Introduction
Myeloid-derived suppressor cells (MDSCs) are a
heterogeneous population of cells that serve an important role in
the negative regulation of the immune response during cancer,
inflammation and infection (1). With
high levels of arginase I and inducible nitric oxide synthase
(iNOS), MDSCs suppress T-cell function through the inhibition of
Janus kinase 3 and signal transducer and activator of transcription
5 functions in T-cells (2), the
inhibition of major histocompatibility complex (MHC) II expression
(3) and the induction of T-cell
apoptosis (4).
As the mechanisms of immune suppression by MDSCs
have been discovered, various drugs and biologic inhibitors aimed
at MDSCs have also been investigated (5), including ATRA, catalase, fluorouracil
and L-N6-(1-Iminoethyl)lysine dihydrochloride (6,7).
Cinnamaldehyde (CA) is a bioactive compound isolated from the stem
bark of Cinnamomum cassia, and has been used as a
Traditional Chinese Medicine (TCM) (8). Studies have demonstrated that CA
exhibits various biological functions, including antibacterial,
immunomodulatory, cytotoxic and antiangiogenic activities (9–11). It is
also known to have marked antitumor effects in vitro and
in vivo through enhancing proapoptotic activity via
anti-topoisomerase I and II, or inhibiting NF-κB and activating
protein 1 (12–14). However, it has yet to be proven
whether CA can reduce immunological suppression induced by
malignant tumors, especially those mediated by MDSCs.
The present study assessed the effect of CA on MDSCs
and the relative molecular mechanism. This may be useful to provide
support for CA as a promising therapeutic compound for treating or
preventing cancer through regulation of the tumor
microenvironment.
Materials and methods
Drugs
CA was purchased from the National Institute for
Food and Drug Control (Beijing, China). Dimethyl sulfoxide (DMSO)
was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany).
Cell culture
The present study used MSC-2 cells (MDSCs
immortalized using a retrovirus encoding the v-myc and v-raf
oncogenes) were provided by Dr Francois Ghiringhelli, (Department
of Medical Oncology, Center GF Leclerc, Dijon, France). CT26 and
RAW 264.7 cells (macrophage cell line) were purchased from the
American Tissue Culture Collection (ATCC, Manassas, VA, USA). MC38
tumor cells (murine colon adenocarcinoma cell line) were provided
by Professor Yangxin Fu (Chinese Academy of Sciences, Beijing).
Cell lines were maintained at 37°C in a 5% CO2
humidified chamber in Dulbecco's modified Eagle's medium (DMEM)
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% fetal bovine serum (FBS) (Pan Biotech, Aidenbach, Bavaria,
Germany) and 100 U/ml penicillin/streptomycin.
Mouse tumor models
BALB/c mice (n=32; age, 6–8 weeks; female; weight,
18–22 g) and C57BL/6 mice (n=20; age, 6–8 weeks; female; weight,
18–22 g) were purchased from the Weitonglihua Company (Beijing,
China). Toll-like receptor 4−/− (TLR4−/−)
mice were purchased from the Model Animal Research Center of
Nanjing University (Nanjing, Jiangsu, China). All mice were
maintained in a specific pathogen-free environment at 21–25°C and
50% relative humidity, with a 12 h light/dark cycle at the
Institute of Biophysics, Chinese Academy of Sciences (Beijing,
China). Three mice were housed in each cage and provided with
sterilized food and water. Mice were grouped randomly, with 8 mice
in each group, and all protocols were approved by the appropriate
authorities. To generate tumor models, 5×105 MC38 tumor
cells or CT26 tumor cells (purchased from ATCC) were injected
subcutaneously in the flank of TLR4−/− C57BL/6 mice and
BALB/c wild-type mice, and splenocytes were isolated after 21 days.
Animal experiments were conducted in accordance with the Guidelines
for the Care and Use of Laboratory Animals of the National
Institute of Health, and were approved by the Biological Research
Ethics Committee (Institute of Biophysics, Chinese Academy of
Sciences). The maximum size that tumors were permitted to grow to
was 1,000 mm3.
Splenocyte isolation
Spleens were dipped into 75% alcohol for 1 sec, then
dipped into PBS for 5 sec. Next, spleens were ground using frosted
glass slides in PBS, and filtered through a mesh filter (150 µm),
centrifuged at 524 × g for 3 min at 4°C. The cell pellets were
resuspended in red blood cell lysis buffer (Cowin Biosciences Co.,
Ltd., Jiangsu, China). After 1 min, 5 ml PBS was added, and cells
were filtered and centrifuged at 524 × g for 3 min at 4°C. The cell
pellets were resuspended with PBS with 2% FBS and prepared for
detection.
Cell survival assay (MTT)
An MTT assay was used to assess the cell viability
of MSC-2 cells and the macrophage RAW264.7 cell line. MSC2 and
RAW264.7 were seeded in 96-well culture plates, respectively, at
3×103 cells/well. Following exposure to various
concentrations (0, 1, 2 and 4 µg/ml) of CA for 72 h at 37°C, 10 µl
MTT (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) solution (5
mg/ml in PBS) was added to each well, and the plates were incubated
for an additional 4 h at 37°C in a CO2 incubator. A
total of 100 µl formazan lysis solution (5% 2-methyl-1-propanol,
10% SDS, 0.012 mol/l HCl) was added to each well. Following a 6-h
incubation at 37°C, the absorbance was read at 570 nm on a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Apoptosis assay
In brief, splenocytes isolated from BALB/c mice
bearing CT26 tumors were cultured in 6-cm plates. Following a 4-h
incubation at 37°C, adherent cells (8×104 cells/well)
were seeded into 24-well plates. Following treatment with serial
concentrations of CA for 48 h, the floating and trypsinized
adherent cells were harvested via centrifugation at 1,500 × g for 2
min at 4°C. The cell pellets were washed with cold PBS and prepared
for detection according to the manufacturer's protocol for an
Annexin V-FITC/PI kit (GenStar, Beijing, China). Samples were
analyzed with a BD FACSCalibur flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA) and FlowJo 7.6 software (FlowJo LLC,
Ashland, OR, USA).
Flow cytometry
MDSCs treated with CA (0, 1, 2 and 4 µg/ml) or
dimethyl sulfoxide (DMSO) were labeled for immunofluorescence and
analyzed by flow cytometry. Antibodies including APC-anti-Gr1 (cat.
no. 553129; dilution, 1:200; BD Biosciences) and PE-anti-CD11b
(cat. no. 553311; dilution, 1:1,200; BD Biosciences), as markers of
MDSCs, from mice bearing CT26 and MC38 tumors, respectively, and
APC-Rat IgG1 isotype antibody (cat. no. R20011-11A; dilution,
1:200; Sungene Biotech, Tianjin, China) and PE-Rat IgG1 isotype
antibody (cat. no. R20011-09A; dilution, 1:200; Sungene Biotech)
were diluted in PBS with 2% fetal calf serum (Pan Biotech), and
incubated for 30 min on ice. Next, the cells were washed with PBS
plus 2% fetal calf serum twice. The data were analyzed using FlowJo
7.6 (FlowJo LLC).
Western blotting
MSC-2 cells were lysed by RIPA buffer [50 mM
Tris-HCl (pH 7.5), 150 mM NaCl, 1.0% Nonidet P-40, 0.5% (w/v)
sodium deoxycholate, 0.1% (w/v) SDS, and 1 mM EDTA] supplemented
with 100 mM phenylmethylsulfonyl fluoride, 25 µg/ml aprotinin, 1 mM
sodium orthovanadate and 50 nM NaF. Total protein was quantified
using a bicinchoninic acid assay, and samples (30 µg/sample) were
separated via 10% SDS-PAGE under denaturing conditions and then
transferred onto a nitrocellulose membrane (GE Healthcare,
Milwaukee, WI, USA) for 1 h at 100 V. The membranes were blocked
with 3% BSA in PBS-T (0.1% Tween-20) for 2 h at 4°C and then
incubated overnight at 4°C with the following primary antibodies:
p53 (cat. no. 2524; dilution, 1:1,000), caspase-3 (cat. no. 9662;
dilution, 1:1,000), caspase-9 (cat. no. 9508; dilution, 1:1,000)
(Cell Signaling Technology, Inc., Danvers, MA, USA). Following
three washes with PBST, the membrane was incubated with
HRP-conjugated goat anti-mouse (cat. no. A3683; dilution, 1:5,000)
or goat anti-rabbit IgG (cat. no. A6154; dilution, 1:3,000)
secondary antibodies (Sigma-Aldrich; Merck KGaA) at room
temperature for 1 h. The membrane was washed with PBST five times
(5 min/time) and then incubated with a chemiluminescent substrate
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 3 min at
room temperature. Specific bands were visualized with a
chemiluminescence imaging system (Clinx Science Instruments Co.,
Ltd., Shanghai, China) and analyzed by Clinx software (http://www.clinx.cn). β-actin (cat. no. 4970S;
dilution, 1:3,000; Cell Signaling Technology, Inc.) was used as an
internal standard.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from stimulated MSC-2 cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. cDNA
was synthesized using the 5X All-in-One RT Master mix (Abcam,
Cambridge, UK). qPCR was determined using SYBR Green II Mix (Thermo
Fisher Scientific, Inc.) on an Applied Biosystems 7500
thermocycler. Primer sequences were as follows: Bax forward,
5′-CCAGGATGCGTCCACCAAG-3′ and reverse, 5′-AAGTAGAAGAGGGCAACCAC-3′;
caspase-9 forward, 5′-TCCTGGTACATCGAGACCTTG-3′ and
5′-AAGTCCCTTTCGCAGAAACAG-3′; caspase-3 forward,
5′-ATGGAGAACAACAAAACCTCAGT-3′ and reverse,
5′-TTGCTCCCATGTATGGTCTTTAC-3′; caspase-8 forward,
5′-TGCTTGGACTACATCCCACAC-3′ and reverse,
5′-TGCAGTCTAGGAAGTTGACCA-3′; p53 forward,
5′-CTCTCCCCCGCAAAAGAAAAA-3′ and reverse,
5′-CGGAACATCTCGAAGCGTTTA-3′; and β-actin forward,
5′-GGAGATTACTGCCCTGGCTCCTA-3′ and reverse,
5′-GACTCATCGTACTCCTGCTTGCTG-3′. The housekeeping gene GAPDH was
used as an internal control. The thermocycling conditions consisted
of an initial denaturation step for 10 min at 95°C, then
amplification for 40 cycles of 15 sec at 95°C and 1 min at 57°C.
The qPCR procedure was repeated three times. qPCR data was analyzed
using the 2−∆∆Cq method (15).
Statistical analysis
All data are presented as the mean values ± standard
deviation and were evaluated by one-way ANOVA with Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference. GraphPad 5.0 (GraphPad Software, Inc., La
Jolla, CA, USA) was used for the analysis.
Results
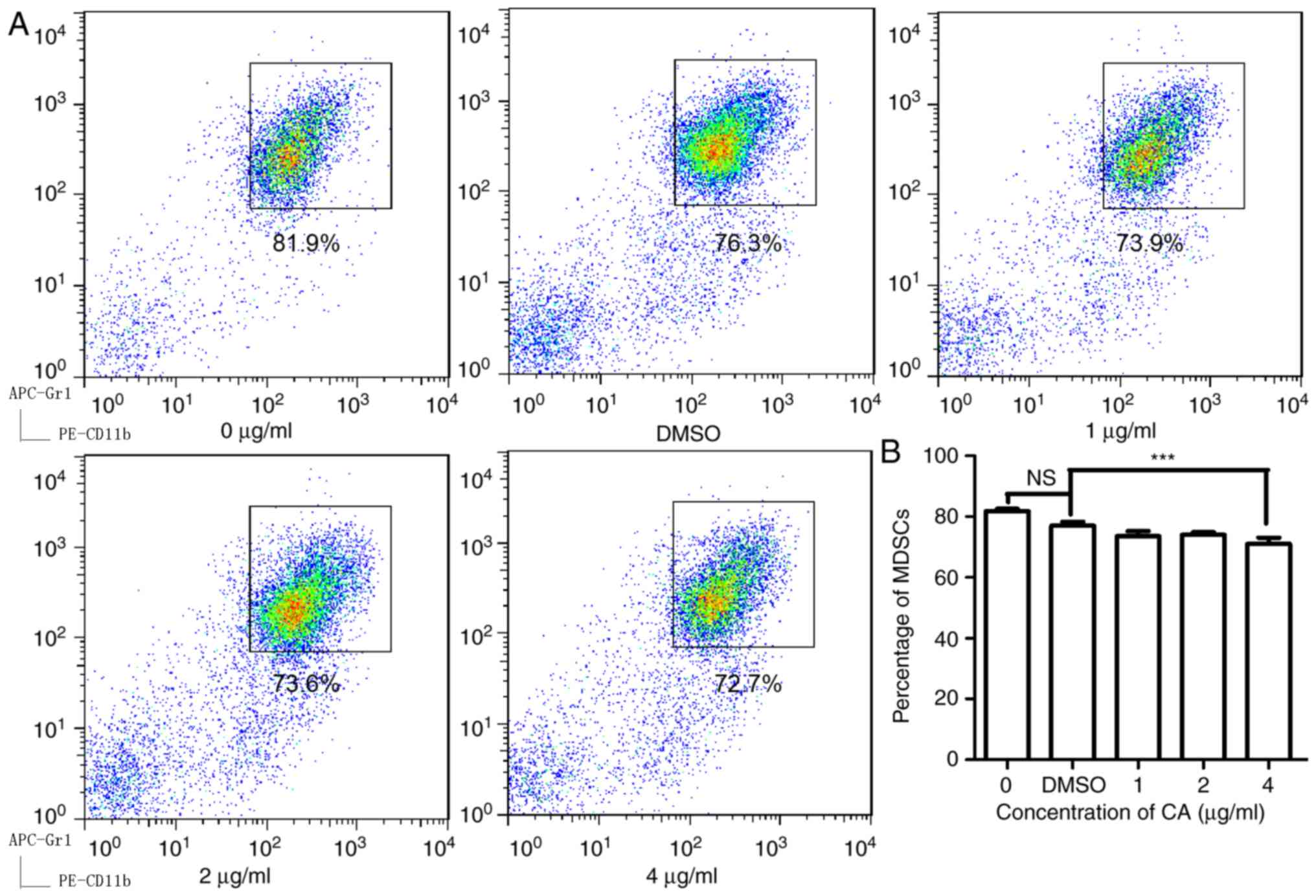
CA decreases the proportion of MDSCs
in splenocytes isolated from mice bearing colon cancer
To examine the effect of CA on MDSCs, isolated
splenocytes from mice bearing CT26 tumors and then treated the
cells with CA at various concentrations (0, 1, 2 and 4 µg/ml) for
24 h. As illustrated in Fig. 1, CA
reduced the MDSC (Gr1+, CD11b+) numbers in a
dose-dependent manner and showed significant suppression at
concentrations of 4 µg/ml (P<0.05).
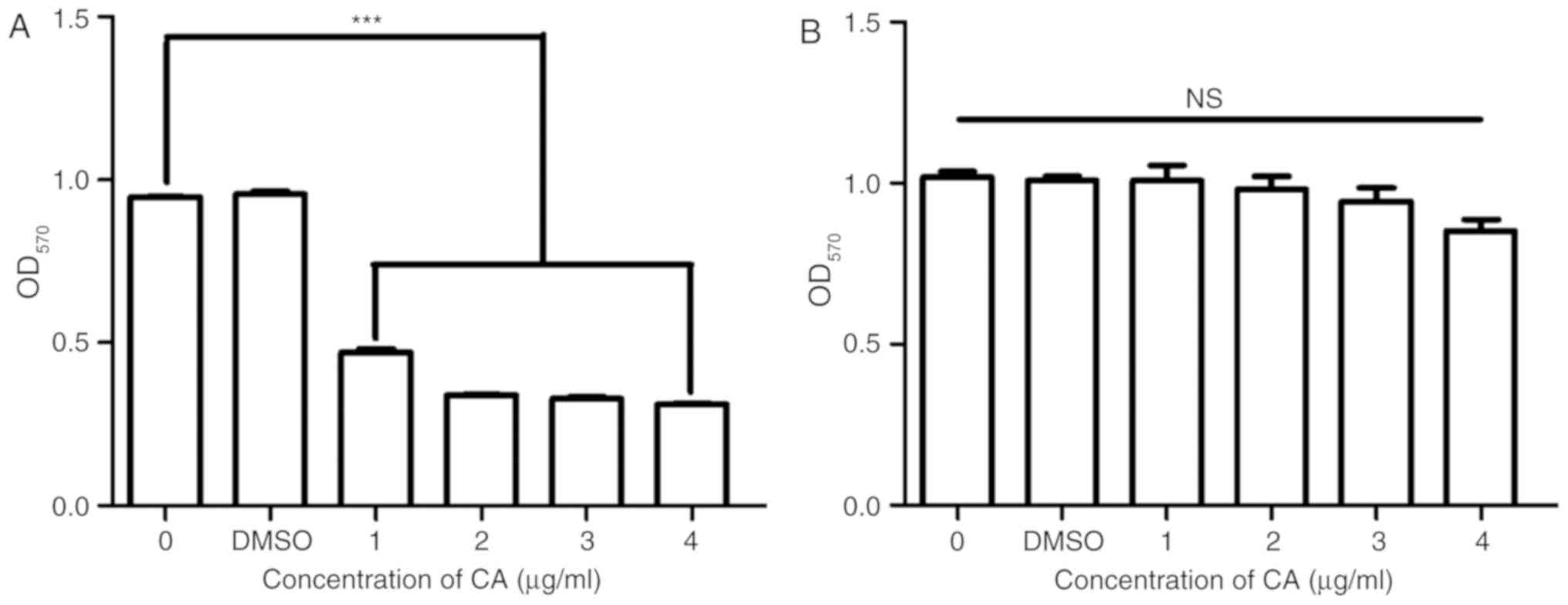
CA selectively inhibits MSC-2 cell
growth
To confirm the inhibition of MDSCs by CA and to
examine the toxicity of CA, the viability and growth of the MSC-2
cells and RAW 264.7 cells was assessed using an MTT assay. The
viability of MSC-2 cells was significantly reduced (P<0.05) by
CA in a dose-dependent manner (Fig.
2A); however, the viability of RAW 264.7 cells was not reduced
by CA (Fig. 2B).
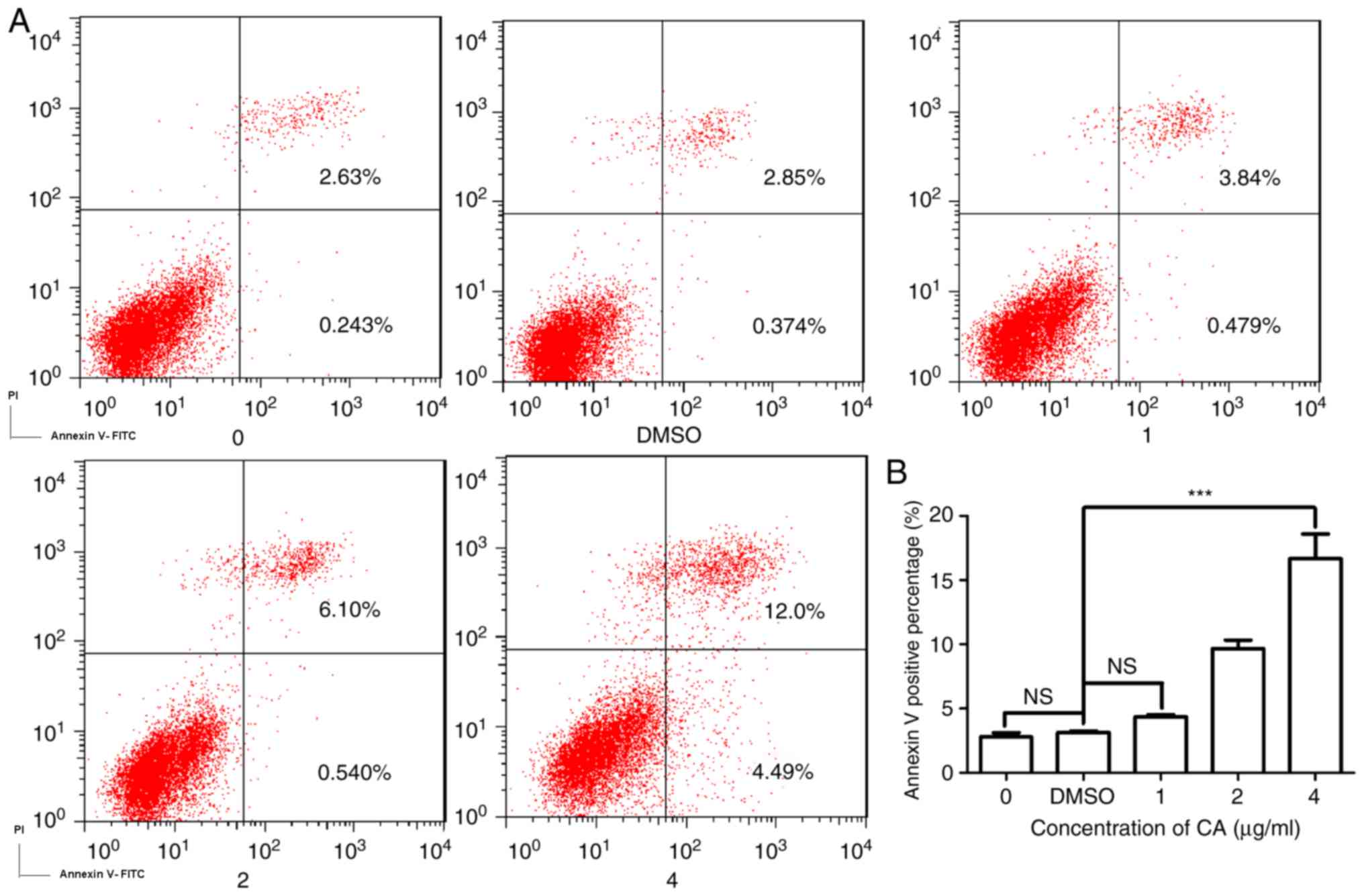
CA induces dose-dependent apoptosis in
MDSCs
As CA was demonstrated to markedly reduce the
survival of MDSCs, the present study investigated the mechanism
underlying this decrease in cell viability. Fig. 3A is a typical quadrant analysis of
the adherent splenocytes isolated from the mice bearing CT26
tumors, treated with CA at different concentrations for 48 h,
double stained with Annexin V-FITC/PI and subjected to flow
cytometry. The percentage of cells in early apoptosis increased
from 0.243 to 4.49% (4 µg/ml). This result demonstrates that the
incubation with CA promoted apoptosis in MDSCs in a dose-dependent
manner (Fig. 3B).
CA regulates the expression of
apoptosis-associated proteins
Apoptosis is classified into two categories
depending on its mediation by the sequential activation of
different caspase proteins (16).
The present study investigated expression levels of various
proteins involved in the intrinsic and extrinsic apoptosis pathways
by western blotting. Caspase-9 and caspase-3 were activated by CA,
as shown in Fig. 4A-C by a
significant time-dependent increase (P<0.05), while the
expression of p53 was also increased. A series of semi-quantitative
and quantitative real-time RT-PCR assays were performed on MSC-2
cells treated with CA (4 µg/ml for different periods of time). The
expression levels of apoptosis factors caspase-9, caspase-3 and Bax
increased in a time-dependent manner. p53 also increased slightly
(Fig. 4B). These results indicated
that CA induced apoptosis in MDSCs via the intrinsic pathway.
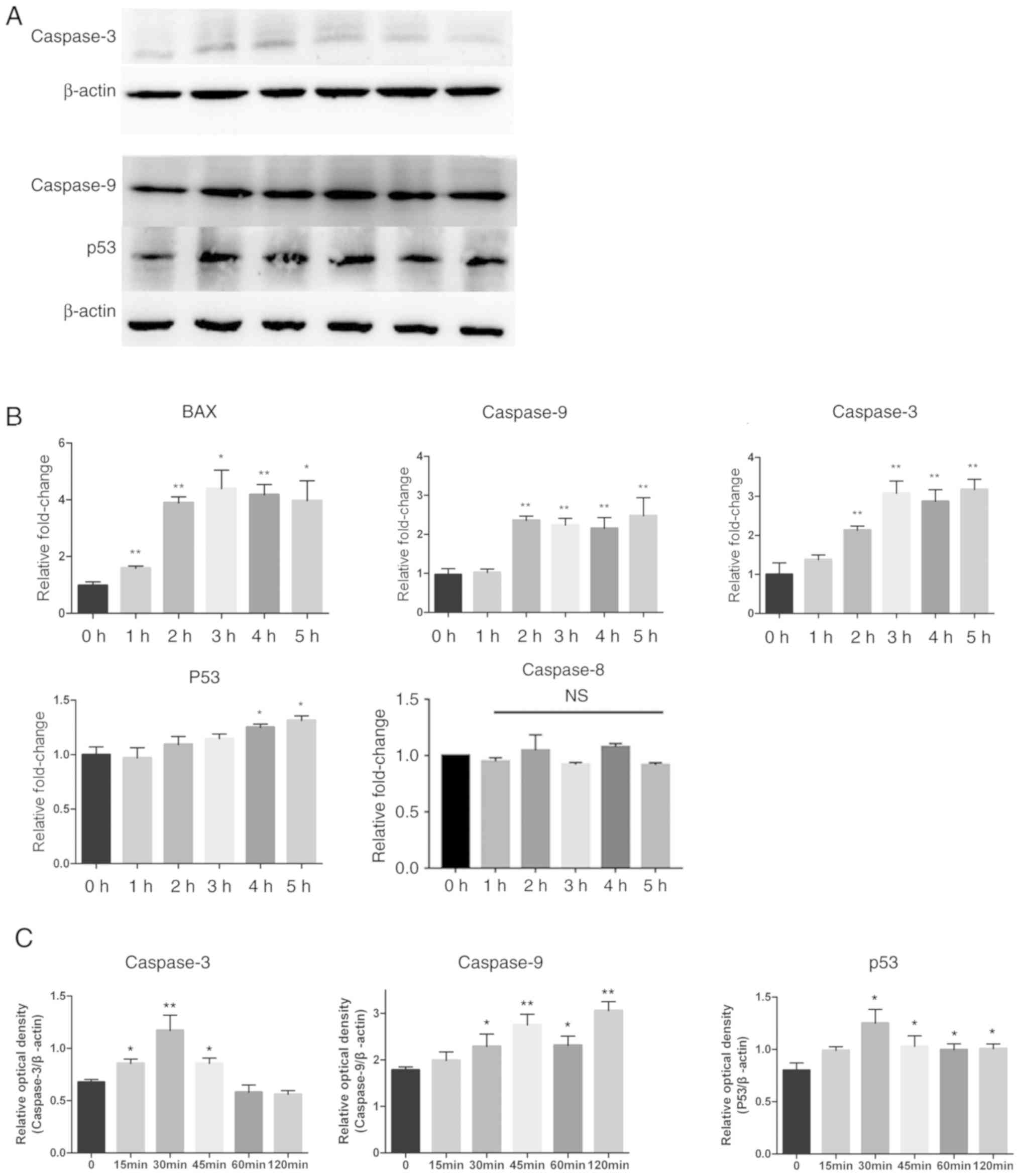 | Figure 4.Expression levels of
apoptosis-associated proteins changed after treatment with CA.
Adherent splenocytes from mice bearing CT26 tumors were treated
with CA (4 µg/ml) for different durations as indicated. (A) Levels
of caspase-9, caspase-3 and p53, in total cell lysates, were
determined by western blotting. The expression of β-actin served as
a control. Lanes 1–6: 0, 15, 30, 45, 60 and 120 min. (B) The Bax,
caspase-9, caspase-3, p53 and caspase-8 mRNA levels were analyzed
by RT-qPCR. The mRNA level of each gene was normalized to β-actin
and presented as a fold increase compared with the control. (C)
Clinx software to analyze the relative optical density of
caspase-3, caspase-9 and p53 compared with β-actin. *P<0.05 and
**P<0.01. RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; NS, not significant; CA, cinnamaldehyde. |
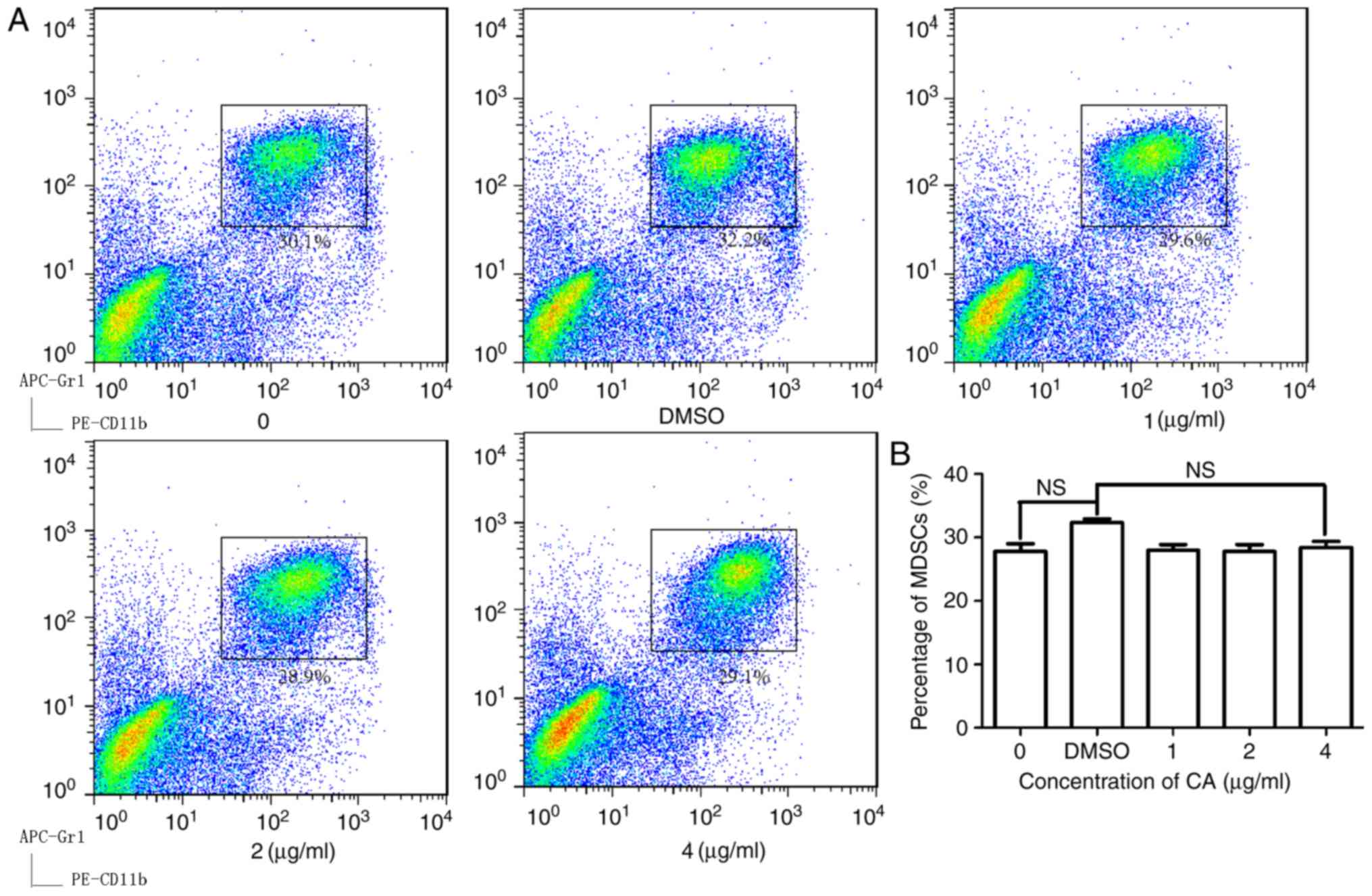
Anti-MDSC activity of CA is
TLR4-dependent
To further clarify the mechanism by which
extracellular CA promotes the intrinsic apoptosis pathway,
splenocytes were isolated from TLR4−/− mice with colon
tumors. As shown in Fig. 5A and B,
in TLR4−/− mice, CA does not decrease the percentage of
MDSCs unlike its effect in WT mice (Fig.
1B). The anti-MDSC activity of CA was abolished in these cells
(Fig. 5A and B), suggesting that CA
reduced MDSCs through a TLR4-dependent pathway.
Discussion
There are two major strategies for reducing the
cancer burden: Active prevention and early therapeutic intervention
(17). Traditional Chinese medicine
has been used to treat human illnesses as part of Chinese
phytotherapy for thousands of years. It has been indicated that
Traditional Chinese Medicine-based treatments may prevent
tumorigenesis, or stabilize tumors and reduce tumor recurrence and
metastasis (18). The present study
aimed to investigate whether CA could inhibit MDSC function. CA has
attracted a great deal of attention for its anticancer properties
and has been demonstrated to inhibit growth and induce apoptosis in
cancer cells as a naturally bioactive substance in a number of
studies (12,19,20). Its
potential in the development of an effective anticancer and
chemopreventive agent has been proven in those studies. MDSCs
promote oncogenesis and metastasis by escaping immunity by
inhibiting T-cell proliferation (21). The present study demonstrated that CA
could selectively decrease the percentage of MDSCs in the spleens
of mice with tumors. Apoptosis serves an important role in
physiological development, homeostasis and pathological
inflammation, infection and tumorigenesis (22–24). The
detection of apoptosis in MDSCs by staining with Annexin V-FITC/PI
in the present study revealed a significant increase in the
percentage of apoptotic MDSCs following treatment with CA. The
levels of caspase-9 and caspase-3, as measured by western blotting,
were increased in a time-dependent manner when the MDSCs were
treated with CA, suggesting that CA promotes apoptosis in MDSCs via
the intrinsic pathway, which is an important type of apoptosis
initiated by the activation of specific receptors.
The p53 protein is an important factor in numerous
processes, such as regulating the repair of cellular DNA, inducing
apoptosis, inhibiting angiogenesis and inducing oxidative shock
(25). In the present study, the
expression of p53 increased in a time-dependent manner in MDSCs,
following exposure to CA (4 µg/ml) for 5 h, supporting the evidence
that CA induces apoptosis via the activation of p53. This research
demonstrated that CA could not decrease the ratio of MDSCs in the
spleens of TLR4-knockout mice with colon tumors after the cells
were exposed to CA (Fig. 5A and B),
suggesting that the apoptosis process is initiated by TLR4.
In conclusion, the results of the present study
demonstrated that CA could selectively decrease the percentage of
MDSCs in the spleens of mice with tumors by promoting apoptosis in
MDSCs, thereby elucidating another facet of its antitumor
properties.
Acknowledgements
The authors would like to thank Miss Mei Jie
(Institute of Biophysics, Chinese Academy of Sciences, Beijing,
China) for providing the cell culture.
Funding
This study was supported by the Zhejiang Province
Natural Science Foundation (grant no. LY15H160003).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NT and CL conceived and designed the experiments.
WH, WZ, YW, QZ and ZW performed the experiments. WZ, QZ, YW, MH and
FM analyzed the data. WH, NT and CL wrote the paper. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by the Biological
Research Ethics Committee (Institute of Biophysics, Chinese Academy
of Sciences, Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bingisser RM, Tilbrook PA, Holt PG and
Kees UR: Macrophage-derived nitric oxide regulates T cell
activation via reversible disruption of the Jak3/STAT5 signaling
pathway. J Immunol. 160:5729–5734. 1998.PubMed/NCBI
|
|
3
|
Harari O and Liao JK: Inhibition of MHC II
gene transcription by nitric oxide and antioxidants. Curr Pharm
Des. 10:893–898. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rivoltini L, Carrabba M, Huber V, Castelli
C, Novellino L, Dalerba P, Mortarini R, Arancia G, Anichini A, Fais
S and Parmiani G: Immunity to cancer: Attack and escape in T
lymphocyte-tumor cell interaction. Immunol Rev. 188:97–113. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baniyash M: Myeloid-derived suppressor
cells as intruders and targets: Clinical implications in cancer
therapy. Cancer Immunol Immunother. 65:857–867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Long AH, Highfill SL, Cui Y, Smith JP,
Walker AJ, Ramakrishna S, El-Etriby R, Galli S, Tsokos MG, Orentas
RJ and Mackall CL: Reduction of MDSCs with all-trans
retinoic acid improves CAR therapy efficacy for sarcomas. Cancer
Immunol Res. 4:869–880. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Waldron TJ, Quatromoni JG, Karakasheva TA,
Singhal S and Rustgi AK: Myeloid derived suppressor cells: Targets
for therapy. Oncoimmunology. 2:e241172013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsai KD, Liu YH, Chen TW, Yang SM, Wong
HY, Cherng J, Chou KS and Cherng JM: Cuminaldehyde from
Cinnamomum verum induces cell death through targeting
topoisomerase 1 and 2 in human colorectal adenocarcinoma COLO 205
cells. Nutrients. 8(pii): E3182016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matan N, Rimkeeree H, Mawson AJ,
Chompreeda P, Haruthaithanasan V and Parker M: Antimicrobial
activity of cinnamon and clove oils under modified atmosphere
conditions. Int J Food Microbiol. 107:180–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim DH, Kim CH, Kim MS, Kim JY, Jung KJ,
Chung JH, An WG, Lee JW, Yu BP and Chung HY: Suppression of
age-related inflammatory NF-kappaB activation by cinnamaldehyde.
Biogerontology. 8:545–554. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh G, Maurya S, DeLampasona MP and
Catalan CA: A comparison of chemical, antioxidant and antimicrobial
studies of cinnamon leaf and bark volatile oils, oleoresins and
their constituents. Food Chem Toxicol. 45:1650–1661. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koppikar SJ, Choudhari AS, Suryavanshi SA,
Kumari S, Chattopadhyay S and Kaul-Ghanekar R: Aqueous cinnamon
extract (ACE-c) from the bark of Cinnamomum cassia causes
apoptosis in human cervical cancer cell line (SiHa) through loss of
mitochondrial membrane potential. BMC Cancer. 10:2102010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong HY, Tsai KD, Liu YH, Yang SM, Chen
TW, Cherng J, Chou KS, Chang CM, Yao BT and Cherng JM:
Cinnamomum verum component 2-methoxycinnamaldehyde: A novel
anticancer agent with both anti-topoisomerase I and II activities
in human lung adenocarcinoma A549 cells in vitro and in vivo.
Phytother Res. 30:331–340. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kwon HK, Hwang JS, So JS, Lee CG, Sahoo A,
Ryu JH, Jeon WK, Ko BS, Im CR, Lee SH, et al: Cinnamon extract
induces tumor cell death through inhibition of NFkappaB and AP1.
BMC Cancer. 10:3922010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagata S and Tanaka M: Programmed cell
death and the immune system. Nat Rev Immunol. 17:333–340. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang CY, Bai XY and Wang CH: Traditional
Chinese medicine: A treasured natural resource of anticancer drug
research and development. Am J Chin Med. 42:543–559. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kwon HK, Jeon WK, Hwang JS, Lee CG, So JS,
Park JA, Ko BS and Im SH: Cinnamon extract suppresses tumor
progression by modulating angiogenesis and the effector function of
CD8+ T cells. Cancer Lett. 278:174–182. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong SH, Ismail IA, Kang SM, Han DC and
Kwon BM: Cinnamaldehydes in cancer chemotherapy. Phytother Res.
30:754–767. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Veglia F, Perego M and Gabrilovich D:
Myeloid-derived suppressor cells coming of age. Nat Immunol.
19:108–119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Youn JI, Nagaraj S, Collazo M and
Gabrilovich DI: Subsets of myeloid-derived suppressor cells in
tumor-bearing mice. J Immunol. 181:5791–5802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu MH, Jin XK, Yu AQ, Zhu YT, Li D, Li WW
and Wang Q: Caspase-mediated apoptosis in crustaceans: Cloning and
functional characterization of EsCaspase-3-like protein from
Eriocheir. Fish Shellfish Immunol. 41:625–632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumar S: Caspase function in programmed
cell death. Cell Death Differ. 14:32–43. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Galluzzi L, Vitale I, Abrams JM, Alnemri
ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry
WS, Fulda S, et al: Molecular definitions of cell death
subroutines: Recommendations of the nomenclature committee on cell
death 2012. Cell Death Differ. 19:107–120. 2012. View Article : Google Scholar : PubMed/NCBI
|