Introduction
Lung cancer is the leading cause of
cancer-associated mortality, and non-small cell lung cancer (NSCLC)
accounts for >80% of lung cancer cases worldwide (1). Despite the fact that the diagnosis and
treatment of lung cancer has improved, the 5-year survival rate of
patients with NSCLC is <15% (2).
Understanding the molecular mechanisms underlying the effects of
lung cancer-associated genes is important for identification of
diagnostic and prognostic markers for the early detection and
targeted treatment of lung cancer. Apoptosis serves an important
role in the development and maintenance of multicellular organisms,
and can remove damaged, aged or autoimmune cells (3). Apoptosis deficiency is one of the main
characteristics of cancer cells. The primary goal of cancer
treatment is to restore and activate apoptosis of cancer cells
(4–6). However, to the best of our knowledge,
an effective treatment that induces lung cancer cell apoptosis is
not currently available. Therefore, identification of an optimal
anti-lung cancer strategy that induces cell apoptosis is
required.
Src homologous and collagen (SHC) is a cell-surface
receptor adapter protein, which can activate growth factor
receptors to signaling pathways, including insulin receptor,
insulin growth factor receptor, epidermal growth factor receptor
and fibroblast growth factor receptor (7–10). SHC
SH2-binding protein 1 (SHCBP1) is a member of the SHC family, and
has been recently identified in several malignant cancers (11). Aurora B-mediated SHCBP1
phosphorylation is essential for proper separation of the cleavage
furrow (12). SHCBP1 has also been
identified as a binding partner of central spindlin by proteome
analysis (13). SHCBP1 activates the
growth factor receptor, which has been indicated to serve a crucial
role in proliferation and is disordered in a number of diseases,
including cancer (14). SHCBP1 has
also been reported to be overexpressed in human hepatocellular
carcinoma (HCC) samples, whereas inhibition of SHCBP1 reduces cell
proliferation and colony formation of HCC cells (15). In addition, SHCBP1 is increased in
breast cancer, and serves a key role in cancer progression and
apoptosis; therefore, it may be considered a potential predictor of
breast cancer prognosis (11).
However, the effect and underlying mechanism of SHCBP1 in lung
cancer cell apoptosis remain unclear. The present study examined
the role of SHCBP1 in lung cancer cell apoptosis.
Phosphatase and tensin homolog (PTEN) is a tumor
suppressor gene on chromosome 10 that inhibits the proliferation,
invasion and metastasis of tumor cells, and induces apoptosis of
tumor cells, including lung cancer (16–18). The
loss or mutation of the PTEN gene occurs in various human
malignancies, and has been reported to be associated with increased
tumor grade and adverse outcomes (16–18).
Therefore, it was hypothesized that SHCBP1 may regulate PTEN in
lung cancer cell apoptosis. The aim of the present study was to
elucidate whether SHCBP1 inhibition induces apoptosis in lung
cancer cells and to determine the relevant mechanisms.
Materials and methods
Patients and tissue samples
In the present study, NSCLC and normal tissue
samples were obtained from 20 patients with NSCLC. A total of 10
patients were male and 10 patients were female; the median age at
diagnosis was 63 years (range, 43–72 years). The samples were
obtained from patients who were diagnosed with stage IA to IIIB
NSCLC (19) and underwent surgery at
the Second Affiliated Hospital of Harbin Medical University
(Harbin, China) between July 2015 and September 2017. None of the
patients received adjuvant chemotherapy, radiation or immunotherapy
prior to surgery. For all patients who participated in this study,
written informed consent was obtained, and this study was approved
by the Ethical Committee of Harbin Medical University.
Immunohistochemistry
Tissue sections (5 µm) were fixed with 10% formalin
at room temperature for 20 min and embedded in paraffin,
deparaffinized in xylene and rehydrated with graded alcohol.
Endogenous peroxidase activity was blocked with 3%
H2O2 for 15 min at room temperature, and
tissue sections were cultured in a humidified atmosphere at 4°C
with SHCBP1 mouse polyclonal antibodies (1:100; cat. no. ab122310;
Abcam). The sections were then incubated at room temperature for 30
min with a secondary biotinylated antibody (1:500; cat. no. 14708;
Cell Signaling Technology, Inc.). Antigen-antibody complexes were
detected with the streptavidin-peroxidase (15 min) method with
diaminobenzidine (DAB) as the chromogen substrate using the
Vectastain elite ABC kit (Vector Laboratories, Inc.), according to
the manufacturer's protocol. Peroxidase signals were observed
visually following treatment with the DAB substrate-chromogen
system for 8 min; finally, the sections were stained with
hematoxylin for 1 min at room temperature. PBS was used in place of
the primary antibody as a negative control. Each slide was examined
in five selected fields of view and 100 cells were observed using a
Fluoview1000 laser scanning confocal microscope at ×200
magnification (Olympus Corporation). Positive reactions were
defined as those that indicated brown immune responses in the
cytoplasm, nucleus and cell membrane.
Cell culture and transfection of
SHCBP1 small interfering RNA (siRNA)
The A549 human lung cancer cell line and the BEAS2B
normal lung epithelial cell line were purchased from Shanghai
Institute of Cell Biology, the Chinese Academy of Sciences. The
cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in an atmosphere containing 5%
CO2.
SHCBP1 siRNA was purchased from Abbexa Ltd. (cat.
no. abx933286) and PTEN siRNA from Santa Cruz Biotechnology, Inc.
(cat. no. sc-29459) Shanghai GenePharma Co., Ltd. synthesized the
negative control (NC) siRNA. The siRNA-NC sequence was as follows:
Forward, 5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3. According to the manufacturer's
protocols, A549 cells were seeded in 6-well plates
(1×105 cells/well) and starved in serum-free medium for
24 h prior to transfection for 72 h at room temperature using
X-treme GENE siRNA transfection reagent (Roche Applied Science,
Penzberg, Germany). The final concentration of SHCBP1 siRNA and
siRNA-NC was 100 nM, and of PTEN siRNA was 200 nM.
Cell viability assay
The viability of A549 cells was determined by MTT
assay (Sigma-Aldrich; Merck KGaA), according to the manufacturer's
protocol. Briefly, cells (2×104 cells/well) were seeded
in a 96-well culture plate. Cells were transfected with SHCBP1
siRNA or PTEN siRNA for 72 h. Following incubation, 5 mg/ml MTT
solution was added and cells were incubated for a further 4 h at
37°C. MTT formazan crystals were solubilized with DMSO and
absorbance was measured at a wavelength of 490 nm using a
microplate reader. Cell viability was measured as percentage of
viable cells relative to the control cells.
Flow cytometry
Apoptotic A549 cells were analyzed using the Alexa
Fluor® 488 Annexin V/Dead Cell Apoptosis kit
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. A549 cells were transfected with SHCBP1
siRNA for 72 h, and were then collected and washed with cold PBS.
Following centrifugation at 450 × g for 4 min at room temperature,
the supernatant was discarded and cell pellets were re-suspended in
1X Annexin-binding buffer to a final concentration of
1×106 cells/ml. Subsequently, 1 µl Alexa
Fluor® 488 Annexin V and 1 µl 100 µg/ml propidium iodide
working solution were added to each 100 µl cell suspension. Cells
were incubated in the dark at room temperature for 15 min. Stained
cells were detected by fluorescence spectrometry at 530 and 575 nm.
The apoptotic rate was calculated by BD FACSDiva software (BD
Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (PCR)
Total RNA from lung tissues, A549 and BEAS2B cells
was extracted with TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The SuperScript IV Reverse Transcriptase kit (Thermo Fisher
Scientific, Inc.) was used to synthesize cDNA according to
manufacturer's protocol. The mRNA expression levels of SHCBP1 and
PTEN were assessed by SYBR Green incorporation on a Roche
LightCycler® 480 real-time PCR system (Roche
Diagnostics, Indianapolis, IN, USA), with GAPDH as an internal
control. The thermocycling conditions were as follows: 120 sec at
95°C, followed by 40 cycles at 95°C for 30 sec and 55°C for 40 sec,
and a final step extension at 72°C for 3 min. Data was collected at
the end of the extension step (72°C). The primer sequences were:
SHCBP1, forward 5′-GCTACCGTGATAAACCAGGTTC-3′, reverse
5′-AGGCTCTGAATCGCTCATAGA-3′; PTEN, forward
5′-TGGATTCGACTTAGACTTGACCT-3′, reverse
5′-GCGGTGTCATAATGTCTCTCAG-3′; and GAPDH, forward
5′-AAGAAGGTGGTGAAGCAGGC-3′ and reverse, 5′-TCCACCACCCAGTTGCTGTA-3′.
The expression levels of the target genes were obtained by
normalizing to the endogenous reference gene and were compared with
a calibrator (average of the control samples), using the
2−ΔΔCq method (20).
Western blot analysis
Protein samples from lung tissues, A549 and BEAS2B
cells were analyzed (21). Protein
samples (70 µg) were extracted with lysis buffer (Beyotime
Institute of Biotechnology) supplemented with 1% protease inhibitor
solution. The concentration of the proteins was determined using a
BCA Protein Assay kit (Beyotime Institute of Biotechnology).
Subsequently, proteins (20 µg) were separated by 10% SDS-PAGE and
transferred to nitrocellulose membranes. The membranes were blocked
in PBS containing 5% non-fat milk for 2 h at room temperature and
incubated at 4°C overnight with the following primary antibodies:
SHCBP1 (1:1,000; cat. no. ab184467; Abcam), PTEN (1:500; cat. no.
ab32199; Abcam) and GAPDH (1:2,000; cat. no. TA802519; OriGene
Technologies, Inc.). Following washing, the membrane was incubated
with secondary anti-rabbit immunoglobulin G (IgG; 1:1,000; cat. no.
A3687; Sigma-Aldrich; Merck KGaA) and anti-mouse IgG (1:1,000; cat.
no. M8770; Sigma-Aldrich; Merck KGaA) antibodies at room
temperature for 1 h. Images were captured on an Odyssey CLx
Infrared Imaging system (LI-COR Biosciences). Subsequently, the
blots were semi-quantified using Odyssey CLx 2.1 software (LI-COR
Biosciences). Data were normalized to GAPDH as an internal
control.
Caspase-3 activity assay
According to the manufacturer's protocol, caspase-3
activity in A549 cells was determined using a colorimetric assay
kit (cat. no. C1116; Beyotime Institute of Biotechnology). A549
cell lysis was performed in ice for 15 min, and the lysate was
subsequently centrifuged at 20,000 × g for 10 min at 4°C.
Supernatant samples (30 µl) were then incubated with 10 µl
substrate (2 mM Ac-DEVD-pNA) in 60 µl assay buffer at 37°C for 2 h.
The absorbance rate was measured at a wavelength of 405 nm.
Statistical analysis
Group data are expressed as the mean ± standard
error of the mean and were analyzed by SPSS 17.0 software (SPSS,
Inc.). Student's t-test was performed for two-group comparisons.
One-way analysis of variance followed by Dunnett's test was used
for multiple-group comparisons. P<0.05 was considered to
indicate a statistically significant difference. Figures were
constructed using GraphPad Prism 5.0 software (GraphPad Software,
Inc.).
Results
SHCBP1 expression is upregulated in
lung cancer tissues
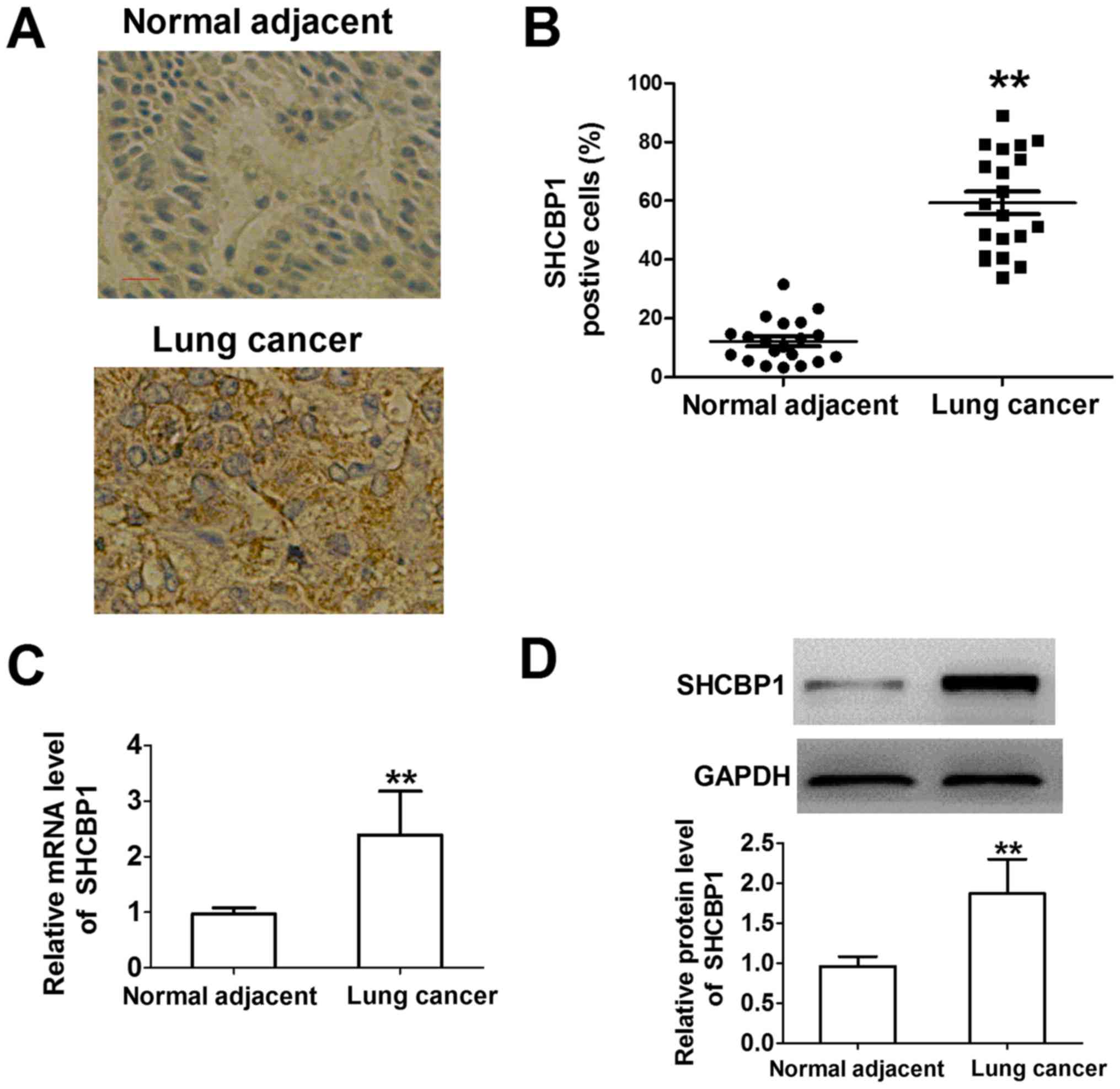
To detect SHCBP1 expression in lung cancer, the
expression of SHCBP1 was compared in 20 lung cancer tissues and
adjacent normal tissues. Human normal lung tissues did not exhibit
SHCBP1 protein expression by immunostaining, whereas the proportion
of SHCBP1-positive cells in lung cancer samples was higher compared
with in the normal lung tissue samples (Fig. 1A and B). SHCBP1 was revealed to be
located in the cytoplasm. These results indicated that SHCBP1 was
overexpressed in lung cancer cells. As shown in Fig. 1C and D, the mRNA and protein
expression levels of SHCBP1 were significantly higher in lung
cancer tissues compared with in adjacent normal tissues. These
results suggested that SHCBP1 may be significantly overexpressed in
lung cancer and may serve an important role in the tumorigenesis of
lung cancer.
Stable SHCBP1 siRNA transfection in
lung cancer cell lines
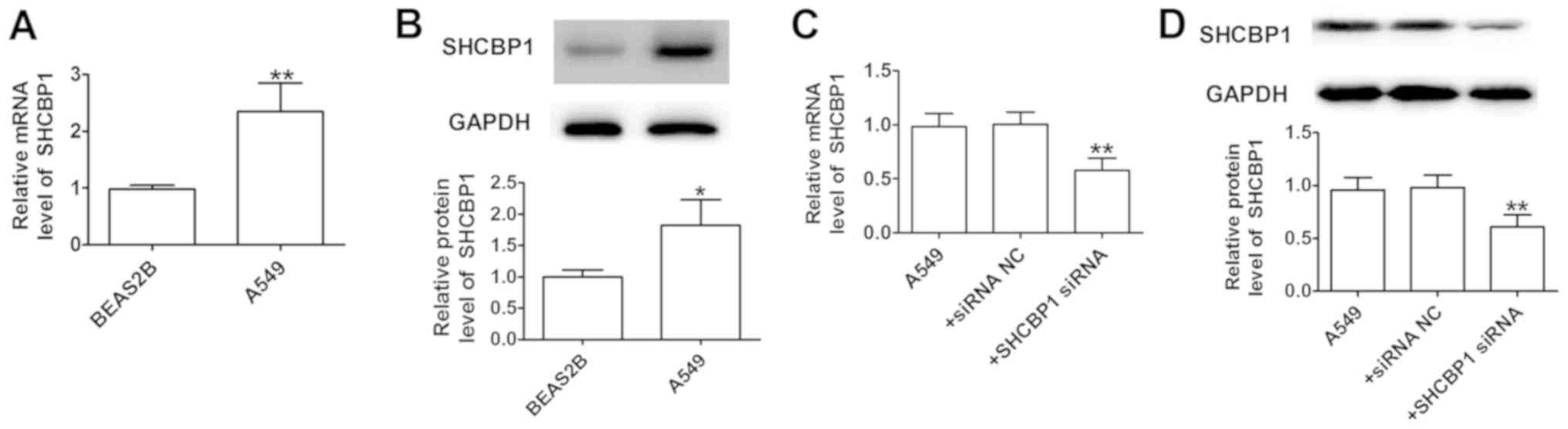
SHCBP1 was measured in A549 cells and the normal
lung cell line, BEAS2B (Fig. 2A and
B). The results indicated that compared with in BEAS2B cells,
SHCBP1 was highly expressed in the tumor cell line A549. The
efficiency of SHCBP1 knockdown by siRNA was verified by detection
of mRNA and protein expression levels, which were significantly
reduced compared with in A549 cells transfected with siRNA-NC at 24
h (Fig. 2C and D). The results
indicated that siRNA can be used to generate stable SHCBP1
knockdown in A549 cells.
Inhibition of SHCBP1 promotes
apoptosis of lung cancer cells
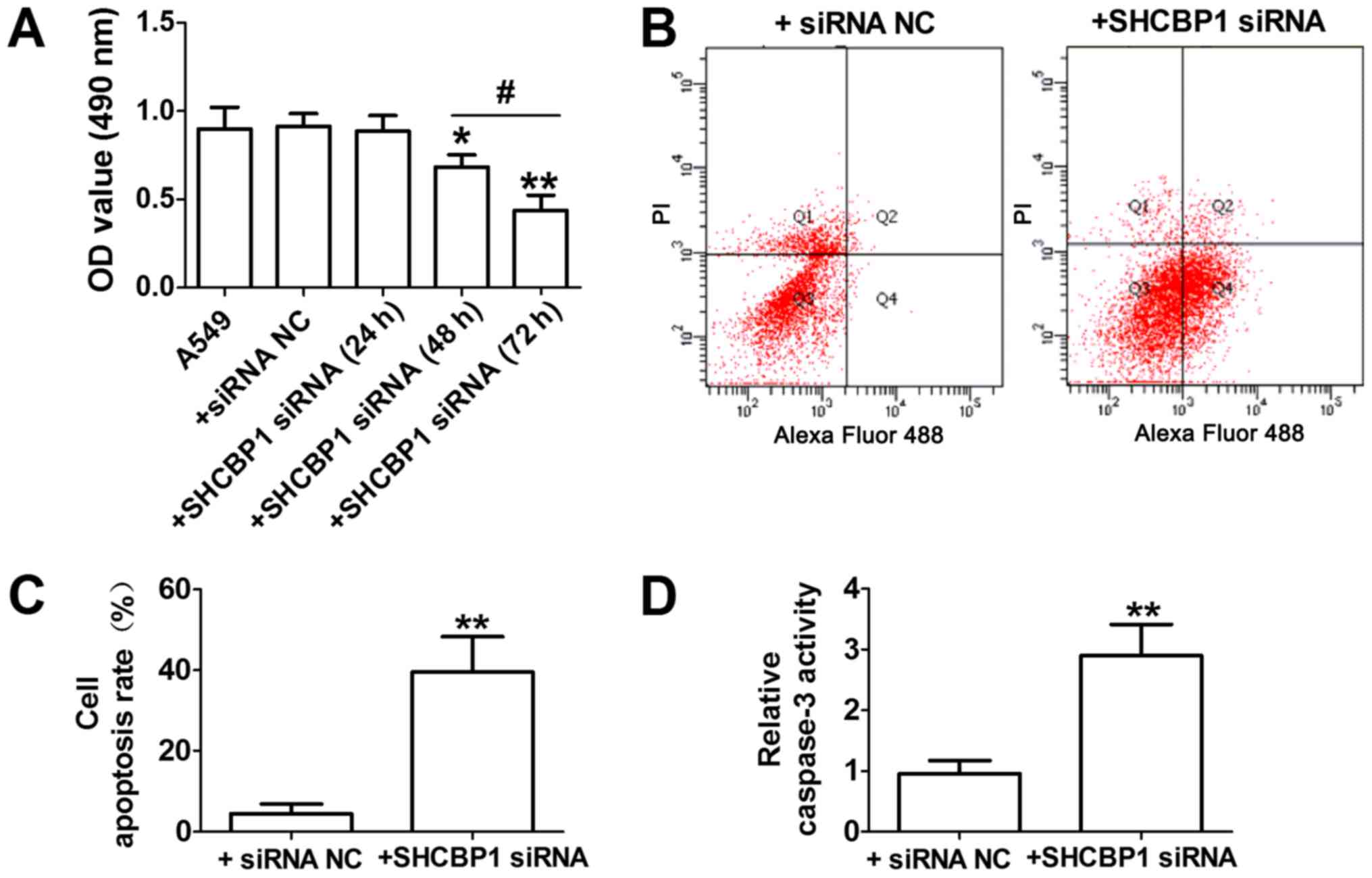
To examine the possible role and underlying
mechanism of SHCBP1 in lung cancer cells, siRNA was used to
suppress SHCBP1 expression, and caspase-3 and cell apoptosis
activity were determined. As shown in Fig. 3A, the viability of A549 cells was
reduced by SHCBP1 siRNA transfection at 24, 48 and 72 h; the most
obvious decrease in cell viability was detected at 72 h. Therefore,
in A549 cells, SHCBP1 siRNA transfection at 72 h was used for
subsequent experiments. SHCBP1 siRNA-induced apoptosis was further
confirmed by flow cytometry, and an increased number of apoptotic
A549 cells was detected at 72 h following SHCBP1 siRNA transfection
(Fig. 3B and C). Furthermore,
alterations in caspase-3 activity, a key downstream protease that
executes the lung cancer apoptotic cascade, were measured;
caspase-3 activity is considered to be the last step of
caspase-dependent apoptosis (6,21,22). As
shown in Fig. 3D, SHCBP1 siRNA
increased the levels of caspase-3 activity compared with siRNA-NC
in A549 cells. Based on these findings, it was concluded that
SHCBP1 siRNA may induce apoptotic effects on lung cancer cells.
SHCBP1 siRNA promotes lung cancer cell
apoptosis partly via PTEN
PTEN serves an important role in apoptosis of
various types of cancer cells, including lung cancer (17,23).
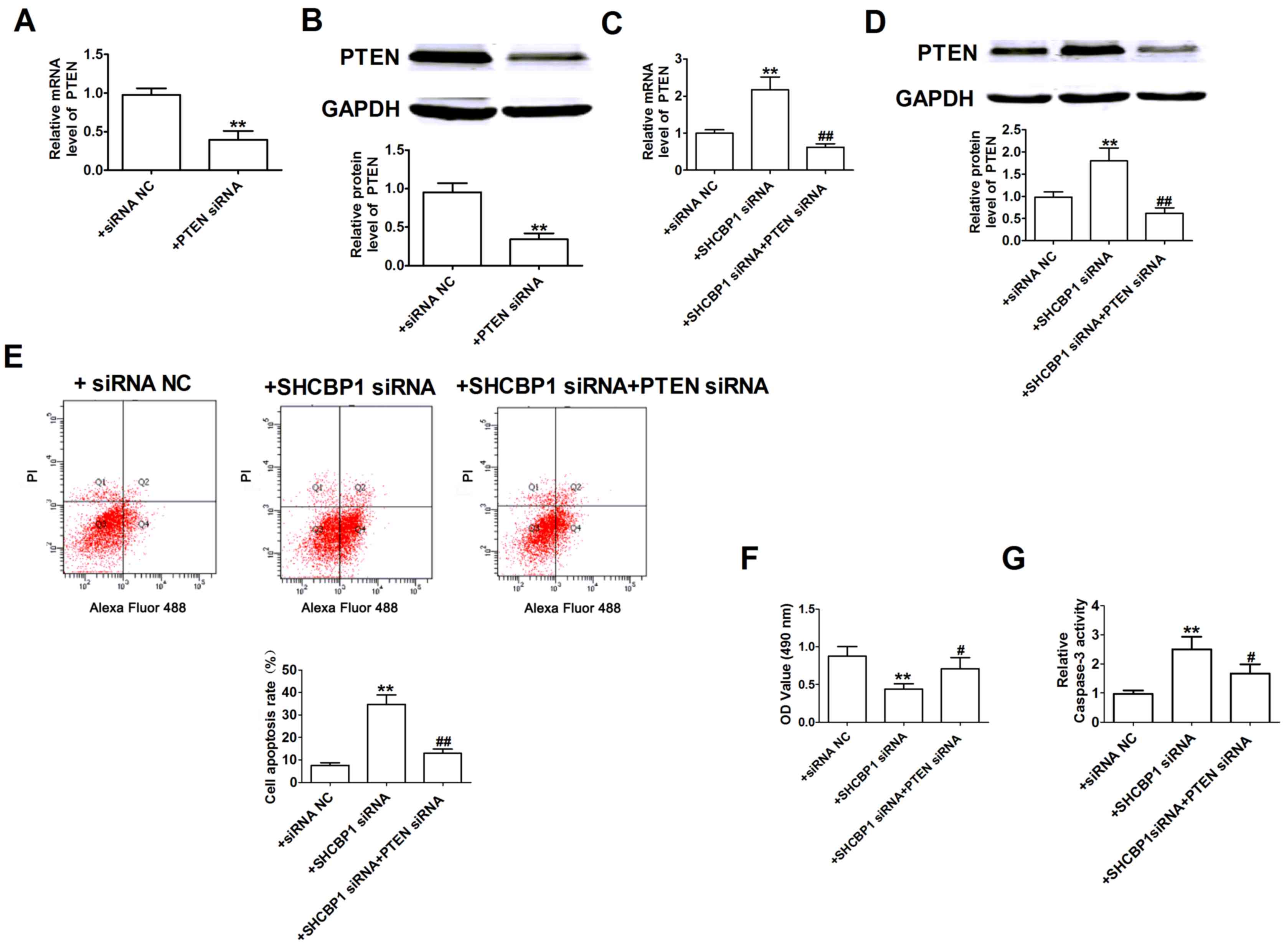
Therefore, the present study investigated whether PTEN was involved
in SHCBP1-mediated apoptosis. The efficiency of PTEN knockdown was
validated by detection of mRNA and protein expression levels, which
were lower in A549 cells compared with in cells transfected with
siRNA-NC (Fig. 4A and B).
Subsequently the effects of SHCBP1 siRNA on PTEN expression were
assessed in A549 cells. As shown in Fig.
4C and D, SHCBP1 siRNA significantly upregulated the expression
levels of PTEN in A549 cells. Following transfection of A549 cells
with SHCBP1 siRNA and PTEN siRNA, PTEN expression was significantly
decreased compared with in cells transfected with SHCBP1 siRNA
alone. The role of PTEN siRNA in SHCBP1 siRNA-induced apoptosis was
further tested. As indicated in Fig.
4E-G, SHCBP1 siRNA increased the number of apoptotic cells,
reduced cell viability and increased caspase-3 activity in A549
cells; these effects that were reversed following PTEN-knockdown
with siRNA. Therefore, SHCBP1 siRNA may promote the apoptosis of
lung cancer cells at least in part through PTEN induction.
Discussion
The present study indicated that SHCBP1 was
overexpressed in lung cancer cell lines and lung cancer tissues.
siRNA-induced knockdown of SHCBP1 significantly increased the
apoptosis of lung cancer cells. In addition, SHCBP1 siRNA
significantly increased the expression of PTEN, whereas inhibition
of PTEN reversed SHCBP1 siRNA-induced apoptosis. These results
suggested that SHCBP1 may be upregulated in lung cancer and may
serve an important role in the apoptosis of lung cancer cells,
which may be associated with PTEN. The present study elucidated a
potential novel effect and mechanism of SHCBP1 on apoptosis of lung
cancer cells.
Lung cancer is one of the most common malignancies
and the leading cause of cancer-associated mortality. It is a
complex disease associated with numerous dysregulated signaling
pathways, including apoptosis (1,3,4). Numerous natural and synthetic compounds
have been reported to exert anticancer effects by inducing various
apoptotic pathways (6,24,25). A
better understanding of the function of lung cancer cell apoptosis
may provide novel therapeutic approaches for disease prevention or
control.
SHCBP1 is a member of the SHC family. SHC genes have
been reported to be important in the regulation of mammalian
apoptosis and resistance (11).
Dysregulated SHCs may lead to uncontrolled proliferation, which is
a hallmark of human cancer (11).
SHCBP1 is overexpressed in HCC, and the loss of SHCBP1 in HCC cells
leads to inhibition of cell proliferation and increased apoptosis
(15). In addition, SHCBP1 is
associated with the spread and apoptosis of breast cancer (11). However, to the best of our knowledge,
the expression of SHCBP1, and the possible role and mechanisms of
SHCBP1 in the apoptosis of lung cancer cells have not been studied.
The present study hypothesized that SHCBP1 may be associated with
lung cancer.
To confirm the role of SHCBP1 in lung cancer cell
apoptosis, the expression levels of SHCBP1 were examined in lung
cancer and matched normal lung tissue samples. The results
indicated a significant increase in SHCBP1 expression in cancer
compared with in normal lung tissue, suggesting that SHCBP1 may be
a candidate oncogene in lung cancer. SHCBP1 siRNA was subsequently
transfected into the lung cancer cell line A549 to inhibit its
expression. The in vitro experiments indicated that
inhibition of SHCBP1 significantly increased apoptosis of lung
cancer cells and caspase-3 activity, and inhibited cell viability.
These results suggested that SHCBP1 siRNA may promote the apoptosis
of lung cancer cells.
PTEN is a well-known tumor suppressor gene, which
has been indicated to serve a crucial role in the spread, apoptosis
and invasion of lung cancer (16,17).
Therefore, the association between SHCBP1 and PTEN was examined in
lung cancer cells. The results indicated that PTEN expression was
significantly increased in SHCBP1-knockdown A549 cells. In
addition, PTEN siRNA reversed the effects of SHCBP1 siRNA on
apoptosis, caspase-3 activity and cell viability. All these results
indicated that SHCBP1 siRNA may promote apoptosis of lung cancer
cells partly by upregulating PTEN. However, the involvement of
other mechanisms that may mediate the pro-apoptotic effects of
SHCBP1 siRNA cannot be excluded. Future research may focus on
disrupting the interactions between SHCBP1 and downstream targets,
which may have important therapeutic implications. Our subsequent
studies will focus on collecting more samples to examine
pathological and clinical data of patients with NSCLC in order to
analyze SHCBP1 further, and will investigate whether it is
significantly associated with invasion depth, lymph node
metastasis, tumor size and survival.
In conclusion, the present study indicated that
SHCBP1 may serve an important role in regulating apoptosis of lung
cancer cells. In addition, it was suggested that this role may be
regulated by PTEN. Therefore, the candidate oncogene SHCBP1 may be
considered an effective novel therapeutic target for the treatment
of lung cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by 2018
Jinan University First Clinical Medical College Research and
Cultivation Fund Project (grant no. 2018104 to JHW).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors contributions
FW and JHW were responsible for the conception and
design of the study. FW, YL, ZZ and JXW performed the experiments.
FW analyzed and interpreted the data. FW and JHW drafted the
article and were responsible for the revision of the manuscript.
JHW gave final approval of the version to be published.
Ethics approval and consent to
participate
For all patients who participated in this study,
written informed consent was obtained, and this study was approved
by the Ethical Committee of Harbin Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nana-Sinkam SP and Powell CA: Molecular
biology of lung cancer: Diagnosis and management of lung cancer,
3rd edition: American college of chest physicians evidence-based
clinical practice guidelines. Chest. 143:e30S–e39S. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reungwetwattana T and Dy GK: Targeted
therapies in development for non-small cell lung cancer. J
Carcinog. 12:222013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Othman N and Nagoor NH: The role of
microRNAs in the regulation of apoptosis in lung cancer and its
application in cancer treatment. Biomed Res Int. 2014:3180302014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsuruo T, Naito M, Tomida A, Fujita N,
Mashima T, Sakamoto H and Haga N: Molecular targeting therapy of
cancer: Drug resistance, apoptosis and survival signal. Cancer Sci.
94:15–21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ding Y and Nguyen TA: Pq1, a quinoline
derivative, induces apoptosis in T47D breast cancer cells through
activation of caspase-8 and caspase-9. Apoptosis. 18:1071–1082.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ornitz DM and Itoh N: The fibroblast
growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol.
4:215–266. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koul HK, Pal M and Koul S: Role of p38 MAP
kinase signal transduction in solid tumors. Genes Cancer.
4:342–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Y, Long H, Wu Z, Jiang X and Ma L:
EGF transregulates opioid receptors through EGFR-mediated GRK2
phosphorylation and activation. Mol Biol Cell. 19:2973–2983. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qu WS, Tian DS, Guo ZB, Fang J, Zhang Q,
Yu ZY, Xie MJ, Zhang HQ, Lu JG and Wang W: Inhibition of EGFR/MAPK
signaling reduces microglial inflammatory response and the
associated secondary damage in rats after spinal cord injury. J
Neuroinflammation. 9:1782012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng W, Li HC, Xu K, Chen YF, Pan LY, Mei
Y, Cai H, Jiang YM, Chen T and Feng DX: SHCBP1 is over-expressed in
breast cancer and is important in the proliferation and apoptosis
of the human malignant breast cancer cell line. Gene. 587:91–97.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Asano E, Hasegawa H, Hyodo T, Ito S, Maeda
M, Chen D, Takahashi M, Hamaguchi M and Senga T: SHCBP1 is required
for midbody organization and cytokinesis completion. Cell Cycle.
13:2744–2751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buckley MW, Arandjelovic S, Trampont PC,
Kim TS, Braciale TJ and Ravichandran KS: Unexpected phenotype of
mice lacking SHCBP1, a protein induced during T cell proliferation.
PLoS One. 9:e1055762014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kupershmidt I, Su QJ, Grewal A, Sundaresh
S, Halperin I, Flynn J, Shekar M, Wang H, Park J, Cui W, et al:
Ontology-based meta-analysis of global collections of
high-throughput public data. PLoS One. 5(pii): e130662010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tao HC, Wang HX, Dai M, Gu CY, Wang Q, Han
ZG and Cai B: Targeting SHCBP1 inhibits cell proliferation in human
hepatocellular carcinoma cells. Asian Pac J Cancer Prev.
14:5645–5650. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carracedo A, Alimonti A and Pandolfi PP:
PTEN level in tumor suppression: How much is too little? Cancer
Res. 71:629–633. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hollander MC, Blumenthal GM and Dennis PA:
PTEN loss in the continuum of common cancers, rare syndromes and
mouse models. Nat Rev Cancer. 11:289–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu XX, Cao LY, Chen X, Xiao J, Zou Y and
Chen Q: PTEN inhibits cell proliferation, promotes cell apoptosis
and induces cell cycle arrest via downregulating the PI3K/AKT/hTERT
pathway in lung adenocarcinoma A549 cells. Biomed Res Int.
2016:24768422016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu S, Xu J, Xu X, Hu S, Li B and Li W: The
expression of astrocyte elevated gene-1 in human non-small-cell
lung cancer and its relationship with postoperative chemotherapy
and radiotherapy. Histopathology. 67:817–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chu C, Liu X, Bai X, Zhao T, Wang M, Xu R,
Li M, Hu Y, Li W, Yang L, et al: MiR-519d suppresses breast cancer
tumorigenesis and metastasis via targeting MMP3. Int J Biol Sci.
14:228–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xue Y, Wu L, Liu Y, Ma Y, Zhang L, Ma X,
Yang Y and Chen J: ENTPD5 induces apoptosis in lung cancer cells
via regulating caspase 3 expression. PLoS One. 10:e01200462015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo Y, Chang H, Li J, Xu XY, Shen L, Yu ZB
and Liu WC: Thymosin alpha 1 suppresses proliferation and induces
apoptosis in breast cancer cells through PTEN-mediated inhibition
of PI3K/Akt/mTOR signaling pathway. Apoptosis. 20:1109–1121. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shimizu S: development of anti-cancer
drugs mediated by apoptosis and autophagy. Nihon Rinsho.
73:1302–1307. 2015.(In Japanese). PubMed/NCBI
|
|
25
|
Gali-Muhtasib H, Hmadi R, Kareh M, Tohme R
and Darwiche N: Cell death mechanisms of plant-derived anticancer
drugs: Beyond apoptosis. Apoptosis. 20:1531–1562. 2015. View Article : Google Scholar : PubMed/NCBI
|