Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies, with ~750,000 people diagnosed with HCC per
year (1), of which nearly 55% occur
in China (2). There are between
600,000 and 700,000 HCC-associated deaths each year worldwide
(3), with ~360,000 deaths in China
alone (4). China has a high
incidence of hepatitis B virus (HBV) infections and chronic HBV
infection is one of the principal causes of HCC.
Surgical resection is the most effective method for
treating patients with HCC at present. For resectable HCC, surgery
is the first consideration. However, a high postoperative
recurrence rate and metastasis rate limit the long-term survival of
patients, even after radical surgery (5,6). In
addition, HCC patients with HBV infection often exhibit cirrhosis,
and HCC resection is limited by the severity of cirrhosis (7). In patients with persistent HBV
infection, there are certain pathological factors that influence
postoperative recurrence (including tumor size, microvascular
invasion (MVI), surgical margin, foci, biological characteristics
and the number of tumors); therefore, in theory, almost all HCC
cases are likely to recur after surgery, and intrahepatic and
extrahepatic metastases can be difficult to treat via surgery
(8). Even if the tumor is completely
resected, small cancerous lesions may exsit in the liver that
cannot be detected by preoperative or intraoperative radiology.
Postoperative recurrence and metastasis of HCC may seriously
decrease a patient's long-term survival (5,6).
Local treatment of the liver may be more effective
than systemic therapies (5). The
prophylactic transcatheter arterial chemoembolization (TACE) method
is the most commonly used local treatment, and is widely used
clinically to improve the efficacy of HCC surgery. Hepatic artery
angiography and subsequent lipiodol computed-tomography (CT)
examination during prophylactic TACE treatment are the most
sensitive methods to detect residual cancer and early recurrence at
an early stage (9). These methods
allow for timely diagnosis and treatment of postoperative residual
cancer or early recurrence (10) and
therefore, adoption of TACE has become more widespread. It is
strongly recommended that patients undergoing radical resection of
HCC are treated with TACE in a timely manner after surgical
resection (11,12). However, to the best of our knowledge,
there are currently no studies evaluating the effectiveness of
postoperative prophylactic TACE treatment for which a large-scale
randomized controlled trial is preferable.
In the present study, a prediction index (PI) model
of the 5-year survival rate of patients with hepatitis B-related
HCC after radical resection was established and the effect of
prophylactic TACE on patient survival was determined. The model may
improve prognosis through individualized prophylactic TACE
intervention after radical resection of HCC.
Materials and methods
Patients
The medical records of patients with hepatitis
B-related HCC, that had received radical resection in The First
Affiliated Hospital of Xinjiang Medical University (Urumqi, China)
from October 2007 to December 2010 were retrospectively reviewed.
There were 201 patients in the final cohort, of which 130 were male
and 71 were female. The age range of the cohort was 38–69 years
with a mean age of 52±8 years. Informed consent was obtained from
each patient and the study was approved by The Ethics Review Board
of The First Affiliated Hospital of Xinjiang Medical
University.
Inclusion and exclusion criteria
The inclusion criteria for patients were: i)
Patients were in good general health and had no obvious heart,
lung, kidney or other vital organ diseases; ii) had a liver
function grade A or B; iii) had chronic hepatitis B infection based
on the Guidelines for Prevention and Treatment of Chronic Hepatitis
B (2005 Edition) (13); and iv) had
surgical radical resection. Radical resection was considered if the
patients had: i) Complete resection of the tumor; ii) no residual
cancer; iii) had ≤3 distinct tumours; iv) no tumor embolus in the
portal vein or branches, primary hepatic duct or branches or in the
hepatic vein or the inferior vena cava; and v) no lymph node or
distant metastases (14).
The exclusion criteria for patients were: i)
Presence of other malignant tumors; ii) other types of viral
hepatitis, drug-induced hepatitis, alcoholic liver diseases,
autoimmune liver diseases or hereditary metabolic liver diseases;
iii) decompensated cirrhosis; iv) a liver function grade of C; v)
major underlying diseases (cardiovascular diseases and respiratory
diseases); or vi) incomplete information.
Follow-ups
Patients were followed up by telephone, clinical
visits and letters. The time of surgery was considered as the
starting point and 5 years after the operation was considered as
end point. Outcome measurement included survival or mortality. At
baseline, patient's age, sex, preoperative examinations (immunity
markers, tumor markers, HBV DNA load, abdominal CT angiography),
surgical procedures, postoperative examinations (liver function,
renal function, rountine blood test results), pathological biopsy
report, anti-virus therapy (AVT), and prophylactic TACE treatment
were recorded. The lower value was extracted if there were two
inconsistent postoperative biochemical blood analyses. The maximum
diameter of a single tumor was defiened as the largest diameter on
the largest cross-sectional area. The sum of the maximum diameters
of all the tumors was termed the ‘diameter of multiple tumors’. The
amount of tumor (AT) was determined based on preoperative imaging,
including CTmagnetic resonance imaging and contrast-enhanced
ultrasound. Detailed variables are listed in Table I.
 | Table I.Univariate Cox regression analysis of
the 5-year survival rate of patients with hepatitis B-related
hepatocellular carcinoma. |
Table I.
Univariate Cox regression analysis of
the 5-year survival rate of patients with hepatitis B-related
hepatocellular carcinoma.
| Variables | Regression β | Standard error | Wald | Levels of
freedom | P-value | Odds ratio | 95% CI |
|---|
| Hepatitis B DNA | 0.44 | 0.23 | 3.85 | 1 | 0.050 | 1.56 | 1.00–2.43 |
| Prealbumin | −0.01 | 0.00 | 4.76 | 1 | 0.029 | 0.99 | 0.98–1.00 |
| PTA | −0.02 | 0.01 | 6.54 | 1 | 0.011 | 0.98 | 0.96–1.00 |
| Tumor envelope | 0.45 | 0.22 | 4.08 | 1 | 0.043 | 1.57 | 1.01–2.43 |
| AVT | −0.56 | 0.23 | 6.00 | 1 | 0.014 | 0.57 | 0.36–0.89 |
| NLR | 0.44 | 0.10 | 18.27 | 1 | <0.001 | 1.55 | 1.27–1.89 |
| MVI | 0.87 | 0.23 | 14.57 | 1 | <0.001 | 2.38 | 1.52–3.71 |
| AT | 0.73 | 0.221 | 10.75 | 1 | 0.001 | 2.07 | 1.34–3.21 |
| PRM | 0.89 | 0.21 | 17.26 | 1 | <0.001 | 2.43 | 1.60–3.68 |
| MTS | 0.68 | 0.22 | 9.24 | 1 | 0.002 | 1.97 | 1.27–3.05 |
| HG | 0.38 | 0.14 | 7.02 | 1 | 0.008 | 1.46 | 1.10–1.92 |
The surgical margin was considered positive if there
were: i) Slightly infiltrating lesions in the surgical margins; ii)
abnormal margins or precancerous lesions; or iii) the margins of
the resected lesions were <0.5 mm.
TACE
TACE was performed 2–12 weeks after surgery and the
dosage regimen was determined based on the angiographic results. If
tumor blood vessel staining was not observed during surgery, the
proper hepatic artery would be infused with 750 mg of
5-fluorouracil and 60 mg of cisplatin, and subsequently embolized
with a suspension of 3 ml of super-liquefied lipiodol and 6 mg of
mitomycin. The procedure was repeated once after 6–8 weeks in
certain cases with risk factors, such as MVI. If tumor blood vessel
staining was observed during surgery, the dose was determined
according to the size and number of lesions and the patient's
condition. CT examination was performed 6 weeks after TACE
treatment to evaluate the intrahepatic condition.
Statistical analysis
SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was
used for statistical analysis. The univariate and multivariate Cox
regression analyses were performed to identify the significant risk
factors and the PI model was established based on the signficant
factors. A receiver operating characteristic (ROC) curve was drawn
and the area under the receiver operating characteristic curve
(AUROC) was determined. The Youden index at the maximum
corresponding values was used to calculate the PI threshold.
Kaplan-Meier survival analysis was used to analyze the cumulative
survival and a Log-Rank test was used to determine differences in
survival. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
A total of 201 HCC cases were included in the
present study. Among the 201 patients, there were 130 males (64.7%)
and 71 females (35.3%), with an age range of 38–69 years (mean age,
52±8 years). The survival time range was 7–60 months. Of the 201
patients, 81 individuals succumbed to the cancer (40.3%) and 120
individuals survived for a minimum of 5 years (59.7%). A total of
95 patients were treated with TACE.
Univariate Cox regression
analysis
Univariate Cox regression analysis showed that among
the 34 related indicators, 11 showed significant differences,
including Hepatitis B DNA, pre-albumin, prothrombin activity, AVT,
neutrophil-to-lymphocyte ratio (NLR), MVI, the maximum size of
tumor (MTS), AT, tumor histological grade (HG), tumor envelope and
positive resection margin (PRM). The above factors may have
affected the patients' survival and prognosis (P<0.05; Table I).
Multivariate Cox regression
analysis
All indicators in univariate analysis were ranked
based on the P-values from the smallest to the largest. The first
20 indexes were further analyzed with the multivariate Cox risk
regression model. After adjusting the confounding variables, seven
independent factors were identified. As shown in Table II, there were six independent risk
factors (NLR, MTS, HG, PRM, MVI, and AT) that may have increased
the 5-year mortality risk of patients and 1 independent protective
factor (AVT). The relative odd ratios (ORs) of NLR, MTS, HG, PRM,
MVI, and AT were 1.38, 1.69, 1.47, 2.50, 2.08 and 1.58. With a
1-unit increase in NLR, the risk of succumbing to the cancer within
5 years was be increased by 38%. Each 1-unit reduction in HG
increased the probability of mortality by 47% within 5 years. AVT
was the independent factor for 5-year mortality reduction (OR,
0.42).
 | Table II.Multivariate Cox regression analysis
of 5-year survival in patients with hepatitis B-related
hepatocellular carcinoma. |
Table II.
Multivariate Cox regression analysis
of 5-year survival in patients with hepatitis B-related
hepatocellular carcinoma.
| Variables | Regression β | Standard error | Wald | Levels of
freedom | P-value | Odds ratio | 95% CI |
|---|
| NLR | 0.32 | 0.11 | 8.80 | 1 | 0.003 | 1.38 | 1.11–1.70 |
| AVT | −0.87 | 0.24 | 13.04 | 1 | <0.001 | 0.42 | 0.26–0.67 |
| MVI | 0.73 | 0.24 | 9.09 | 1 | 0.003 | 2.08 | 1.29–3.35 |
| AT | 0.46 | 0.23 | 4.03 | 1 | 0.045 | 1.58 | 1.01–2.46 |
| PRM | 0.92 | 0.22 | 16.80 | 1 | <0.001 | 2.50 | 1.61–3.88 |
| MTS | 0.53 | 0.23 | 5.42 | 1 | 0.020 | 1.69 | 1.09–2.63 |
| HG | 0.39 | 0.15 | 6.91 | 1 | 0.009 | 1.47 | 1.10–1.97 |
Cox risk regression analysis of
postoperative TACE treatment
Cox regression univariate and multivariate analyses
of postoperative TACE treatment were performed. In the adjusted Cox
regression model, postoperative TACE treatment demonstrated a
significant difference, suggesting that TACE may have a significant
effect on the 5-year survival of these patients (Table III). The corrected OR value was
0.51, thus, the postoperative risk of mortality within 5 years was
1.96 times greater for patients who did not receive TACE treatment
compared with patients that did receive it (P<0.05; Table III).
 | Table III.Cox risk proportional regression
analysis of postoperative TACE treatment. |
Table III.
Cox risk proportional regression
analysis of postoperative TACE treatment.
| Variables | Regression β | Standard error | Wald | Levels of
freedom | P-value | Odds ratio | 95% CI |
|---|
| Before
adjustment | −0.46 | 0.23 | 4.16 | 1 | 0.041 | 0.63 | 0.40–0.98 |
| After
adjustment | −0.67 | 0.24 | 8.14 | 1 | 0.004 | 0.51 | 0.32–0.81 |
Establishment of the 5-year survival
prediction model
The PI model developed, based on the seven
significant factors identified by the multivariate analysis, was:
PI=0.32 × NLR + 0.39 × HG (high=1, medium=2, low=3) + 0.92 × PRM
(yes=1, no=0) + 0.87 × MVI (yes=1, no=0) + 0.73 × AT (single=0,
many=1) + 0.53 × MTS (≥5 cm=1, <5 cm=0)-0.87 × AVT (yes=1,
no=0). The PI values of the patients in the present study ranged
between 0.72 and 4.78. The larger the PI value, the higher the
mortality rate within 5 years; the smaller the PI value, the lower
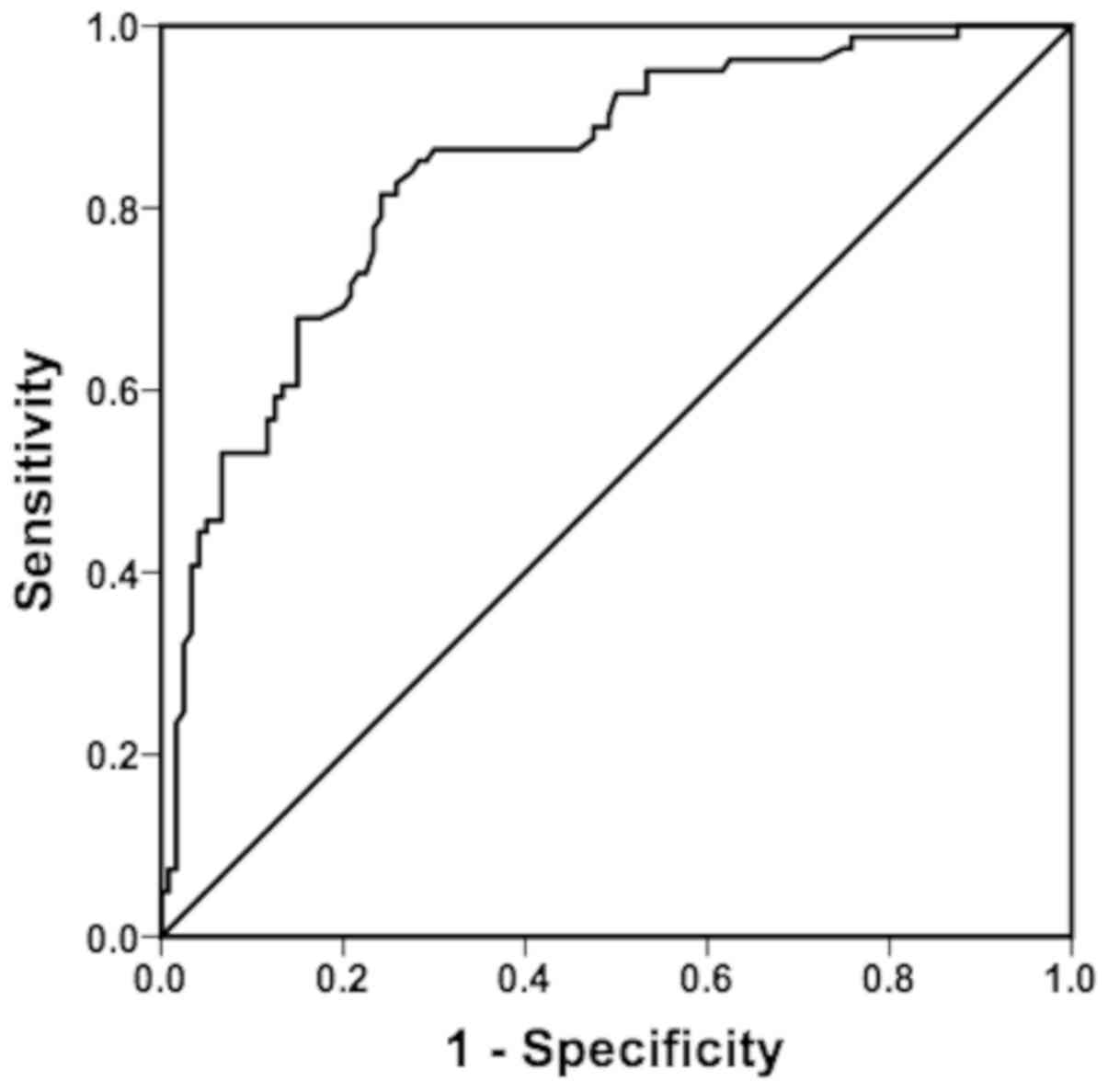
the mortality rate within 5 years. Fig.
1 shows that the AUROC of PI was 0.841, suggesting that the
predictive value of PI was moderate. The PI cut-off value, which
corresponded to the maximum Youden index, was 2.75. The PI value
was analyzed by the Cox risk regression model. After adjusting the
factors, the PI values became independent predictors, and for each
additional PI unit, the risk of death within 5 years was increased
by 1.72 times (P<0.05; Table
IV).
 | Table IV.Cox risk proportional regression
analysis of PI model predicting 5-year survival in patients with
hepatitis B-related hepatocellular carcinoma. |
Table IV.
Cox risk proportional regression
analysis of PI model predicting 5-year survival in patients with
hepatitis B-related hepatocellular carcinoma.
| Variables | Regression β | Standard error | Wald | Levels of
freedom | P-value | Odds ratio | 95% CI |
|---|
| Before
adjustment | 1.00 | 0.12 | 66.78 | 1 | 0.000 | 2.72 | 2.14–3.46 |
| After
adjustment | 1.00 | 0.12 | 66.78 | 1 | 0.000 | 2.72 | 2.14–3.46 |
Survival analysis
The patients with a PI value >2.75 were defined
as the high-risk group and those with a PI value <2.75 as the
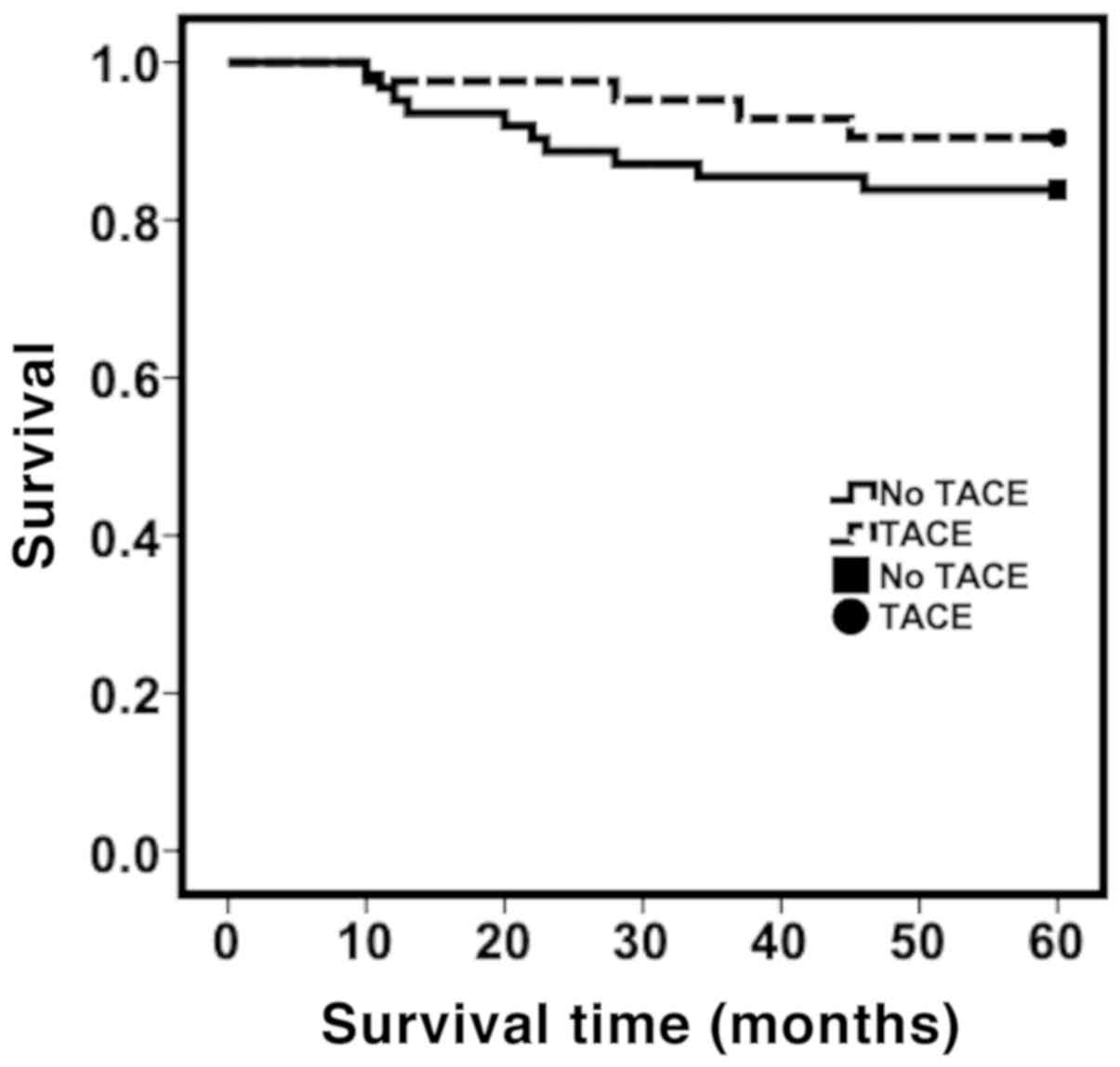
low-risk group. Kaplan-Meier survival analysis showed that the 1, 3
and 5-year cumulative survival rate of the group treated with TACE
was 97.6, 95.2 and 90.5% respectively. In the low-risk group (PI
<2.75), the 1, 3 and 5-year cumulative survival rate was 95.2,
85.5 and 83.9%, respectively, for patients who did not receive TACE
treatment (Fig. 2). However, there
was no significant difference between the two groups in the
cumulative survival rates at all stages (P>0.05). For patients
in the high risk group, the cumulative survival rate at all stages
showed significant differences between patients who received >3
TACE treatments compared with that received <3 TACE treatments
(P<0.05; Fig. 3). The cumulative
survival rates at 1, 3 and 5-years were as follows: ≥3 TACE
treatments were: 100; 80.0; and 53.3% respectively, for patients
who received <3 TACE treatments: 81.8; 40.9; and 36.4%
repectively, and for patients who did not receive TACE treatment:
73.3; 13.3; and 13.3% respectively (P<0.05; Fig. 3).
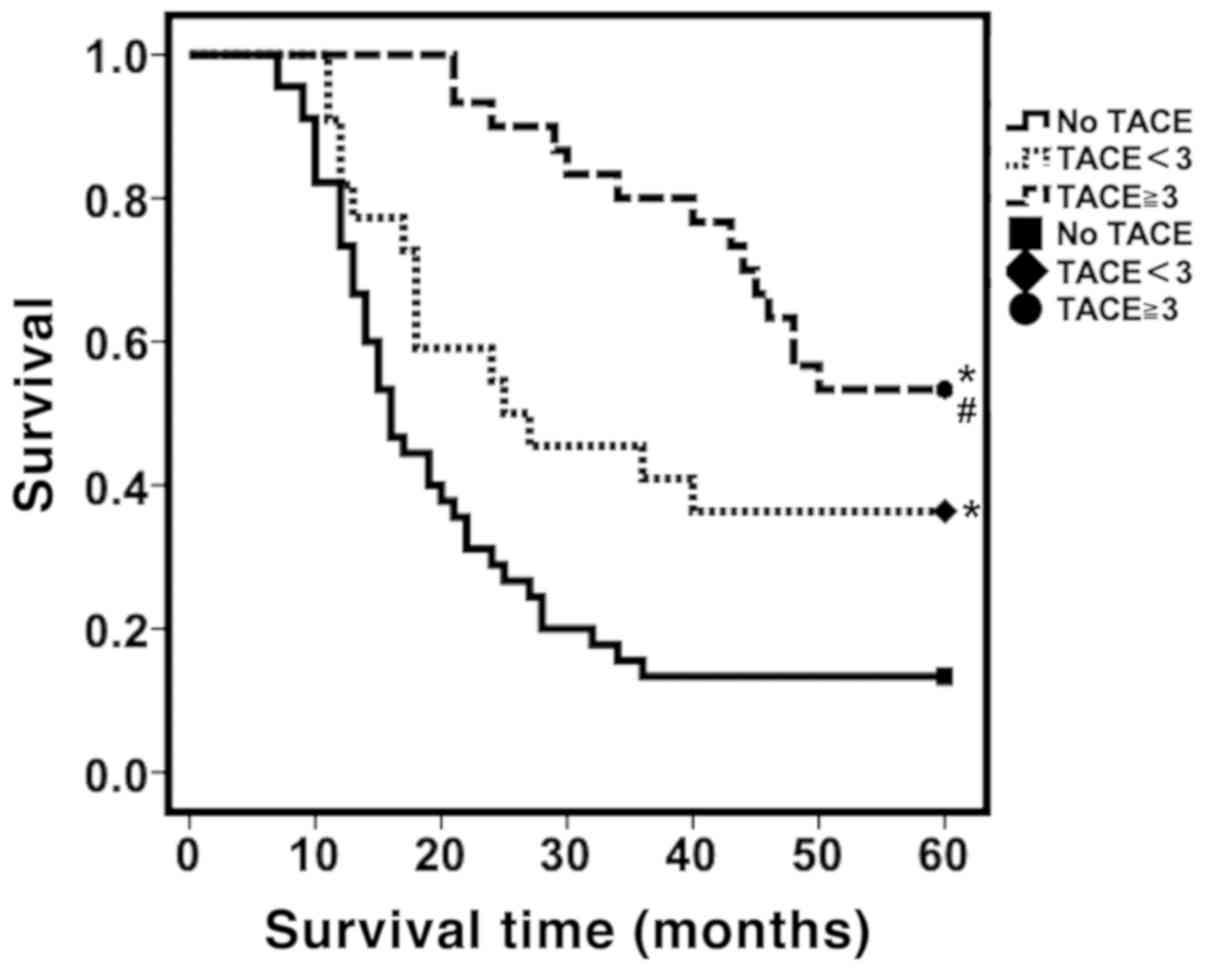 | Figure 3.Distribution of survival curves for
patients treated with TACE ≥3 times, <3 times or no TACE
treatment for the high-risk group. Kaplan-Meier survival analysis
was used to analyze the cumulative survival and the log-rank test
was used to determine differences in survival. In the high-risk
group, patients who received ≥3 TACE treatments, TACE <3 times
or no TACE treatment had significant differences in survival curve
distribution and the cumulative survival rates at each stage. The 1
year, 3 years and 5 years cumulative survival rates were: TACE ≥3:
100, 80.0, 53.3%; TACE <3: 81.8, 40.9, 36.4%; no TACE treatment:
73.3, 13.3, 13.3%. *P<0.05 compared with no TACE treatment at
all stages. #P<0.05 compared with TACE <3 times at
all stages. TACE, transcatheter arterial chemoembolization. |
Discussion
HCC is a blood vessel-rich tumor enabling easier
extravasation into blood vessels, and metastasis to the hepatic
sinusoids, and portal vein through the formation of micro-venous
intrahepatic metastases (15).
Therefore, MVI is often considered a major risk factor for
postoperative recurrence and intrahepatic metastasis (15–18). MVI
is additionally a risk factor for early recurrence (19). Postoperative TACE therapy in
MVI-positive patients can prevent the establishment of a blood
supply, preventing or at least limiting the proliferation of
residual tumor cells, reducing the risk of early recurrence and
metastasis, and thus, increasing disease-free and overall
survival.
The size and number of tumors are important factors
affecting postoperative recurrence (20–24) as
they can reflect the aggressiveness of the tumor. Large tumors,
especially tumors larger than 5 cm, significantly increases the
risk of postoperative recurrence. Previous studies have
demonstrated that the incidence of intrahepatic metastases and
portal vein invasion in tumors larger than 5 cm was significantly
higher compared with those smaller than 5 cm (25), and the larger the tumor, the earlier
postoperative recurrence occurred (26).
Tumor diameter, number, positive surgical margins,
and MVI were the risk factors that significantly influenced
postoperative recurrence and metastasis. For patients with these
risk factors, TACE should be performed at 2–12 weeks after surgery
based on the results of the multivariate Cox regression analysis.
To determine the individualized TACE treatment regimen for
patients, a PI model was established. The Cox regression analysis
showed that a one unit increase in PI value increased the risk of
mortality withing 5 years by 1.72 times. The final Cox multivariate
regression model was refined by accounting for the number of times
TACE was performed. For high-risk patients with a PI >2.75, the
survival curves showed that ≥3 TACE treatments significantly
improved overall survival. Based on the PI model, the early use of
prophylactic interventional therapy for high-risk HCC patients
after radical surgery may improve the 5-year survival rate. The PI
model may provide evidence for the early intervention treatment
after radical resection of liver cancer.
For the low-risk group, although the cumulative
survival rates in each phase of the TACE group were higher than
those in the non-TACE group, no statistical significance was found.
Based on the data, it may be hypothesized that there are certain
risk factors (such as positive surgical margins, larger tumor
volume, multiple lesions and microvascular thrombosis) which
contribute to metastasis (15–26). The
postoperative TACE treatment may help eliminate these risk factors
and improve survival. For those with limited tumor or no tumor
thrombus, radical resection is usually achieved and TACE treatment
in not needed (13). Regular
follow-ups should be performed. Therefore, based on the results of
the present study, it is recommended that low-risk patients receive
1–2 TACE treatments after surgery. Multiple TACE treatments may
increase postoperative liver load, prolong the postoperative
immunosuppressive period, and thus, reduce the quality of life
(10,11).
The risk factors that affected the 5-year survival
in hepatitis B-related HCC were investigated and a predictive model
to identify the high-risk groups with low 5-year survival rates
after HCC radical resection was developed in the present study. The
prediction model demonstrated that 1–3 TACE treatments with TACE
for high-risk patients significantly improved the 5-year survival
rate. For the low-risk group, there was no significant difference
in the 5-year survival rate between the TACE group and non-TACE
group. These results suggest that individualized early TACE
treatment after radical HCC surgery is recommended.
The present study has certain limitations. The
sample size was relatively small and recurrence-free survival was
not analyzed. In future studies, larger sample sizes will be
required to optimize the PI model and to evaluate the
recurrence-free survival.
In conclusion, postoperative prophylactic TACE
should be considered after radical resection of HCC. Based on the
PI model developed in the present study, ≥3 prophylactic TACE
treatments will have the most significant improvements in patient
outcome for high-risk patients (PI >2.75), and for low-risk
patients or patients with ≤2 risk factors (PI <2.75), <3
prophylactic TACE treatments is recommended after surgery. TACE
treatment is not recommended for low-risk patients without any risk
factors. The PI model developed in the present study may be used as
a guide to determine whether treatment with TACE is recommended or
a per-patient basis following radical resection of HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 81360138).
Availability of data and materials
The data used and analyzed during the present study
are available from the corresponding author on reasonable
request.
Authors contributions
FH designed the study and reviewed the manuscript.
SH, XF, HM, HX and AR performed the experiments. JF and XS provided
help with data analysis and critically revised the manuscript. SH
analyzed data and wrote the manuscript. All authors have read and
approved the final manuscript.
Ethical approval
All procedures performed in the present study
involving human participants were approved by The Ethics Review
Board of The First Affiliated Hospital of Xinjiang Medical
University and conducted in accordance with the ethical standards
of the Ethics Review Board of The First Affiliated Hospital of
Xinjiang Medical University and with the 1964 Helsinki declaration
and its later amendments or comparable ethical standards. Informed
consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PI
|
prediction index
|
|
HCC
|
hepatocellular carcinoma
|
|
ROC
|
receiver operating characteristic
|
|
TACE
|
transcatheter arterial
chemoembolization
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang XF, Qi X, Meng B, Liu C, Yu L, Wang
B and Lv Y: Prognosis evaluation in alpha-fetoprotein negative
hepatocellular carcinoma after hepatectomy: Comparison of five
staging systems. Eur J Surg Oncol. 36:718–724. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amarapurkar D, Han KH, Chan HL and Ueno Y;
Asia-Pacific Working Party on Prevention of Hepatocellular C, :
Application of surveillance programs for hepatocellular carcinoma
in the Asia-Pacific Region. J Gastroenterol Hepatol. 24:955–961.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lai EC and Lau WY: The continuing
challenge of hepatic cancer in Asia. Surgeon. 3:210–215. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lau WY, Lai EC, Leung TW and Yu SC:
Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable
hepatocellular carcinoma: A prospective randomized trial-update on
5-year and 10-year survival. Ann Surg. 247:43–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cucchetti A, Zanello M, Cescon M, Ercolani
G, Del Gaudio M, Ravaioli M, Grazi GL and Pinna AD: Improved
diagnostic imaging and interventional therapies prolong survival
after resection for hepatocellular carcinoma in cirrhosis: The
university of bologna experience over 10 years. Ann Surg Oncol.
18:1630–1637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie H, Wang H, An W, Ma W, Qi R, Yang B,
Liu C, Gao Y, Xu B and Wang W: The efficacy of radiofrequency
ablation combined with transcatheter arterial chemoembolization for
primary hepatocellular carcinoma in a cohort of 487 patients. PLoS
One. 9:e890812014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin Z, Ren Z and Xia J: Appraisal of
postoperative transcatheter arterial chemoembolization (TACE) for
prevention and treatment of hepatocellular carcinoma recurrence.
Zhonghua Zhong Liu Za Zhi. 22:315–317. 2000.(In Chinese).
PubMed/NCBI
|
|
10
|
Lee JK, Chung YH, Song BC, Shin JW, Choi
WB, Yang SH, Yoon HK, Sung KB, Lee YS and Suh DJ: Recurrences of
hepatocellular carcinoma following initial remission by
transcatheter arterial chemoembolization. J Gastroenterol Hepatol.
17:52–58. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Portolani N, Coniglio A, Ghidoni S,
Giovanelli M, Benetti A, Tiberio GA and Giulini SM: Early and late
recurrence after liver resection for hepatocellular carcinoma:
Prognostic and therapeutic implications. Ann Surg. 243:229–235.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Samuel M, Chow PK, Chan Shih-Yen E, Machin
D and Soo KC: Neoadjuvant and adjuvant therapy for surgical
resection of hepatocellular carcinoma. Cochrane Database Syst Rev.
21:CD0011992009.
|
|
13
|
Association HBaIDBoCM: Guidelines for
prevention and treatment of chronic hepatitis B (2010 Edition).
Chin J Hepatology Electronic Edition. 3:40–56. 2011.
|
|
14
|
Sun HC, Zhang W, Qin LX, Zhang BH, Ye QH,
Wang L, Ren N, Zhuang PY, Zhu XD, Fan J and Tang ZY: Positive serum
hepatitis B e antigen is associated with higher risk of early
recurrence and poorer survival in patients after curative resection
of hepatitis B-related hepatocellular carcinoma. J Hepatol.
12:684–690. 2007. View Article : Google Scholar
|
|
15
|
Roayaie S, Blume IN, Thung SN, Guido M,
Fiel MI, Hiotis S, Labow DM, Llovet JM and Schwartz ME: A system of
classifying microvascular invasion to predict outcome after
resection in patients with hepatocellular carcinoma.
Gastroenterology. 137:850–855. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ardiles V, Sánchez Clariá R, Mazza OM,
Ciardullo MA, Pekolj J and De Santibañes E: Prognostic factors
after resection of hepatocellular carcinoma in the non-cirrhotic
liver: Presentation of 51 cases. Cir Esp. 87:148–154. 2010.(In
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sumie S, Kuromatsu R, Okuda K, Ando E,
Takata A, Fukushima N, Watanabe Y, Kojiro M and Sata M:
Microvascular invasion in patients with hepatocellular carcinoma
and its predictable clinicopathological factors. Ann Surg Oncol.
15:1375–1382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian YH, Li JD, Peng Y, Li QL, Li DX and
Li Q: Postoperative survival analysis of patients without liver
cirrhosis. Chin J Digestive Surg. 10:305–306. 2011.
|
|
19
|
Yamamoto J, Kosuge T, Takayama T, Shimada
K, Yamasaki S, Ozaki H, Yamaguchi N and Makuuchi M: Recurrence of
hepatocellular carcinoma after surgery. Br J Surg. 83:1219–1222.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Geschwind JF, Kudo M, Marrero JA, Venook
AP, Chen XP, Bronowicki JP, Dagher L, Furuse J, Ladrón de Guevara
L, Papandreou C, et al: TACE treatment in patients with
sorafenib-treated unresectable hepatocellular carcinoma in clinical
practice: Final analysis of GIDEON. Radiology. 279:630–640. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hua YF, Li CD, Qiu F, Yu WM, Wu SD, Zhang
GJ, Peng T and Yang HT: Transcatheter arterial chemoembolization
after liver resection for hepatocellular carcinoma with portal vein
tumor thrombus. Chin J Hepatobiliary Surg. 18:357–360. 2012.(In
Chinese).
|
|
22
|
Shah SA, Cleary SP, Wei AC, Yang I, Taylor
BR, Hemming AW, Langer B, Grant DR, Greig PD and Gallinger S:
Recurrence after liver resection for hepatocellular carcinoma: Risk
factors, treatment and outcomes. Surgery. 141:330–339. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsurusaki M and Murakami T: Surgical and
locoregional therapy of HCC: TACE. Liver Cancer. 4:165–175. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu Q, Qiao GL, Yan JJ, Wu MC and Yan YQ:
Prognosis after resection of early hepatocellular carcinoma in
HBV-related cirrhotic patients. Chin J Hepatobiliary Surg.
20:258–264. 2014.
|
|
25
|
Otto G, Heuschen U, Hofmann WJ, Krumm G,
Hinz U and Herfarth C: Survival and recurrence after liver
transplantation versus liver resection for hepatocellular
carcinoma: A retrospective analysis. Ann Surg. 227:424–432. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pawlik TM, Delman KA, Vauthey JN, Nagorney
DM, Ng IO, Ikai I, Yamaoka Y, Belghiti J, Lauwers GY, Poon RT and
Abdalla EK: Tumor size predicts vascular invasion and histologic
grade: Implications for selection of surgical treatment for
hepatocellular carcinoma. Liver Transpl. 11:1086–1092. 2005.
View Article : Google Scholar : PubMed/NCBI
|