Introduction
Although the incidence rate of gastric cancer (GC)
has decreased ~3% per year in the last few decades, GC has been
reported as the second leading cause of cancer-associated mortality
worldwide, behind lung cancer in 2012 (1). Unfortunately, the majority of GC cases
are diagnosed at an advanced stage; therefore, the clinical outcome
of patients with GC remains poor and the 5-year survival rate of
patients with advanced metastatic disease is <30% (2). Therefore, the most effective treatment
strategies for patients with advanced GC include chemotherapy and
novel targeted therapies (2). Unlike
other tumor types that have a number of therapeutic options based
on the molecular characteristic of the tumor, the genomic landscape
of GC is highly heterogeneous (3).
Therefore, it can be difficult to target the entire GC tumor as
sub-clones of GC cells possess different biological behaviors
(4). In addition to Trastuzumab, an
anti-human epidermal growth factor receptor 2 (HER2) antibody, and
Ramucirumab, an anti-vascular endothelial growth factor receptor
antibody (5), the Food and Drugs
Administration (FDA) has recently approved Pembrolizumab, an
anti-programmed cell death protein-1 (PD-1) antibody, for the
treatment of patients with metastatic gastric cancer and high PD-L1
immunohistochemical expression (6,7).
Immunotherapy has revolutionized cancer treatment by introducing
new therapies, including checkpoint inhibitors that target the host
immune system instead of tumor cells (8).
The PD-1 and programmed death-ligand 1 (PD-L1)
pathway is one of the most widely studied immune checkpoint
pathways as it is crucial in physiological conditions for the
maintenance of self-tolerance and preventing autoimmune diseases
(9), and for evading antitumor
immunity (10). PD-1 is an
immune-inhibitory receptor that is constitutively expressed by
activated T lymphocytes and macrophages, whereas other non-T
lymphocytes, including B cells and natural killer cells, only
express PD-1 following cytokine-induced stimulation (11,12). In
addition to being constitutively expressed by T cells, B cells,
macrophages and dendritic cells, PD-L1 is also expressed on tumor
cells (TCs) in several types of cancer. The interaction between
PD-1 on T cells and PD-L1 on TCs inhibits the cluster of
differentiation 8 (CD8)+ T cell-mediated immune
response, which induces an immunosuppressive microenvironment
within the tumor and promotes evasion of immune destruction
(12). PD-1/PD-L1 inhibitors can
restore antitumor immunity and immunotherapy using immune
checkpoint inhibitors has been reported to be effective in certain
cancer types, including malignant melanoma (13) and non-small cell lung cancer
(14). However, the sensitivity to
Pembrolizumab may not be the same in all patients eligible for
immunotherapy; therefore, it may be useful to identify adjunctive
biomarkers associated with the expression of PD-L1, which may allow
a more accurate and targeted selection of eligible patients.
In colorectal cancer, it has been demonstrated that
the majority of patients with mismatch repair deficiency (MMR-D)
are good responders to anti-PD-1/PD-L1 therapy (15,16). The
mismatch repair (MMR) pathway is a DNA repair mechanism that
recognizes and removes DNA replication errors. The loss of MMR
proteins leads to an accumulation of DNA replication errors, which
is termed microsatellite instability (MSI) (17). Proteins translated from genes with
DNA replication errors can be immunogenic and provoke an antitumor
immune response by increasing immune cell infiltration, which
improves the sensitivity to PD-1/PD-L1 blockade therapy (15,16).
The Cancer Genome Atlas Research Network revealed
that PD-L1 gene amplification was higher in Epstein Barr virus
(EBV)+ GC (18). In 1990,
Burke et al (19) first
reported an association between EBV status and the occurrence of GC
with a characteristic lymphoepithelioma-like histology.
The expression of PD-L1 on the surface of TCs and in
immune cells can be evaluated by immunohistochemistry (IHC) and
serve as a predictive biomarker to identify patients that may
benefit from immunotherapy; however, it has been identified that
not all patients with a PD-L1+ status respond well to
immunotherapy (20). Therefore,
PD-L1 expression on TCs is currently considered an imperfect
predictor of the response to immune checkpoint inhibitor therapy
(15). For this reason, a number of
studies have begun to investigate the tumor microenvironment,
particularly focusing on the extent of tumor immune cell
infiltration (7,15,21–23). An
immunological classification of tumors into four different tumor
microenvironment immune types (TMITs) based on PD-L1 status and
low/high CD8+ tumor infiltrating lymphocyte (TIL)
density has been proposed and validated in melanoma (24,25).
However, to the best of our knowledge, few studies have applied
this classification in GC (26,27). To
promote a more accurate selection of patients, the present study
evaluated PD-L1 expression in the tumor microenvironment and
quantified tumor infiltrating CD8+ T cell density in a
number of GC cases characterized by MMR-D, HER2 and EBV status.
Materials and methods
Patients and tumor
characteristics
A total of 46 males and 24 females (median age, 65.8
years; age range, 34–83 years) who underwent a curative gastrectomy
for primary GC at the National Institute of Gastroenterology ‘S. de
Bellis’ (Castellana Grotte, Italy) between 2014 and 2017 were
included in the current study. The inclusion criteria included no
previous chemotherapy, radiotherapy, Trastuzumab therapy or
anti-PD-1/PD-L1 treatment prior to surgery. The pathological and
clinical features of the patients are presented in Table I. The tumor site was proximal
(cardias, corpus and fundus) in 37 patients and distal
(antrum/pylorus) in 33 patients. According to the Lauren
classification (21,22,28), the
histological types of the 70 GC cases included 36 diffuse and 34
intestinal. Other pathological characteristics of the GC cases are
summarized in Table I. The present
study was approved by the Review Board of National Institute of
Gastroenterology (Castellana Grotte, Italy) and was conducted in
accordance with the Declaration of Helsinki. Prior to enrollment,
all participants provided written informed consent.
 | Table I.Association between PD-L1 expression
and clinicopathological characteristics. |
Table I.
Association between PD-L1 expression
and clinicopathological characteristics.
|
|
| PD-L1 TCs | PD-L1 IICs |
|---|
|
|
|
|
|
|---|
| Characteristic | Total (n=70), n
(%) | Positive (n=8), n
(%) | Negative (n=62), n
(%) | P-value | Positive (n=28), n
(%) | Negative (n=42), n
(%) | P-value |
|---|
| Sex |
|
|
| NSb |
|
| NSb |
|
Female | 24 (34.00) | 3 (12.50) | 21 (87.50) |
| 9
(37.50) | 15 (62.50) |
|
|
Male | 46 (66.00) | 5 (11.00) | 41 (89.00) |
| 19 (41.00) | 27 (59.00) |
|
| Age,
yearsa | 65.83±10.63 | 70.75±11.40 | 65.19±10.45 | NSd | 67.39±10.99 | 65.12±10.27 | NSd |
| Tumor site |
|
|
| NSb |
|
| NSb |
|
Distal | 33 (47.00) | 3 (9.00) | 30 (91.00) |
| 13 (39.00) | 20 (61.00) |
|
|
Proximal | 37 (53.00) | 5 (13.50) | 32 (86.50) |
| 15 (40.50) | 22 (59.50) |
|
| Histological
type |
|
|
| NSb |
|
|
<0.05b |
|
Diffuse | 36 (51.00) | 5 (14.00) | 31 (86.00) |
| 9
(25.00) | 27 (75.00) |
|
|
Intestinal | 34 (49.00) | 3 (9.00) | 31 (91.00) |
| 19 (56.00) | 15 (44.00) |
|
| Tumor grade
(65) |
|
|
| NSc |
|
| NSb |
|
G1+G2 | 12 (17.00) | 0 (0.00) | 12
(100.00) |
| 6
(50.00) | 6
(50.00) |
|
| G3 | 58 (83.00) | 8 (14.00) | 50 (86.00) |
| 22 (38.00) | 36 (62.00) |
|
| Pattern of
growth |
|
|
| NSb |
|
|
<0.05b |
|
Pushing | 14 (20.00) | 3 (21.00) | 11 (79.00) |
| 9
(64.00) | 5
(36.00) |
|
|
Infiltrating | 56 (80.00) | 5 (9.00) | 51 (91.00) |
| 19 (34.00) | 37 (66.00) |
|
| Tumor Budding |
|
|
| NSb |
|
| NSb |
|
Absent | 25 (36.00) | 2 (8.00) | 23 (92.00) |
| 13 (52.00) | 12 (48.00) |
|
|
High | 45 (64.00) | 6 (13.00) | 39 (87.00) |
| 15 (33.00) | 30 (67.00) |
|
| IICs |
|
|
|
<0.05b |
|
| NSb |
|
Mild | 45 (64.00) | 2 (4.00) | 43 (96.00) |
| 15 (33.00) | 30 (67.00) |
|
|
Marked | 25 (36.00) | 6 (24.00) | 19 (76.00) |
| 13 (52.00) | 12 (48.00) |
|
| pT status |
|
|
| NSc |
|
| NSb |
|
T1-T2 | 10 (14.00) | 0 (0.00) | 10 (100.00) |
| 4
(40.00) | 6
(60.00) |
|
|
T3-T4 | 60 (86.00) | 8 (13.00) | 52 (87.00) |
| 24 (40.00) | 36 (60.00) |
|
| pN status |
|
|
| NSb |
|
| NSb |
| N0 | 13 (19.00) | 3 (23.00) | 10 (77.00) |
| 7 (54.00) | 6
(46.00) |
|
| N+ | 57 (81.00) | 5 (9.00) | 52 (91.00) |
| 21 (37.00) | 36 (63.00) |
|
| MMR status |
|
|
|
<0.05b |
|
|
<0.05c |
|
Deficient | 7
(10.00) | 4 (57.00) | 3
(43.00) |
| 7 (100.00) | 0 (0.00) |
|
|
Proficient | 63 (90.00) | 4 (6.00) | 59 (94.00) |
| 21 (33.00) | 42 (67.00) |
|
| HER2 status |
|
|
| NSb |
|
| NSb |
|
Positive | 19 (27.00) | 2 (10.50) | 17 (89.50) |
| 8
(42.00) | 11 (58.00) |
|
|
Negative | 51 (73.00) | 6 (12.00) | 45 (88.00) |
| 20 (39.00) | 31 (61.00) |
|
|
CD8+ |
|
|
|
<0.05b |
|
| NSb |
|
High | 16 (23.00) | 5 (31.00) | 11 (69.00) |
| 7
(44.00) | 9
(56.00) |
|
|
Low | 54 (77.00) | 3 (5.50) | 51 (94.50) |
| 21 (39.00) | 33 (61.00) |
|
| PDL1 TCs |
|
|
| – |
|
|
<0.05b |
|
Positive | 8
(11.00) | – | – |
| 6
(75.00) | 2
(25.00) |
|
|
Negative | 62 (89.00) | – | – |
| 22 (35.00) | 40 (65.00) |
|
Paraffin-embedded sections (4-µm-thick) were fixed
with 10% neutral buffered formalin at room temperature for 24–48 h,
immunostained, and in situ hybridization assays were
performed. For each tumor, the histological subtype and tumor stage
were reevaluated from hematoxylin and eosin (H&E)-stained
slides (29) using a light
microscope (magnification, ×5-40). Age, sex, nodal status and
quantification of infiltrating immune cells (IICs), which encompass
intratumoral and peritumoral lymphocytes, macrophages and plasma
cells, were obtained from pathology reports and review of H&E
slides. IICs were evaluated as ‘mild’ when few cells (30–40 cells)
were stained within the tumor and/or at the tumor-stroma interface
(not deforming the distance between the glands), and ‘marked’
(>100 cells) when the infiltrate exhibited a greater density
with a tendency to flow into plaques and infiltrate the neoplastic
epithelium, deforming the distance between the glands.
Survival time was defined as the time from the date
of surgery to the date of mortality or of the last successful
interview. The median follow-up time was 24 months (range, 3–168
months). Patients who succumbed to the disease due to complications
of the surgical procedure during the perioperative period or
contact was lost prior to the first interview were excluded from
the survival analysis.
IHC staining
IHC was performed on 10% neutral buffered
formalin-fixed (room temperature for 24–48 h), paraffin-embedded
sections (4-µm-thick). IHC with anti-PD-L1 (cat. no. 13684S, clone
E1L3N; Cell Signaling Technology, Inc., Danvers, MA, USA) diluted
1:800 at room temperature for 30 min was performed as previously
described (23).
IHC with anti-CD8 (cat. no. M7103, Mab C8/144B;
1:200; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA),
anti-MutL homolog 1 (MLH1; cat. no. M3640, Mab ES05; 1:50; Dako
Agilent Technologies, Inc. Santa Clara, CA, USA) and anti-HER2
(polyclonal antibody, cat. no. A0485; 1:200; Dako; Agilent
Technologies, Inc.) was analyzed on an automated autostainer (Dako;
Agilent Technologies, Inc.). Briefly, sections were dewaxed in an
oven for 20 min at 60°C, followed by two washes with xylene. The
sections were then rehydrated in a graded alcohol series (ethanol
99, 95, 70%) and incubated with 3% hydrogen peroxide for 10 min at
room temperature to block endogenous peroxidase activity. Sections
were incubated with EDTA buffer (pH 8) at 98°C for 30 min for CD8
and MLH1 staining, and with 10 mM citrate buffer (pH 6) at 98°C for
30 min for HER2 staining. Monoclonal antibodies against CD8 and
MLH1, and a polyclonal antibody against HER2 were incubated with
the tissue sections (30 min at room temperature). PBS alone was
used as the negative control. Dako Real ENVISION (cat. no. K5007;
Dako; Agilent Technologies, Inc.) was used as visualization reagent
(30 min at room temperature) and 3,3′-diaminobenzidine as chromogen
(10 min at room temperature), according to the manufacturer´s
protocol.
PD-L1 immunoreactivity was randomly evaluated
separately for TCs and IICs. Cells were counted in 5
randomly-selected fields. PD-L1 positivity was defined as ≥5%
positive cells with membrane staining of any intensity (30). Cytoplasmic staining was not
considered in the present study. For the evaluation of CD8, five
fields (radius, 150 µm) in the intratumoral and peritumoral areas
were selected. The number of positive cells was counted and the
median number of CD8+ T lymphocytes was used as the
cut-off value to classify low/high CD8+ TIL cases of GC
(31). Tumors with no MLH1 staining
in the TC nuclei were defined as MMR deficient, while tumors with
nuclear staining were classified as MMR proficient (MMR-P). HER2
status was evaluated as previously described by Hofmann et
al (32). All IHC staining was
independently scored by two pathologists, Dr Raffaele Armentano and
Dr Maria Lucia Caruso from the Department of Pathology (National
Institute of Gastroenterology ‘S. de Bellis’, Research Hospital,
Castellana Grotte, Italy).
HER2 chromogenic in situ hybridization
(CISH)
Cases with an IHC score of 2+ for HER2 were
subjected to CISH for the evaluation of HER2 gene amplification.
CISH was performed using the ZytoDot2C SPEC ERBB2/CEN17 probe kit
(ZytoVision GmBH, Bremerhaven, Germany). Tissue sections (4
µm-thick) were deparaffinized, incubated in pretreatment buffer for
15 min at 98–100°C and then washed with distilled water. Following
enzymatic digestion for 15 min at room temperature, sections were
dehydrated and incubated with 15 µl HER2 probe at 80°C for 5 min
for denaturation and then at 37°C overnight for hybridization in a
Thermobrite Hybridizer (StatSpin, Norwood, MA, USA). To prevent
evaporation during incubation, standard coverslips and rubber
cement were used. Following removal of the coverslips, the slides
were washed in stringent wash buffer for 5 min at 75°C. HER2 gene
amplification was detected by sequential incubation with
anti-DIG/DNP-mix, HRP/AP-Polymer-mix, AP-Red solution and HRP-Green
solution. Finally, the slides were counterstained with hematoxylin
(1 min at room temperature). Sections of breast cancer tissue known
to be HER2-amplified were included as the positive control in each
run.
The CISH signal was evaluated using a light
microscope (magnification, ×40) following review of the H&E and
IHC stained slides to identify areas of invasive GC with the
strongest intensity of HER2 expression. The TC nuclei were
evaluated to calculate the ratio of HER2 to centromere 17 (CEN-17)
signals. HER2 amplification was defined as a HER2/CEN-17 ratio ≥2.
If the ratio was <2, an average number of HER2 signals per cell
count <4 indicated no HER2-amplification and an average number
of signals per cell count >6 indicated HER2 amplification. A
mean average ≥4 and <6 indicated an equivocal result. For an
equivocal result performing IHC or CISH in a different patient
sample was recommended (33). For
the evaluation of HER2 status, cases with an IHC score of 3+ or 2+
with gene amplification were regarded as HER2+ and the
remaining cases were defined as HER2−.
EBV status
CISH for EBV-encoded RNA (EBER) was performed using
an RNAscope detection kit (Advanced Cell Diagnostics, Newark, CA,
USA), according to the manufacturer's protocol. Tissue sections
(4-µm-thick) were baked at 60°C for 1 h and then deparaffinized.
Deparaffinized slides were boiled for 10 min with citric buffer (pH
6.0) for antigen retrieval. Slides were further permeabilized by
protease treatment at 40°C for 30 min in a HybEZ hybridization oven
(Advanced Cell Diagnostics), followed by hybridization with the
target probe, amplification and detection by adding Amp1-4.
Chromogenic detection was performed using 3,3′-diaminobenzidine
followed by counterstaining with hematoxylin (1 min at room
temperature). The kit included slides with HeLa cultured cells that
were used as a positive or negative control depending on the probes
adopted for hybridization. Samples stained brown in any TC nuclei
were considered positive by using a light microscope
(magnification, ×10 and ×20).
Statistical analysis
Continuous variables are presented as the mean ±
standard deviation and categorical variables are presented as the
relative frequency (%). To investigate associations between the
categorical variables a χ2 test or Fisher's exact test
were used, depending on the sample size. For non-parametric
variables, analyses were performed by Mann-Whitney U test or
Kruskal-Wallis test. The post hoc test used was Mann Whitney U with
Bonferroni's correction test. For survival analysis, Kaplan-Meier
analysis followed by a Wilcoxon-Breslow test and log-rank test was
used to evaluate the importance of single variables as prognostic
factors associated with survival. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analysis was performed using STATA v.12.1 statistical software
(StataCorp LP, College Station, TX, USA).
Results
PD-L1 immunohistochemical
expression
Different percentages of PD-L1 were observed on TCs
and IICs. All non-neoplastic gastric epithelia were identified to
be PD-L1−. Among the 70 patients, 8 cases (11%) were
revealed to possess PD-L1+ TCs (Table I), the majority of which revealed a
patchy pattern and rarely a diffused one. PD-L1 immunoreactivity on
TCs was significantly associated with a greater density of IICs
(P<0.05).
Furthermore, PD-L1 immunoreactivity was detected in
IICs in 40% of GC cases (Table I)
and was present within the tumor and at the interface between the
tumor and surrounding stroma, with a prevalently patchy pattern.
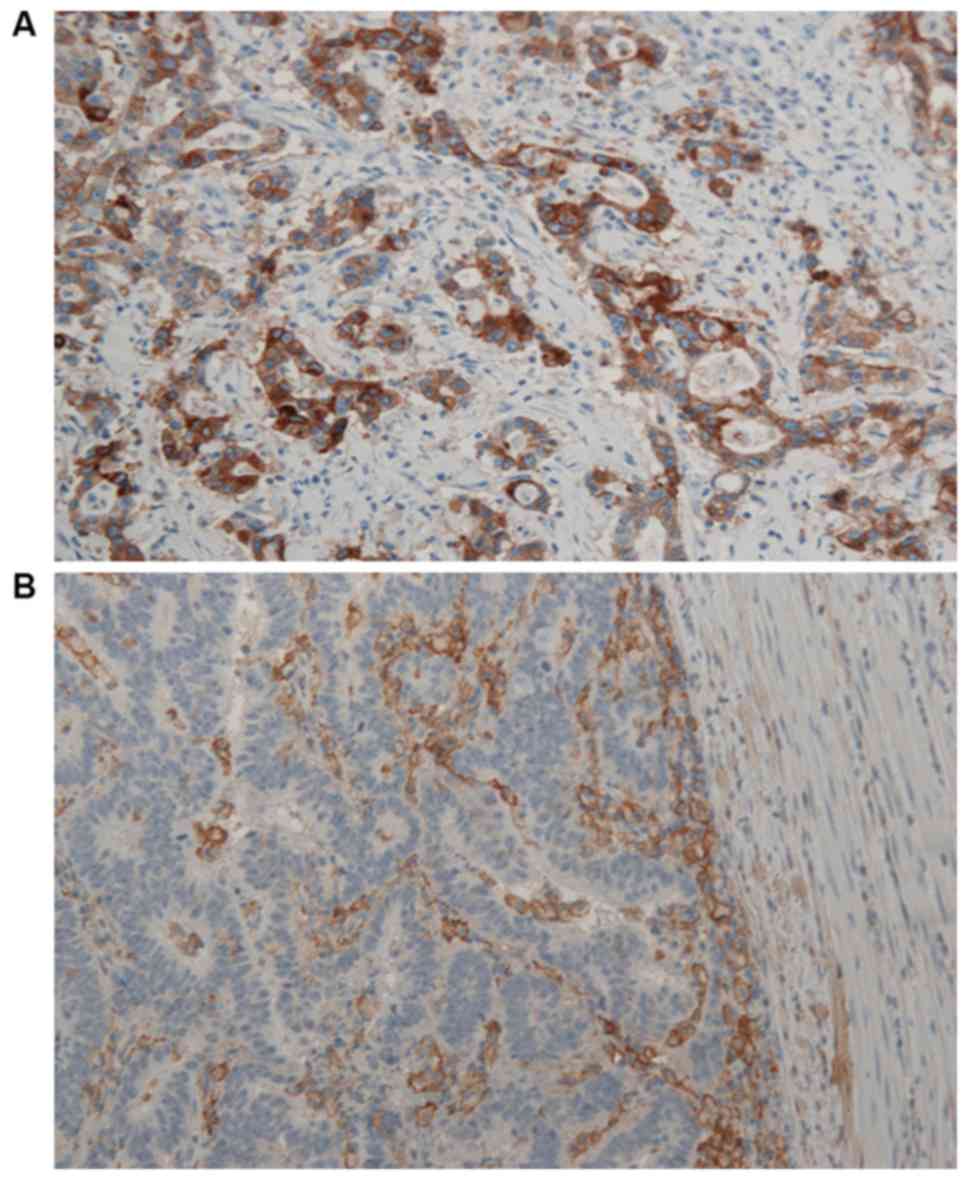
Representative images of PD-L1 immunohistochemical expression on
TCs and IICs are presented in Fig. 1A
and B, respectively. A total of six (75%) TC PD-L1+
GC cases were also IIC PD-L1+, while 35% of
PD-L1− GCs were IIC PD-L1+ (P<0.05;
Table I). PD-L1 positivity in IICs
was significantly higher in the intestinal-type cases of GC
compared with the diffuse-type cases (56 vs. 25%, P<0.05) and
significantly higher in GC cases with a pushing pattern of growth
compared with cases with an infiltrating pattern of growth (64 vs.
34%; P<0.05; Table I).
HER2 immunohistochemical expression
and association with PD-L1 staining
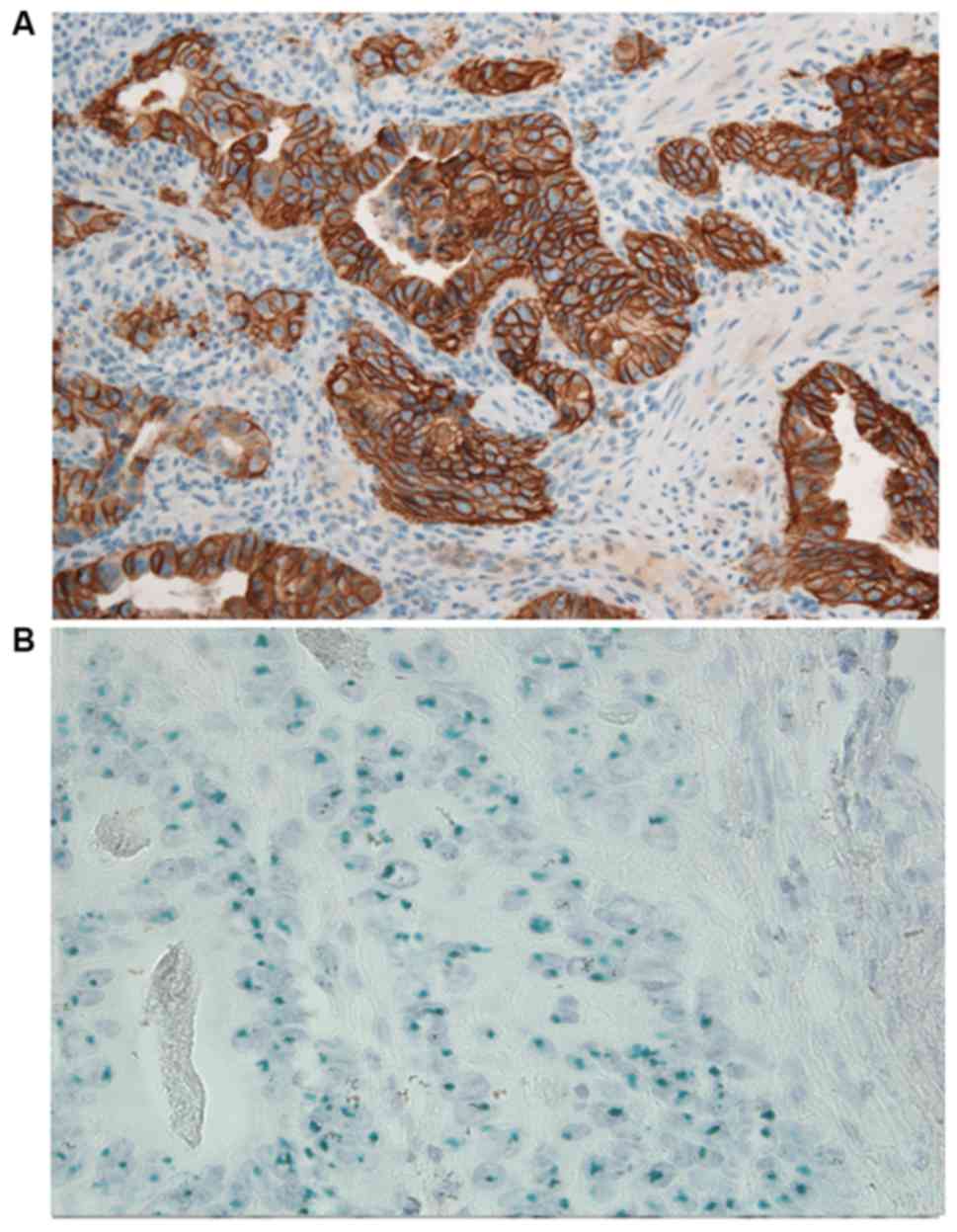
HER2 positivity was observed in 19 (27%) samples (13
IHC score, 3+; 6 IHC score, 2+/gene amplified; Fig. 2A and B). A total of 51 HER2- tumors
(25 IHC score, 0; 23 IHC score, 1; three IHC score, 2+ not
amplified) were identified (Table
II). Among the HER2+ samples, one (5%) sample was
PD-L1+ on TCs and IICs, seven (37%) were positive only
on IICs, and eleven (58%) were negative on the two. No significant
association was revealed between PD-L1 expression and HER2 status
(Table I).
 | Table II.Association between EBV status, PD-L1
expression and CD8+ TILs density. |
Table II.
Association between EBV status, PD-L1
expression and CD8+ TILs density.
|
|
| PD-L1 TCs | PD-L1 IICs | CD8+ TILs |
|---|
|
|
|
|
|
|
|---|
| Characteristic | Total (n=70), n
(%) | Positive (n=8), n
(%) | Negative (n=62), n
(%) |
P-valuea | Positive (n=28), n
(%) | Negative (n=42), n
(%) |
P-valueb | High (n=16), n
(%) | Low (n=54), n
(%) |
P-valuea |
|---|
| EBV status |
|
|
| <0.05 |
|
| NS |
|
| <0.05 |
|
Positive | 2 (3) | 2 (100) | 0 (0) |
| 1
(50) | 1
(50) |
| 2 (100) | 0 (0) |
|
|
Negative | 68 (97) | 6 (0) | 62
(100) |
| 27 (40) | 41 (60) |
| 14 (21) | 54 (79) |
|
MLH1 immunohistochemical expression
and association with PD-L1 staining
MLH1 deficiency was observed in seven (10%) cases
and was more frequent in PD-L1+ GC cases compared with
PD-L1− GC cases. Four (57%) GC cases that were
PD-L1+ on TCs were MLH1 deficient, whereas 43% of
PD-L1− TC GC cases were MLH1 deficient (P<0.05;
Table I). All GC cases that were MMR
deficient were PD-L1+ on IICs, whereas 33% of MMR
proficient GC cases were PD-L1+ on IICs (P<0.05;
Table I).
EBV status
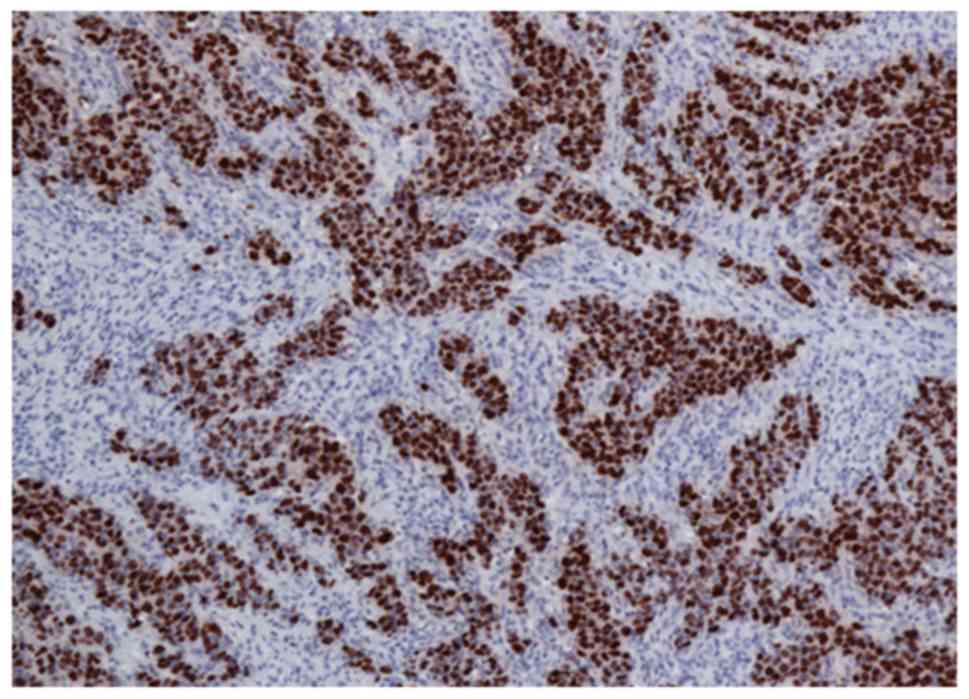
Among the 70 patients, two (3%) were positive for
EBV infection. The histological features demonstrated poorly
differentiated adenocarcinomas with a relatively rich lymphocyte
infiltration. EBV+ cases demonstrated intense nuclear
staining. EBV positivity was observed in all tumoral cells
aggregated in cords, which was clearly surrounded by
EBV− lymphocytes (Fig.
3). No cases revealed positivity for EBV in the non-tumoral
mucosa. The two EBV+ samples were PD-L1+ on
TCs (P<0.05; Table II).
CD8 immunohistochemical expression and
TMITs
CD8+ lymphocytes were present within
tumor-cell nests and intratumoral and peritumoral stroma. In total,
16 (23%) GC cases with a high CD8+ TILs density were
identified and 54 (77%) GC cases were revealed to have a low
CD8+ TILs density. The cases that were PD-L1+
on TCs more frequently featured a high CD8+ TILs density
compared with the PD-L1− cases (P<0.05; Table I), and the two EBV+
samples had a high CD8+ high density (P<0.05;
Table II).
Based on the results of immunohistochemical
expression of PD-L1 on TCs and CD8+ TILs density, the
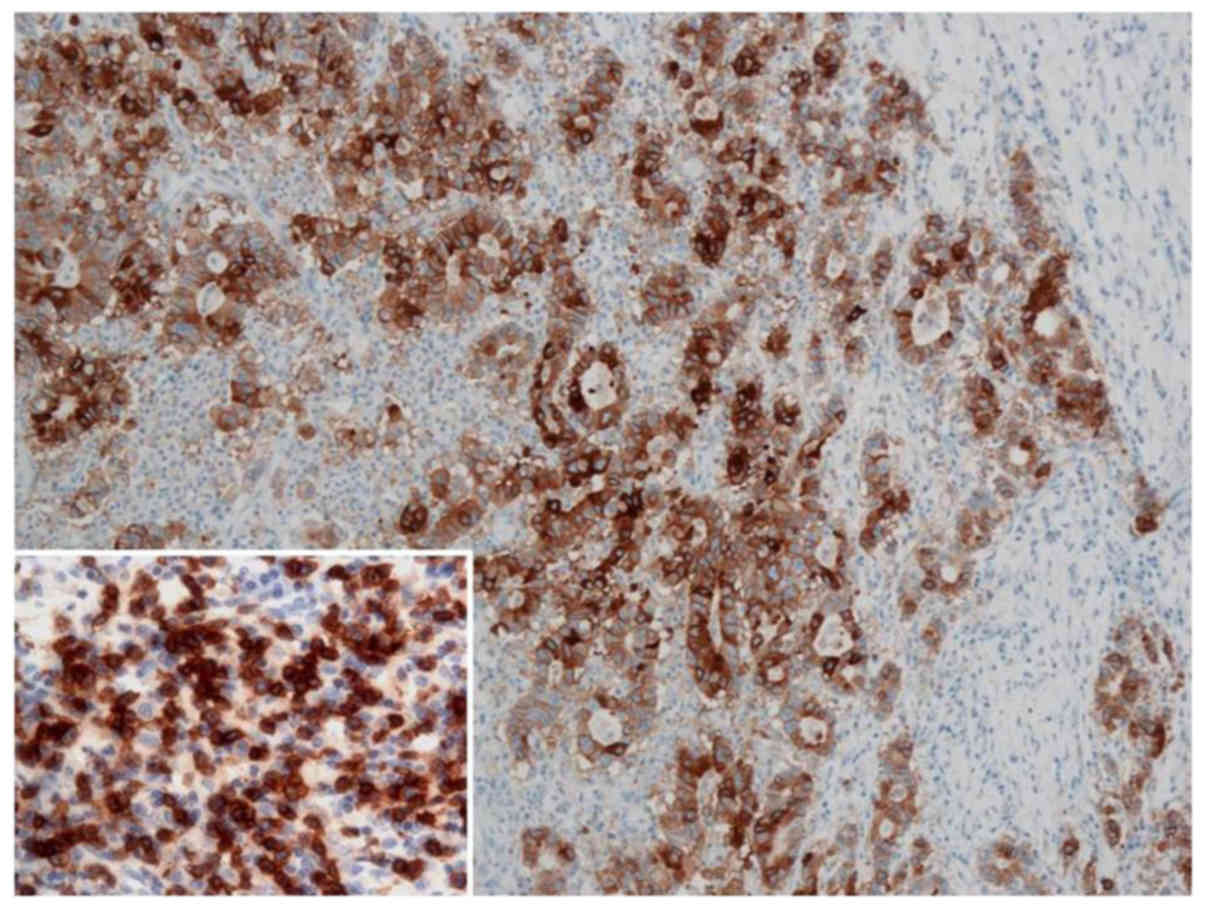
patients were categorized into the TMIT subgroups I–IV (34). The number and proportion in each TMIT
were as follows: five (7%) type I
(PD-L1+/CD8+ high; Fig. 4), 51 (73%) type II
(PD-L1−/CD8+ low), three (4%) type III
(PD-L1+/CD8+ low); eleven (16%) type IV
(PD-L1−/CD8+ high). TMITs were significantly
associated with pattern of growth, MMR status and EBV status
(Table III).
 | Table III.Association between TMIT and
clinicopathological characteristics. |
Table III.
Association between TMIT and
clinicopathological characteristics.
|
|
| TMIT |
|---|
|
|
|
|
|---|
| Characteristic | Total (n=70), n
(%) | I (n=5), n (%) | II (n=51), n
(%) | III (n=3), n
(%) | IV (n=11), n
(%) | P-value |
|---|
| Sex |
|
|
|
|
| NSb |
|
Female | 24 (34.00) | 1 (4.00) | 15 (62.50) | 2 (8.00) | 6 (25.50) |
|
|
Male | 46 (66.00) | 4 (9.00) | 36 (78.00) | 1 (2.00) | 5 (11.00) |
|
| Age,
yearsa | 65.83±10.63 | 65.50±14.39 | 64.60±10.66 | 76.00±4.76 | 67.67±9.53 | NSc |
| Tumor site |
|
|
|
|
| NSb |
|
Distal | 33 (47.00) | 1 (3.00) | 24 (73.00) | 2 (6.00) | 6 (82.00) |
|
|
Proximal | 37 (53.00) | 4
(11.00) | 27 (73.00) | 1 (3.00) | 5 (13.00) |
|
| Histological
type |
|
|
|
|
| NSb |
|
Diffuse | 36 (51.00) | 4
(11.00) | 25 (69.00) | 1 (3.00) | 6 (17.00) |
|
|
Intestinal | 34 (49.00) | 1 (3.00) | 26 (76.00) | 2 (6.00) | 5 (15.00) |
|
| Tumor grade
(65) |
|
|
|
|
| NSd |
|
G1+G2 | 12 (17.00) | 0 (0.00) | 12
(100.00) | 0 (0.00) | 0 (0.00) |
|
| G3 | 58 (83.00) | 5 (9.00) | 39 (70.00) | 3 (5.00) | 11 (16.00) |
|
| Pattern of
growth |
|
|
|
|
|
<0.05d |
|
Pushing | 14 (20.00) | 1 (7.00) | 9
(64.00) | 3
(22.00) | 1 (7.00) |
|
|
Infiltrating | 56 (80.00) | 4 (7.00) | 42 (75.00) | 0 (0.00) | 10 (18.00) |
|
| Tumor budding |
|
|
|
|
| NSd |
|
Absent | 25 (36.00) | 0 (0.00) | 20 (80.00) | 2 (8.00) | 3 (12.00) |
|
|
High | 45 (64.00) | 5
(11.00) | 31 (69.00) | 1 (2.00) | 8 (18.00) |
|
| pT status |
|
|
|
|
| NSd |
|
T1-T2 | 10 (14.00) | 0 (0.00) | 9
(90.00) | 0 (0.00) | 1
(10.00) |
|
|
T3-T4 | 60 (86.00) | 5 (8.00) | 42 (70.00) | 3 (5.00) | 10 (17.00) |
|
| pN status |
|
|
|
|
| NSb |
| N0 | 13 (19.00) | 1 (8.00) | 8
(61.50) | 1 (8.00) | 3 (22.50) |
|
| N+ | 57 (81.00) | 4 (7.00) | 43 (75.00) | 2 (3.50) | 8 (14.50) |
|
| MMR status |
|
|
|
|
|
<0.05d |
|
Deficient | 7
(10.00) | 2
(28.50) | 3 (43.00) | 2
(28.50) | 0 (0.00) |
|
|
Proficient | 63 (90.00) | 3 (5.00) | 48 (76.00) | 1 (2.00) | 11 (17.00) |
|
| HER2 status |
|
|
|
|
| NSb |
|
Positive | 19 (27.00) | 1 (5.00) | 15 (79.00) | 1 (5.00) | 2 (10.50) |
|
|
Negative | 51 (73.00) | 4 (8.00) | 36 (71.00) | 2 (4.00) | 9 (17.00) |
|
| EBV status |
|
|
|
|
|
<0.05d |
|
Positive | 2 (3.00) | 2 (100.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
|
|
Negative | 68 (97.00) | 3 (4.00) | 51 (75.00) | 3 (4.00) | 11 (17.00) |
|
Survival analysis
Survival data were available for 32 patients. The
median follow-up time was 24 months (range, 3–168 months). During
follow-up, 19 patients succumbed to GC and 13 were alive at the
last date of follow-up. No significant associations were revealed
with survival time (data not shown).
Discussion
Certain studies have demonstrated that PD-L1 is
highly expressed on TCs and IICs in GC (26,35–38).
However, the prognostic significance of PD-L1 remains controversial
and its role as a therapeutic predictor requires further
investigation.
A recent immune checkpoint blockade has received
much attention for prolonging survival of patients with GC
(39). In the KEYNOTE-059 study, a
phase II trial, 259 patients with advanced refractory GC received
Pembrolizumab until progression. The overall response rate was
15.5% in PD-L1+ patients versus 5.5% in
PD-L1− patients. The threshold of PD-L1
immunohistochemical positivity was 1% and took into account
staining on TCs and IICs (39).
Following the results of this trial, the FDA approved Pembrolizumab
for the treatment of patients with locally advanced or metastatic
gastric, or gastroesophageal junction cancer in which TCs express
PD-L1 (cut-off >1%) (6). Since
not all patients who are determined as eligible for a targeted
therapy demonstrate equal sensitivity, patient selection may be
improved by taking into account PD-L1 expression in addition to
other available biomarkers.
Furthermore, in accordance with the strategy of
combining immunotherapy and targeted therapy, two recent
multicenter studies have investigated the antitumor activity and
safety of Pembrolizumab in combination with anti-HER2 agents in
patients with HER2+ GC. The available data are at an
early stage; however, high response rates and the preliminary
overall survival data justify the interest associated with PD-1
inhibitors, which indicates immunotherapy may serve a significant
role in GC (40).
To gain more in-depth information, the present study
investigated PD-L1 expression in the tumor gastric
microenvironment, and evaluated its association with HER2, MMR and
EBV status. In agreement with previous studies (21,35,36), the
current study identified a higher expression level of PD-L1 on IICs
(40%) compared with TCs (11%). In previous studies, PD-L1 on TCs
was detected in 14–74% of patients with GCs (22,26,34,38,41)
compared with 30–88% on IICs (21,35). The
wide range of PD-L1 expression reported in previous studies may be
explained by the different antibodies used to detect PD-L1 or the
evaluation methods used to define PD-L1 positivity. The present
study used the monoclonal antibody E1L3N from Cell Signaling
Technology, Inc. as it is considered to have a higher sensitivity
compared with other PD-L1 antibodies (42). The cut-off value for PD-L1 remains
controversial. However, the majority of studies investigating GC
evaluated the expression of PD-L1 using a 1 or 5% cut-off value
(26,35,36,38). The
latter value was used in the current study since minimal PD-L1
expression may have no effect on tumor biology (35).
Although the present study had a number of intrinsic
limitations, our group has ample experience and has published
various articles regarding different aspects of the PD-1/PD-L1
pathway (23,43). A number of previous studies indicate
that immunohistochemical positivity should be considered with
caution (32,33), particularly when it is used for the
selection of patients suitable for therapy. Previously, extensive
evaluations of markers with a cutoff >1% have been replaced by
different markers as they were ineffective, for example, the use of
epidermal growth factor receptor and K-Ras has evolved over time in
targeted therapy with Cetuximab (44,45).
Furthermore, certain studies consider cytoplasmic
positivity for the purpose of evaluating positive cases (46,47). By
contrast, it has repeatedly been stressed that membrane positivity
is the only indicator of a receptor's presence (48). Unlike the strict immunohistochemical
evaluation of HER2 in mammary and gastric carcinomas, to the best
of our knowledge, precise guidelines do not exist for PD-L1,
including for the evaluation of PD-L1 expression on biopsy
material. However, the prevalent focal pattern of PD-L1 suggests
immunohistochemical analysis should be performed on multiple
biopsies to avoid underestimating the expression of PD-L1.
In the present study, the majority of patients who
were PD-L1+ on TC demonstrated PD-L1 positivity on IICs,
which suggests that PD-L1 expression in GCs is predominantly
controlled by an adaptive immune resistance induced by immune cells
via interferon-γ secretion rather than by an intrinsic pathway
(25). However, 31% of GCs were only
PD-L1+ on IICs. PD-L1 is constitutively expressed in certain immune
cells, including lymphocytes, macrophages and dendritic cells
(49); however, it can also be
induced by inflammatory cytokines (25). This likely reflects the combined
effect of innate and adaptive, and cellular and soluble factors
present in the tumor microenvironment (50). Certain studies have reported a
positive association between PD-L1 expression on IICs and the
response to PD-1 inhibitor treatment, which supports the use of
PD-L1 expression as a predictive marker for immunotherapy (42,51).
The positive association between PD-L1 expression on
IICs and intestinal type GC may be due to the different
pathogenesis of intestinal and diffuse histological types (52). The present study suggests that the
multistep carcinogenic pathway, caused by carcinogens, from chronic
gastric inflammation to intestinal type cancer could exert a
greater and more prolonged stimulation of the immune system,
including mucosa-associated lymphoid tissue, which eventually
becomes self-limited and induces the expression of PD-L1.
In agreement with Kawazoe et al (47), the present study demonstrated that
PD-L1 expression on TCs and/or IICs was not associated with
clinicopathological characteristics of poor prognosis. In addition,
PD-L1 expression on TCs was identified to be significantly
associated with high CD8+ TIL density, which is
associated with adaptive resistance. This indicates that the tumor
cells expressing PDL-1 in response to the cytokines produced by the
TILs are able to escape the immune response of the host through the
binding to PD-1 in T cells (25). By
contrast, no association was revealed between PD-L1 expression on
IICs and high CD8+ TILs, which indicates that the IICs
are not only composed of CD8+ T lymphocytes.
Furthermore, the current study evaluated the MMR
status only by an assessment of MLH1 protein expression, as it was
lost in >90% of GCs with MSI (4).
In the present study, the percentage of GCs with MMR-D (10%) was
consistent with a previous study that reported a frequency of
10–20% (4). In agreement with
previous studies (35,43), the current study demonstrated a
significant association between MMR-D and PD-L1 expression on TCs
and IICs. This finding can be explained as MMR deficient tumors are
considered to be highly immunogenic due to the heavy
mutation-associated-neoantigens burden, which is responsible for
adaptive immune resistance (15,16).
A number of studies support the hypothesis that TILs
exhibit a tumor prognostic value and an ‘immunoscore’, which takes
into account CD3+ and CD8+ TILs, has been
demonstrated to be a powerful prognostic and therapeutic indicator
(21,53). To identify which patients are
suitable for immunotherapy, a new classification of tumors has
recently been proposed, based on PD-L1 status on TCs and the
presence/absence of TILs (24,25).
This classification was developed to improve understanding of the
tumor immune microenvironment. However, the tumor microenvironment
is particularly complex and several critical issues have to be
considered for the interaction between PD-L1 and TILs, including
density, location and lymphocytes subpopulations. This
classification was considered too simplistic and was later modified
using immunohistochemical positivity for CD8 as a surrogate marker
for TILs (34). The new
classification consists of the four following TMITs: I
(PD-L1+/CD8+ high, adaptive immune
resistance), II (PD-L1−/CD8+ low, immune
ignorance type), III (PD-L1+/CD8+ low,
intrinsic induction of PD-L1) and IV
(PD-L1−/CD8+ high, tolerant tumors), which
indicates the role of other suppressors in promoting immune
tolerance (24). The proportion of
various cancer types that fit into each of these types likely
depends on other genetic aberrations and oncogene drivers, as well
as the type of tissue in which they originate (25).
Malignant melanoma has been extensively studied and
a high proportion of type I and II microenvironments has been
reported (25). Clinical studies
have demonstrated that ~38% of patients with advanced melanoma with
a type I profile are sensitive to anti-PD-L1 treatment. By
contrast, patients with melanoma with a type II tumor
microenvironment are not responsive to checkpoint blockade
(25). To the best of our knowledge,
similar information regarding GC remains unknown.
The present study classified patients with GC into
the TMITs I–IV based on the results of PD-L1 expression on TCs and
CD8+ TIL density. In agreement with previous studies
(21,54), it was identified that the majority of
GC cases belong to TMIT II (73%), which is the immune ignorance
type. Previous studies that have described TMITs in GC revealed
that the highest percentage of tumors were type II (21,27);
however, a higher frequency was identified in the present study.
This may be due to the cut-off value of 5% used in the current
study, which can lead to the identification of a larger number of
negative cases compared with studies that used a cut-off value of
1%. A low level of CD8+ TILs may partially explain the
high mortality rate of patients with GC and restrict the use of
antibodies that target immune checkpoints.
In agreement with previous studies (21,27), the
current study demonstrated that TMIT I GC cases are associated with
EBV infection and MSI status, which are characterized by heavy
lymphocytic infiltration (19).
EBERs are the most abundant latency-associated transcripts in
EBV-infected cells (55). Notably,
EBERs are very stable in formalin-fixed paraffin-embedded tissues,
which makes them sensitive EBV markers (55). CISH of EBER has been reported to be
beneficial for the detection of EBV-infected cells (56). The RNAscope is a novel RNA-ISH
platform that makes use of a novel probe design strategy and a
hybridization-based signal amplification system that can
theoretically yield up to 8,000 labels for each target RNA
molecule, resulting in high definition signals (57). The percentage of positive EBER GC
cases identified in the present study (3%) was lower compared with
that reported in a previous study (7–10%) (18), which may be due to the limited number
of lymphoepithelioma-like cases. In the current study, EBER was
exclusively detected in the TC compartment, and not in lymphocytes
and stromal cells, which is consistent with a previous study
(18). Furthermore, a high rate of
infiltration of CD8+ T cells is a characteristic of
EBV+ GC cases (58). The
EBV+ GC cases in the present study were associated with
high densities of CD8+ TILs and belonged to TMIT I. TMIT
I status involves adaptive immune escape responses and previous
studies suggest that GC cases with this signature can be reversed
by immune checkpoint blockade (24,59).
TMIT III was identified as the least frequent
category, which demonstrates that constitutive expression of PD-L1
on TCs through oncogenic signaling is not an important phenomenon
in GC. This group could include patients who exhibit positive PD-L1
expression on neoplastic cells but do not respond to therapy
(25). GC cases of TMIT IV contain a
high CD8+ TILs density, however, do not demonstrate
obvious adaptive resistance, as neoplastic gastric cells do not
express PD-L1.
Tumeh et al (60) reported that for patients with stage
III malignant melanoma, a predictive marker of clinical response to
PD-1 blockade is the density of CD8+ TILs and not PD-L1
expression itself. This could explain the responsiveness to therapy
of patients with negative PD-L1 expression on TCs.
Furthermore, in the present study, an association
between PD-L1 and HER2 was not identified. Li et al
(22) reported that PD-L1 expression
on GC TCs is associated with HER2 expression; however, the study
did not specify if the samples were HER2− or
HER2+. Oki et al (61) demonstrated that PD-L1+ GC cases were
associated with an increasing HER2 score. These previous studies
did not make a distinction between HER2+ versus
HER2− samples. Despite correctly evaluating the
positivity of HER2, according to Hofmann's criteria (32), Böger et al (35), Ju et al (30) and Wang et al (36) reported contradictory results
regarding the association between PD-L1 and HER2.
In agreement with a previous study (62), the present study revealed that 10.5%
of HER2+ GC samples versus 12% of HER− GC
samples exhibited PD-L1 expression on TCs. No HER2+
sample was identified to be MMR deficient. The Research Network of
The Cancer Genome Atlas data demonstrated that HER2 amplification
was more common in tumors with chromosomal instability compared
with microsatellite instability (18). Therefore, the positive association
between PD-L1 and MMR status may explain the low probability of
identifying GC samples positive for PD-L1 and HER2.
In GC, the association between PD-L1 expression and
survival remains to be fully understood (35,41,63,64).
Fang et al (41) demonstrated
that PD-L1 expression on TCs was not associated with overall
survival, while patients with PD-L1 expression on TILs had a
significantly shorter 5-year overall survival rate compared those
with negative PD-L1 expression. A recent study of a large cohort of
Caucasian patients with GC revealed that PD-L1 expression in tumor
and stromal immune cells was associated with improved survival
(34), while previous studies of an
Asian population revealed a poor prognostic role of PD-L1 (63,64).
Apart from the difference of ethnicity, these studies were
performed in heterogeneous populations, which contained patients
with various stages of cancer that were undergoing different
treatment strategies in different clinical settings.
In the present study, the small number of samples
did not allow any significant differences in survival time to be
identified between PD-L1+ and PDL1− patients,
even when taking into account the TMITs. In conclusion, the current
results, which require validation with a larger sample size, may
highlight a subset of patients with GC who exhibit PD-L1 expression
on TCs, a high density of CD8+ TILs and MMR deficiency.
To the best of our knowledge, for the first time the present study
demonstrated that a positivity of EBER, detected using the RNAscope
method, may be useful for predicting the effectiveness of
anti-PD-1/PD-L1 antibody therapy.
Acknowledgements
Not applicable
Funding
This study was supported by the Italian Ministry of
Health, Ricerca Corrente 2018.
Availability of data and materials
All data analyzed during this study are included in
this article.
Authors contributions
AMV and MLC conceived and designed the study. FDP
and SC collected samples for immunohistochemical analysis and
performed the immunohistochemical staining. AMV and RA were
responsible for immunohistochemical evaluation. VG analyzed the
data. FDP prepared the figures. AMV and MLC wrote the paper. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board (National Institute of Gastroenterology ‘S. de
Bellis’, Research Hospital, Castellana Grotte, Bari, Italy) and was
conducted in accordance with the Declaration of Helsinki. Prior to
enrollment, all parti cipants provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GC
|
gastric carcinoma
|
|
IHC
|
immunohistochemistry
|
|
IIC
|
infiltrating immune cell
|
|
MMR
|
mismatch repair
|
|
MMR-D
|
mismatch repair deficiency
|
|
MMR-P
|
mismatch repair proficient
|
|
MSI
|
microsatellite instability
|
|
PD-1
|
programmed cell death protein-1
|
|
PD-L1
|
programmed death-ligand 1
|
|
TC
|
tumor cell
|
|
TIL
|
tumor infiltrating lymphocyte
|
|
TMIT
|
tumor microenvironment immune
type
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yazici O, Sendur MA, Ozdemir N and Aksoy
S: Targeted therapies in gastric cancer and future perspectives.
World J Gastroenterol. 22:471–489. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wong SS, Kim KM, Ting JC, Yu K, Fu J, Liu
S, Cristescu R, Nebozhyn M, Gong L, Yue YG, et al: Genomic
landscape and genetic heterogeneity in gastric adenocarcinoma
revealed by whole-genome sequencing. Nat Commun. 5:54772014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee J and Kim KM: Biomarkers for gastric
cancer: Molecular classification revisited. Precis Future Med.
1:59–68. 2017. View Article : Google Scholar
|
|
5
|
Setia N, Agoston AT, Han HS, Mullen JT,
Duda DG, Clark JW, Deshpande V, Mino-Kenudson M, Srivastava A,
Lennerz JK, et al: A protein and mRNA expression-based
classification of gastric cancer. Mod Pathol. 29:772–784. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fashoyin-Aje L, Donoghue M, Chen H, He K,
Veeraraghavan J, Goldberg KB, Keegan P, McKee AE and Pazdur R: FDA
Approval Summary: Pembrolizumab for Recurrent Locally Advanced or
Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma
Expressing PD-L1. Oncologist. 24:103–109. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kulangara K, Zhang N, Corigliano E,
Guerrero L, Waldroup S, Jaiswal D, Ms MJ, Shah S, Hanks D, Wang J,
et al: Clinical Utility of the Combined Positive Score for
Programmed Death Ligand-1 Expression and the Approval of
Pembrolizumab for Treatment of Gastric Cancer. Arch Pathol Lab Med.
143:330–337. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Topalian SL, Drake CG and Pardoll DM:
Immune checkpoint blockade: A common denominator approach to cancer
therapy. Cancer Cell. 27:450–461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishimura H and Honjo T: PD-1: An
inhibitory immunoreceptor involved in peripheral tolerance. Trends
Immunol. 22:265–268. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boussiotis VA: Molecular and biochemical
aspects of the PD-1 checkpoint pathway. N Engl J Med.
375:1767–1778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fanoni D, Tavecchio S, Recalcati S, Balice
Y, Venegoni L, Fiorani R, Crosti C and Berti E: New monoclonal
antibodies against B-cell antigens: Possible new strategies for
diagnosis of primary cutaneous B-cell lymphomas. Immunol Lett.
134:157–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Terme M, Ullrich E, Aymeric L, Meinhardt
K, Desbois M, Delahaye N, Viaud S, Ryffel B, Yagita H, Kaplanski G,
et al: IL-18 induces PD-1-dependent immunosuppression in cancer.
Cancer Res. 71:5393–5399. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hino R, Kabashima K, Kato Y, Yagi H,
Nakamura M, Honjo T, Okazaki T and Tokura Y: Tumor cell expression
of programmed cell death-1 ligand 1 is a prognostic factor for
malignant melanoma. Cancer. 116:1757–1766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sui H, Ma N, Wang Y, Li H, Liu X, Su Y and
Yang J: Anti-PD-1/PD-L1 Therapy for Non-Small-Cell Lung Cancer:
Toward Personalized Medicine and Combination Strategies. J Immunol
Res. 2018:69849482018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Llosa NJ, Cruise M, Tam A, Wicks EC,
Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS,
et al: The vigorous immune microenvironment of microsatellite
instable colon cancer is balanced by multiple counter-inhibitory
checkpoints. Cancer Discov. 5:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao Y and Freeman GJ: The microsatellite
instable subset of colorectal cancer is a particularly good
candidate for checkpoint blockade immunotherapy. Cancer Discov.
5:16–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Caruso ML: Role of mismatch repair
proteins and microsatellite instability in colon carcinoma.
Immunohistochemistry and in situ hybridization of human carcinomas.
Hayat MA: Elsevier Academic Press; New York, NY: pp. 215–226.
2005
|
|
18
|
Bass AJ, Thorsson V, Shmulevich I,
Reynolds SM, Miller M, Bernard B, Hinoue T, Laird PW, Curtis C,
Shen H, et al Cancer Genome Atlas Research Network, : Comprehensive
molecular characterization of gastric adenocarcinoma. Nature.
513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burke AP, Yen TS, Shekitka KM and Sobin
LH: Lymphoepithelial carcinoma of the stomach with Epstein-Barr
virus demonstrated by polymerase chain reaction. Mod Pathol.
3:377–380. 1990.PubMed/NCBI
|
|
20
|
Tie Y, Ma X, Zhu C, Mao Y, Shen K, Wei X,
Chen Y and Zheng H: Safety and efficacy of nivolumab in the
treatment of cancers: A meta-analysis of 27 prospective clinical
trials. Int J Cancer. 140:948–958. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim KJ, Yang HK, Kim WH and Kang GH:
Combined prognostic effect of PD-L1 expression and immunoscore in
microsatellite-unstable advanced gastric cancers. Oncotarget.
8:58887–58902. 2017.PubMed/NCBI
|
|
22
|
Li Z, Lai Y, Sun L, Zhang X, Liu R, Feng
G, Zhou L, Jia L, Huang X, Kang Q, et al: PD-L1 expression is
associated with massive lymphocyte infiltration and histology in
gastric cancer. Hum Pathol. 55:182–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Valentini AM, Di Pinto F, Cariola F,
Guerra V, Giannelli G, Caruso ML and Pirrelli M: PD-L1 expression
in colorectal cancer defines three subsets of tumor immune
microenvironments. Oncotarget. 9:8584–8596. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Taube JM1, Anders RA, Young GD, Xu H,
Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL
and Chen L: Colocalization of inflammatory response with B7-h1
expression in human melanocytic lesions supports an adaptive
resistance mechanism of immune escape. Sci Transl Med.
4:127ra372012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Teng MWL, Ngiow SF, Ribas A and Smyth MJ:
Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer
Res. 75:2139–2145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koh J, Ock CY, Kim JW, Nam SK, Kwak Y, Yun
S, Ahn SH, Park DJ, Kim HH, Kim WH, et al: Clinicopathologic
implications of immune classification by PD-L1 expression and
CD8-positive tumor-infiltrating lymphocytes in stage II and III
gastric cancer patients. Oncotarget. 8:26356–26367. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma J, Li J, Hao Y, Nie Y, Li Z, Qian M,
Liang Q, Yu J, Zeng M and Wu K: Differentiated tumor immune
microenvironment of Epstein-Barr virus-associated and negative
gastric cancer: Implication in prognosis and immunotherapy.
Oncotarget. 8:67094–67103. 2017.PubMed/NCBI
|
|
28
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Feldman AT and Wolfe D: Tissue processing
and hematoxylin and eosin staining. Methods Mol Biol. 1180:31–43.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ju X, Shen R, Huang P, Zhai J, Qian X,
Wang Q and Chen M: Predictive relevance of PD-L1 expression with
pre-existing TILs in gastric cancer. Oncotarget. 8:99372–99381.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang W, Wang K, Chen Z, Chen L, Guo W,
Liao P, Rotroff D, Knepper TC, Liu Z, Zhang W, et al:
Immunoclassification characterized by CD8 and PD-L1 expression is
associated with the clinical outcome of gastric cancer patients.
Oncotarget. 9:12164–12173. 2018.PubMed/NCBI
|
|
32
|
Hofmann M, Stoss O, Shi D, Büttner R, van
de Vijver M, Kim W, Ochiai A, Rüschoff J and Henkel T: Assessment
of a HER2 scoring system for gastric cancer: Results from a
validation study. Histopathology. 52:797–805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bartley AN, Washington MK, Colasacco C,
Ventura CB, Ismaila N, Benson AB III, Carrato A, Gulley ML, Jain D,
Kakar S, et al: HER2 Testing and Clinical Decision Making in
Gastroesophageal Adenocarcinoma: Guideline From the College of
American Pathologists, American Society for Clinical Pathology, and
the American Society of Clinical Oncology. J Clin Oncol.
35:446–464. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ock CY, Keam B, Kim S, Lee JS, Kim M, Kim
TM, Jeon YK, Kim DW, Chung DH and Heo DS: Pan-Cancer Immunogenomic
Perspective on the Tumor Microenvironment Based on PD-L1 and CD8
T-Cell Infiltration. Clin Cancer Res. 22:2261–2270. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Böger C, Behrens HM, Mathiak M, Krüger S,
Kalthoff H and Röcken C: PD-L1 is an independent prognostic
predictor in gastric cancer of Western patients. Oncotarget.
7:24269–24283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang L, Zhang Q, Ni S, Tan C, Cai X, Huang
D and Sheng W: Programmed death-ligand 1 expression in gastric
cancer: Correlation with mismatch repair deficiency and
HER2-negative status. Cancer Med. 7:2612–2620. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu Y, Cao D, Qu L, Cao X, Jia Z, Zhao T,
Wang Q and Jiang J: PD-1 and PD-L1 co-expression predicts favorable
prognosis in gastric cancer. Oncotarget. 8:64066–64082.
2017.PubMed/NCBI
|
|
38
|
Harada K, Dong X, Estrella JS, Correa AM,
Xu Y, Hofstetter WL, Sudo K, Onodera H, Suzuki K, Suzuki A, et al:
Tumor-associated macrophage infiltration is highly associated with
PD-L1 expression in gastric adenocarcinoma. Gastric Cancer.
21:31–40. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T,
Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al: Safety and
Efficacy of Pembrolizumab Monotherapy in Patients With Previously
Treated Advanced Gastric and Gastroesophageal Junction Cancer:
Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 4:e1800132018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Apicella M, Corso S and Giordano S:
Targeted therapies for gastric cancer: Failures and hopes from
clinical trials. Oncotarget. 8:57654–57669. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fang W, Chen Y, Sheng J, Zhou T, Zhang Y,
Zhan J, Liu L, Huang J, Peng P and Zhang L: Association between
PD-L1 Expression on Tumour-Infiltrating Lymphocytes and Overall
Survival in Patients with Gastric Cancer. J Cancer. 8:1579–1585.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ying L, Yan F, Meng Q, Yuan X, Yu L,
Williams BRG, Chan DW, Shi L, Tu Y, Ni P, et al: Understanding
immune phenotypes in human gastric disease tissues by multiplexed
immunohistochemistry. J Transl Med. 15:2062017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cavalcanti E, Armentano R, Valentini AM,
Chieppa M and Caruso ML: Role of PD-L1 expression as a biomarker
for GEP neuroendocrine neoplasm grading. Cell Death Dis.
8:e30042017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Saltz LB, Meropol NJ, Loehrer PJ Sr,
Needle MN, Kopit J and Mayer RJ: Phase II trial of cetuximab in
patients with refractory colorectal cancer that expresses the
epidermal growth factor receptor. J Clin Oncol. 22:1201–1208. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cunningham D, Humblet Y, Siena S, Khayat
D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype
C, et al: Cetuximab monotherapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gu L, Chen M, Guo D, Zhu H, Zhang W, Pan
J, Zhong X, Li X, Qian H and Wang X: PD-L1 and gastric cancer
prognosis: A systematic review and meta-analysis. PLoS One.
12:e01826922017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kawazoe A, Kuwata T, Kuboki Y, Shitara K,
Nagatsuma AK, Aizawa M, Yoshino T, Doi T, Ohtsu A and Ochiai A:
Clinicopathological features of programmed death ligand 1
expression with tumor-infiltrating lymphocyte, mismatch repair, and
Epstein-Barr virus status in a large cohort of gastric cancer
patients. Gastric Cancer. 20:407–415. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Udall M, Rizzo M, Kenny J, Doherty J, Dahm
S, Robbins P and Faulkner E: PD-L1 diagnostic tests: A systematic
literature review of scoring algorithms and test-validation
metrics. Diagn Pathol. 13:122018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kwon D, Kim S, Kim PJ, Go H, Nam SJ, Paik
JH, Kim YA, Kim TM, Heo DS, Kim CW, et al: Clinicopathological
analysis of programmed cell death 1 and programmed cell death
ligand 1 expression in the tumour microenvironments of diffuse
large B cell lymphomas. Histopathology. 68:1079–1089. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Scognamiglio G, De Chiara A, Di Bonito M,
Tatangelo F, Losito NS, Anniciello A, De Cecio R, D'Alterio C,
Scala S, Cantile M, et al: Variability in Immunohistochemical
Detection of Programmed Death Ligand 1 (PD-L1) in Cancer Tissue
Types. Int J Mol Sci. 17:E7902016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Romano E and Romero P: The therapeutic
promise of disrupting the PD-1/PD-L1 immune checkpoint in cancer:
Unleashing the CD8 T cell mediated anti-tumor activity results in
significant, unprecedented clinical efficacy in various solid
tumors. J Immunother Cancer. 3:152015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Grabsch HI and Tan P: Gastric cancer
pathology and underlying molecular mechanisms. Dig Surg.
30:150–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jiang Y, Zhang Q, Hu Y, Li T, Yu J, Zhao
L, Ye G, Deng H, Mou T, Cai S, et al: ImmunoScore Signature: A
Prognostic and Predictive Tool in Gastric Cancer. Ann Surg.
267:504–513. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Choi YY, Bae JM, An JY, Kwon IG, Cho I,
Shin HB, Eiji T, Aburahmah M, Kim HI, Cheong JH, et al: Is
microsatellite instability a prognostic marker in gastric cancer? A
systematic review with meta-analysis. J Surg Oncol. 110:129–135.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shinozaki-Ushiku A, Kunita A and Fukayama
M: Update on Epstein-Barr virus and gastric cancer (review). Int J
Oncol. 46:1421–1434. 2015.(review). View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Niedobitek G, Young LS, Sam CK, Brooks L,
Prasad U and Rickinson AB: Expression of Epstein-Barr virus genes
and of lymphocyte activation molecules in undifferentiated
nasopharyngeal carcinomas. Am J Pathol. 140:879–887.
1992.PubMed/NCBI
|
|
57
|
Wang F, Flanagan J, Su N, Wang LC, Bui S,
Nielson A, Wu X, Vo HT, Ma XJ and Luo Y: RNAscope: A novel in situ
RNA analysis platform for formalin-fixed, paraffin-embedded
tissues. J Mol Diagn. 14:22–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nishikawa J, Iizasa H, Yoshiyama H,
Shimokuri K, Kobayashi Y, Sasaki S, Nakamura M, Yanai H, Sakai K,
Suehiro Y, et al: Clinical Importance of Epstein-Barr
Virus-Associated Gastric Cancer. Cancers (Basel). 10(pii):
E1672018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Taube JM, Klein A, Brahmer JR, Xu H, Pan
X, Kim JH, Chen L, Pardoll DM, Topalian SL and Anders RA:
Association of PD-1, PD-1 ligands, and other features of the tumor
immune microenvironment with response to anti-PD-1 therapy. Clin
Cancer Res. 20:5064–5074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Oki E, Okano S, Saeki H, Umemoto Y,
Teraishi K, Nakaji Y, Ando K, Zaitsu Y, Yamashita N, Sugiyama M, et
al: Protein Expression of Programmed Death 1 Ligand 1 and HER2 in
Gastric Carcinoma. Oncology. 93:387–394. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gonzalez RS, Messing S, Tu X, McMahon LA
and Whitney-Miller CL: Immunohistochemistry as a surrogate for
molecular subtyping of gastric adenocarcinoma. Hum Pathol.
56:16–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Eto S, Yoshikawa K, Nishi M, Higashijima
J, Tokunaga T, Nakao T, Kashihara H, Takasu C, Iwata T and Shimada
M: Programmed cell death protein 1 expression is an independent
prognostic factor in gastric cancer after curative resection.
Gastric Cancer. 19:466–471. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang L, Qiu M, Jin Y, Ji J, Li B, Wang X,
Yan S, Xu R and Yang D: Programmed cell death ligand 1 (PD-L1)
expression on gastric cancer and its relationship with
clinicopathologic factors. Int J Clin Exp Pathol. 8:11084–11091.
2015.PubMed/NCBI
|
|
65
|
Ohman U, Wetterfors J and Moberg A:
Histologic grading of gastric cancer. Acta Chir Scand. 138:384–390.
1972.PubMed/NCBI
|