Introduction
Cervical cancer is one of the most common
gynecological cancers. In China, cervical cancer is the second most
common cancer in females after breast cancer (1). In 2012, ~52,7600 new cases of cervical
cancer were diagnosed worldwide and 265700 patients succumbed
(2). The average age of diagnosis
for cervical cancer is 50 years, which is younger than that of
other types of cancer, including endometrial and ovarian cancers
(3). Pretreatment evaluation of
patients with cervical cancer, including staging and assessment of
prognostic factors, is required for predicting the prognosis and
determining the optimal treatment regimen (4).
Previous studies have revealed that patient age,
clinical symptoms, degree of pathological differentiation,
International Federation of Gynecology and Obstetrics (FIGO) stage,
status of lymph node metastasis and tumor size affect the prognosis
of patients with cervical cancer (5,6). Imaging
diagnosis of cervical cancer may assist clinical decision making.
Positron emission tomography (PET) and magnetic resonance (MR)
imaging serve important roles in the diagnosis and treatment of
tumors. Diffusion-weighted imaging (DWI) is an imaging method that
reflects the diffusion of water molecules through the apparent
diffusion coefficient (ADC), which is negatively correlated with
tumor cellularity. This method can reflect the diffusion limitation
and molecular density of tumor tissue (7). It is frequently used in tumor diagnosis
and prognosis evaluation (8).
18F-fluorodeoxyglucose (18F-FDG) PET reflects
glucose metabolism of the tumor, as the faster the tumor cells
proliferate, the higher their metabolic rate. Patients with
cervical cancer manifest lesions with marked elevations in
metabolic rate on PET images (9).
The standardized uptake value (SUV) is used to measure the glucose
uptake within a region of interest (ROI), and may be used to assess
tumor aggressiveness and prognosis (10). The maximum SUV (SUVmax)
may be used as an independent prognostic factor (11). SUV and ADC values are associated with
a number of pathological prognostic factors and may be used to
assess the prognosis of patients. The two aforementioned parameters
may serve a complementary role in describing tumor characteristics
and assessing the prognosis of cervical cancer. Unlike PET and MR
imaging, integrated PET/MR imaging provides multiparametric
functional images in a single examination, including a DWI
(7). Additionally,
18F-FDG PET/MR imaging provides metabolic information.
PET/MR imaging provides comprehensive information about tumor
characteristics, including the location, size and extent of the
lesion (12,13). Furthermore, deviations caused by
physiological changes during the time interval between PET and MR
imaging are avoided (7). Previous
studies have revealed that integrated PET/MR provides comprehensive
information and excellent image quality in the diagnosis and
staging of different types of cancers, and is superior to PET alone
(14–16).
Previous studies have revealed that the maximum
standard uptake value (SUVmax) has a negative
correlation with the minimum ADC (ADCmin) on PET/MR
imaging for various types of cancer, including endometrial
(11), rectal (17), cervical (18) and breast cancer (19). However, there are currently few
studies on the combination of DWI and PET functional imaging for
the detection of cervical cancer (13,18). The
aim of the current study was to evaluate the correlation between
the SUVmax and the ADCmin using an integrated
PET/MR imaging system, and to establish the correlation with
pathological prognostic factors.
Materials and methods
Patients
This study was approved the Ethics Committee of the
Chinese PLA General Hospital and all patients signed informed
consent forms. Patients were enrolled in the Chinese PLA General
Hospital (Beijing, China) between April 2016 and December 2017, and
follow up was conducted one time six months following surgery. All
patients had undergone PET/MR imaging examinations prior to
surgery, including total hysterectomy, bilateral pelvic lymph node
dissection and paraaortic lymph node dissection. A total of 46
patients were pathologically confirmed to have cervical cancer
(mean age, 56.5±12.1; range, 31–74). The inclusion criteria were as
follows: i) Clinical suspicion of cervical cancer; and ii)
histopathological diagnosis of cervical cancer following PET/MR
imaging. The exclusion criteria were as follows: i) Pregnant
patients; ii) patients with metal implants; iii) patients with
claustrophobia, iv) patients with impaired renal function; and v)
patients with poor quality PET/MR images.
Following tumor resection, tissues were fixed with
10% formaldehyde for 24 h, dehydrated in an ethanol gradient (100,
95, 85 and 75% ethanol for 5, 4, 3 and 2 min, respectively),
wax-impregnated and embedded in paraffin. Serial sections (5 µm
thick) were prepared and dried at 50°C for 30 min. Sections were
stained with hematoxylin for 5 min and 1% eosin for 30–60 sec at
room temperature. Histopathological evaluations of these sections
were assessed using a light microscope (magnification, 40×). The
patients received surgical treatment and the tumor size, grade,
stage and the status of lymph node metastasis were recorded. The
histopathologic findings were reviewed as previously described
(20,21).
PET/MR examination
Whole-body PET/MR examination was performed using an
integrated PET/MR machine (Biograph mMR; Siemens Healthineers).
Prior to the examination, patients fasted for >6 h, and their
fasting blood glucose levels were monitored. Prior to image
acquisition, 18F-FDG (Sumitomo HM-20 Cyclotron)
2.22–4.44 MBq/kg was injected intravenously at rest. Images were
acquired 50–60 min following injection. The acquisition time was 3
min per bed position, and the accumulated acquisition time was 40
min/person. The attenuation correction technique was used based on
the Dixon sequence: Images were reconstructed with an iterative
algorithm (3 iterations; 21 subsets; and a Gaussian filter with a
full width at half maximum=4 mm for scatter correction)
incorporating a point spread function (22). MR image C was acquired using the
complete Matrix coils to cover most of the trunk (from the neck to
the middle segment of the femur). The sequences included 3D
volumetric interpolated breath-hold T1-weighted sequence in the
horizontal axis, and the T2-weighted turbo spin-echo imaging
(T2WI-TSE) sequence in the horizontal axis. DWI with b values of 50
and 800 in the horizontal axis was performed. Patients with
cervical cancer were scanned with the sagittal T2WI-TSE
sequence.
Image analysis
Two nuclear medicine physicians independently
measured the ADCmin and SUVmax of primary
tumors. For the ADC value, the large ROIs were drawn manually to
cover the lesions (with abnormal enhancement) on the DWI. The
lowest ADC value was defined as the ADCmin. The ROI was
manually delineated by the substantial part of abnormal enhancement
within the lesion on the PET image, and syngo MMWP software
(version VE40A; Siemens Healthineers) automatically calculated the
SUV value of the lesion. The highest SUV value of the ROI was
defined as the SUVmax.
Data analysis
All data were analyzed using SPSS software (version
19.0; IBM Corp., Armonk, NY, USA). Continuous data were presented
as the mean ± standard deviation. Pearson's correlation analysis
was used to evaluate the correlation between imaging biomarkers and
the tumor size. The Mann-Whitney U test was used to evaluate the
associations between the imaging biomarkers (SUVmax and
ADCmin) and pathological factors (histological grade,
FIGO stage and lymph node metastasis) (18,23). The
sensitivity and specificity of the imaging biomarkers for
pathological factors were determined by measuring the area under
the curve (AUC). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient clinical data
This study enrolled a total of 46 patients with a
definitive pathological diagnosis of cervical cancer. The patients
had a mean age of 56.5±12.1 years (range, 31–74). Detailed clinical
data of the patients are presented in Table I. A total of 40 patients had squamous
carcinoma and 6 had adenocarcinoma. A total of 23 patients had
poorly differentiated carcinoma, 23 had moderately differentiated
carcinoma and none had well differentiated carcinoma. The mean
tumor size was 10.3±9.8 mm3 (maximum size, 60
mm3). All 46 patients underwent FIGO staging, which
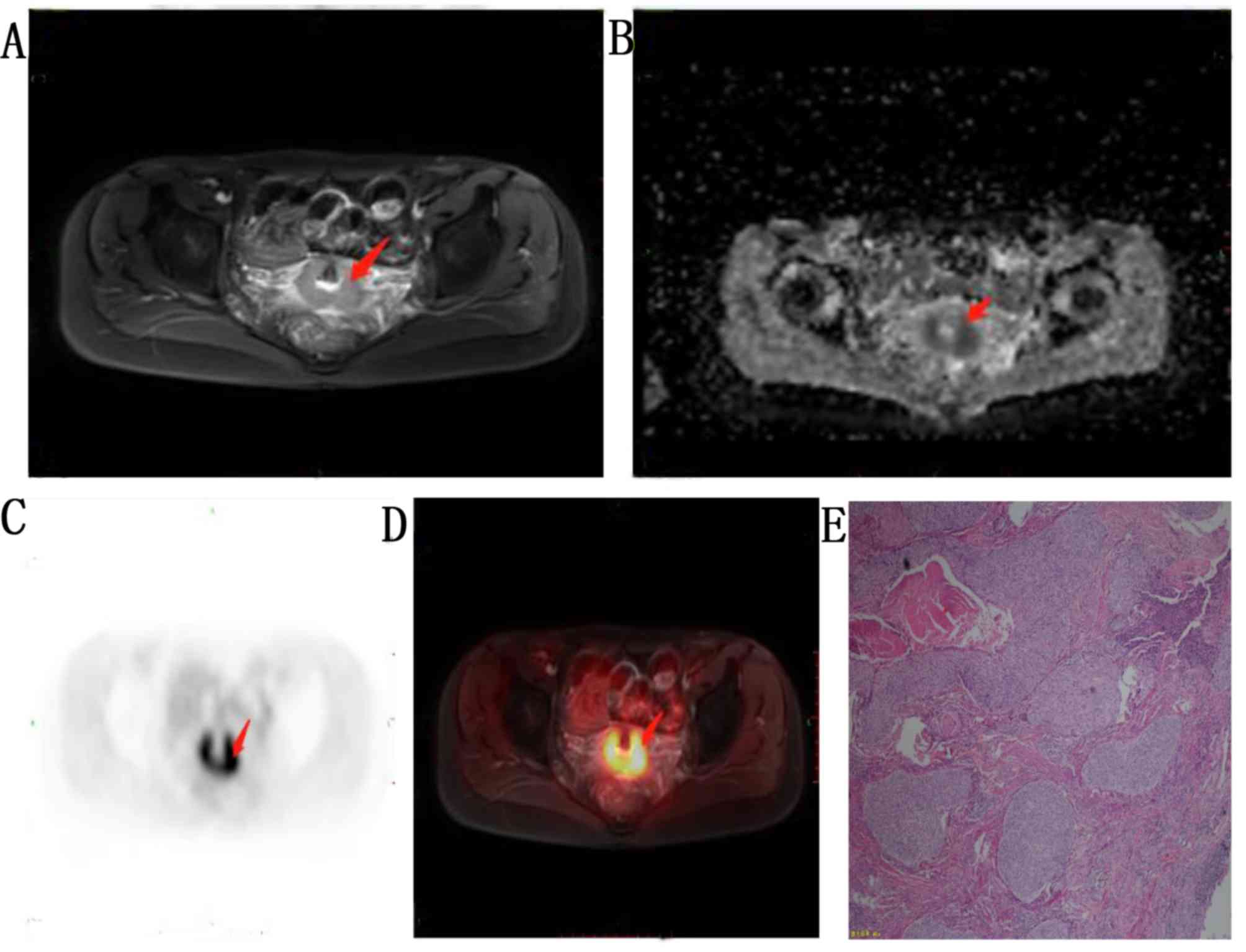
revealed stage IB in 25 patients (moderately differentiated;
Fig. 1), stage IIA in 10 patients
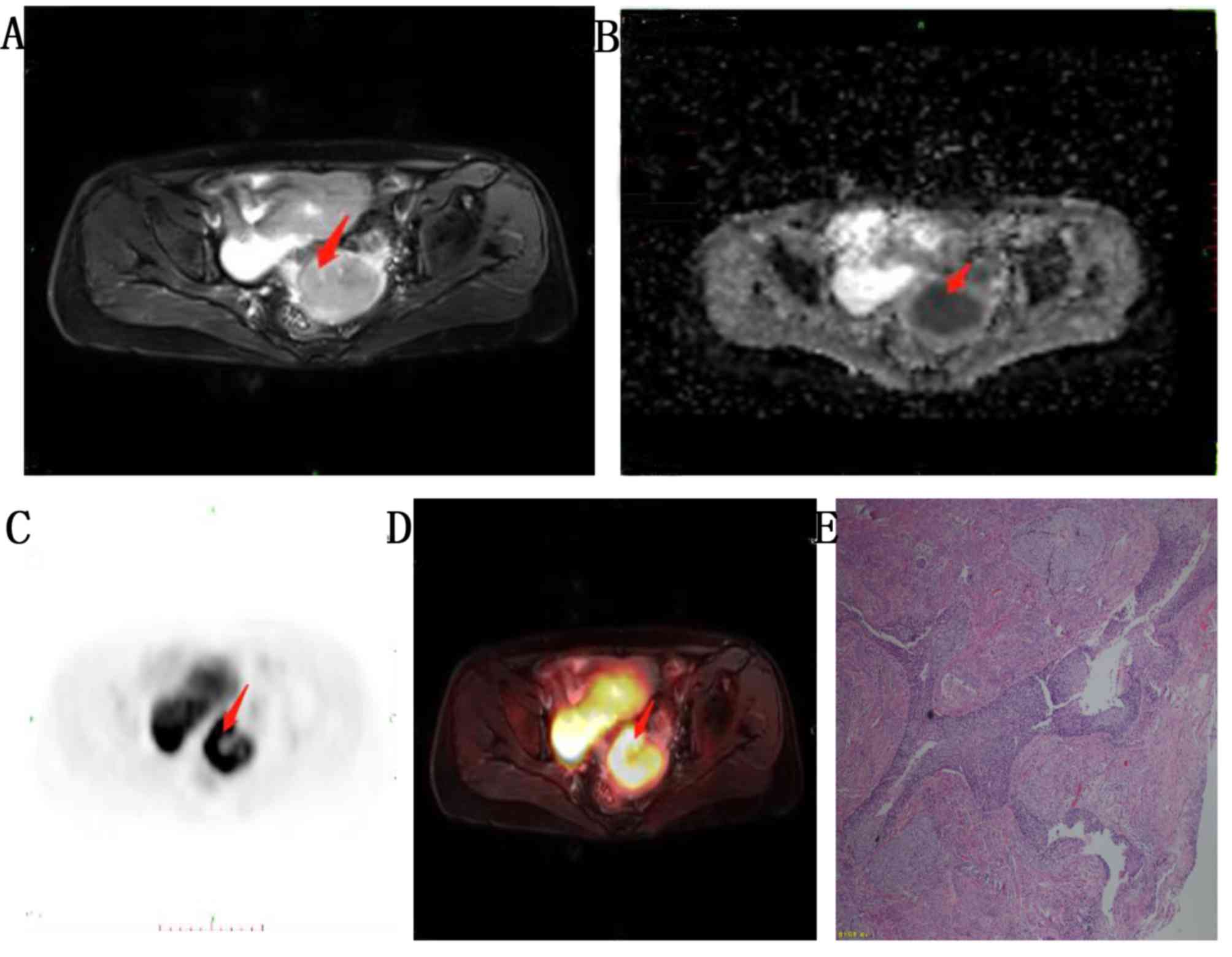
(moderately differentiated; Fig. 2)
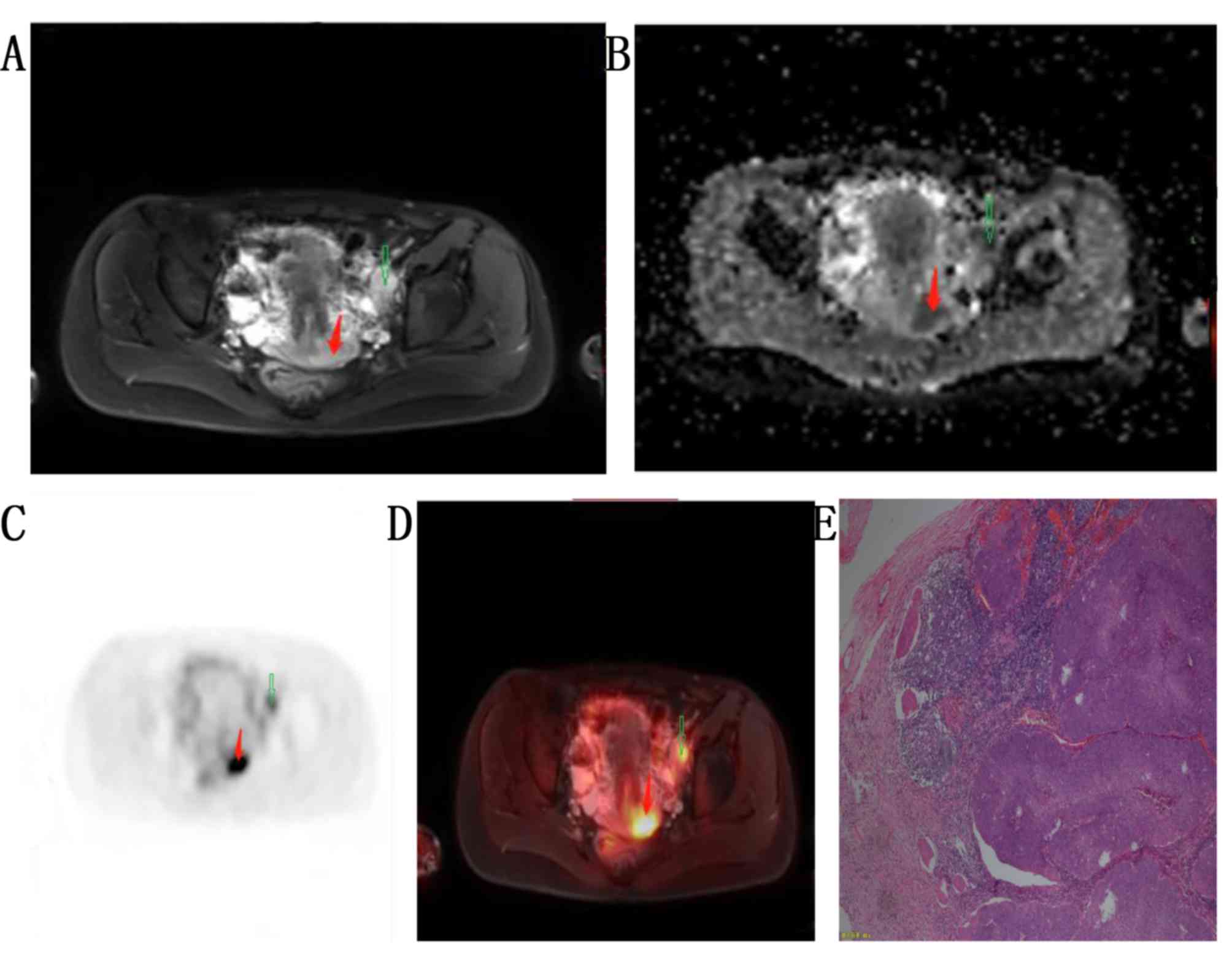
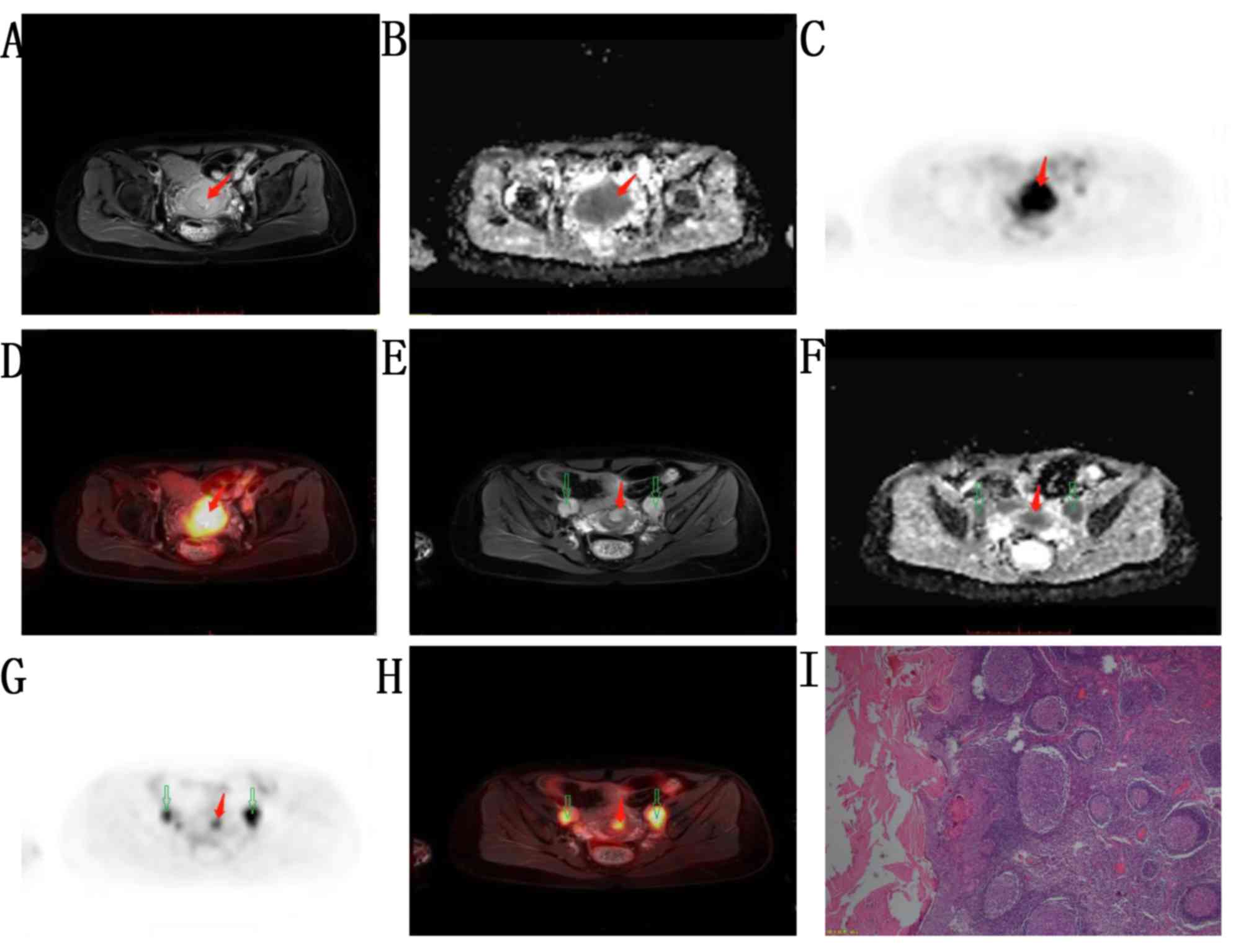
and stage IIB in 11 patients. A total of 18 patients had cervical
invasion with lymph node metastasis, including left pelvic lymph
node metastasis (poorly differentiated; stage IIA; Fig. 3) and bilateral pelvic lymph node
metastasis (poorly differentiated; stage IIB; Fig. 4).
 | Table I.Clinical data of the 46 patients with
cervical cancer in the present study. |
Table I.
Clinical data of the 46 patients with
cervical cancer in the present study.
|
Characteristics | Value |
|---|
| Age (years) | 56.5±12.1
(31–74) |
| Tumor
size (mm3) | 10.3±9.8
(0.72–60) |
| Histology |
|
|
Adenocarcinoma | 6 (13) |
|
Squamous carcinoma | 40 (87) |
| Histology
grade |
|
|
Well-differentiated | 0 (0) |
|
Moderately-differentiated | 23 (50) |
|
Poorly-differentiated | 23 (50) |
| International
Federation of Gynecology and Obstetrics stage |
|
| IB | 25 (54) |
|
IIA | 10 (22) |
|
IIB | 11 (24) |
| Lymph node
metastasis |
|
|
Absent | 28 (61) |
|
Present | 18 (39) |
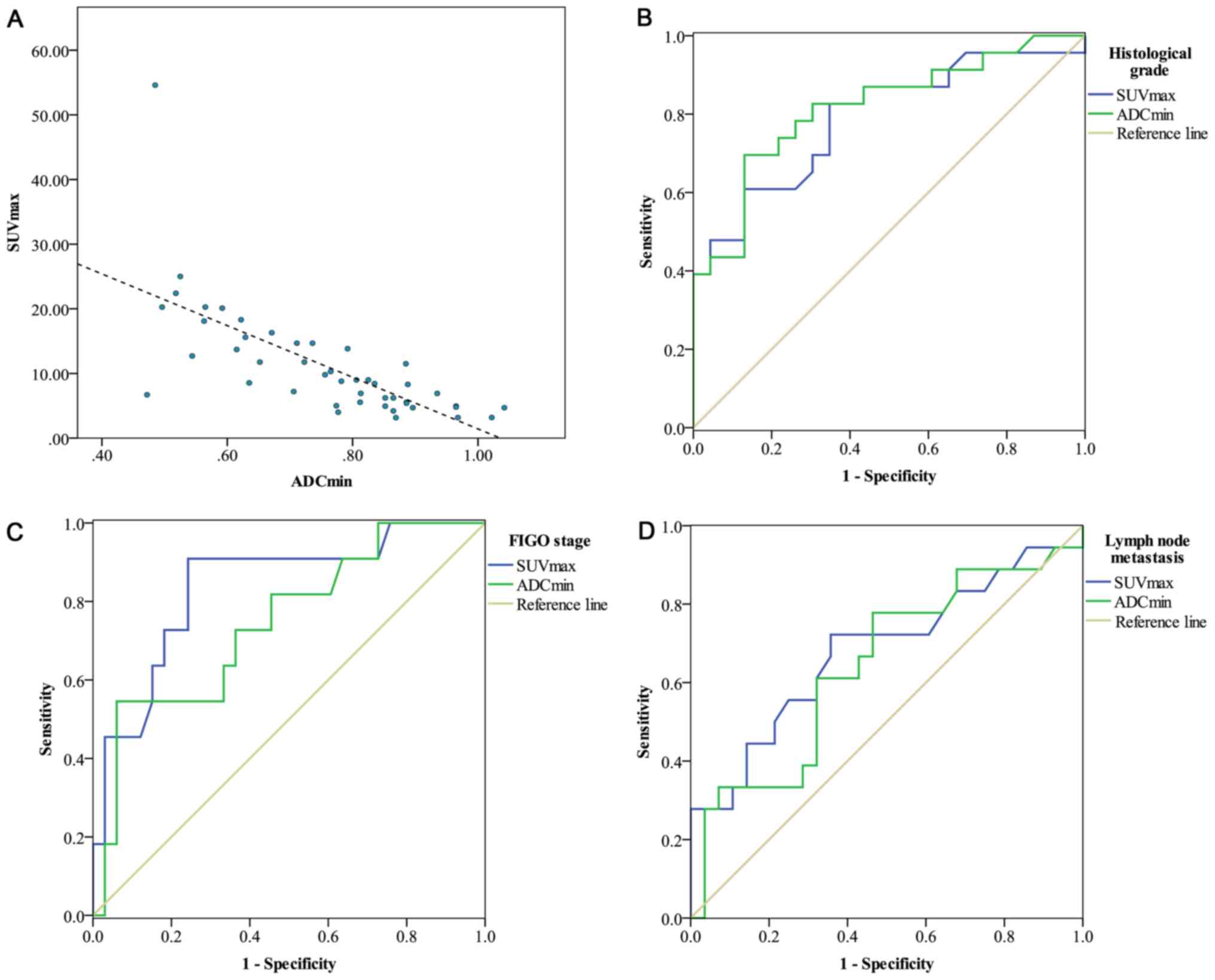
Analysis of the correlations between
SUVmax, ADCmin and the tumor size
Following integrated PET/MR imaging for the 46
patients, the mean SUVmax was 11.1±8.7 (range,
3.16–51.6) and the mean ADCmin was
0.76±0.15×10−3 mm2/s (range,
0.47–1.04×10−3 mm2/s). The SUVmax
had a significant negative correlation with the ADCmin
(r=−0.700; P=<0.001; Fig. 5A).
Statistical analysis revealed no significant difference between the
tumor size and the SUVmax (r=0.286; P=0.054), or between
the tumor size and the ADCmin (r=−0.231; P=0.122). The
results of correlation analyses are detailed in Table II.
 | Table II.Correlations between
SUVmax, ADCmin and tumor size. |
Table II.
Correlations between
SUVmax, ADCmin and tumor size.
| Parameter |
SUVmax |
ADCmin | Tumor size |
|---|
|
SUVmax |
| r | 1 | −0.700 | 0.286 |
|
P-value |
| <0.001 | 0.054 |
|
ADCmin |
| r |
| 1 | −0.231 |
| P-value |
|
| 0.122 |
| Tumor size |
| r |
|
| 1 |
| P-value |
|
|
|
Association between
SUVmax/ADCmin/tumor size and tumor
differentiation/FIGO stage/lymph node metastasis
The SUVmax was significantly increased in
patients with poorly differentiated tumors (P=0.001), patients with
lymph node metastasis (P=0.040) and patients with FIGO stage IIB
(P=0.005) compared with patients with moderately differentiated
tumors, patients with no lymph node metastasis and patients with
early-stage (IB-IIA), respectively. The ADCmin was
significantly decreased in patients with poorly differentiated
tumors (P<0.001) and patients with FIGO stage IIB (P=0.017),
compared with patients with moderately differentiated tumors and
patients with FIGO stage IB-IIA, respectively. The
ADCmin was decreased in patients with lymph node
metastasis compared with those without lymph node metastasis, but
the difference was not statistically significant (P=0.105). The
tumor sizes were slightly larger in patients with poorly
differentiated tumors (P=0.553), patients with FIGO stage IIB
(P=0.193), and patients with lymph node metastasis (P=0.386),
compared with patients with moderately differentiated tumors, FIGO
stage IB-IIA and without lymph node metastasis, respectively, but
the differences were not statistically significant (Table III).
 | Table III.Correlation between the
SUVmax/ADCmin/tumor size and tumor
differentiation/FIGO stage/lymph node metastasis. |
Table III.
Correlation between the
SUVmax/ADCmin/tumor size and tumor
differentiation/FIGO stage/lymph node metastasis.
|
| Histological
grade | FIGO stage | Lymph node
metastasis |
|---|
|
|
|
|
|
|---|
| Parameter | Poorly
differentiated (n =23) | Moderately
differentiated (n =23) | P-value | (IB-IIA)
(n=35) | IIB (n=11) | P-value | Absent (n=28) | Present (n=18) | P-value |
|---|
|
SUVmax | 14.9±10.6 | 7.3±3.6 |
0.001a | 8.6±4.9 | 18.9±13.1 |
| 8.79±4.83 | 14.7±11.9 | 0.040 |
|
| (3.16–54.6) | (3.18–15.6) |
| (3.16–22.4) | (4.94–54.6) | 0.005 | (3.18–20.1) | (3.16–54.) |
|
|
ADCmin | 0.67±0.14 | 0.84±0.11 |
0.000a | 0.79±0.14 | 0.66±0.15 | 0.017 | 0.78±0.14 | 0.72±0.17 | 0.105 |
| (×10−3
mm2/s) | (0.47–0.97) | (0.62–1.04) |
| (0.47–1.04) | (0.48–0.97) |
| (0.47–1.02) | (0.48–1.04) |
|
| Tumor size | 11.6±12.2 | 9.0±6.7 | 0.553 | 9.6±10.4 | 12.5±7.9 | 0.193 | 8.61±5.96 | 12.9±13.7 | 0.386 |
|
(mm3) | (2.89–60.0) | (0.72–25.0) |
| (0.72–60) | (2.89–25) |
| (3.0–25) | (0.72–60.0) |
|
Sensitivity and specificity of SUVmax and
ADCmin for histological grade, FIGO stage and lymph node
metastasis are presented in Fig. 5.
SUVmax exhibited a higher AUC value for predicting FIGO
stage (AUC=0.837) and lymph node metastasis (AUC=0.681) compared
with ADCmin for predicting FIGO stage (AUC=0.745) and
lymph node metastasis (AUC=0.643). ADCmin was more
useful for predicting the grade of tumor differentiation
(AUC=0.816) compared with SUVmax (AUC=0.788).
Discussion
The present study demonstrated that PET/MR imaging
provides valuable imaging data for patients with cervical cancer.
These PET/MR results were associated with various pathological
factors and may serve a role in the evaluation of the prognosis of
patients with cervical cancer. The SUV represents the metabolism of
glucose in tumor cells (15,23). The invasive ability of tumors may be
estimated from the SUV, and this may be used to predict the
prognosis to some extent (24). The
ADC presents tumor cellularity and may reflect tumor invasiveness
(25). The ADC may be used to
indicate tumor characteristics, including tumor size, number of
metastatic lymph nodes, portal vein invasion and extrapancreatic
nerve plexus invasion (25).
Previous studies have revealed that the SUVmax and the
ADCmin may be used as tumor imaging biomarkers, and they
have a significantly negative correlation in different types of
cancer, including endometrial (11),
rectal (17), cervical (18) and breast cancer (19). As was the case with the
aforementioned studies, the current study demonstrated that the
SUVmax and the ADCmin had a significantly
negative correlation, which may present a biological association
between tumor cellularity and glucose metabolic activity. Previous
studies relied on SUV and ADC values measured separately by
distinct PET and MR devices (19,23). The
time interval between the two examinations may impact the analysis.
By contrast, an integrated PET/MR system provides the two types of
data at the same time (26). The
current study revealed that the SUVmax and the
ADCmin had no significant correlation with the tumor
size, as did a previous study by Kidd et al (27). Notably, a P-value close to 0.05 was
obtained (P=0.054), and this warrants further investigation by
expanding the sample size in future studies, as it may be used for
guiding clinical diagnosis.
The grade of pathological differentiation and the
FIGO stage are key factors affecting prognosis of patients with
cervical cancer (28). The
SUVmax and ADCmin are associated with
pathological prognostic factors in various types of cancer
(11,17,25).
Previous studies revealed that tumor FDG uptake was associated with
the grade of pathological differentiation: Poorly differentiated
tumors had a higher SUVmax than moderately and well
differentiated tumors (9,19,23,27). A
previous study revealed that patients with poorly differentiated
tumors had a decreased ADC compared with patients with well
differentiated tumors (29). In
addition, a previous study revealed that the SUV was positively
correlated with the FIGO stage (4).
The current study revealed a significantly increase in the
SUVmax and a significant decrease in the ADC value in
patients with poorly differentiated tumors compared with those with
moderately differentiated tumors, as well as significant
differences in the SUVmax and ADCmin values
between patients with early cervical cancer (stage IB-IIA) and
patients with mid-stage cervical cancer (stage IIB). The SUV in
patients with stage (IIB-IIA) was decreased compared with patients
with stage IIB. These results may be due to an increase in glucose
uptake caused by the rapid growth and active proliferation of
tumors, resulting in an increased FDG uptake in patients with
poorly differentiated tumors.
The presence or absence of lymph node metastasis is
an important prognostic factor in patients with early cervical
cancer (9). Previous studies
revealed SUV is significantly associated with lymph node metastasis
(6,30). A previous study revealed that
patients with cervical cancer with lymph node metastasis, had an
increased SUV in their primary lesions compared with patients
without lymph node metastasis (31).
The current study demonstrated that a higher SUV was positively
associated with lymph node metastasis, which indicated that high
FDG uptake may predict local invasion of tumor cells. There was no
significant difference in ADCmin between patients with
lymph node metastasis and those without lymph node metastasis in
the current study, contrary to observations made by Chen et
al (32), who demonstrated that
patients with lymph node metastasis had a significantly decreased
ADCmin compared with patients without lymph node
metastasis. This difference may be attributed to a smaller sample
size.
Surgery is the preferred treatment for patients with
early cervical cancer (4,13). By analyzing imaging markers to
evaluate prognostic factors, surgical approaches may be optimized
for individual patients (31,33). The
current study demonstrated that PET had a higher diagnostic value
for lymph node metastasis and FIGO staging than MR imaging alone,
whereas MR imaging had a higher diagnostics value for the grade of
pathological differentiation compared with PET. Thus, the
comprehensive image data provided by an integrated PET/MR imaging
system may have greater clinical significance than the data
provided by MR imaging or PET alone.
There are two limitations for the current
retrospective study. Firstly, no analysis was performed for the
cervical cancer based on the pathological types due to the small
sample size and future studies with a larger sample are required.
Secondly, due to short follow-up time subsequent to the examination
the postoperative survival of the patients was not included in the
current study. The prognostic significance of the SUV and ADC were
therefore not demonstrated.
In conclusion, the results of the current study
revealed that there was a significant negative correlation between
SUVmax and ADCmin. These two imaging
biomarkers were associated with pathological prognostic factors.
Compared with PET or MR imaging alone, integrated PET/MR imaging
may provide higher value data for the diagnosis of cervical
cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors contributions
JG and NWa designed the study and drafted the
manuscript. WF and YM participated in the design of the study and
provided guidance. MY performed the imaging examinations. NWa and
MF collected the patient data. NWe, MF, LB and MW analyzed the
data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Chinese PLA General Hospital and study participants
provided written informed consent.
Patient consent for publication
Study participants provided their consent for the
publication of data. All identifying patient data and images were
removed.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SUVmax
|
maximum standardized uptake value
|
|
ADCmin
|
minimum apparent diffusion
coefficient
|
|
18F-FDG
|
18F-fluorodeoxyglucose
|
|
PET/MR
|
positron emission tomography/magnetic
resonance
|
|
AUC
|
area under the curve
|
|
FIGO
|
International Federation of Gynecology
and Obstetrics
|
|
ROI
|
region of interest
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petry KU: HPV and cervical cancer. In: HPV
and cervical cancer. Springer; Berlin, Germany: pp. 59–62. 2012
|
|
4
|
Bhatla N, Aoki D, Sharma DN and
Sankaranarayanan R: Cancer of the cervix uteri. Int J Gynaecol
Obstet. 2 (Suppl 143):S22–S36. 2018. View Article : Google Scholar
|
|
5
|
Khiewvan B, Torigian DA, Emamzadehfard S,
Paydary K, Salavati A, Houshmand S, Shamchi SP, Werner TJ, Aydin A,
Roy SG, et al: Update of the role of PET/CT and PET/MRI in the
management of patients with cervical cancer. Hell J Nucl Med.
19:254–268. 2016.PubMed/NCBI
|
|
6
|
Tangjitgamol S, Anderson BO, See HT,
Lertbutsayanukul C, Sirisabya N, Manchana T, Ilancheran A, Lee KM,
Lim SE, Chia YN, et al: Management of endometrial cancer in Asia:
Consensus statement from the asian oncology summit 2009. Lancet
Oncol. 10:1119–1127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huellner MW, Appenzeller P, Kuhn FP,
Husmann L, Pietsch CM, Burger IA, Porto M, Delso G, von Schulthess
GK and Veit-Haibach P: Whole-body nonenhanced PET/MR versus PET/CT
in the staging and restaging of cancers: Preliminary observations.
Radiology. 273:859–869. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Preda L, Conte G, Bonello L, Giannitto C,
Travaini LL, Raimondi S, Summers PE, Mohssen A, Alterio D, Cossu
Rocca M, et al: Combining standardized uptake value of FDG-PET and
apparent diffusion coefficient of DW-MRI improves risk
stratification in head and neck squamous cell carcinoma. Eur
Radiol. 26:4432–4441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Viswanathan C, Faria S, Devine C, Patnana
M, Sagebiel T, Iyer RB and Bhosale PR:
[18F]-2-Fluoro-2-Deoxy-D-glucose-PET assessment of cervical cancer.
PET Clin. 13:165–177. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grant P, Sakellis C and Jacene HA:
Gynecologic Oncologic Imaging With PET/CT. Semin Nucl Med.
44:461–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamura K, Joja I, Fukushima C, Haruma T,
Hayashi C, Kusumoto T, Seki N, Hongo A and Hiramatsu Y: The
preoperative SUVmax is superior to ADCmin of
the primary tumour as a predictor of disease recurrence and
survival in patients with endometrial cancer. Eur J Nucl Med Mol
Imaging. 40:52–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Collins CD: PET/CT in oncology: For which
tumours is it the reference standard? Cancer Imaging 7 Spec No A.
S77–S87. 2007. View Article : Google Scholar
|
|
13
|
Ashwin KR and Somashekhar SP: Management
of early stage cervical cancer. Rev Recent Clin Trials. 10:302–308.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kjær A, Loft A, Law I, Berthelsen AK,
Borgwardt L, Löfgren J, Johnbeck CB, Hansen AE, Keller S, Holm S
and Højgaard L: PET/MRI in cancer patients: First experiences and
vision from copenhagen. MAGMA. 26:37–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Platzek I, Beuthien-Baumann B, Langner J,
Popp M, Schramm G, Ordemann R, Laniado M, Kotzerke J and van den
Hoff J: PET/MR for therapy response evaluation in malignant
lymphoma: Initial experience. MAGMA. 26:49–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Drzezga A, Souvatzoglou M, Eiber M, Beer
AJ, Fürst S, Martinez-Möller A, Nekolla SG, Ziegler S, Ganter C,
Rummeny EJ and Schwaiger M: First clinical experience with
integrated whole-body PET/MR: Comparison to PET/CT in patients with
oncologic diagnoses. J Nucl Med. 53:845–855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Er HÇ, Erden A, Küçük NÖ and Geçim E:
Correlation of minimum apparent diffusion coefficient with maximum
standardized uptake on fluorodeoxyglucose PET-CT in patients with
rectal adenocarcinoma. Diagn Interv Radiol. 20:105–109.
2014.PubMed/NCBI
|
|
18
|
Grueneisen J, Beiderwellen K, Heusch P,
Buderath P, Aktas B, Gratz M, Forsting M, Lauenstein T, Ruhlmann V
and Umutlu L: Correlation of standardized uptake value and apparent
diffusion coefficient in integrated whole-body PET/MRI of primary
and recurrent cervical cancer. PLoS One. 9:e967512014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baba S, Isoda T, Maruoka Y, Kitamura Y,
Sasaki M, Yoshida T and Honda H: Diagnostic and prognostic value of
pretreatment SUV in 18F-FDG/PET in breast cancer: Comparison with
apparent diffusion coefficient from diffusion-weighted MR imaging.
J Nucl Med. 55:736–742. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kurman RJ, Carcangiu ML, Herrington CS and
Young RH: WHO classification of tumours of female reproductive
organs. 4th. IARC; Lyon: 2014
|
|
21
|
Stock RJ, Zaino R, Bundy BN, Askin FB,
Woodward J, Fetter B, Paulson JA, DiSaia PJ and Stehman FB:
Evaluation and comparison of histopathologic grading systems of
epithelial carcinoma of the uterine cervix: Gynecologic Oncology
Group studies. Int J Gynecol Pathol. 13:99–108. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martinez-Möller A, Eiber M, Nekolla SG,
Souvatzoglou M, Drzezga A, Ziegler S, Rummeny EJ, Schwaiger M and
Beer AJ: Workflow and scan protocol considerations for integrated
whole-body PET/MRI in oncology. J Nucl Med. 53:1415–1426. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shih IL, Yen RF, Chen CA, Chen BB, Wei SY,
Chang WC, Sheu BC, Cheng WF, Tseng YH, Chen XJ, et al: Standardized
uptake value and apparent diffusion coefficient of endometrial
cancer evaluated with integrated whole-body PET/MR: Correlation
with pathological prognostic factors. J Magn Reson Imaging.
42:1723–1732. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chung HH, Nam BH, Kim JW, Kang KW, Park
NH, Song YS, Chung JK and Kang SB: Preoperative [18F]FDG
PET/CT maximum standardized uptake value predicts recurrence of
uterine cervical cancer. Eur J Nucl Med Mol Imaging. 37:1467–1473.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hayano K, Miura F, Amano H, Toyota N, Wada
K, Kato K, Sano K, Takeshita K, Aoyagi T, Shuto K, et al:
Correlation of apparent diffusion coefficientmeasured by
diffusionweighted MRI and clinicopathologic features in pancreatic
cancer patients. J Hepatobiliary Pancreat Sci. 20:243–248. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Small W Jr, Strauss JB, Jhingran A, Yashar
CM, Cardenes HR, Erickson-Wittmann BA, Gullett N, Kidd E, Lee LJ,
Mayr NA, et al: ACR Appropriateness Criteria® definitive
therapy for early-stage cervical cancer. Am J Clin Oncol.
35:399–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kidd EA, Spencer CR, Huettner PC, Siegel
BA, Dehdashti F, Rader JS and Grigsby PW: Cervical cancer histology
and tumor differentiation affect 18F-fluorodeoxyglucose
uptake. Cancer. 115:3548–3554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singh N and Arif S: Histopathologic
parameters of prognosis in cervical cancer-a review. Int J Gynecol
Cancer. 14:741–750. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Razek AA and Nada N: Correlation of
choline/creatine and apparent diffusion coefficient values with the
prognostic parameters of head and neck squamous cell carcinoma. NMR
Biomed. 29:483–489. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jung NY, Kim SH, Kang BJ, Park SY and
Chung MH: The value of primary tumor (18)F-FDG uptake on
preoperative PET/CT for predicting intratumoral lymphatic invasion
and axillary nodal metastasis. Breast Cancer. 23:712–717. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Monteil J, Maubon A, Leobon S, Roux S,
Marin B, Renaudie J, Genet D, Fermeaux V, Aubard Y and
Tubiana-Mathieu N: Lymph node assessment with
18F-FDG-PET and MRI in uterine cervical cancer.
Anticancer Res. 31:3865–3871. 2011.PubMed/NCBI
|
|
32
|
Chen YB, Liao J, Xie R, Chen GL and Chen
G: Discrimination of metastatic from hyperplastic pelvic lymph
nodes in patients with cervical cancer by diffusion-weighted
magnetic resonance imaging. Abdom Imaging. 36:102–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sala E, Rockall AG, Freeman SJ, Mitchell
DG and Reinhold C: The added role of MR imaging in treatment
stratification of patients with gynecologic malignancies: What the
radiologist needs to know. Radiology. 266:717–740. 2013. View Article : Google Scholar : PubMed/NCBI
|