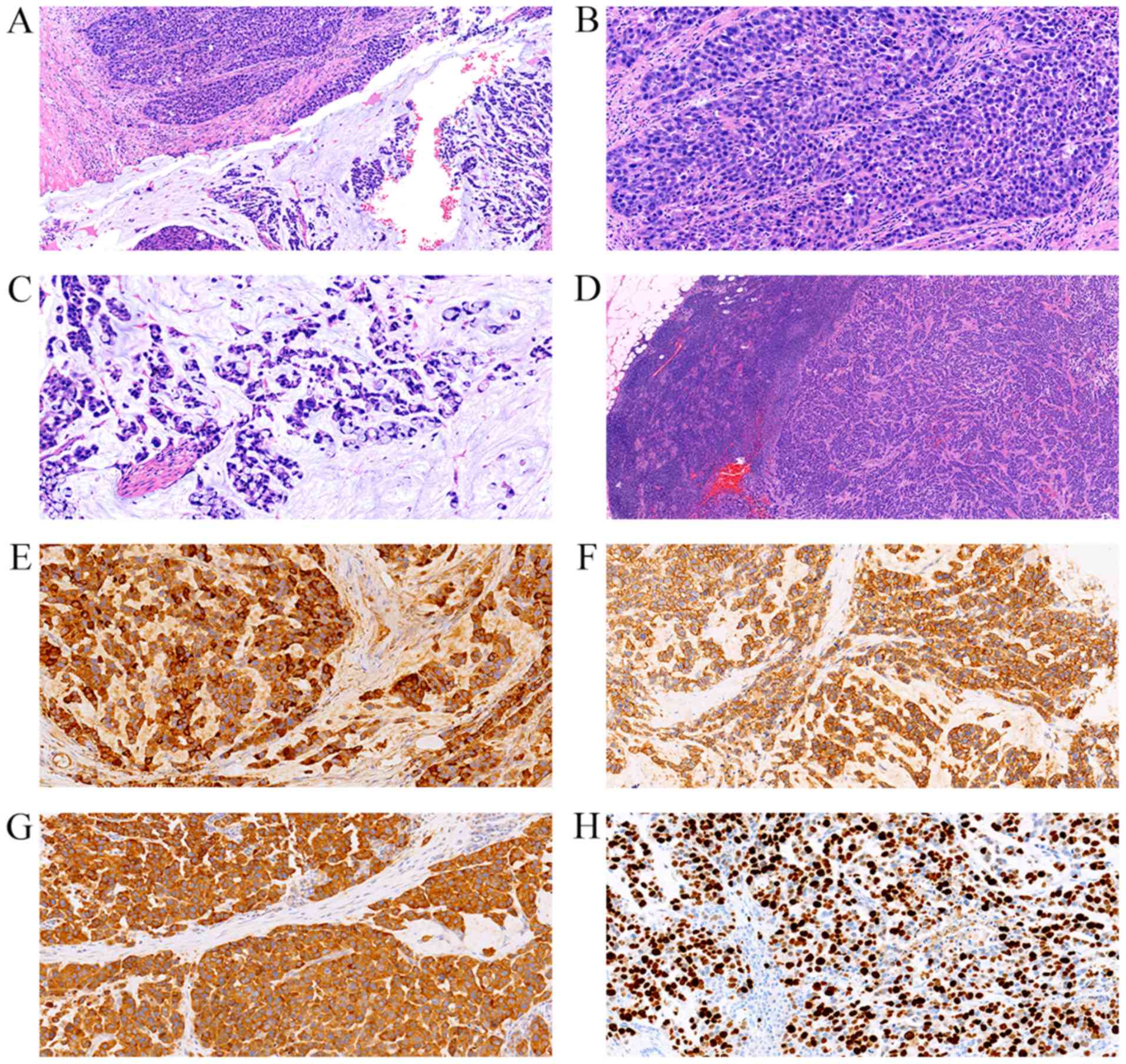 | Figure 2.Pathological analysis of the surgical
specimen. (A) Tumor was composed of adenocarcinoma and
neuroendocrine components, with each occupying >30% of the
lesion. The two components were arranged in a clearly separated
pattern (H&E; magnification, ×100). (B) Neuroendocrine
component was formed by small tumor cells with scant cytoplasm and
hyperchromatic nuclei (H&E; magnification, ×200). (C) Mucin
pool and signet ring-like cell clusters were noted in the
adenocarcinoma component (H&E; magnification, ×200). (D)
Metastatic lesions in the lymph nodes were predominantly
neuroendocrine carcinoma (H&E; magnification, ×40).
Immunohistochemical staining revealed that the neuroendocrine
component was strongly positive for (E) chromogranin A
(magnification, ×200), (F) cluster of differentiation 56
(magnification, ×200) and (G) synaptophysin (magnification, ×200),
with a (H) Ki-67 labeling index >50% (magnification, ×200).
H&E, hematoxylin and eosin. |
Following surgery, the patient recovered and was
discharged from the hospital after 2 weeks. However, repeated
imaging studies postoperatively over 8 months revealed multiple
intrahepatic and pulmonary metastases. The patient succumbed to
disease 12 months after the surgery.
Discussion
MANECs predominately occur in the colon, appendix
and stomach, where neuroendocrine cells are diffusely distributed
(4). MANECs arising from the EHBT
are extremely rare, with a total of eight cases reported in the
medical literature since the category was introduced in 2010
(13–20). The histogenesis of biliary MANEC
remains under debate due to the scarcity of enterochromaffin
(Kultchisky) cells in the normal bile duct (21). To explain this issue, the following
theories have been formulated.
As mentioned in the presented case and other
studies, intestinal metaplasia is a frequent and well-documented
event in MANEC (22,23). A case of biliary NEC has been
described in which histopathological analysis revealed concurrent
dysplasia with intestinal and neuroendocrine differentiations in
the biliary tracts within and adjacent to the invasive NEC
(9). Given the close
histopathological associations, intestinal metaplasia of the
biliary epithelium may be involved in the development of MANEC
following a sequence of metaplasia-dysplasia-carcinoma.
Using surgical specimens of biliary MANECs, the
expression levels of Notch1, Jagged1 and hes family bHLH
transcription factor 1 (Hes1) have been demonstrated to be constant
in the adenocarcinoma component, but decreased or absent in the
neuroendocrine component (24).
Additionally, disruption of the Notch1-Hes1 signaling axis
significantly increases the expression profiles of neuroendocrine
protein markers in a cultured CCA cell line (24). Collectively, this evidence suggests
that biliary MANEC may be associated with the transdifferentiation
of adenocarcinoma (24). A
hypothesis was proposed that it may result from proliferation of a
common precursor stem cell, which is capable of divergent
differentiation. This hypothesis was supported by the observation
that prominin 1, a biomarker of cancer stem cells, is expressed in
63.6% of cases of digestive MANECs (25). Evidence obtained from next-generation
sequencing in nonbiliary digestive MANECs also suggests a
monoclonal origin of the two histological components (26).
Histopathologically, the glandular component of
MANEC is generally detected at the tumor surface, while the
neuroendocrine component is located in the deep stroma; the latter
is typically responsible for tumor invasiveness (23). The two tumoral phases of MANEC may be
arranged in either clearly separated (collision) or tightly mingled
(combined) patterns; much less frequently, the tumor cells exhibit
a mixed adenocarcinomatous-neuroendocrine (amphicrine) phenotype
(27).
NEN in the EHBT represents an uncommon disease
accounting for 0.1–0.2% of all gastroenteropancreatic NENs.
However, patients with this unusual entity exhibit a wide spectrum
of oncology outcomes, ranging from curative following tumor
excision to a poor prognosis even following multidisciplinary
treatment (10,28). Accumulating clinical data suggests
that the prognostic heterogeneity may be associated with
pathological classification (10,11,23,26).
However, a straightforward comparison among the three pathological
types is currently lacking due to the rarity of the disease. To
gain an improved understanding of NEN in the EHBT by incorporating
all pathological types and to identify prognostic predictors, a
literature review of pertinent publications in the English
literature was conducted.
PubMed (www.ncbi.nlm.nih.gov/pubmed) was searched for English
language studies that described NEN in the EHBT between 2010 and
2018 using medical terms, including ‘carcinoid’, ‘mixed
adenoendocrine carcinoma’, ‘neuroendocrine neoplasm’,
‘neuroendocrine tumor’, ‘neuroendocrine carcinoma’, ‘biliary duct’
and ‘bile duct’. In the present study, 2010 was selected as the
starting year since this is when the latest classification system
of NEN was introduced. Cases of NENs located in the gallbladder,
cystic duct and the ampulla of Vater were excluded. Aggregated data
for patients with biliary NEN from series studies were also
excluded where patient-level information was not available. The
search identified 37 patients with NEN in the EHBT since 2010.
Eventually, a total of 38 cases, including the present case, were
analyzed (Table I) (9,10,13–20,28–53).
Clinical characteristics and survival outcomes among different
pathological types were compared, and they are summarized in
Table II.
 | Table I.Summary of cases of neuroendocrine
neoplasms in the extrahepatic biliary tract. |
Table I.
Summary of cases of neuroendocrine
neoplasms in the extrahepatic biliary tract.
| First author,
year | Country | Age (years),
sex | Symptoms | Location | Size (cm) | Treatment | Classification | IHC markers | Metastasis | Survival | (Refs.) |
|---|
| Sasatomi et
al, 2013 | America | 76, F | Jaundice | CHD | 5.0 | BTR + LNR +
hepatectomy + hepaticojejunostomy | NEC | Syn, Chg: (+);
Ki-67 80–90% | LN, PO8d | Deceased,
PO21d | (9) |
| Zhang et al,
2018 | China | 62, M | Jaundice | CHD | 2.0 | BTR + LNR +
hepaticojejunostomy | NEC | Syn, Chg, CD56 (+);
Ki-67 >80% | LN, PP, liver,
PO2m | Deceased, PO6m | (10) |
| Izumo et al,
2017 | Japan | 66, M | Anorexia,
fatigue | CBD | 1.0 | PD | MANEC | Syn, Chg (+); Ki-67
30% | LN, PP | Survived, PO
30m | (13) |
| Komo et al,
2017 | Japan | 82, M | Asymptomatic | CBD | 1.8 | PD | MANEC | Syn, Chg (+); Ki-67
37% | None | Survived, PO7m | (14) |
| Lee et al,
2014 | Korea | 75, M | Jaundice | CBD | 2.0 | BTR + LNR +
hepatectomy + hepaticojejunostomy | MANEC | Syn, Chg, CD56
(+) | None | Survived,
PO11m | (15) |
| Linder et
al, 2013 | Israel | 82, M | Jaundice, pain,
WL | CBD | 1.9 | PD | MANEC | Syn, Chg, CD56
(+) | LN, PO | Survived, PO6m | (16) |
| Masui et al,
2011 | Japan | 82, M | Jaundice, anorexia,
WL | CBD | 2.5 | BTR + LNR +
hepaticojejunostomy | MANEC | Syn, Chg, CD56: (+)
Ki-67 30–40% | Liver, PO3m | Deceased, PO6m | (17) |
| Onishi et
al, 2013 | Japan | 74, F | Jaundice,
fever | CBD | 2.0 | PD | MANEC | Syn (+), chg, CD56
(−) | None | NA | (18) |
| Priyanka et
al, 2016 | India | 76, M | Jaundice, WL | CHD | 4.0 | BTR + LNR+
hepaticojejunostomy | MANEC | Syn, CD56 (+) Chg
(−) Ki-67 90% | None | NA | (19) |
| Wysocki et
al, 2014 | America | 65, M | Jaundice, WL,
nausea | CHD-CBD | 5.0 | BTR + LNR
hepaticojejunostomy | MANEC | Syn, Chg, CD56 (+);
Ki-67 80% | NA | Deceased, PO5m | (20) |
| Liu et al,
2018 | China | 57, F | Fever | CBD | 6.0 | BTR + LNR+
hepaticojejunostomy | NET | Syn, CD56 (+);
Chg(−); Ki-67 12% | None | Survived, PO8m | (28) |
| Squillaci et
al, 2010 | Italy | 52, M | Jaundice | CHD-CBD | 2.0 | BTR + LNR+
hepaticojejunostomy | NET | Syn, Chg: (+);
Ki-67 <2% | None | Survived,
PO41m | (29) |
| Squillaci et
al, 2010 | Italy | 70, M | Pain | CHD | 4.5 | BTR + LNR +
hepatectomy + hepaticojejunostomy | NET | Syn, Chg: (+) | LN, PP | Survived,
PO59m | (29) |
| Zhan et al,
2010 | China | 10, M | Jaundice, pain | CBD | 2.0 | PD | NET | Chrom (+) | None | Survived,
PO12m | (30) |
| Cappell et
al, 2011 | America | 42, M | Jaundice, pain,
WL | CBD | 1.8 | PD | NET | Syn, Chg: (+) | None | Survived, PO9y | (31) |
| Lee et al,
2011 | Korea | 59, M | Jaundice | CBD | 2.5 | BTR +
hepaticojejunostomy + radiotherapy | NET | NA | None | Deceased, PO5m | (32) |
| Bhalla et
al, 2012 | India | 28, F | Pain, WL | CHD | 2.0 | BTR + LNR +
hepaticojejunostomy | NET | Syn, Chg: (+);
Ki-67 3% | LN, PP | Survived, PO4m | (33) |
| De Luca et
al, 2013 | Italy | 78, M | Jaundice | CBD | 3.0 | PD | NET | Syn, Chg, CD56 (+);
Ki-67<20% | None | NA | (34) |
| Jethava et
al, 2013 | America | 42, M | Pain,
dyspepsia | CBD | 1.7 | PD | NET | Syn, Chg, CD56
(+) | None | Survived, PO6m | (35) |
| Yasuda et
al, 2013 | Japan | 60, F | Asymptomatic | CHD | 2.5 | BTR + LNR +
hepaticojejunostomy | NET | Syn, Chg, CD56
(+) | None | Survived, PO2y | (36) |
| Ayllon-Teran et
al, 2014 | Spain | 19, F | Jaundice, WL,
anorexia | CBD | 2.0 | BTR + LNR +
hepaticojejunostomy | NET | Ki-67 10% | None | NA | (37) |
| Khuroo et
al, 2014 | India | 56, F | Jaundice, pain,
WL | CHD | 1.7 | BTR + LNR +
chemoradiotherapy + hepaticojejunostomy | NET | Chg (−) | None | Survived,
PO18m | (38) |
| Park et al,
2014 | Korea | 75, F | Jaundice,
nausea | CBD | 2.7 | BTR + LNR+
hepaticojejunostomy | NEC | Syn, Chg (+) | LN, PP, liver,
PO7m | Decceased,
PO12m | (39) |
| Yalav et al,
2014 | Turkey | 16, M | Jaundice | CBD | NA | BTR +
hepaticojejunostomy | NET | Syn, Chg (+) | None | Survived,
PO40m | (40) |
| Kihara et
al, 2015 | Japan | 70, F | Jaundice | CHD | 3.0 | BTR + LNR +
chemotherapy + hepatectomy + choledochojejunostomy | NEC | Syn, Chg, CD56 (+);
Ki-67 70% | LN, PP | Survived,
PO10m | (41) |
| Banerjee et
al, 2016 | India | 45, F | Jaundice, WL | CBD | 3.2 | PD | NET | Syn (+), Chg (−);
Ki-67 <1% | LN, PP | NA | (42) |
| Hosoda et
al, 2016 | Japan | 35, M | Asymptomatic | CBD | 1.1 | BTR + LNR+
hepaticojejunostomy | NET | Syn, Chg (+); Ki-67
2.5% | None | Survived, PO1m | (43) |
| Murakami et
al, 2016 | Japan | 80, M | Jaundice,
anorexia | CBD | 2.4 | BTR + LNR +
chemotherapy hepaticojejunostomy | NEC | Syn, CD56 (+);
Chg(−); Ki-67 72% | Lung, liver,
PO2.5m | Deceased, PO3m | (44) |
| Oshiro et
al, 2016 | Japan | 75, M | Jaundice | CBD | 3.0 | BTR + LNR +
chemotherapy hepaticojejunostomy | NEC | Syn, CD56 (+) Ki-67
56.2% | LN, PP, liver,
PO3m | Survived, PO7m | (45) |
| Raspanti et
al, 2016 | Italy | 51, F | Jaundice | CBD | 1.5 | BTR + LNR +
hepaticojejunostomy | NET | Syn, Chg, CD56 (+);
Ki-67 <2% | None | Survived, PO1m | (46) |
| Abe et al,
2017 | Japan | 57, F | Asymptomatic | CBD | 3.0 | BTR + LNR +
hepaticojejunostomy | NET | Syn, Chg, CD56 (+);
Ki-67 2% | None | Survived,
PO34m | (47) |
| Costin et
al, 2017 | Italy | 37, F | Jaundice | CBD | 2.5 | BTR + LNR +
hepaticojejunostomy | NET | Syn, Chg (+); Ki-67
2% | None | Survived, PO2y | (48) |
| Hoepfner et
al, 2017 | America | 45, M | Jaundice, pain | CHD-CBD | 4.0 | BTR + LNR +
hepaticojejunostomy | NET | Ki-67 4% | LN, PP | Survived, PO6m | (49) |
| Khan et al,
2017 | America | 64, M | Jaundice, WL | CHD-CBD | 1.3 | BTR + LNR +
hepaticojejunostomy | NET | Syn, Chg, CD56 (+);
Ki-67 <5% | None | NA | (50) |
| Sanchez et
al, 2017 | France | 38, M | Jaundice | CBD | 2.0 | BTR + LNR +
hepaticojejunostomy | NET | Syn, CD56 (+);
Ki-67 15% | None | Survived, PO6d | (51) |
| Sano et al,
2017 | Japan | 30, F | Abdominal
discomfort | CHD | 2.4 | BTR + LNR +
hepaticojejunostomy | NET | Syn, Chg, CD56
(+); | None | Survived,
PO18d | (52) |
| Koo et al,
2018 | Korea | 77, F | Jaundice, pain | CHD | 1.0 | Radiotherapy | NEC | Syn, Chg, CD56 (+);
Ki-67 60% Ki-67 6.6% | LN, liver,
AD1m | NA | (53) |
| Present study | China | 64, F | Jaundice, pain | CBD | 4.5 | PD | MANEC | Syn, Chg, CD56 (+);
Ki-67 >50% | Liver, PO5m | Deceased, PO1y | – |
 | Table II.Comparison of NET, NEC and MANEC in
the extrahepatic biliary tract. |
Table II.
Comparison of NET, NEC and MANEC in
the extrahepatic biliary tract.
| Variable | NEN | NET | NEC | MANEC |
P-valuea |
|---|
| No. of cases | 38 | 22 | 7 | 9 |
|
| Male/female | 23/15 | 13/9 | 3/4 | 7/2 |
|
| Age, years
(IQR) | 62.0
(43.5–75.0) | 45.0
(36.0–58.0) | 75.0
(70.0–77.0) | 75.0
(65.5–82.0) | <0.001 |
| Symptoms, no. |
|
|
|
|
|
|
Jaundice | 27 | 14 | 7 | 7 |
|
|
Abdominal pain | 9 | 7 | 1 | 2 |
|
| Weight
loss | 2 | 1 | 0 | 1 |
|
|
Fever | 10 | 6 | 0 | 4 |
|
|
Asymptomatic | 4 | 3 | 0 | 1 |
|
|
Anorexia/nausea/dyspepsia/fatigue/discomfort | 8 | 3 | 2 | 3 |
|
| Tumor size, cm
(IQR) | 2.4 (1.9–3.0) | 2.0 (1.8–3.0) | 2.7 (2.0–3.0) | 2.0 (1.9–4.3) | 0.369 |
| Tumor location |
|
|
|
| 0.41c |
| CHD
involved | 15 | 9 | 4 | 2 |
|
| Only CBD
involved | 23 | 13 | 3 | 7 |
|
| Mean follow-up
time, months (IQR) | 7.0 (5–24) | 10.0
(3.3–35.5) | 6.5 (2.5–10.5) | 7.0 (6.0–12.0) | 0.611 |
| Mortality, no.
(%) | 8
(26%) | 1 (5%) | 4 (66.7%) | 3 (42.9%) |
|
| Recurrent events,
no. (%) | 6
(19.4%) | 0 | 5 (71.4%) | 2 (28.6%) |
|
| Survival,
months |
|
|
|
| 0.006b |
|
Mean | 72.2 | 100 | 7.7 | 16.6 |
|
|
Median | – | – | 6 | 12 |
|
Preliminary Shapiro-Wilk tests demonstrated the
skewed distributions of quantitative variables, which were
therefore expressed as median and interquartile range (IQR) and
compared by Kruskal-Wallis tests. χ2 tests were used for
categorical variables. The survival analysis was conducted by the
Kaplan-Meier method, and the log rank test was used for comparisons
among groups. Notably, Cox proportional hazards regression analysis
was not performed due to the limited data for survival analysis
(n=31). Statistical analysis was performed using IBM SPSS
Statistics v19.0 software (IBM Corp., Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Of the 38 cases of NEN in the EHBT (including the
present case), the majority were NET (n=22, 57.9%). Biliary NECs
and MANECs were less common, with a total of seven (18.4%) and nine
(23.7%) cases identified, respectively. The median age of patients
at diagnosis was 62 years (IQR 43.5–75.0), and males were slightly
predominant (n=23, 60.5%). The tumor size ranged between 1.0 and
6.0 cm, with a median of 2.4 cm (IQR 1.9–3.0). The involvement of
the perihilar biliary tract was noted in 15 cases. Patients with
biliary MANEC had a median age of 75 years at diagnosis, which was
comparable to that of patients with NEC (median 75 years) but
significantly higher than that of patients with NET (median 45
years; P<0.001). Males appeared to be predominant in the MANEC
and NET groups, with a male:female ratio of 7:2 and 13:9,
respectively, while no gender discrepancy was noted in NEC (3:4).
Compared with NET and MANEC, NEC tended to exhibit a larger tumor
size and to be more frequently associated with the involvement of
the perihilar biliary duct; however, these differences did not
reach statistical significances (P=0.369 and P=0.41,
respectively).
Fairly well recognized gastroenteropancreatic NENs
were characterized by the capability to produce bioactive
substances that cause characteristic hormonal symptoms. It has been
estimated that the carcinoid syndrome (including flushing,
hypotension, diarrhea and bronchospasm) is presented in ~1/3
patients with small intestinal NETs; 40–55% of pancreatic NETs may
be classified as functional tumors (54). Nonetheless, in this literature
review, symptoms caused by biliary NEN were mostly due to the mass
effect; functional symptoms tended to be absent in all pathological
subtypes. The most common symptom of biliary NEN was jaundice (27
patients, 71.1%), followed by fever (10 patients, 26.3%), abdominal
pain (9 patients, 23.7%), anorexia/nausea
dyspepsia/fatigue/discomfort (8 patients, 21.1%) and weight loss
(two patients, 5.3%); 4 patients were asymptomatic (10.5%) at
diagnosis. In parallel with the lack of symptoms, biliary NEN was
rarely identified to be associated with abnormal hormone levels.
Biochemical tests have frequently highlighted the elevated levels
of serum carcinoembryonic antigen and carbohydrate antigen 19-9, as
described in 3/21 cases (14.3%) and 15/36 cases (41.7%),
respectively (data not shown). Although efforts have been made, the
measurement of serum hormone levels was not technically feasible
for the presented case. In the majority of cases reported in the
literature, the hormone levels are within the normal range,
contributing to the high rate of misdiagnosis of these cases as CCA
(8–11,13–20,28–53).
Imaging results for biliary NEN generally overlap
with those of CCA, leading to a high rate of misdiagnosis.
Generally, a computed tomography scan depicts a hypodense,
well-vascularized and heterogeneously enhanced lesion. Upstream
bile-duct dilation and lymph-node enlargement are common findings.
Biliary NENs on magnetic resonance imaging mostly appear as nodular
(45%) and intraductal-growing (45%) shapes and less frequently as
periductal-infiltrating (9%) type (55). In positron emission tomography, NEN
usually demonstrates high glucose metabolism, particularly for
poorly differentiated NEC (44,56).
Due to the unspecific clinical and imaging
characteristics, an accurate preoperative diagnosis of biliary NEN
is extremely difficult. In the majority of cases, histopathological
analysis completed by IHC investigations of surgically resected
specimens is required to achieve a definitive diagnosis.
Macroscopic examinations of tumors usually reveal a nodular,
infiltrating or polypoid mass. Histopathologically, tumors tend to
exhibit cord, nest or trabeculae growth patterns. Perineural and
lymphovascular invasions have been frequently observed. To confirm
the neuroendocrine phenotype and the grade of the NENs, IHC
investigation is required. Among the commonly used markers,
synaptophysin and chromogranin are two of the most reliable
neuroendocrine markers (57). NEN
usually stains diffusely for synaptophysin due to the presence of
small clear vesicles in tumor cells and for chromogranin due to
large neurosecretory granules. Neuron-specific enolase and CD56
have been identified to exhibit a lower specificity. The Ki-67
staining index and mitotic count are crucial for tumor grading, as
defined in the classification systems (3). Specifically, the Ki-67 index is
generally more accurate and reproducible when compared with mitotic
count.
The standardized management protocols for NEN in the
EHBT have not been well developed due to limited experience with
this uncommon disease. Radical surgery represents a potentially
curative option, and it has been recommended for cases of all
pathological types where imaging examinations suggest that complete
resection is feasible. The surgical procedures heavily depend on
the primary tumor sites. Of the cases considered in the present
study, pancreaticoduodenectomy was performed in 10 cases, mainly
for tumors located in the distal CBD. Surgical resections for
perihilar NENs involved bile-duct excision, lymph-node dissection,
and Roux-en-Y hepaticojejunostomy or hepaticoduodenostomy, with or
without hepatic lobectomy, as performed in 27 cases. In 6/31 cases
(19.4%), the tumor recurred following surgical resection; four were
NECs and two were MANECs. Notably, no tumor recurrence was noted in
cases of biliary NETs over a median follow-up period of 10
months.
Surgical resection usually provides a curative
chance for patients with biliary NET. Adjuvant therapies are not
required for completely resected well-differentiated NET (58).
For the cases of NECs in the EHBT considered in this
study, systematic chemotherapy was frequently employed (3/7 cases,
42.9%) and aimed to improve resectability or to control tumor
progression. There is no standard chemotherapy regimen established
for biliary NEC. The most common chemotherapy regime consists of a
combination of cisplatin and etoposide, borrowing from treatment
experiences with pulmonary NEC. A higher chemotherapy response rate
for advanced NEC may be expected when the tumor presents with a
higher Ki-67 proliferative index (>55%) (59).
The chemotherapy regimen selection for MANEC remains
a large clinical dilemma, since it is complicated by the mixture of
distinctive malignant histologies. Without clear consensus based on
evidence, attempts at adjuvant therapies have been seldom made in
patients with biliary MANEC. However, as recurrent events were
noted in 2/9 patients (22.2%), adjuvant chemotherapy may be
justified. Further studies are required to tailor chemotherapy
strategies, and to determine which component to target to obtain
the best therapeutic benefits.
During a median follow-up period of 7 months, a
total of eight patients succumbed to disease, of which one, four
and three were diagnosed with NET (12.5%), NEC (50%) and MANEC
(37.5%), respectively. To identify parameters useful for prognostic
stratification, Kaplan-Meier analysis and a log-rank test were
performed for 31 patients with survival data.
As anticipated, the survival outcome of patients
with NEN in the EHBT varied significantly by pathological type. The
median overall survival for patients diagnosed with NET, NEC and
MANEC was 100 (data not shown), 7.7, and 16.6 months, respectively
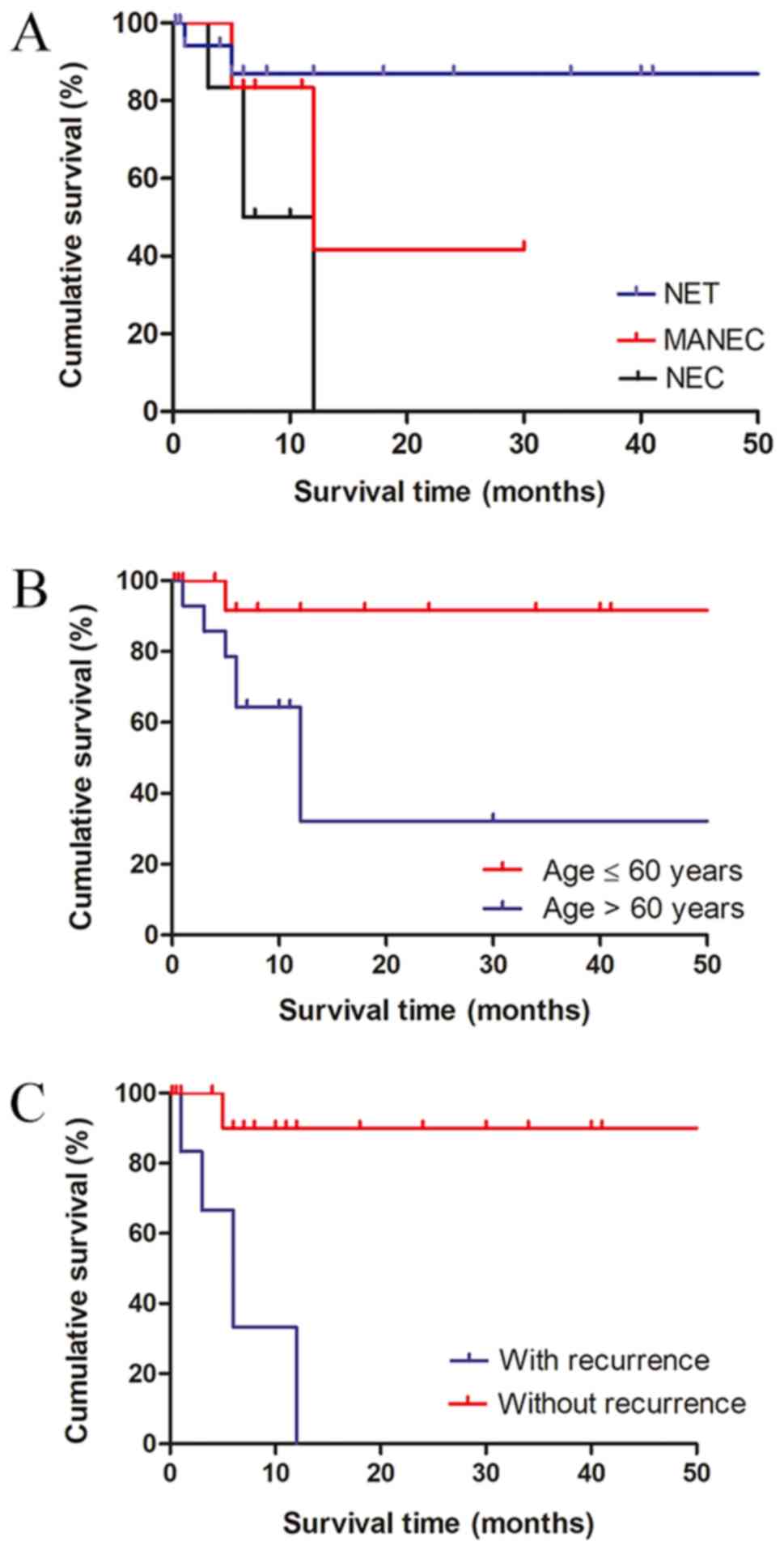
(P<0.001, Fig. 3A; the X-axis was
shortened regarding the great difference of median overall patient
survival among three pathological types). Additionally, old age and
tumor recurrence were identified to negatively affect clinical
outcomes (Fig. 3B and C). Patients
aged >60 years exhibited significantly worse survival than those
who were ≤60 years old (P=0.033). A significantly shorter overall
survival was observed in patients with postoperative tumor
recurrence than those without (P<0.001). Sex, tumor size and
location were identified to not be associated with survival
outcomes (data not shown).
The findings of the present study were consistent
with the results from prior studies that investigated NENs from
other primary sites, and once again, they highlighted the pivotal
role of histological classification in predicting tumor biological
behavior and clinical outcome. A national surveillance,
epidemiology and end results (SEER) survey of appendiceal MANECs
demonstrated that MANEC is associated with a significantly worse
prognosis than NET, with a median overall survival of 6.5 and 39.4
years (P<0.001), respectively (60). In the same study, patients with MANEC
were revealed to be older compared with patients with NET (58 vs.
40 years; P<0.001) (60).
However, the discrepancy between MANEC and NEC prognoses may vary
by primary sites. Similar to the findings of the present study,
which suggested that the prognosis of biliary MANEC was superior to
that of NEC, patients with gastric MANEC exhibit an improved
survival rate compared with those with pure NEC (61). Conversely, in a previous study no
significant survival differences were identified in patients with
colorectal MANEC and NEC (62).
Furthermore, the negative effect of older age on patient survival
has also been documented. In line with the results of the present
study, a SEER survey comprising 35,618 patients with
gastrointestinal NEN demonstrated that age is a strong predictor of
survival duration; those aged >60 years exhibit the worst
outcome at all disease stages (P<0.001) (7).
Since the present analysis was mostly based on case
reports in the literature, a reporting bias may potentially exclude
the publication of similar cases and thus underestimate the true
incidences of NET and NEC in the EHBT. A previous collection of
cases of NETs in the EHBT from 1959 to 2012 yielded a total of 150
cases in the medical literature (8).
Similarly, our previous study collected a total of 21 cases of
biliary NECs from the literature (10). These studies, however, only focused
on a single pathological subtype. Distinctively, the present survey
included all pathological types from prior publications, and
centralized data is therefore provided in brief. Nonetheless, the
limited number of cases could potentially weaken the statistical
power, and it hampered the Cox proportional hazards model analysis
for independent prognostic factors. However, this is inevitable, as
the disease itself is uncommon. Additionally, the heterogeneity of
management protocols among the centers of different authors would
constitute a prominent confounding bias that affects patient
prognosis. The absence of consistent descriptions among these case
reports restricted the extended analysis for other potential
parameters, including the Ki-67 index, tumor stage and
surgery-associated variables. To explore their prognostic
relevance, further studies based on a large series of complete data
are required.
In conclusion, NEN infrequently occurs in the EHBT,
with NET representing the predominant type. NEN in the EHBT is
challenging to diagnose preoperatively due to its tendency to mimic
conventional CCA. Unlike NET, where a favorable prognosis can be
expected following surgical resection, NEC and MANEC were
associated with poor clinical outcomes. Furthermore, old age
(>60 years) and the presence of tumor recurrence were associated
with a decreased survival rate.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 31671019) and the
National S&T Major Project (grant no. 2017ZX10203205).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ assessed the clinical findings of the case and
was responsible for the conception of the study. HX, LZ and ML
designed the research and were responsible for quality control of
data. ZY, QC, DW and XZ made substantial contributions to
acquisition, analysis and interpretation of data. LZ, ZY and QC
wrote the manuscript. HX and SZ provided constructive discussions
and revised the manuscript critically for important intellectual
content. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for publication of this
case report was obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CBD
|
common bile duct
|
|
CCA
|
cholangiocarcinoma
|
|
EHBT
|
extrahepatic biliary tract
|
|
ERCP
|
endoscopic retrograde
cholangiopancreatography
|
|
GCDFP-15
|
gross cystic disease fluid
protein-15
|
|
IHC
|
immunohistochemical
|
|
IQR
|
interquartile range
|
|
MANEC
|
mixed adenoendocrine carcinoma
|
|
MMG
|
mammaglobin
|
|
NEC
|
neuroendocrine carcinoma
|
|
NEN
|
neuroendocrine neoplasm
|
|
NET
|
neuroendocrine tumor
|
|
TTF-1
|
thyroid transcription factor-1
|
References
|
1
|
Modlin IM, Oberg K, Chung DC, Jensen RT,
de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA,
Krenning EP, et al: Gastroenteropancreatic neuroendocrine tumours.
Lancet Oncol. 9:61–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uccella S, La Rosa S, Volante M and
Papotti M: Immunohistochemical biomarkers of gastrointestinal,
pancreatic, pulmonary, and thymic neuroendocrine neoplasms. Endocr
Pathol. 29:150–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO Classification of Tumours of the Digestive System.
Fourth Edition. IARC; Lyon: 2010
|
|
4
|
La Rosa S, Marando A, Sessa F and Capella
C: Mixed adenoneuroendocrine carcinomas (MANECs) of the
gastrointestinal tract: An update. Cancers (Basel). 4:11–30. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vakili K and Pomfret EA: Biliary anatomy
and embryology. Surg Clin North Am. 881159–1174. (vii)2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rizvi S, Khan SA, Hallemeier CL, Kelley RK
and Gores GJ: Cholangiocarcinoma-evolving concepts and therapeutic
strategies. Nat Rev Clin Oncol. 15:95–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A and Evans
DB: One hundred years after ‘carcinoid’: Epidemiology of and
prognostic factors for neuroendocrine tumors in 35,825 cases in the
United States. J Clin Oncol. 26:3063–3072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Michalopoulos N, Papavramidis TS,
Karayannopoulou G, Pliakos I, Papavramidis ST and Kanellos I:
Neuroendocrine tumors of extrahepatic biliary tract. Pathol Oncol
Res. 20:765–775. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sasatomi E, Nalesnik MA and Marsh JW:
Neuroendocrine carcinoma of the extrahepatic bile duct: Case report
and literature review. World J Gastroenterol. 19:4616–4623. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Wan D, Bao L, Chen Q, Xie H, Xu S
and Lin S: Neuroendocrine carcinoma in the extrahepatic biliary
tract: A case report and literature review. Medicine (Baltimore).
97:e114872018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Acosta AM and Wiley EL: Primary biliary
mixed adenoneuroendocrine carcinoma (MANEC): A short review. Arch
Pathol Lab Med. 140:1157–1162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adsay V: Ki67 labeling index in
neuroendocrine tumors of the gastrointestinal and pancreatobiliary
tract: To count or not to count is not the question, but rather how
to count. Am J Surg Pathol. 36:1743–1746. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Izumo W, Higuchi R, Yazawa T, Uemura S,
Matsunaga Y, Shiihara M, Furukawa T and Yamamoto M: A long-term
recurrence-free survival of a patient with the mixed
adeno-neuroendocrine bile duct carcinoma: A case report and review
of the literature. Int J Surg Case Rep. 39:43–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Komo T, Kohashi T, Nakashima A, Ohmori I,
Hihara J, Mukaida H, Kaneko M and Hirabayashi N: Mixed
adenoneuroendocrine carcinoma of the distal bile duct: A case
report. Int J Surg Case Rep. 39:203–207. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee SW, Lee IS, Cho YK, Park JM, Kim SW,
Choi MG, Choi KY, Lee MA, Hong TH, You YK and Jung ES: A case of
mixed adenoneuroendocrine carcinoma of the common bile duct:
Initially diagnosed as cholangiocarcinoma. Korean J Pathol.
48:445–448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Linder R, Dorfman T, Ben-Ishay O,
Kakiashvili E, Velodavsky E and Kluger Y: Mixed neuroendocrine
tumor of the common bile duct. JOP. 14:71–73. 2013.PubMed/NCBI
|
|
17
|
Masui T, Doi R, Kawaguchi Y, Iwanaga Y,
Ito T, Koizumi M and Uemoto S: Adenoendocrine cell carcinoma of the
extrahepatic bile duct: A case report and review of the literature.
Clin J Gastroenterol. 4:174–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Onishi I, Kitagawa H, Harada K, Maruzen S,
Sakai S, Makino I, Hayashi H, Nakagawara H, Tajima H, Takamura H,
et al: Intraductal papillary neoplasm of the bile duct accompanying
biliary mixed adenoneuroendocrine carcinoma. World J Gastroenterol.
19:3161–3164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Priyanka Akhilesh S, Kamal Sunder Y,
Chandralekha T, Samir P and Prasad Kashinath W: Common hepatic duct
mixed adenoneuroendocrine carcinoma masquerading as
cholangiocarcinoma. Case Rep Gastrointest Med.
2016:48270502016.PubMed/NCBI
|
|
20
|
Wysocki J, Agarwal R, Bratton L, Nguyen J,
Weidenhaft MC, Shores N and Kimbrell HZ: Mixed large cell
neuroendocrine carcinoma and adenocarcinoma with spindle cell and
clear cell features in the extrahepatic bile duct. Case Rep Pathol.
2014:3479492014.PubMed/NCBI
|
|
21
|
Dancygier H, Klein U, Leuschner U, Hübner
K and Classen M: Somatostatin-containing cells in the extrahepatic
biliary tract of humans. Gastroenterology. 86:892–896.
1984.PubMed/NCBI
|
|
22
|
Oshiro H, Matsuo K, Mawatari H, Inayama Y,
Yamanaka S, Nagahama K, Endo I, Shimada H, Nakajima A and Kubota K:
Mucin-producing gallbladder adenocarcinoma with focal small cell
and large cell neuroendocrine differentiation associated with
pancreaticobiliary maljunction. Pathol Int. 58:780–786. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harada K, Sato Y, Ikeda H, Maylee H,
Igarashi S, Okamura A, Masuda S and Nakanuma Y: Clinicopathologic
study of mixed adenoneuroendocrine carcinomas of hepatobiliary
organs. Virchows Arch. 460:281–289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harada K, Sato Y, Ikeda H, Hsu M, Igarashi
S and Nakanuma Y: Notch1-Hes1 signalling axis in the tumourigenesis
of biliary neuroendocrine tumours. J Clin Pathol. 66:386–391. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mia-Jan K, Munkhdelger J, Lee MR, Ji SY,
Kang TY, Choi E and Cho MY: Expression of CD133 in neuroendocrine
neoplasms of the digestive tract: A detailed immunohistochemical
analysis. Tohoku J Exp Med. 229:301–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scardoni M, Vittoria E, Volante M, Rusev
B, Bersani S, Mafficini A, Gottardi M, Giandomenico V, Malleo G,
Butturini G, et al: Mixed adenoneuroendocrine carcinomas of the
gastrointestinal tract: Targeted next-generation sequencing
suggests a monoclonal origin of the two components.
Neuroendocrinology. 100:310–316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lewin K: Carcinoid tumors and the mixed
(composite) glandular-endocrine cell carcinomas. Am J Surg Pathol.
11 (Suppl 1):S71–S86. 1987. View Article : Google Scholar
|
|
28
|
Liu Z, Zhang DY, Lu Z, Zhang P, Sun WL, Ma
X, Wu H, Wu BQ and Zhou S: Neuroendocrine tumor of the common bile
duct: A case report and review of the literature. Onco Targets
Ther. 11:2295–2301. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Squillaci S, Marchione R, Piccolomini M,
Colombo F, Bucci F, Bruno M and Bisceglia M: Well-differentiated
neuroendocrine carcinoma (malignant carcinoid) of the extrahepatic
biliary tract: Report of two cases and literature review. APMIS.
118:543–556. 2010.PubMed/NCBI
|
|
30
|
Zhan J, Bao G, Hu X, Gao W, Ruo X, Gong J,
Zhu Q and Liu Y: Carcinoid tumor of the common bile duct in
children: A case report. J Pediatr Surg. 45:2061–2063. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cappell MS, Killeen TC and Jury R: Common
bile duct carcinoid mimicking the clinical, EUS, and ERCP findings
of cholangiocarcinoma: A rare but potentially curable cause of
obstructive jaundice. Clin Gastroenterol Hepatol. 9:e112–e113.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee JH, Lee KG, Oh YH, Paik SS, Park HK
and Lee KS: Carcinoid tumors of the extrahepatic biliary tract:
Report of four cases. Surg Today. 41:430–435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bhalla P, Powle V, Shah RC and Jagannath
P: Neuroendocrine tumor of common hepatic duct. Indian J
Gastroenterol. 31:144–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
De Luca L, Tommasoni S, de Leone A,
Bianchi ML, de Nictolis M and Baroncini D: Neuroendocrine tumor of
the extrahepatic bile duct: A tumor in an unusual site visualized
by cholangioscopy. Endoscopy. 45 (Suppl 2):E338–E339. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jethava A, Muralidharan V, Mesologites T,
Stoica-Mustafa E and Dasanu CA: An unusual presentation of a
carcinoid tumor of the common bile duct. JOP. 14:85–87.
2013.PubMed/NCBI
|
|
36
|
Yasuda T, Imai G, Takemoto M, Yamasaki M,
Ishikawa H, Kitano M, Nakai T and Takeyama Y: Carcinoid tumor of
the extrahepatic bile duct: Report of a case. Clin J Gastroenterol.
6:177–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ayllon-Teran MD, Valverde-Martinez A,
Diaz-Nieto R, Ciria-Bru R, Luque-Molina A, López-Cillero P and
Briceño-Delgado J: Carcinoid tumor of the common bile duct. Rev Esp
Enferm Dig. 106:560–561. 2014.PubMed/NCBI
|
|
38
|
Khuroo S, Rashid A, Bali RS, Mushtaque M
and Khuroo F: Carcinoid Klatskin tumour: A rare cause of
obstructive jaundice. Australas Med J. 7:243–246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park SB, Moon SB, Ryu YJ, Hong J, Kim YH,
Chae GB and Hong SK: Primary large cell neuroendocrine carcinoma in
the common bile duct: First Asian case report. World J
Gastroenterol. 20:18048–18052. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yalav O, Ulku A, Demiryurek H and Doran F:
A rare cause of bile duct obstruction in adolescence:
Neuroendocrine tumor. Turk J Gastroenterol. 25 (Suppl 1):S311–S312.
2014. View Article : Google Scholar
|
|
41
|
Kihara Y, Yokomizo H, Urata T, Nagamine M
and Hirata T: A case report of primary neuroendocrine carcinoma of
the perihilar bile duct. BMC Surg. 15:1252015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Banerjee JK, Saranga Bharathi R,
Shrivastava S and Ranjan P: Neuroendocrine tumor of distal bile
duct. Med J Armed Forces India. 72 (Suppl 1):S101–S104. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hosoda K, Kobayashi A, Shimizu A, Kitagawa
N, Ito T, Yamada A and Miyagawa S: Neuroendocrine tumor of the
common bile duct. Surgery. 160:525–526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Murakami M, Katayama K, Kato S, Fujimoto
D, Morikawa M, Koneri K, Hirono Y and Goi T: Large-cell
neuroendocrine carcinoma of the common bile duct: A case report and
a review of literature. Surg Case Rep. 2:1412016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Oshiro Y, Gen R, Hashimoto S, Oda T, Sato
T and Ohkohchi N: Neuroendocrine carcinoma of the extrahepatic bile
duct: A case report. World J Gastroenterol. 22:6960–6964. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Raspanti C, Falco N, Silvestri V, Rotolo
G, Bonventre S and Gulotta G: Neuroendocrine tumor of the common
bile duct: Case report. G Chir. 37:275–280. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Abe T, Nirei A, Suzuki N, Todate Y, Azami
A, Waragai M, Sato A, Takano Y, Nishino N, Sakuma H and Teranishi
Y: Neuroendocrine tumor of the extrahepatic bile duct: A case
report. Int J Surg Case Rep. 40:6–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Costin AI, Păun I, Păun M, Constantin VD
and Vârcuş F: Primary neuroendocrine tumors-an extremely rare cause
of obstruction of extrahepatic bile ducts: A case report. Rom J
Morphol Embryol. 58:641–644. 2017.PubMed/NCBI
|
|
49
|
Hoepfner L and White JA: Primary
extrahepatic bile duct neuroendocrine tumor with obstructive
jaundice masquerading as a Klatskin tumor. J Surg Case Rep.
2017:rjx1042017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Khan FA, Stevens-Chase A, Chaudhry R,
Hashmi A, Edelman D and Weaver D: Extrahepatic biliary obstrution
secondary to neuroendocrine tumor of the common hepatic duct. Int J
Surg Case Rep. 30:46–49. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sanchez Cabus S, Pittau G, Sebagh M and
Cherqui D: Primary non-functioning neuroendocrine tumor of the
extrahepatic bile duct. Rev Esp Enferm Dig. 109:228–229.
2017.PubMed/NCBI
|
|
52
|
Sano I, Kuwatani M, Sugiura R, Kato S,
Kawakubo K, Ueno T, Nakanishi Y, Mitsuhashi T, Hirata H, Haba S, et
al: Hepatobiliary and pancreatic: A rare case of a
well-differentiated neuroendocrine tumor in the bile duct with
spontaneous regression diagnosed by EUS-FNA. J Gastroenterol
Hepatol. 32:112017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Koo JY, Kim KH and Kim TN: Primary large
cell neuroendocrine carcinoma of the common hepatic duct mimicking
a Klatskin tumor. Korean J Intern Med. 34:452–453. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Oberg K, Knigge U, Kwekkeboom D and Perren
A; ESMO Guidelines Working Group, : Neuroendocrine
gastro-entero-pancreatic tumors: ESMO clinical practice guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 23 (Suppl
7):vii124–vii130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hong N, Kim HJ, Byun JH, Kim SY, Kim KW,
Kim JH and Hong SM: Neuroendocrine neoplasms of the extrahepatic
bile duct: Radiologic and clinical characteristics. Abdom Imaging.
40:181–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sundin A, Arnold R, Baudin E, Cwikla JB,
Eriksson B, Fanti S, Fazio N, Giammarile F, Hicks RJ, Kjaer A, et
al: ENETS consensus guidelines for the standards of care in
neuroendocrine tumors: Radiological, nuclear medicine & hybrid
imaging. Neuroendocrinology. 105:212–244. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Perren A, Couvelard A, Scoazec JY, Costa
F, Borbath I, Delle Fave G, Gorbounova V, Gross D, Grossma A, Jense
RT, et al: ENETS consensus guidelines for the standards of care in
neuroendocrine tumors: Pathology: Diagnosis and prognostic
stratification. Neuroendocrinology. 105:196–200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kaltsas G, Caplin M, Davies P, Ferone D,
Garcia-Carbonero R, Grozinsky-Glasberg S, Hörsch D, Tiensuu Janson
E, Kianmanesh R, Kos-Kudla B, et al: ENETS consensus guidelines for
the standards of care in neuroendocrine tumors: Pre- and
perioperative therapy in patients with neuroendocrine tumors.
Neuroendocrinology. 105:245–254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sorbye H, Welin S, Langer SW, Vestermark
LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K,
et al: Predictive and prognostic factors for treatment and survival
in 305 patients with advanced gastrointestinal neuroendocrine
carcinoma (WHO G3): The NORDIC NEC study. Ann Oncol. 24:152–160.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Brathwaite S, Yearsley MM, Bekaii-Saab T,
Wei L, Schmidt CR, Dillhoff ME, Frankel WL, Hays JL, Wu C and
Abdel-Misih S: Appendiceal mixed Adeno-Neuroendocrine carcinoma: A
Population-based study of the surveillance, epidemiology, and end
results registry. Front Oncol. 6:1482016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Rayhan N, Sano T, Qian ZR, Obari AK and
Hirokawa M: Histological and immunohistochemical study of composite
neuroendocrine-exocrine carcinomas of the stomach. J Med Invest.
52:191–202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
La Rosa S, Marando A, Furlan D, Sahnane N
and Capella C: Colorectal poorly differentiated neuroendocrine
carcinomas and mixed adenoneuroendocrine carcinomas: Insights into
the diagnostic immunophenotype, assessment of methylation profile,
and search for prognostic markers. Am J Surg Pathol. 36:601–611.
2012. View Article : Google Scholar : PubMed/NCBI
|