Introduction
Breast cancer is a malignant tumor with the highest
incidence in women worldwide, and is the leading cause of mortality
among women 40–55 years old in developed countries, including China
(1). When diagnosed and treated,
patients with early breast cancer may have positive outcomes,
therefore, early detection is essential for decreasing breast
cancer mortality (2). Although great
progress has been made in the treatment of breast cancer, the
effect is not satisfactory owing to its recurrence, metastasis and
drug resistance in some patients (3). Cancer stem cell theory hypothesizes
that cancer stem cells serve an important role in the formation and
growth of cancer (4), and ignoring
the treatment of cancer stem cells may result in tumor recurrence
and metastasis (5). Phage display
peptide library screening technology is a method for identifying
high-specificity protein molecules (6). By screening with any target molecules,
the sequence of the polypeptide or protein, which interacts with
the target protein, is rapidly obtained (7,8). It may
also be used to analyze tumor epitopes to identify new tumor
surface markers and prepare tumor vaccines (9,10).
Previous studies have demonstrated that phage display technology
can identify multiple specific surface markers in triple negative
breast cancer in human epidermal growth factor receptor 2-related
breast cancer research (7,11,12). In
a breast cancer study, single-chain variable fragment 173, a
specific binding protein capable of binding to the PM-1 breast
cancer cell line, and Wiiger, a polypeptide specifically binding to
breast cancer cells (13), were
identified using phage display peptide library technology (14). Phage display technology is a mature
technology for the screening of tumor-specific peptides that has
been used to screen a variety of tumor-specific peptides (8,15), but
the application of this technology to identify breast cancer stem
cell-specific peptides requires further investigation.
Materials and methods
Cells, phage peptide library and host
bacteria
Human breast cancer cell lines MDA-MB-231 and MCF-7,
and human mammary gland cell line hs578bst (all purchased from the
Type Culture Collection of the Chinese Academy of Sciences) were
used in the present study. The bacteriophage random 12-peptide
library kit (Ph.D.™−12 Phage Display Peptide Library
kit; cat. no. E8110S) was purchased from New England BioLabs, Inc.,
and 100 µl bacteriophage was stored in TBS containing 50% glycerol
at −20°C. The following antibodies were used for the identification
of breast cancer stem cells: Flow cytometry (FCM); FITC anti-human
CD44 (cat. no. 338804; BioLegend, Inc.); phycoerythrin-conjugated
anti-human CD24 (cat. no. 311106; BioLegend, Inc.); and Alexa Fluor
647 anti-human CD326 antibody (cat. no. 324212; BioLegend,
Inc.).
Enrichment and identification of
breast cancer stem cells
Cells were cultured at 37°C in 5% CO2 in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc., cat. no. 16000-044), 100 U/ml
penicillin and 100 U/ml streptomycin with regular media changes and
frequent subculture. Stem cells were enriched in serum-free medium
supplemented with epidermal growth factor, basic fibroblast growth
factor and B27 supplement, and the microspheres were observed under
an inverted microscope (Leica Microsystems, Inc., cat. no. CTR6000;
magnification, ×100). Centrifugation at 300 g for 10 min at 24°C
was conducted to change the medium every 2–3 days. Following 1 week
of serum starvation, cells were cultured medium with 10% FBS for
one passage to remove dead cells. The cells were cultured
alternately with ‘serum and serum-free’ culture medium to enrich
the breast cancer stem cells, and were observed under an inverted
microscope (Leica Microsystems, Inc., cat. no. CTR6000;
magnification, ×100). Subsequently, cells from the microspheres
were labeled with following FITC-, PE-, Alexa Fluor 647-,
conjugated mouse anti-human antibodies in fluorescence activated
cell sorter buffer (BD Biosciences) for 30 min at room temperature.
The FITC anti-human CD44 antibody (cat. no. 338804), PE anti-human
CD24 antibody (cat. no. 311106) and Alexa Fluor 647(APC) anti-human
CD326 (ESA) (cat. no. 324212) antibody were purchased from
BioLegend, Inc. The
CD44+/CD24−/lowESA+ phenotype
cancer stem cells were sorted using a flow cytometer, then analyzed
using FACS Canto II instruments (BD Biosciences) and BD Dive
software 6.0 to determine the percentage of positive cells.
Screening phage random peptide
library
The hs578bst cells, the MCF7 and MDA-MB-231 breast
cancer cells and respectively enriched breast cancer stem cells
were seeded in polylysine-coated petri dishes with serum-free DMEM
for 2 h and blocked with 0.5% bovine serum albumin (BSA; Tianjin
Umbrella Science & Technology Co., Ltd.) for 1 h. On day 1,
cells were trypsinized to obtain a single cell suspension and cells
(5×105) were seeded on a polylysine-coated plate.
Adherent cells were cultured in DMEM with 10% FBS and the stem cell
microspheres were cultured in DMEM/Nutrient Mixture F-12 (Gibco;
Thermo Fisher Scientific, Inc.) with 10% FBS. Cells were cultured
with serum-free medium the next day for 2 h, blocked with 0.5%
immunoglobulin G-free BSA-free medium for 1 h and washed with 0.1%
TBS + 0.1% Tween-20 (TBST) buffer three times. For the first round
of selection, 25 µl of the original library was diluted with 1 ml
TBST buffer and added to the negative selection cells, MCF7 and
MDA-MB-231. Subsequently, the cells were incubated at 37°C for 1 h
and the supernatant was transferred to the negative selection
cells. The phages were removed by washing with 1 ml 0.2 M
glycine-HCl (pH 2.2) buffer. The cell supernatant was collected in
a centrifuge tube after incubation for 10 min and neutralized with
150 µl Tris-HCl (pH 9.1). The product was amplified and titrated
for the next round of selection. In the next round of selection,
the total amount of each initial phage was 1×1011, the
time for positive selection was 30 min and 0.2% (v/v) Tween-20 was
used for washing; other conditions remained unchanged. In the third
round of screening, conditions were unchanged with two exceptions:
The positive selection time was 15 min and 0.3% (v/v) Tween-20 was
used for washing.
Phage amplification
ER2738 Escherichia coli were incubated in 10
ml lysogeny broth (LB)-tetracycline-10 (Oxoid, Ltd.) medium
overnight to prepare for the second day of phage amplification. The
ER2738 cell suspension was diluted in 20 ml LB medium at a ratio of
1:100, shaken and subsequently cultured at 37°C for 1–2 h to reach
early logarithmic growth phase [optical density at 600 nm
(OD600), 0.3–0.4]. The phage were added in
1×1011 pfu and cultured at 37°C with vigorous shaking by
200 rpm for 4.5 h. The culture product was transferred to a
centrifuge tube and centrifuged at 10,000 × g for 10 min at 4°C.
The supernatant was transferred to another centrifuge tube and
centrifuged again under the same conditions. The upper part (80%)
of the supernatant was transferred to a new tube 1/6 volume of
PEG/NaCl was added and left to precipitate overnight at 4°C.
Subsequently, the sample was centrifuged at 10,000 × g for 15 min
at 4°C. The supernatant was discarded, the pellet was centrifuged
briefly and the residual supernatant was aspirated. The pellet was
resuspended in 1 ml TBS, transferred to a microcentrifuge tube and
centrifuged at 10,000 g, 4°C for 5 min to precipitate the residual
cells.
The supernatant was transferred to a new centrifuge
tube, re-precipitated with 1/6 volume of PEG/NaCl and incubated on
ice for 15–60 min. Following centrifugation at 10,000 × g, 4°C for
10 min, the supernatant was discarded and briefly centrifuged
again. The residual supernatant was aspirated using a micropipette.
The pellet was resuspended in 200 µl TBS and centrifuged at 4°C,
10,000 × g for 1 min. The supernatant was subsequently transferred
to a fresh tube as the amplification product.
Phage titration
The phage was titrated with a LB/isopropyl
β-D-1-thiogalactopyranoside (IPTG)/5-bromo-4-chloro-3-
indolyl-β-D-galactoside (X-gal) tablet: ER2738 single colonies were
inoculated in 5–10 ml LB medium, shaken and cultured to
mid-logarithmic growth phase (OD600, ~0.5). The upper
agar was melted in the microwave and divided it into 3 ml aliquots
in sterilized tubes, one tube per phage dilution. The tubes were
stored at 45°C for future use. A LB/IPTG/X-gal plate was prewarmed
at 37°C for each phage dilution. A 10-fold serial dilution of phage
in LB was prepared. The titers were in the range of
101−1011. The phage at the logarithmic phase
was divided into 200 µl aliquots in microcentrifuge tubes, one tube
per phage dilution. A total of 10 µl phage was added in different
tubes, mixed rapidly and thoroughly shaken. Subsequently, the
samples were incubated at room temperature for 1–5 min. Infected
cells were added to the 45°C prewarmed upper agar culture tube,
mixed rapidly and put onto the 37°C prewarmed LB/IPTG/X-gal plate.
The plate was tilted to allow the spread of the upper agar.
Following cooling for 5 min, the plate was incubated overnight at
37°C. The number of blue spots with ~102 plaques was
counted and multiplied by the dilution fold to obtain the plaque
forming unit (pfu) titer per 10 µl phage.
Positive phage identification
The enriched breast cancer stem cells, MCF7 and
MDA-MB-231, were seeded in 96-well plates (1×104
cells/well) with serum-free DMEM for 2 h following adherence. The
cells were fixed with 4% paraformaldehyde at 24°C for 15 min and
washed with PBS. The cells were treated with 0.1% Triton X-100 for
10 min and washed with PBS + 0.5% Tween-20 (PBST) three times.
Following 1 h of blocking with 2% PBS-BSA at 37°C, the cells were
incubated with the amplified monoclonal phage for 2 h at 37°C and
washed three times with PBST. Following incubation at 24°C with
horseradish peroxidase (HRP)-conjugated anti-M13 antibody (cat. no.
405210; BioLegend, Inc.; dilution, 1:5,000 in 2% PBS-BSA) for 1 h
cells were washed with PBST three times. Then
3,3′,5,5′-tetramethylbenzidine (TMB) was added to proceed with the
chromogenic reaction. HCl was added to terminate the TMB
chromogenic reaction and the absorbance was read at 450 nm using a
microplate reader. A phage plaque was randomly selected as a
control, and the value of OD phage clone/OD control >2 was
regarded as positive. Normal breast cells (hs578bst), breast cancer
and enriched breast cancer stem cells, MCF7 and MDA-MB-231, were
seeded in a 24-well plate (1×105 cells/well), and a
similar process was repeated using a DAB HRP chromogenic kit
instead of TMB, and HCl was replaced with distilled water following
a 10 min incubation. Cells were counterstained with Mayer's
hematoxylin solution and observed under an inverted microscope
(Leica Microsystems, Inc., cat. no. CTR6000; magnification,
×100).
Positive phage sequencing
Single E. coli ER2738 colonies were
inoculated into 20 ml LB medium, shaken and cultured at 200 rpm,
37°C to early logarithmic growth phase. A total of 10 µl positive
phage clone KL-6 stock solution at 1×1011 pfu was added
to the ER2738 solution, shaken and centrifuged at 37°C and 250 rpm
for 3.5 h. Following centrifugation at 10,000 × g, 24°C for 5 min,
the supernatant was added to 1/6 volume of 20% polyethylene glycol
(PEG)/NaCl to precipitate at room temperature for 1 h and
centrifuged at 10,000 × g, 24°C for 10 min. The supernatant was
removed, and the precipitate was resuspended with 1 ml TBS and
stored at 4°C. During plaque amplification, 500 µl phage-containing
supernatant was transferred to a new tube following the first
centrifugation. A total of 200 µl PEG/NaCl was added, and the
mixture was inverted and mixed. Subsequently, the mixture was
allowed to stand at room temperature for 10 min. The sample was
centrifuged at 10,000 × g, 24°C for 10 min and the supernatant was
discarded. The sample was centrifuged briefly again, and the
residual supernatant was aspirated. The pellet was completely
resuspended in 100 µl sodium iodide buffer, 250 µl absolute ethyl
alcohol was added and incubated at room temperature for 10 min to
precipitate single stranded phage DNA. Following the incubation,
the sample was centrifuged at 10,000 × g, 24°C for 10 min and the
supernatant was discarded. The precipitate was washed with 70%
ethanol and briefly vacuum dried. The pellet was resuspended in 30
µl Tris-EDTA and the resulting suspension was used as the template
solution for sequencing. MDA-MB-231 cell clone B1-B10 sequences
were as follows: B1, LYAVDLSPKSRY; B3, HLAVRPISTNSR; B4,
ITRSYPPLPLPP; B5, TNSFHAIAGYQS; B6, TMHYKGTAASES; B7, YSDGVRAPRTVE;
B8, TNSFHGIAGYQS; B9: KLTALVTTWPWT. As a control, two negative
peptide sequences were obtained from the selected three blue
plaques: B11, GMLSWTKERPNT; B13, NHKTINYQNDAT. MCF7 cell clone
A1-A10 sequences were as follows: A1, DSMHHSLKPPYS; A2,
FKPTWVVPINVA; A3, NPGTDLYRPPHN; A5, NPSRSGPANWPD; A8, HRTLDPQPLTHP;
A9, VDWYDISRLRRT. As a control, two negative peptide sequences were
obtained from the selected three blue plaques: A11, GSYTAYSSIATA;
A13, SFHASMRPHTST. The sequencing primer was −96 gIII,
5′-HOCCCTCATAGTTAGCGTAACG-3′ (Tianjin Purui Biological Engineering
Co. Ltd).
Synthesis and determination of
polypeptides in vitro
Polypeptides were synthesized based on the
sequencing results and labeled with FITC. The breast cancer cells
and breast cancer stem cells MCF7 and MDA-MB-231 were incubated at
24°C for 15 min with the polypeptides. Subsequently, the
distribution of the FITC-tagged polypeptides was observed in
different cell lines and images were captured under an inverted
microscope (Leica Microsystems, Inc., cat. no. CTR6000;
magnification, ×100).
Specificity identification of
polypeptide in vivo
A total of 10 nude BALB/c female mice (6–8 weeks,
average weight 20 g) were used to establish an animal model of
breast cancer with the approval of the Ethics Committee of Shandong
Cancer Hospital and Institute. The mice were purchased from Nanjing
Animal Center. The ad libitum-fed mice were kept at 22°C in a SPF
environment (45% humidity; 20 Pa pressure difference) with a 14/10
h light/dark cycle. MDA-MB-231 stem cells were centrifuged and the
concentration of breast stem cells was adjusted to 1×108
cells/ml. The cells were implanted subcutaneously under the right
breast pad of nude BALB/c mice. Palpable tumor nodules first
appeared 3 weeks after tumor injection and they rapidly grew and
reached 8–10 mm in diameter in 3 weeks. The polypeptide labeled
with FITC (120 µl, 1 mMol) was injected into the tail vein of nude
mice, and the mice were sacrificed by cervical dislocation and
dissected after 2 h. The tumor and liver control tissue were cut to
<1 mm, added with PBS, moved to 12-hole plate with cover glass,
cultured at 37°C for 1 h in an incubator. Then, cells were fixed
with 4% paraformaldehyde (Tianjin Umbrella Science & Technology
Co., Ltd. at 24°C for 1 h, washed with PBS, then treated by 0.3%
Triton (Tianjin Umbrella Science & Technology Co., Ltd.) for 20
min and washed again with PBS. A total of 100 µl DAPI
(Sigma-Aldrich; Merck KGaA; cat. no. D9542) diluted as 10:1 by PBS
contained 3% BSA (Tianjin Umbrella Science & Technology Co.,
Ltd.), was added and kept at 10 min to complete nuclear staining.
The tumor and liver cells were examined using a microscope (Zeiss
confocal Microscope 880; magnification, ×60, excitation wavelength
488 nm) and images were obtained.
Results
Breast cancer stem cell primary
culture
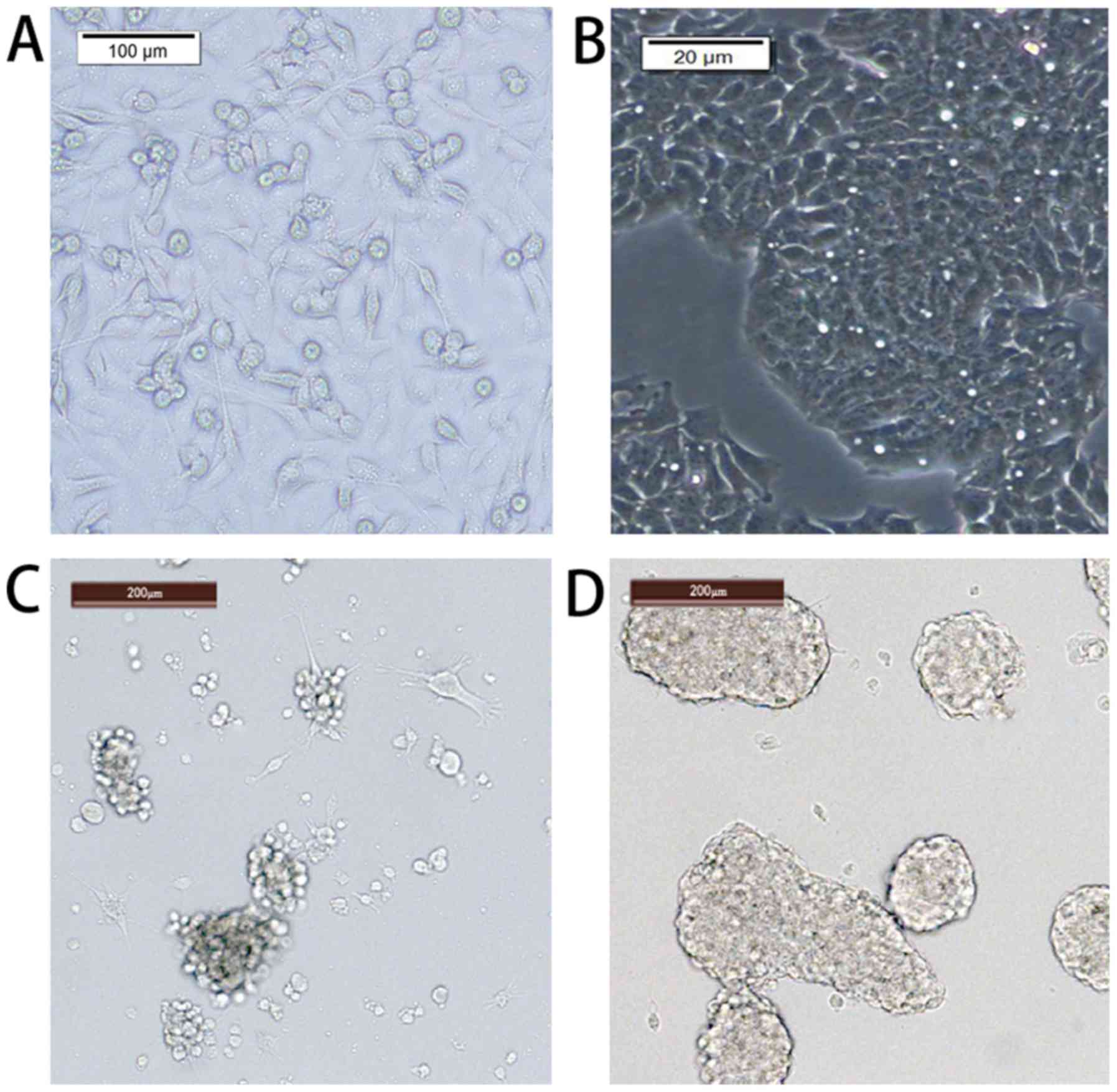
The pretreated primary breast cancer cells were
cultured overnight to adhere to the surface. Elongated flat cells
were observed under a microscope. Following 30 days of serum and
serum-free culture, the cells were suspended in the culture medium
under the microscope and appeared bright and round (Fig. 1). The cells formed balloon-like
shapes and were able to grow in complete medium. The volume and
number of cells increased as the culture time increased (×100;
Fig. 1).
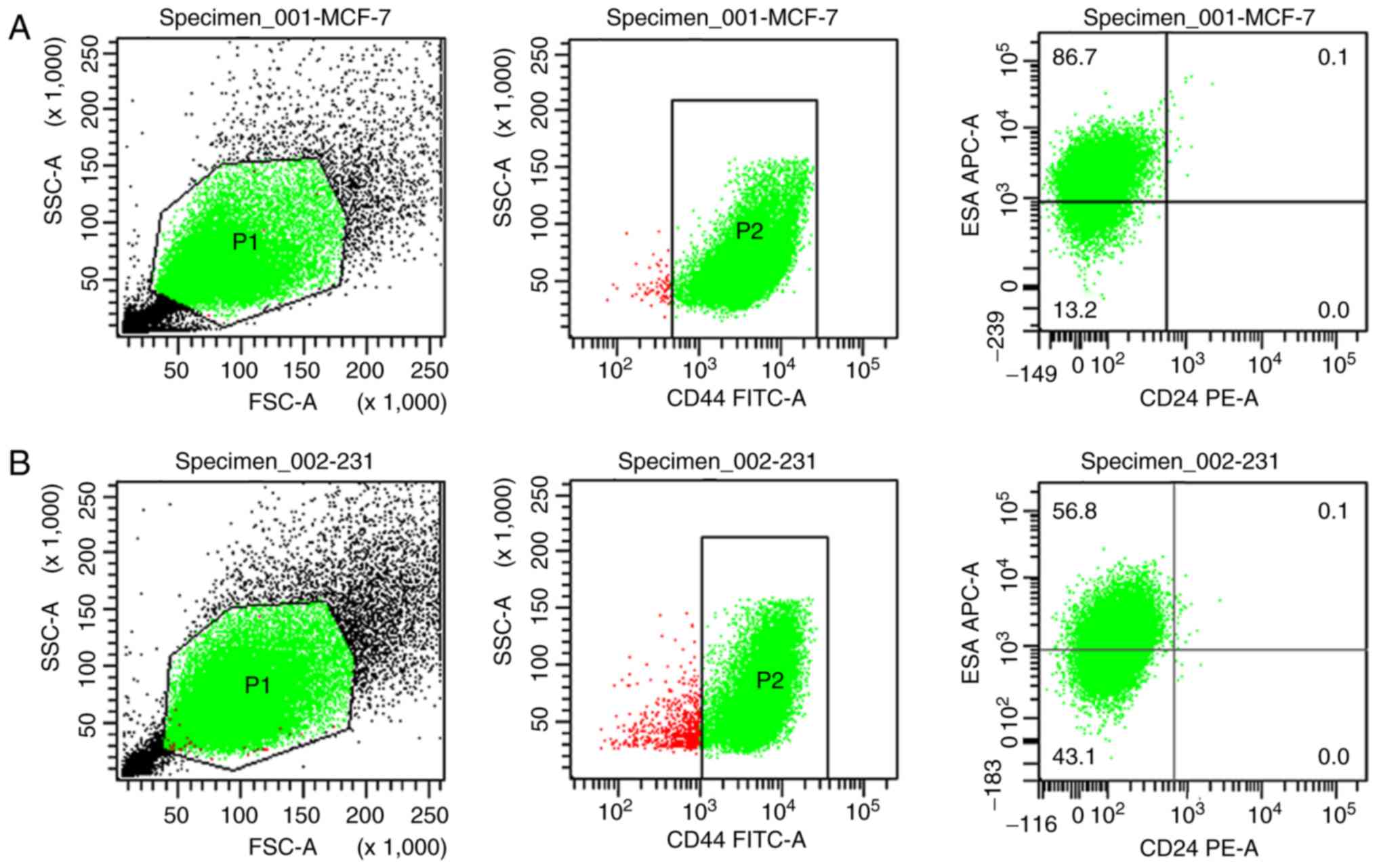
Flow cytometry for breast stem cell
identification
Breast cancer stem cells were sorted by flow
cytometry to identify the
CD44+/CD24−/lowESA+ cell group.
The average proportions of induced MCF7 and MDA-MB-231 breast
cancer stem cells were 89.4 and 57.8%, respectively (Fig. 2).
Screening and enrichment analysis of
phage peptide library
The phage peptide library was subjected to three
rounds of ‘affinity-elutriation-amplification’ affinity screening.
Following three rounds of elutriation, the phage recovery rate
increased and stabilized in the third round (Tables I and II), which indicated that the phage may
specifically bind to human breast cancer cells. The third round of
screening enriched the phage ~200 times more compared with the
first round, and the titer was monitored for screening products. A
total of 10 µl phage was titrated and the enrichment prior to and
following each screening was compared. On the final round of
titration, 10 blue plaques and two monoclonal phages were randomly
selected.
 | Table I.Results of three rounds of phage
screening and enrichment in MCF-7 breast cancer stem cells. |
Table I.
Results of three rounds of phage
screening and enrichment in MCF-7 breast cancer stem cells.
| Round | Initial phage volume
(pfu/ml) | Elution phage volume
(pfu/ml) | Enrichment rate
(%) |
|---|
| Round 1 |
2.00×1010 |
1.05×102 | 0.007 |
| Round 2 |
2.00×1010 |
1.20×104 | 0.005 |
| Round 3 |
2.00×1010 |
1.12×106 | 0.004 |
 | Table II.Results of three rounds of phage
screening and enrichment in MDA-MB-231 breast cancer stem
cells. |
Table II.
Results of three rounds of phage
screening and enrichment in MDA-MB-231 breast cancer stem
cells.
| Round | Initial phage volume
(pfu/ml) | Elution phage volume
(pfu/ml) | Enrichment rate
(%) |
|---|
| Round 1 |
2.00×1010 |
6.60×102 | 0.0441 |
| Round 2 |
2.00×1010 |
7.60×104 | 0.0022 |
| Round 3 |
2.00×1010 |
9.80Ex105 | 0.0015 |
Clone sequencing
A total of 10 clones were randomly selected from the
final screening products and sequenced. In total, 10 positive
clones were obtained through MDA-MB-231 and MCF7 screening, and
eight and six sequencing results were obtained, respectively. The
screening result was considered reliable and credible owing to the
complexity of the cell surface. A total of six distinct polypeptide
sequences with no similar or identical sequences were obtained in
the 10 sequences from the MCF7 cell screening.
The results of the B8 positive clone sequencing
were:
3′-TCAGACGTTAGTAATGAATTTTCTGTATGGGATTTTGCTAAACAACTTTCAACAGTTTCGGCCGAACCTCCACCCGACTGATAACCCGCAATACCATGAAAAGAATTAGTAGAGTGAGAATAGAAAGGTACCACTAAAGGAATTGCGAATAAACCCTAAAAAAA-5′;
the complementary sequence was
5′-ACTAATTCTTTTCATGGTATTGCGGGTTATCAGTCG-3′ (TNSFHGIAGYQS;). The
results of the A3 positive clone sequencing were:
3′-CCGACGTTAGTAAATGAATTTTCTGTATGGGATTTTGCTAAACAACTTTCAACAGTTTCGGCCGAACCTCCACCATTATGAGGAGGACGATAAAGATCCGTACCAGGATTAGAGTGAGAATAGAAAGGTACCACTAAAGGAATTGCGAATAAAACCACGCTGTTTTGACCGCTCCGTACGCGAACCTGCGGTCGTCCCCTTATTGATATGCTCGATCTCAGTGGTATTTGTCACGTTAACGCTAACATGAATCTCTCTGTTAGAGCGGATGTGTGAGGTTGTAGAAATTCCGTGTGTACTGCGGTGAGCAAATGTATCTTATGGGACTGTAACTGTCCCGATGTGTTCTGGGAAATCACCACATTACCCCAAGTTATTTAAGTGACATTCACTTCTGTTTTCGGCATGAACTTTCGCAAATGTCGCAGGATCTTCATCTACCCAAACCTGTTGGTTCTTGCCAGATCTGTGTCTATGCTCACCCTGGAATACGTAATCATGTCATAGCCGTTTCCTGTGGGAAACTGTTATCCGCTCACAATTCCACAAT-5′;
the complete sequence was
5′-AATCCGGGTACGGATCTTTATCGTCCTCCTCATAAT-3′ (NPGTDLYRPPHN).
Determination of polypeptides
According to the sequencing results, the peptides
were synthesized and labeled with FITC. The peptides were
identified to be specific for breast cancer stem cells; the cells
were observed and imaged under a fluorescence microscope following
co-incubation in vitro.
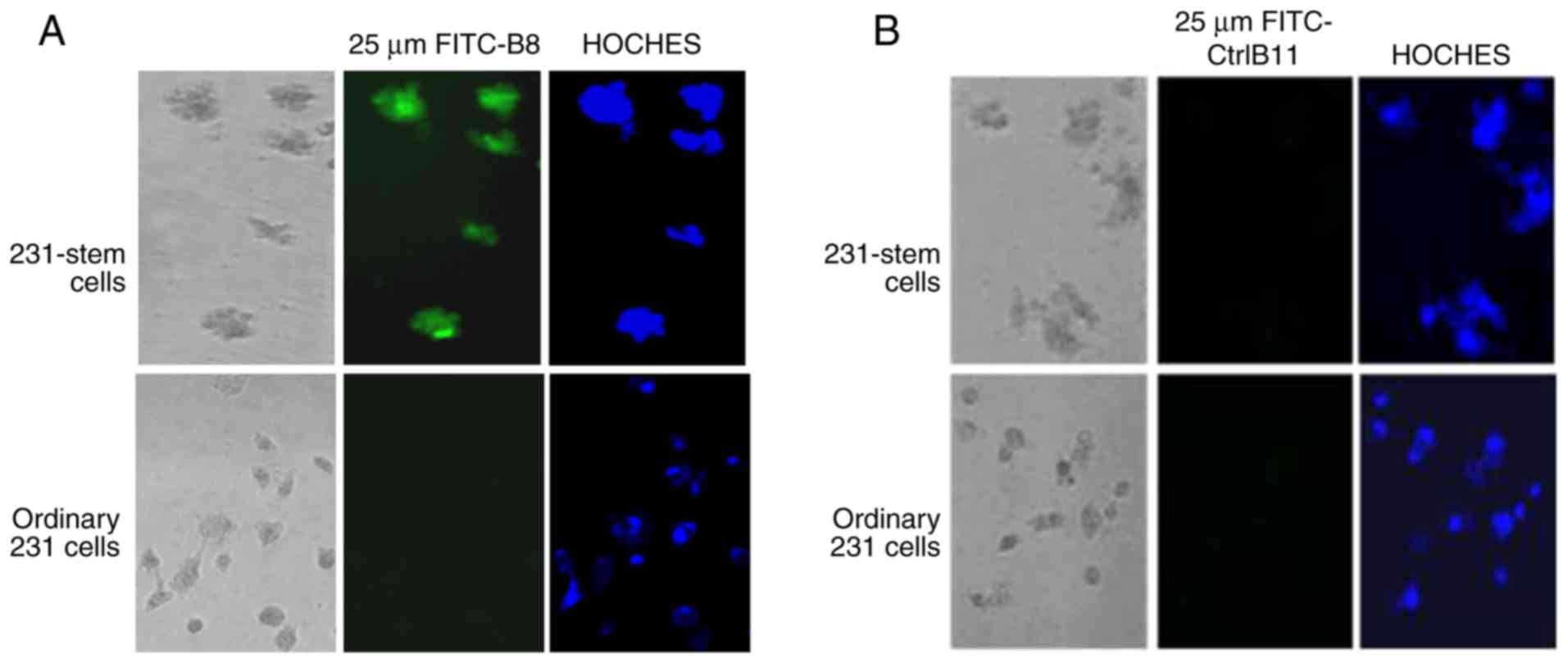
Polypeptide B8 (sequenced twice) was able to
specifically bind to the MDA-MD-231 stem cells without binding to
the original MDA-MD-231 cells (Fig.
3A), whereas the negative control polypeptide B11 did not bind
to the MDA-MD-231 stem cells or to the original MDA-MB-231 cells
(Fig. 3B).
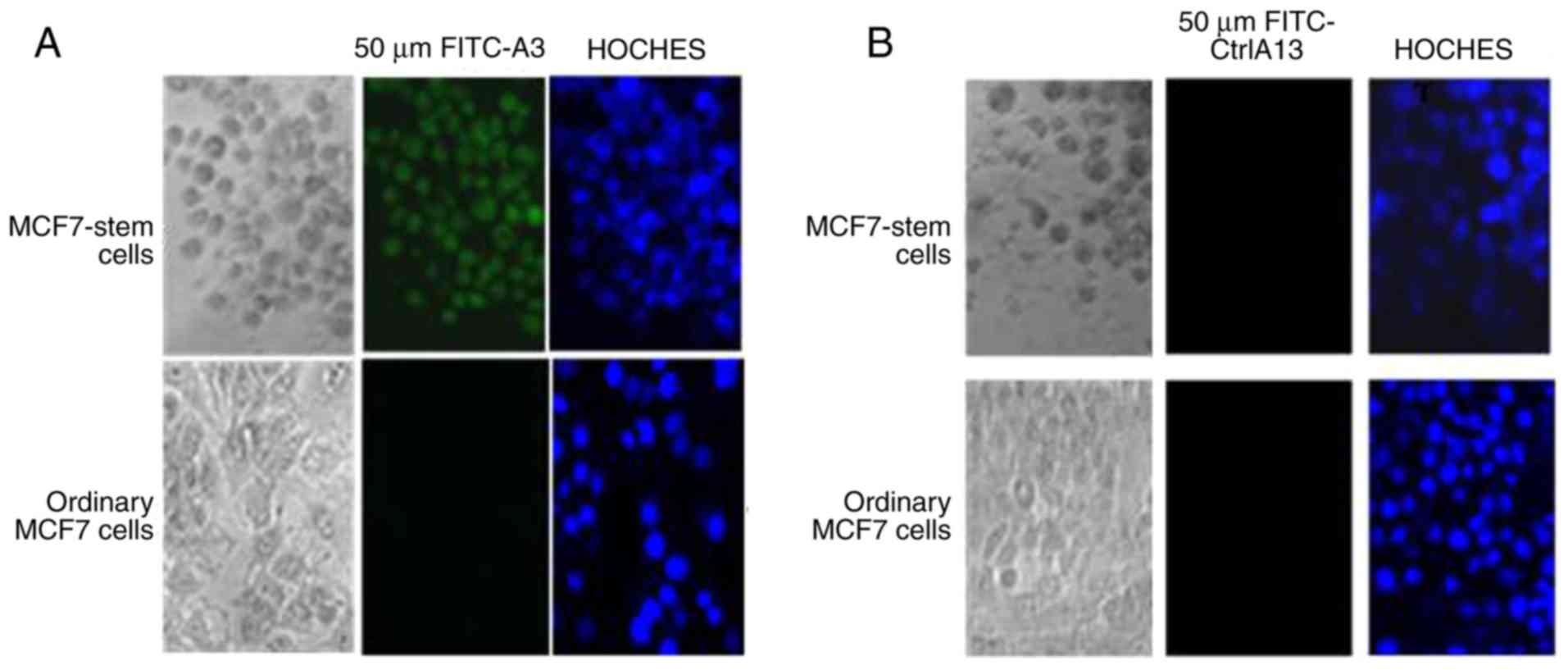
Polypeptide A3 (sequenced once) was able to
specifically bind to the MCF7 stem cells without binding to the
original MCF7 cells (Fig. 4A),
whereas the negative control peptide A13 did not bind to the MCF7
stem cells or to the original MCF7 cells (Fig. 4B).
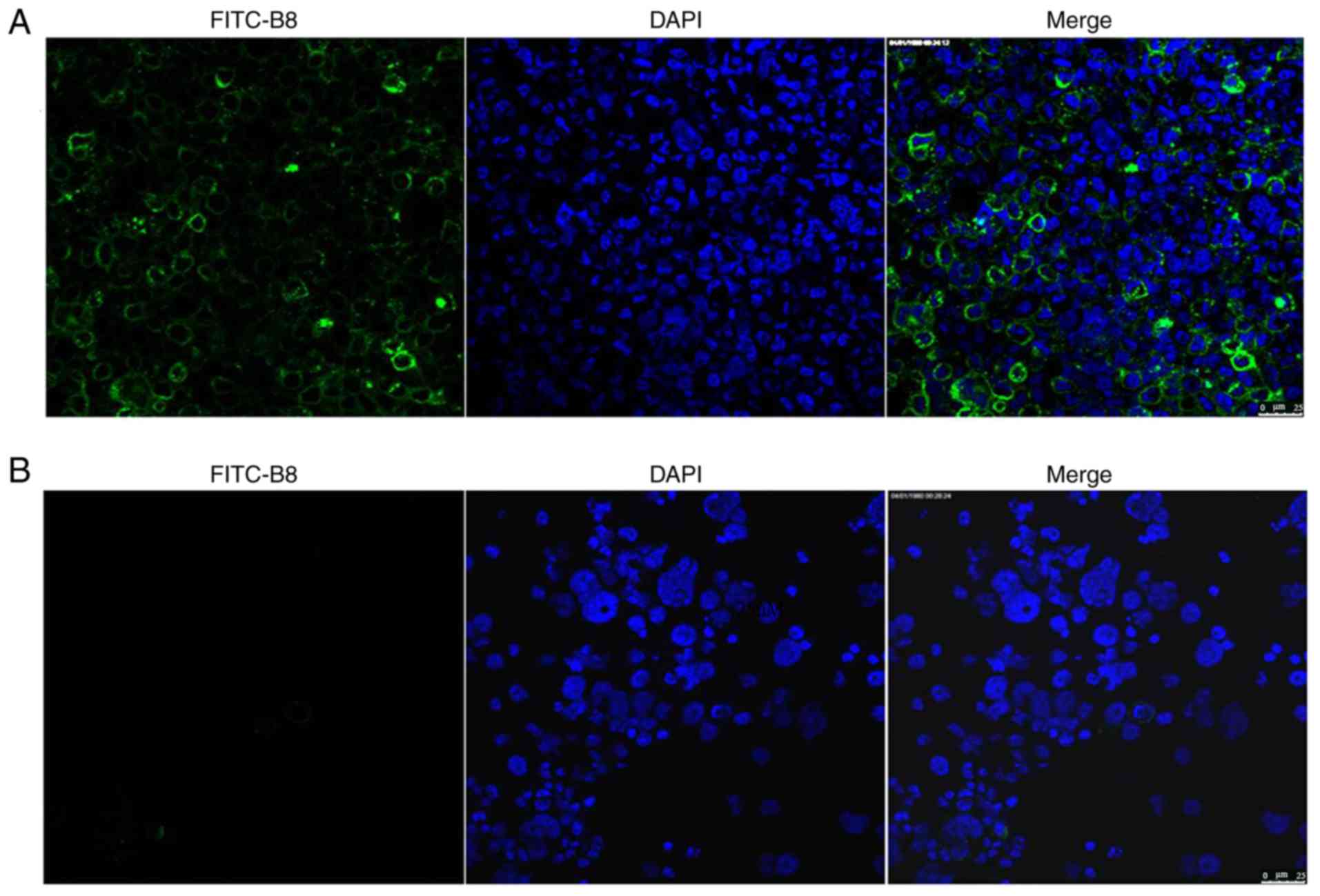
Determination of polypeptide B8
distribution in nude mice
Tumor tissues and control livers tissues of the nude
mice injected with MDA-MB-231 stem cells and polypeptide B8 were
observed under a microscope. FITC labeling of B8 was abundant in
tumor tissue, but not detected in control liver tissue (Fig. 5A and B, respectively).
Discussion
Traditional treatments of breast cancer include
surgery, chemotherapy, hormonal therapy, target therapy,
radiotherapy and immunotherapy (16). Although there have been improvements
in the survival of patients with breast cancer using multi-modal
methods, recurrence and metastasis still occur in a number of
patients (1). Breast cancer stem
cells serve a decisive role during the process of tumorigenesis and
recurrence (17). Previous studies
on breast cancer stem cells have investigated their relationship
with viruses (18), expression of
estrogen (19) and radiation
treatment (20). The generation of
highly selective ligands to breast cancer cells is important for
precise diagnosis and treatment. The screening of phage display
libraries provides an economic and easy method to generate a
variety of selective and highly specific ligands to target cells
(18) The development of targeted
peptides for breast cancer stem cells may reduce drug dosage and
unwanted effects of chemotherapy drugs on normal cells, and may
represent a radical method to cure breast cancer (14).
In the present study, the ‘serum and serum-free’
culture method, also known as dual-subtract biopanning, was used to
enrich breast cancer stem cells. This method can reduce the
disadvantages of the serum-free culture method, which does not
enrich a large number of stem cells, and has minimal damage to stem
cells. In breast cancer, a population of CD44+ cancer
stem cell surface markers that is highly enriched for
cancer-initiating cells has been isolated (21). The CD44+ marker is one of
the most promising markers for early breast cancer detection. CD133
is one of the numerous markers used for screening targets for
cancer stem cells, but the roles of CD133 and its natural ligand in
breast cancer stem cells have not yet been determined. CD44 and
CD24 were selected as cell surface markers for breast cancer stem
cells following enrichment, and their expression was detected by
flow cytometry.
Peptides have advantages as detection probes for
imaging and targeting (17). They
have a smaller size and better tissue penetration compared with
other antibodies. Furthermore, the specific binding of tumor cells
using high affinity and highly selective peptides combined with
traditional chemotherapeutics enables the use of low doses,
eliminating the toxic effects of chemotherapeutics (14). The display of peptide libraries on
the surface of bacteriophages is a standard technology for
selecting peptides with specific binding properties (22).
Park et al (14) used a peptide library to screen novel
diverse peptides that bind to CD44 with high affinity, however,
this study not only screened the phage specifically binding to
breast cancer stem cells, but also identified their specificity
in vitro and in vivo. In this study, a total of 10
positive phage clones were obtained from MDA-MB-231 and MCF-7
breast stem cells, and eight and six sequencing results were
obtained, respectively. A random positive sequence result for
MDA-MB-231 and MCF-7 breast cancer stem cells was selected and the
peptides were synthesized. The peptides were subsequently labeled
with FITC. The peptides were identified to be specific for breast
cancer stem cells. Finally, a breast cancer model was established
using MDA-MB-231 breast cancer stem cells in nude mice, and the
mice were injected with the FITC-labeled polypeptide B8. The
polypeptide B8 FITC labeling with was abundant in the tumor tissue,
but not observed in the control liver tissue. This suggested that
the polypeptide B8 may be specific to breast cancer cells. However,
in this study a breast cancer model in nude mice was not developed
by injecting MCF-7 stem cells; therefore, further research in this
area is required.
In conclusion, several phage sequences specific to
breast cancer stem cells were identified and peptides that can be
used, coupled with various drugs and enzymes, to target breast
cancer cells or tissues were synthesized. Therefore, these peptides
may have broad prospects in the treatment of breast cancer.
Acknowledgments
The authors would like to thank Dr Wenwen Yu from
the Immunoassay Laboratory of Tianjin Medical University, Key
Laboratory of Ministry of Education of China (Tianjin, China) for
flow cytometric analysis.
Funding
The present study was funded by The Shandong
Province Natural Science Foundation Project (grant no.
ZR2014HL076).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
TL conducted the experiments, data analysis and the
writing of the manuscript. BL designed the project and analyzed the
data. LZ conducted the experiments. CZ and YJ analyzed the data. YW
designed and guided the project. All authors have checked and
confirmed the data of the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The experimental protocol was approved by Ethics
Committee of Shandong Cancer Hospital and Institute.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Feuer EJ, Wun LM, Boring CC, Flanders WD,
Timmel MJ and Tong T: The lifetime risk of developing breast
cancer. J Natl Cancer Inst. 85:892–897. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
So WK, Choi KC, Chan CW and Chair SY:
Age-related differences in the quality of life of Chinese women
undergoing adjuvant therapy for breast cancer. Res Gerontol Nurs.
29–26. 2011.
|
|
3
|
Zhu D, Lam DH, Purwanti YI, Goh SL, Wu C,
Zeng J, Fan W and Wang S: Systemic delivery of fusogenic membrane
glycoprotein-expressing neural stem cells to selectively kill tumor
cells. Mol Ther. 21:1621–1630. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luo M, Clouthier SG, Deol Y, Liu S,
Nagrath S, Azizi E and Wicha MS: Breast cancer stem cells: Current
advances and clinical implications. Methods Mol Biol. 1293:1–49.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hyvönen M and Laakkonen P: Identification
and characterization of homing peptides using in vivo peptide phage
display. Methods Mol Biol. 1324:205–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tiede C, Tang AA, Deacon SE, Mandal U,
Nettleship JE, Owen RL, George SE, Harrison DJ, Owens RJ, Tomlinson
DC and McPherson MJ: Adhiron: A stable and versatile peptide
display scaffold for molecular recognition applications. Protein
Eng Des Sel. 27:145–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ajani JA, Song S, Hochster HS and
Steinberg IB: Cancer stem cells: The promise and the potential.
Semin Oncol. 42 (Suppl 1):S3–S17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X and Mao C: Using phage as a platform
to select cancer cell-targeting peptides. Methods Mol Biol.
1108:57–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang T, Hartner WC, Gillespie JW, Praveen
KP, Yang S, Mei LA, Petrenko VA and Torchilin VP: Enhanced tumor
delivery and antitumor activity in vivo of liposomal doxorubicin
modified with MCF-7-specific phage fusion protein. Nanomedicine.
10:421–430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pouyanfard S, Bamdad T, Hashemi H,
Bandehpour M and Kazemi B: Induction of protective anti-CTL epitope
responses against HER-2-positive breast cancer based on multivalent
T7 phage nanoparticles. PLoS One. 7:e495392012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Finlay-Schultz J, Cittelly DM, Hendricks
P, Patel P, Kabos P, Jacobsen BM, Richer JK and Sartorius CA:
Progesterone downregulation of miR-141 contributes to expansion of
stem-like breast cancer cells through maintenance of progesterone
receptor and Stat5a. Oncogene. 34:3676–3687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lv YG, Wang T, Yuan SF, Li NL, Chen JH,
Zhao AZ, Ling R and Wang L: T-vector and in vivo recombination as
tools to construct a large antibody library of breast cancer.
Hybridoma (Larchmt). 29:251–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Petrenko VA and Jayanna PK: Phage
protein-targeted cancer nanomedicines. FEBS Lett. 588:341–349.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park HY, Lee KJ, Lee SJ and Yoon MY:
Screening of peptides bound to breast cancer stem cell specific
surface marker CD44 by phage display. Mol Biotechnol. 51:212–220.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nemudraya AA, Kuligina EV, Ilyichev AA,
Fomin AS, Stepanov GA, Savelyeva AV, Koval OA and Richter VA:
Selection of antitumor displayed peptides for the specific delivery
of the anticancer drug lactaptin. Oncol Lett. 12:4547–4555. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang H, Brand JS, Fang F, Chiesa F,
Johansson AL, Hall P and Czene K: Time-dependent risk of
depression, anxiety, and stress-related disorders in patients with
invasive and in situ breast cancer. Int J Cancer. 140:841–852.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang F, Xu J, Tang L and Guan X: Breast
cancer stem cell: The roles and therapeutic implications. Cell Mol
Life Sci. 74:951–966. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Asad AS, Moreno Ayala MA, Gottardo MF,
Zuccato C, Nicola Candia AJ, Zanetti FA, Seilicovich A and Candolfi
M: Viral gene therapy for breast cancer: Progress and challenges.
Exp Opin Biol Ther. 17:945–959. 2017. View Article : Google Scholar
|
|
19
|
Alferez DG, Simões BM, Howell SJ and
Clarke RB: The role of steroid hormones in breast and effects on
cancer stem cells. Curr Stem Cell Rep. 4:81–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wolfe AR and Woodward WA: Breast cancer
stem cell correlates as Predicative factors for radiation therapy.
Semin Radiat Oncol. 25:251–259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
To K, Fotovati A, Reipas KM, Law JH, Hu K,
Wang J, Astanehe A, Davies AH, Lee L, Stratford AL, et al: Y-box
binding protein-1 induces the expression of CD44 and CD49f leading
to enhanced self-renewal, mammo-sphere growth, and drug resistance.
Cancer Res. 70:2840–2851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu F, Qi CL, Kong M, Liu TT, Li L and Li
BJ: Screening specific polypeptides of breast cancer stem cells
from a phage display random peptide library. Oncol Lett.
12:4727–4731. 2016. View Article : Google Scholar : PubMed/NCBI
|