Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide. Non-small cell lung cancer
(NSCLC) accounts for >80% of all cases of lung cancer, and the
majority of patients present with advanced disease at diagnosis
(1). Despite recent advances in
multi-modal therapy, the overall 5-year survival rate for NSCLC
remains poor, ranging between 8 and 12%. Surgery continues to be
the primary treatment option for localized NSCLC. However, the
results of surgical treatment remain unsatisfactory, with 35–50% of
patients experiencing disease relapse within 5 years.
RAB proteins are members of the Ras superfamily of
GTPases that regulate intracellular trafficking (2). It has been revealed that a point
mutation in the GTP-binding domain of RAB38 is responsible
for human Hermansky-Pudlak syndrome, which is characterized by
oculocutaneous albinism, bleeding diathesis and pulmonary fibrosis
(3). Studies have investigated the
potential role in regulating tumor progression (4); several RAB proteins have been
identified in transcriptomic studies, exhibiting deregulated
expression in malignant cells compared with normal tissues
(5,6). However, the exact functions of these
proteins in tumor biology remain to be fully elucidated.
Accumulating evidence has demonstrated that RABs are overexpressed
or upregulated in several types of cancer, including hepatoma,
tongue, breast, pancreatic and prostate cancer (7–12); by
contrast, the downregulation of certain RAB genes as a
result of hypermethylation has been identified in colon, gastric
and endometrial cancer (13,14).
RAB38 is a recently identified member of the RAB
family of proteins and its expression is tissue-specific. RAB38 is
predominantly expressed in melanocytes and melanoma tissues, but
not in other normal tissues, serving as a melanocyte
differentiation antigen (15). In
gliomas, the expression levels of RAB38 have been associated
with disease progression (16).
However, the biological effects of RAB38 in other types of cancer
remain unclear.
Utilizing two microarray platforms, a four-gene
signature, including LCN2, PTHLH, RAB38 and FJX1, was
identified as being significantly associated with tumor recurrence
in patients with NSCLC (17). In the
present study, the association between the expression of
RAB38 and survival rate was further evaluated in patients
with NSCLC. The results demonstrated that the expression of
RAB38 was negatively associated with patient survival rate,
and that knockdown of the expression of RAB38 attenuated
tumor invasion in vitro. These results suggest that
RAB38 may be used as an important prognostic predictor in
NSCLC.
Materials and methods
Sample acquisition
Tumor tissues were retrospectively acquired from the
Tissue Bank of Chang Gung Memorial Hospital (Taoyuan, Taiwan)
following the standard procedure, and written informed consent was
obtained from all patients. The tissue sections were reviewed by a
pathologist, and only those comprising >50% tumor area were used
(confirmed by hematoxylin and eosin staining). A total of 60
patients were enrolled in the present study and frozen specimens
were available from all patients. The present study was approved by
the Institutional Review Board of Chang Gung Memorial Hospital.
RNA extraction and semi-quantitative
reverse transcription-polymerase chain reaction (RT-PCR)
analysis
The patients were divided into two groups: Group R
comprised patients who had tumor recurrence within 4 years
following surgery, and Group NR comprised patients who remained
disease-free 4 years following initial surgery. A total of 40 Group
R and 20 Group NR cases of NSCLC were included. Following
mechanical tissue disruption, total tumor RNA was extracted using
the RNeasy Mini kit (Qiagen GmbH, Hilden, Germany) according to the
manufacturer's protocol. Subsequently, 2–5 µg of total RNA was
converted to cDNA using the SuperScript® III kit
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
according to the manufacturer's protocol. The RAB38 and
control GAPDH genes were amplified by PCR. The primers were
as follows: RAB38, forward 5′-AGGCTGCGCTTCCCTGGTCA-3′ and
reverse 5′-CCACGCCCAGGTCGCCAATC-3′; GAPDH, forward
5′-GACAACAGCCTCAAGATCATCA-3′ and reverse
5′-GGTCCACCACTGACACGTTG-3′. PCR amplification was performed in a
total volume of 20 µl, containing 2–5 µg of cDNA. Following
denaturation at 94°C for 5 min, a total of 30 thermal cycles were
performed at 94°C for 30 sec, 55°C for 30 sec and 72°C for 30 sec.
The amplified samples were mixed with gel loading dye and subjected
to electrophoresis using a 1% agarose gel, containing 0.5 µg/ml
ethidium bromide in 1X tris-borate-EDTA buffer. The gel was
visualized and analyzed using ChemiCapt 3000 software (version
5.03; Vilber Lourmat, Marne-la-Vallée, France), and the expression
levels of the PCR products were quantified using Image J v4.0
software (National Institutes of Health). The relative
semi-quantitative RT-PCR density of RAB38 to GAPDH
was quantified. High expression was defined as a ratio of
RAB38 to GAPDH ≥0.5.
Cell culture and stable knockdown
The following human lung cancer cell lines were
purchased from the American Type Culture Collection: HCC827, which
expresses high levels of RAB38 and mutated epidermal growth factor
receptor (EGFR: deletion, E746-A750) RNA, and A549, which expresses
low levels of RAB38 and non-mutated EGFR RNA. The cells were
maintained in RPMI 1640 (complete medium; Invitrogen; Thermo Fisher
Scientific, Inc.). The Lentiviral Expression Vector system (dual
function of TOOLSilent shRNA vector; Biotools Co., Ltd., New Taipei
City, Taiwan) was used to establish stable RAB38-knockdown sublines
with Lipofectamine® 3000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) (target sequence,
5′-AATTCAAAAAAGCAATGAGTGTGACCTACTCTTGAACATTAGGTCACACTCATTTGCG-3′).
The stable transfectants were selected using G418 for 2 weeks. The
basal expression of RAB38 and knockdown efficiency were
determined by RT-PCR, as described above.
Western blot analysis
The expression levels of the RAB38 were also
detected by western blot analysis. The protein was extracted using
ice-cold lysis buffer (20 mM Tris-base pH8.0, 150 mM NaCl, 1%
NP-40) supplemented with a cocktail of protease inhibitor (cat. no.
ab65621; Abcam) and the concentration of protein was determined
using a Bradford protein assay. Subsequently, the protein (35 µg)
was separated by a 4–15% gradient SDS-PAGE and transferred onto a
nitrocellulose membrane. The blotted membrane was washed with tris
buffered saline (TBS) for 5 min at room temperature and incubated
in blocking buffer (1X TBS containing 5% non-fat milk) for 1 h at
room temperature. The membrane was then incubated overnight at 4°C
with a monoclonal antibody direct against human β-actin (1:10,000
dilution, cat. no. ab8224; Abcam, Cambridge, MA, USA) and RAB38
(1:100 dilution, cat. no. GTX51060; GeneTex, Inc., Irvine, CA,
USA). Following washing three times for 5 min with 1X TBS
containing 0.1% Tween-20, the membrane was incubated with a
horseradish peroxidase-conjugated secondary antibody (1:5,000
dilution; RAB38 cat. no. ab97051; β-actin cat. no. 97023; Abcam)
for 2 h at room temperature. The antigen-antibody interaction was
traced with a chemiluminescence detection system. The blot signals
were detected using a digital image system. These data were
quantified by densitometric analysis with Image J v4.0 software,
and the relative expression level was normalized by the internal
standard β-actin.
In vitro invasiveness
The transfected cells were seeded into the upper
wells of a BioCoat Matrigel invasion chamber (Corning, Bedford, MA,
USA) in serum-free medium containing 0.1% BSA (Corning). The lower
wells were filled with complete medium. Following incubation for 48
h at 37°C, a cotton swab was applied to remove the non-invaded
cells in the upper chamber; those that had migrated through the
filter pores were fixed and stained with 0.5% (w/v) crystal violet
(cat. no. C6158; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in
methanol. Images were captured using a light microscope (IX70,
Olympus Corporation, Tokyo, Japan), and analyzed with PAX–IT image
analysis software (Midwest Information Systems, Villa Park, IL,
USA).
Statistical analysis
Semiquantitative results are presented as the mean ±
standard deviation. Differences in the expression of RAB38
were analyzed using Student's t-test. Categorical data were
analyzed by Pearson's χ2 method. Disease-free survival
(DFS) was defined as the period of time following primary surgical
treatment until recurrence of NSCLC. Overall survival (OS) was
defined as the date of diagnosis until mortality, or the most
recent follow-up. The DFS and OS were estimated using Kaplan-Meier
survival analysis, and the log-rank test was used to assess the
difference between these two groups. For validation of the value of
prognosticator, univariate and multivariate Cox proportional
hazards analyses were also performed. Statistical analyses were
performed with IBM SPSS version 20.0 software (IBM Corp., Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
A total of 60 patient samples were assessed. The
patients' characteristics are shown in Table I. The mean patient age was 69.5 years
in Group R and 71.5 years in Group NR. The sex ratio (male/female)
was 2.08 in Group R and 2.33 in Group NR. The percentages of
adenocarcinoma, adenosquamous cell carcinoma and squamous cell
carcinoma were 62.5, 5.0 and 32.5% in Group R and 50.0, 10.0 and
40.0% in Group NR, respectively. There was no difference between
the R and NR groups, with the exception that there was a higher
number of cases of cancer stage III in Group R. In Group R, the
stage distribution of I, II and III was 40.0, 17.5 and 42.5%;
whereas in Group NR the stage distribution of I, II and III was
70.0, 25.0 and 5.0%.
 | Table I.Demographic data of 60 patients with
non-small cell lung cancer (R=40; NR=20). |
Table I.
Demographic data of 60 patients with
non-small cell lung cancer (R=40; NR=20).
| Variable | Group R, n (%) | Group NR, n
(%) |
P-valuea |
|---|
| Age (years) | 69.5 (40–89) | 71.5 (31–90) | 0.469 |
| Gender
(male:female) | 27:13 | 14:6 | 0.844 |
| Stage |
|
| 0.011 |
| I | 16 (40.0) | 14 (70.0) |
|
| II | 7 (17.5) | 5 (25.0) |
|
|
III | 17 (42.5) | 1 (5.0) |
|
| Histology |
|
| 0.585 |
| AC | 25 (62.5) | 10 (50.0) |
|
|
ASC | 2 (5.0) | 2 (10.0) |
|
|
SqCC | 13 (32.5) | 8 (40.0) |
|
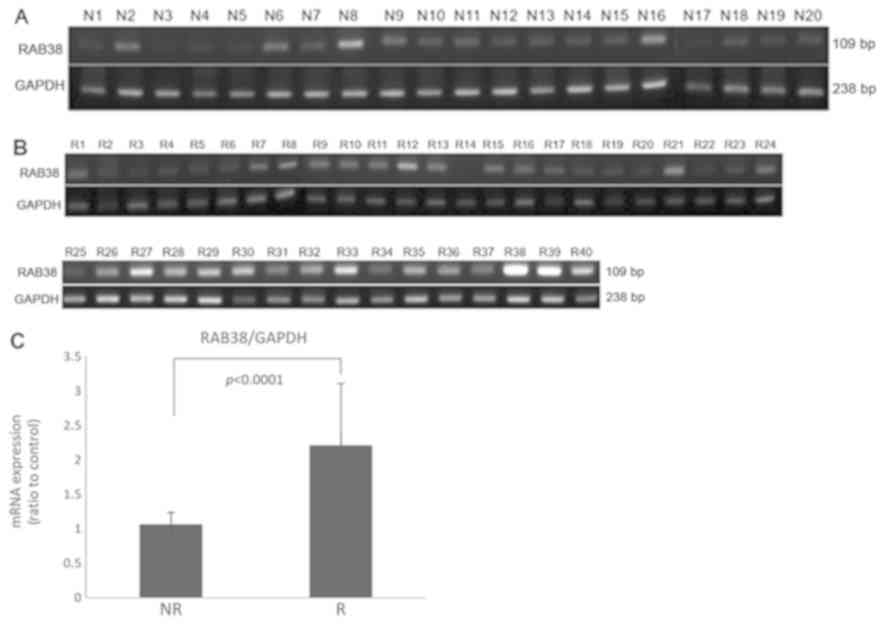
Expression of RAB38 is higher in Group
R samples compared with those in Group NR
In the present study, the expression of levels of
RAB38 in 40 Group R (Fig. 1A)
and 20 Group NR (Fig. 1B) frozen
tumor tissues were assessed using semi-quantitative RT-PCR
analysis. The results revealed that the expression levels of
RAB38 were significantly higher in Group R samples compared
with those in Group NR samples (P<0.0001; Fig. 1C).
RAB38 may be a prognostic factor in
NSCLC
The expression level of the RAB38 was
significantly associated with DFS and OS in patients with NSCLC. As
shown in Fig. 2A, the median DFS was
not reached in the RAB38 low expression group, whereas in
the RAB38 high expression group, the DFS was 19.9 months
[95% confidence interval (CI)=4.2–35.6 months; P<0.001]. The
median OS was not reached in the RAB38 low expression group,
whereas the OS of the RAB38 high expression group was 31.6
months (95% CI=15.0–48.2 months; P=0.001; Fig. 2B).
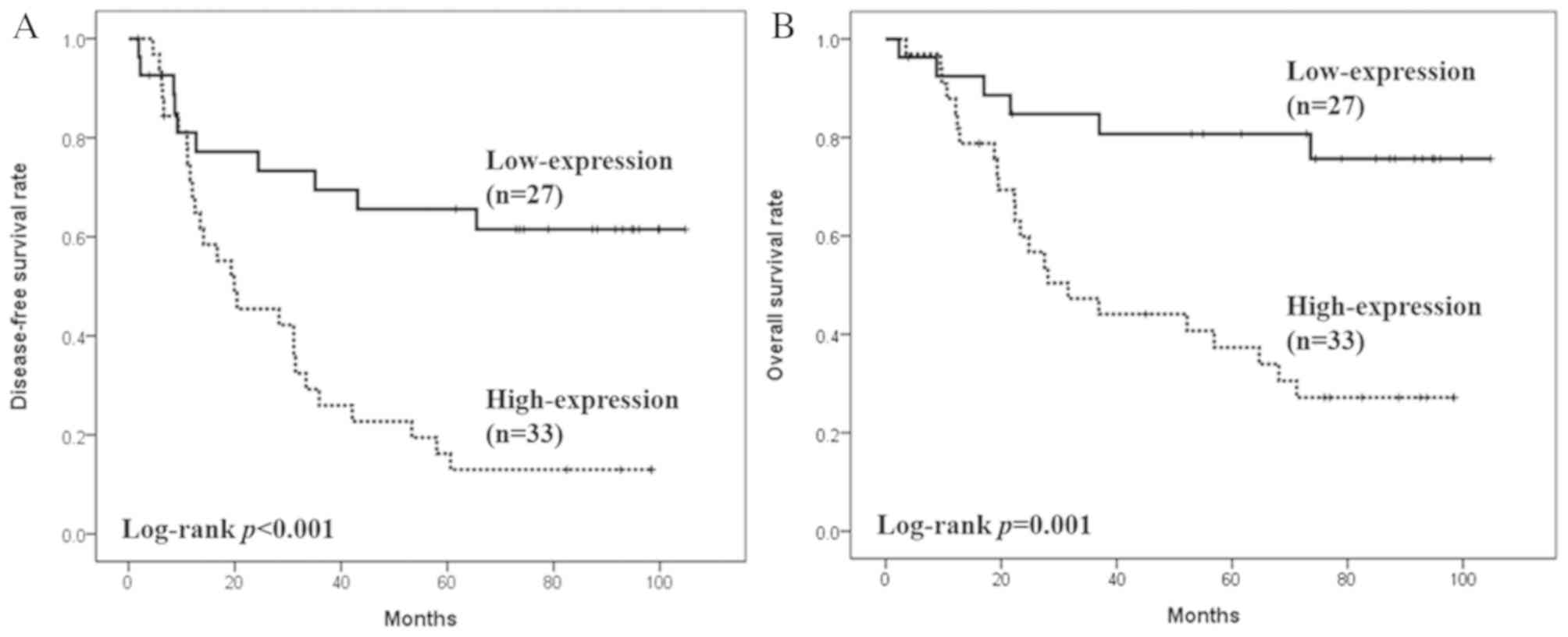 | Figure 2.Effects of RAB38 on patient
survival rates. (A) The median DFS was 19.9 months (95%
CI=4.2–35.6) and was not reached in patients whose tumors expressed
high and low RAB38, respectively (HR=0.28, 95%
CI=0.133–0.589, P<0.001). (B) The median OS was 31.6 months (95%
CI=15.0–48.2) and was not reached in patients whose tumors
expressed high and low RAB38, respectively (HR=0.23, 95%
C=0.094–0.576, P=0.001). DFS, disease-free survival; OS, overall
survival; CI, confidence interval; HR, hazard ratio; RAB38,
Ras-related protein Rab-38. |
Consistent with previous results (17), the expression level of RAB38
was correlated with tumor recurrence. While 29 of the 33 patients
(87.8%) with high expression levels of RAB38 developed
recurrence, it was apparent in only 11 of the 27 patients (40.7%)
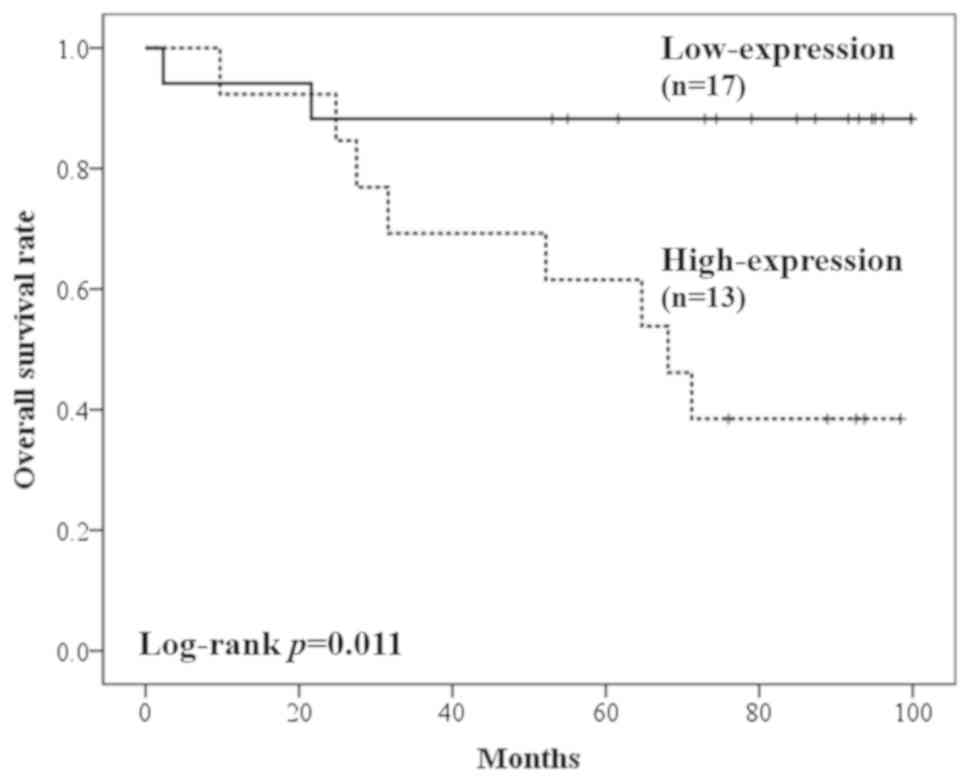
with low expression levels of RAB38 (P=0.001). In Group R,
the mRNA expression level of RAB38 was associated with poor
OS, although this was not statistically significant (median OS,
37.0 vs. 27.5 months; P=0.335). Although the higher number of stage
III cases in Group R may lead to a higher recurrence rate and poor
prognosis in patients with a high expression of RAB38, poor
prognosis was also observed in stage I cases (Fig. 3) in tumors with high expression of
RAB38 (median OS, not reached vs. 68.1 months, P=0.011). The
results of the univariate and multivariate analyses are shown in
Table II. The univariate analysis
revealed that only cancer stage (P=0.004) and the expression level
of RAB38 (P=0.002) were prognostic factors for NSCLC. Cox
multivariate analysis indicated that the expression level of RAB38
(P=0.010) was a more robust prognostic factor than stage status
(P=0.053).
 | Table II.Univariate and multivariate overall
survival analyses of 60 (R=40, NR=20) patients with non-small cell
lung cancer using the Cox proportional hazards model. |
Table II.
Univariate and multivariate overall
survival analyses of 60 (R=40, NR=20) patients with non-small cell
lung cancer using the Cox proportional hazards model.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI |
P-valuea | HR | 95% CI |
P-valuea |
|---|
| Age (years) |
|
≤65 | 0.635 | 0.271–1.487 | 0.295 |
|
|
|
|
>65 | 1 |
|
|
|
|
|
| Gender |
|
Male | 0.810 | 0.382–1.717 | 0.582 |
|
|
|
|
Female | 1 |
|
|
|
|
|
| Histology |
|
| 0.919 |
|
|
|
| AC | 0.855 | 0.396–1.846 | 0.691 |
|
|
|
|
ASC | 0.982 | 0.217–4.432 | 0.891 |
|
|
|
| SqCC | 1 |
|
|
|
|
|
| Stage |
|
| 0.004 |
|
| 0.053 |
|
III | 4.227 | 1.813–9.856 | 0.001 | 2.718 | 1.137–6.497 | 0.025 |
| II | 2.029 | 0.735–5.596 | 0.172 | 2.495 | 0.890–6.994 | 0.082 |
| I | 1 |
|
| 1 |
|
|
| RAB38 |
|
High | 4.29 | 1.736–10.603 | 0.002 | 3.650 | 1.367–9.743 | 0.010 |
|
Low | 1 |
|
| 1 |
|
|
Expression of RAB38 is associated with
the invasiveness of lung cancer cells
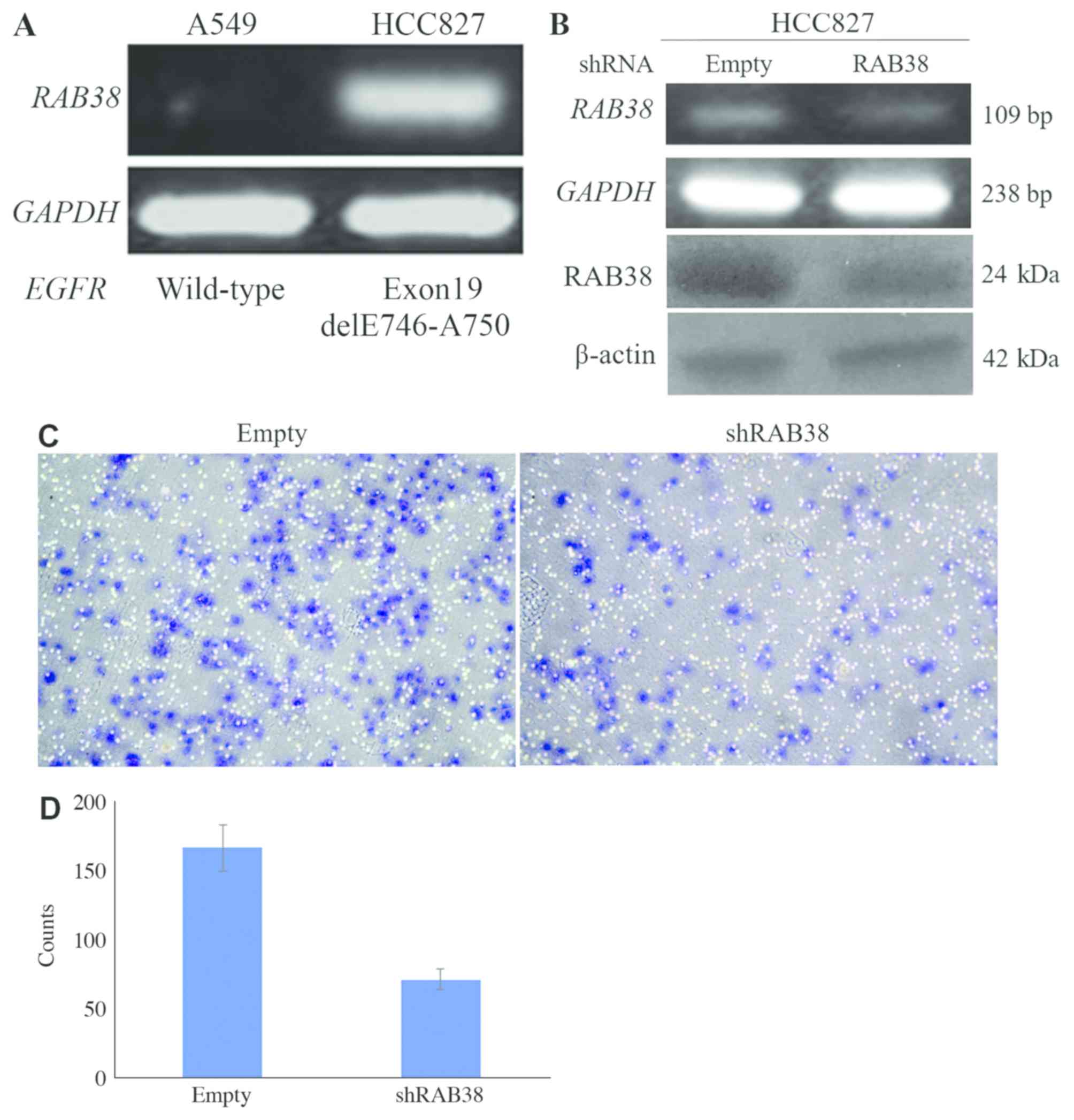
The expression of RAB38 was further analyzed
in two human NSCLC cell lines. RAB38 was expressed at a
higher level in HCC827 cells harboring an active EGFR
mutation, compared with that in A549 cells expressing wild-type
EGFR (Fig. 4A). Additionally,
RAB38 silencing in the HCC827 cells (with a reduction of
20–30% according to RT-PCR and western blot data, Fig. 4B) substantially inhibited Matrigel
invasiveness (with a reduction of 60%, Fig. 4C and D), indicating that the
expression of RAB38 may be positively associated with lung
cancer metastasis.
Discussion
In the present study, the gene expression of
RAB38 was evaluated in patients with NSCLC; it was
demonstrated that high levels of RAB38 were more frequently
detected in stage III NSCLC, and that this was significantly
associated with tumor recurrence and poorer OS. However, the mRNA
expression level of RAB38 was also associated with poor OS
in patients with stage I NSCLC. The small sample size may have
contributed to this observation, thus further investigation with a
larger sample number is required to confirm these conclusions. To
the best of our knowledge, this is the first report describing the
prognostic role of RAB38 in human NSCLC.
Although RAB proteins have been reported to be
overexpressed in numerous types of cancer, the functionality of
these proteins in cancer progression remains to be clarified. The
tissue-specific expression pattern of RAB38 has previously
been demonstrated. Jäger et al (18) reported that high RT-PCR signals were
detected in cultured melanocytes and adrenal gland tissues; whereas
weak to moderate signals were observed in the testes, kidney,
uterus, prostate and pancreas. RAB38 mRNA was expressed in
80–90% of melanoma cases, but showed minimal expression in
non-melanocytic malignancies. Although not detectable in lung
tissues, the expression of RAB38 was observed in one of four
lung cancer cell lines assessed, suggesting that it may serve a
role in lung tumorigenesis (18).
This study further demonstrated that RAB38 is strongly immunogenic,
leading to spontaneous antibody responses in a significant
proportion of patients with melanoma, and indicated that it may
serve as a potential therapeutic target (19).
Chen et al (20) established a paired human lung
adenocarcinoma cell line from primary tumor site tissues and
metastatic lymph nodes and analyzed the gene expression profiles by
microarray. The expression of RAB38 was markedly increased
in tumor cells derived from metastatic lymph nodes compared with
those from the primary tumor site. The present study revealed that
gene silencing markedly reduced tumor cell invasion in HCC827
cells. Collectively, these findings suggest that RAB38 may
be involved in lung cancer metastasis.
By contrast, in searching for potential tumor
suppressor genes in lung cancer, Wu et al (21) reported that RAB37 was
frequently downregulated in tumor tissues, compared with that in
normal non-cancerous tissues, by promoter methylation, and that the
low expression of RAB37 was associated with tumor
metastasis. In addition, the expression of RAB37 was shown
to be inversely correlated with cell motility in lung cancer cell
lines (22), indicating the
differential functionality of RAB proteins in lung cancer
metastasis.
Crosstalk between integrin and growth factor
receptor pathways has been reported to serve a prominent role in
tumor progression (23,24). β1-integrin is required for EGFR
signaling, and the silencing of β1-integrin substantially impairs
the EGF-induced activation of EGFR in lung cancer cells (25). Ectopic expression of RAB25 was
demonstrated to increase the expression of β1-integrin and
subsequent activation of EGFR in breast cancer cells in
vitro, and to increase tumorigenesis and pulmonary metastasis
in ovarian cancer cells in vivo (26). The present study demonstrated that
RAB38 was upregulated in cases of NSCLC associated with an
active EGFR mutation, compared with those associated with
wild-type EGFR, suggesting that the expression of
RAB38 may be associated with EGFR status. However,
only two cell lines were analyzed, and further investigation is
required to confirm this association. Furthermore, the collection
of additional tumor specimens is required to determine this
association in patient tissues. Investigation of the functional
analysis of RAB38 in NSCLC, in vitro and in
vivo, is currently in progress.
In conclusion, the present study demonstrated that
RAB38 is an important prognostic factor and potential
therapeutic target in NSCLC.
Acknowledgements
The authors would like to thank Professor Alex YC
Chang (Johns Hopkins Singapore) for data evaluation and as a
consultant for manuscript writing. The authors also wish to thank
the Laboratory of Dr Cheng-Hsu Wang (Chang Gung Memorial Hospital,
Keelung branch) for their technical support.
Funding
The present study was supported by grants to JC
(grant nos. CMRPG380761 and CMRPG3B0081~3) and MH (grant no.
CMRPG3F1261) from Chang Gung Memorial Hospital, Taiwan.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH and HC performed data analyses and wrote the
manuscript. YS and MH collected the dataset, and contributed to
data analyses and manuscript revision. JC and TH conceived and
designed the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
In the original creation of the datasets, the
Institutional Review Board of Chang Gung Memorial Hospital approved
the study and informed consent to participate was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
References
|
1
|
Silvestri GA and Rivera MP: Targeted
therapy for the treatment of advanced non-small cell lung cancer: A
review of the epidermal growth factor receptor antagonists. Chest.
128:3975–3984. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Recchi C and Seabra MC: Novel functions
for Rab GTPases in multiple aspects of tumor progression. Biochem
Soc Trans. 40:1398–1403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Osanai K and Voelker DR: Analysis and
expression of Rab38 in oculocutaneous lung disease. Methods
Enzymol. 438:203–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
MAQC Consortium, ; Shi L, Reid LH, Jones
WD, Shippy R, Warrington JA, Baker SC, Collins PJ, de Longueville
F, Kawasaki ES, et al: The MicroArray Quality Control (MAQC)
project shows interplatform reproducibility of gene expression
measurements. Nat Biotechnol. 24:1151–1161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ho JR, Chapeaublanc E, Kirkwood L, Nicolle
R, Benhamou S, Lebret T, Allory Y, Southgate J, Radvanyi F and Goud
B: Deregulation of Rab and Rab effector genes in bladder cancer.
PLoS One. 7:e394692012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dong W, Cui J, Yang J, Li W, Wang S, Wang
X, Li X, Lu Y and Xiao W: Decreased expression of Rab27A and Rab27B
correlates with metastasis and poor prognosis in colorectal cancer.
Discov Med. 20:357–367. 2015.PubMed/NCBI
|
|
7
|
He H, Dai F, Yu L, She X, Zhao Y, Jiang J,
Chen X and Zhao S: Identification and characterization of nine
novel human small GTPases showing variable expressions in liver
cancer tissues. Gene Expr. 10:231–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimada K, Uzawa K, Kato M, Endo Y, Shiiba
M, Bukawa H, Yokoe H, Seki N and Tanzawa H: Aberrant expression of
RAB1A in human tongue cancer. Br J Cancer. 92:1915–1921. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Culine S, Honore N, Closson V, Droz JP,
Extra JM, Marty M, Tavitian A and Olofsson B: A small GTP-binding
protein is frequently overexpressed in peripheral blood mononuclear
cells from patients with solid tumours. Eur J Cancer 30A. 670–674.
1994. View Article : Google Scholar
|
|
10
|
Amillet JM, Ferbus D, Real FX, Antony C,
Muleris M, Gress TM and Goubin G: Characterization of human Rab20
overexpressed in exocrine pancreatic carcinoma. Hum Pathol.
37:256–263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kotzsch M, Sieuwerts AM, Grosser M, Meye
A, Fuessel S, Meijer-van Gelder ME, Smid M, Schmitt M, Baretton G,
Luther T, et al: Urokinase receptor splice variant
uPAR-del4/5-associated gene expression in breast cancer:
Identification of rab31 as an independent prognostic factor. Breast
Cancer Res Treat. 111:229–240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan PY, Chang CW, Chng KR, Wansa KD, Sung
WK and Cheung E: Integration of regulatory networks by NKX3-1
promotes androgen-dependent prostate cancer survival. Mol Cell
Biol. 32:399–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mori Y, Yin J, Sato F, Sterian A, Simms
LA, Selaru FM, Schulmann K, Xu Y, Olaru A, Wang S, et al:
Identification of genes uniquely involved in frequent
microsatellite instability colon carcinogenesis by expression
profiling combined with epigenetic scanning. Cancer Res.
64:2434–2438. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shibata D, Mori Y, Cai K, Zhang L, Yin J,
Elahi A, Hamelin R, Wong YF, Lo WK, Chung TK, et al: RAB32
hypermethylation and microsatellite instability in gastric and
endometrial adenocarcinomas. Int J Cancer. 119:801–806. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zippelius A, Gati A, Bartnick T, Walton S,
Odermatt B, Jaeger E, Dummer R, Urosevic M, Filonenko V, Osanai K,
et al: Melanocyte differentiation antigen RAB38/NY-MEL-1 induces
frequent antibody responses exclusively in melanoma patients.
Cancer Immunol Immunother. 56:249–258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H and Jiang C: RAB38 confers a poor
prognosis, associated with malignant progression and subtype
preference in glioma. Oncol Rep. 30:2350–2356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang JW, Wei NC, Su HJ, Huang JL, Chen
TC, Wu YC, Yu CT, Hou MM, Hsieh CH, Hsieh JJ, et al: Comparison of
genomic signatures of non-small cell lung cancer recurrence between
two microarray platforms. Anticancer Res. 32:1259–1265.
2012.PubMed/NCBI
|
|
18
|
Jäger D, Stockert E, Jäger E, Güre AO,
Scanlan MJ, Knuth A, Old LJ and Chen YT: Serological cloning of a
melanocyte rab guanosine 5′-triphosphate-binding protein and a
chromosome condensation protein from a melanoma complementary DNA
library. Cancer Res. 60:3584–3591. 2000.PubMed/NCBI
|
|
19
|
Walton SM, Gerlinger M, de la Rosa O,
Nuber N, Knights A, Gati A, Laumer M, Strauss L, Exner C, Schäfer
N, et al: Spontaneous CD8 T cell responses against the melanocyte
differentiation antigen RAB38/NY-MEL-1 in melanoma patients. J
Immunol. 177:8212–8218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Wang M, Zu L, Li W, Wang W, Li Y
and Liu H: Abstract 4966: Establishment and characterization of a
paired human non-small cell lung cancer cell lines from primary
cancer and metastasis lymph node. Cancer Res. 74:2014.
|
|
21
|
Wu CY, Tseng RC, Hsu HS, Wang YC and Hsu
MT: Frequent down-regulation of hRAB37 in metastatic tumor by
genetic and epigenetic mechanisms in lung cancer. Lung Cancer.
63:360–367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tzeng HT, Tsai CH, Yen YT, Cheng HC, Chen
YC, Pu SW, Wang YS, Shan YS, Tseng YL, Su WC, et al: Dysregulation
of Rab37-mediated cross-talk between cancer cells and endothelial
cells via thrombospondin-1 promotes tumor neovasculature and
metastasis. Clin Cancer Res. 23:2335–2345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Subramani D and Alahari SK:
Integrin-mediated function of Rab GTPases in cancer progression.
Mol Cancer. 9:3122010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huck L, Pontier SM, Zho DM and Muller WJ:
Beta1-integrin is dispensable for the induction of ErbB2 mammary
tumors but plays a critical role in the metastatic phase of tumor
progression. Proc Natl Acad Sci USA. 107:15559–15564. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morello V, Cabodi S, Sigismund S,
Camacho-Leal MP, Repetto D, Volante M, Papotti M, Turco E and
Defilippi P: β1 integrin controls EGFR signaling and tumorigenic
properties of lung cancer cells. Oncogene. 30:4087–4096. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeong BY, Cho KH, Jeong KJ, Park YY, Kim
JM, Rha SY, Park CG, Mills GB, Cheong JH and Lee HY: Rab25 augments
cancer cell invasiveness through aβ1 integrin/EGFR/VEGF-A/Snail
signaling axis and expression of fascin. Exp Mol Med. 50:e4352018.
View Article : Google Scholar : PubMed/NCBI
|