Introduction
Pancreatic cancer is a malignancy that originates
from cells in the pancreas, and can be divided into a number of
subtypes, according to pathological features (1). These subtypes include intraductal
papillary-mucinous neoplasm, acinar cell carcinoma, mucinous cystic
neoplasm with an invasive adenocarcinoma and adenocarcinoma
(1). Pancreatic adenocarcinoma, as
the predominant type of pancreatic cancer, accounts for >85% of
all cases (1). Despite advances in
the treatment and prevention of this disease, pancreatic cancer
remains a major cause of cancer-associated mortalities and, in
2015, it was reported to be directly responsible for >200,000
mortalities worldwide annually (2).
The 5-year survival rate in 2012 in certain regions, including
China was <5%, or <1% in particular areas, for example the
Southeast (3). In addition, the
incidence rate of pancreatic adenocarcinoma has increased in the
last 5 years worldwide (4). At
present, surgical resection is the only radical treatment for
pancreatic adenocarcinoma (4).
However, it is difficult to detect early stage pancreatic
adenocarcinoma and the majority of patients are diagnosed at
advanced stages, making surgical resection an inappropriate
treatment, leading to poor prognosis and high mortality rates
(5). Male gender, older age, a poor
diet, diabetes mellitus, obesity and smoking have been proven to be
risk factors for pancreatic adenocarcinoma (6). However, the current understanding on
the pathogenesis of pancreatic cancer is limited.
The development of pancreatic cancer is a complex
process with numerous genetic factors involved. Additionally to
messenger RNAs (mRNAs), which encode proteins, the human genome
also transcribes a number of non-coding RNAs (ncRNAs) that can
participate in physiological processes and pathological changes
(7). As a subtype of ncRNAs, long
ncRNAs (lncRNAs) have been demonstrated to be involved in the
pathogenesis of several human diseases, including various types of
malignacy (8). Since hox transcript
antisense RNA (HOTAIR) was reported to function as an oncogene in
breast cancer by modifying chromatin structure, this lncRNA has
been demonstrated to participate in the occurrence, development and
progression of various types of cancer, including pancreatic
adenocarcinoma (9,10). However, the diagnostic and prognostic
values of HOTAIR for pancreatic adenocarcinoma, as well as its
involvement in cancer cell energy metabolism requires further
investigation.
Materials and methods
Patients
Between August 2009 and August 2012, a total of 78
patients with pancreatic adenocarcinoma at the Department of
Oncology, Affiliated Hospital of School of Medicine, Southeast
University (Nanjing, China) were enrolled to the present study.
Pancreatic adenocarcinoma was confirmed in patients by pathological
and imaging examinations. A total of 48 males and 30 females, with
an mean age of 45.0±16.1 years (range, 19–78 years), were included.
Primary tumors were staged according to following standards: i) T1,
tumor confined to the pancreas with a maximum diameter <2 cm;
ii) T2, tumor confined to the pancreas with a diameter of 2–4cm;
iii) T3, tumor confined to the pancreas with diameter >4 cm; and
iv) T4, celiac axis or the superior mesenteric artery were
involved. The cohort included 12 cases of stage 1, 14 cases of
stage 2, 25 cases of stage 3 and 27 cases of stage 4 (AJCC)
(11). Among these patients, 51
received surgical resection, and tumor tissues, as well as adjacent
healthy tissues, were collected during the procedure. All tissues
were confirmed by pathological examinations. Additionally, 30
healthy volunteers (sex, 18 male and 12 female; age range, 20–79
years; mean, 45.3±15.2 years) with similar age and gender
distributions were enrolled, to serve as a control group. The
Ethics Committee of the Affiliated Hospital of School of Medicine,
Southeast University approved this study. All patients and
volunteers provided signed informed consent.
Preparation of serum samples
Fasting blood (20 ml) was collected from all 78
patients and 30 healthy controls on the morning following their
admission. The blood samples were incubated at room temperature for
1.5 h, followed by centrifugation at 1,400 × g at room temperature
for 15 min to separate the serum, which was then stored at −80°C
prior to use.
Cell lines and cell culture
Human pancreatic adenocarcinoma cell lines BxPC-3
and Capan-2 were purchased from the American Type Culture
Collection (ATCC; Manassas, VA, USA). All cell lines were cultured
according to the supplier's protocols. The BxPC-3 cells were
cultured in ATCC-formulated RPMI-1640 medium containing 10% fetal
bovine serum (FBS) (both ATCC). The Capan-2 cells were cultured in
ATCC-formulated McCoy's 5a medium (ATCC) containing 10% FBS. All
incubations were performed at 37°C. Cells were harvested during
logarithmic growth phase for subsequent experiments.
Establishment of HOTAIR- and
hexokinase-2 (HK2)-overexpressing cell lines
HOTAIR (sequence ID, NR_047517.1) and HK2 (GeneBank
ID, M23115.1) expression vectors (pcDNA3.1) were established by
inserting an EcoRI fragment containing full-length HOTAIR or
HK2 cDNA into the pcDNA3.1 vector (Clontech Laboratories, Inc.,
Mountainview, CA, USA). BxPC-3 and Capan-2 cells were cultured
overnight to ensure they reached 80–90% confluence prior to
transfection. The experiment was performed using
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) to transfect 10
nM vector into 106 cells, according to the
manufacturer's protocol. Empty vector without HOTAIR or HK2 cDNA
was used as negative control. The interval between transfection and
subsequent experiments was 24 h.
Cell proliferation assay
For the cell proliferation assays, a Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) was used. BxPC-3 and Capan-2 cells were plated into a
96-well plate (100 µl cell suspension containing 4×104
cells), cultured at 37°C, and CCK-8 solution (10 µl) was added into
each well at 24, 48, 72 and 96 h post-cell seeding. Following the
addition of CCK-8 solution, cells were cultured at 37°C for an
additional 4 h, and a Fisherbrand™ accuSkan™ GO UV/Vis microplate
spectrophotometer (Thermo Fisher Scientific, Inc.) was used to
measure optical density (OD) values at a wavelength of 450 nm.
Lactate production, glucose uptake and
ATP production assays
The ATP assay kit, Celltiter-Glo Luminescent Cell
Viability assay (Promega Corporation, Madison, WI, USA) was used to
measure ATP levels, according to the manufacturer's protocols. For
the glucose uptake assays, BxPC-3 and Capan-2 cells were cultured
in 6-well plates for 24 h at a density of ~5×105 cells
per well. The cell supernatant was collected and OD values at 570
nm were measured with a microplate reader, using fresh culture
medium as a control. Glucose uptake was calculated by subtracting
the concentration of glucose in the tested samples from the initial
concentration of glucose in the fresh culture medium. Lactate
production was determined according to a standard curve. Lactate
production and glucose uptake were normalized to the cellular
protein levels.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific Inc.) was used to extract total RNA from tumor
tissues, adjacent healthy tissues, serum and in vitro
cultured cells. Tumor tissues and adjacent healthy tissues were
ground in liquid nitrogen prior to the addition of TRIzol. RNA
quality was tested using gel electrophoresis, and the RNA samples
were subjected to RT for the synthesis of cDNA using AMV Reverse
Transcriptase XL kit (Takara Bio, Inc., Otsu, Japan). The
temperature conditions were 25°C for 5 min, 55°C for 15 min and
75°C for 10 min. SYBR® Green Real-Time PCR master mix
(Thermo Fisher Scientific, Inc.) was used for qPCR reactions with
the cDNA, according to the manufacturer's protocols. The following
primers were used: HOTAIR forward, 5′-AACGATGTGTGTGTGCCTTGAT-3′;
and reverse, 5′-TGGTCCGACAGGGTGAATT-3′; HK2 forward,
5′-GGGCATCTTGAAACAAG-3′; and reverse, 5′-GGTCTCAAGCCCTAAG-3′; and
β-actin forward, 5′-GACCTCTATGCCAACACAGT-3′; and reverse,
5′-AGTACTTGCGCTCAGGAGGA-3′. The thermocycling conditions were: 95°C
for 50 s, followed by 40 cycles of 95°C for 12 s and 60°C for 30 s.
The data were processed using the 2−ΔΔCq method
(12). The expression levels of
HOTAIR and HK2 were normalized to the endogenous β-actin control
levels.
Western blot analysis
Radioimmunoprecipitation assay buffer (Thermo Fisher
Scientific, Inc.) was used to extract total protein from BxPC-3 and
Capan-2 cells, and protein quantity was determined by bicinchoninic
acid assay. Gel electrophoresis was performed using 10% SDS-PAGE
with 25 µg per lane and the proteins were transferred onto
polyvinylidene difluoride membranes. The membranes were blocked
with 5% skimmed milk for 2 h at room temperature, followed by
incubation with primary antibodies including rabbit anti-HK2
(dilution, 1:2,000; cat. no. ab37593) and anti-GAPDH primary
antibody (dilution, 1:1,000; cat. no. ab8245) (both Abcam,
Cambridge, MA, USA) overnight at 4°C. The following day, membranes
were washed in triplicate with PBS for 15 min, and incubated with
horseradish peroxidase-conjugated anti-rabbit IgG secondary
antibody (dilution, 1:1,000; cat. no. MBS435036; MyBioSource, Inc.,
San Diego, CA, USA) at room temperature for 2 h, followed by signal
detection using an enhanced chemiluminescence kit (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). Signals were scanned using MYECL™
Imager (Thermo Fisher Scientific, Inc.), and ImageJ v.1.48 software
(National Institutes of Health, Bethesda, MD, USA) was used to
normalize the relative expression level of HK2 to the endogenous
GAPDH control levels.
Statistical analysis
All data were processed using SPSS version 19.0 (IBM
Corp., Armonk, NY USA). Count data were processed using a
χ2 test. Measurement data were expressed as mean ±
standard deviation, and comparisons between two groups were
performed using Student's paired t-test. Comparisons among multiple
groups were performed using analysis of variance (one-way) and a
least significant difference post hoc test. Receiver operating
characteristic (ROC) curve analysis was performed to evaluate the
diagnostic value of serum HOTAIR and HK2 levels in pancreatic
adenocarcinoma. Patients were divided into high- and low-expression
groups according to the median serum levels of HOTAIR and HK2.
Survival curves were plotted using the Kaplan-Meier method and were
compared using a log rank test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of HOTAIR and HK2 in tumor
and adjacent healthy tissues of patients with pancreatic
adenocarcinoma
The expression levels of HOTAIR and HK2 in tumor and
adjacent healthy tissues of 51 patients with pancreatic
adenocarcinoma, who had undergone surgical resection, were detected
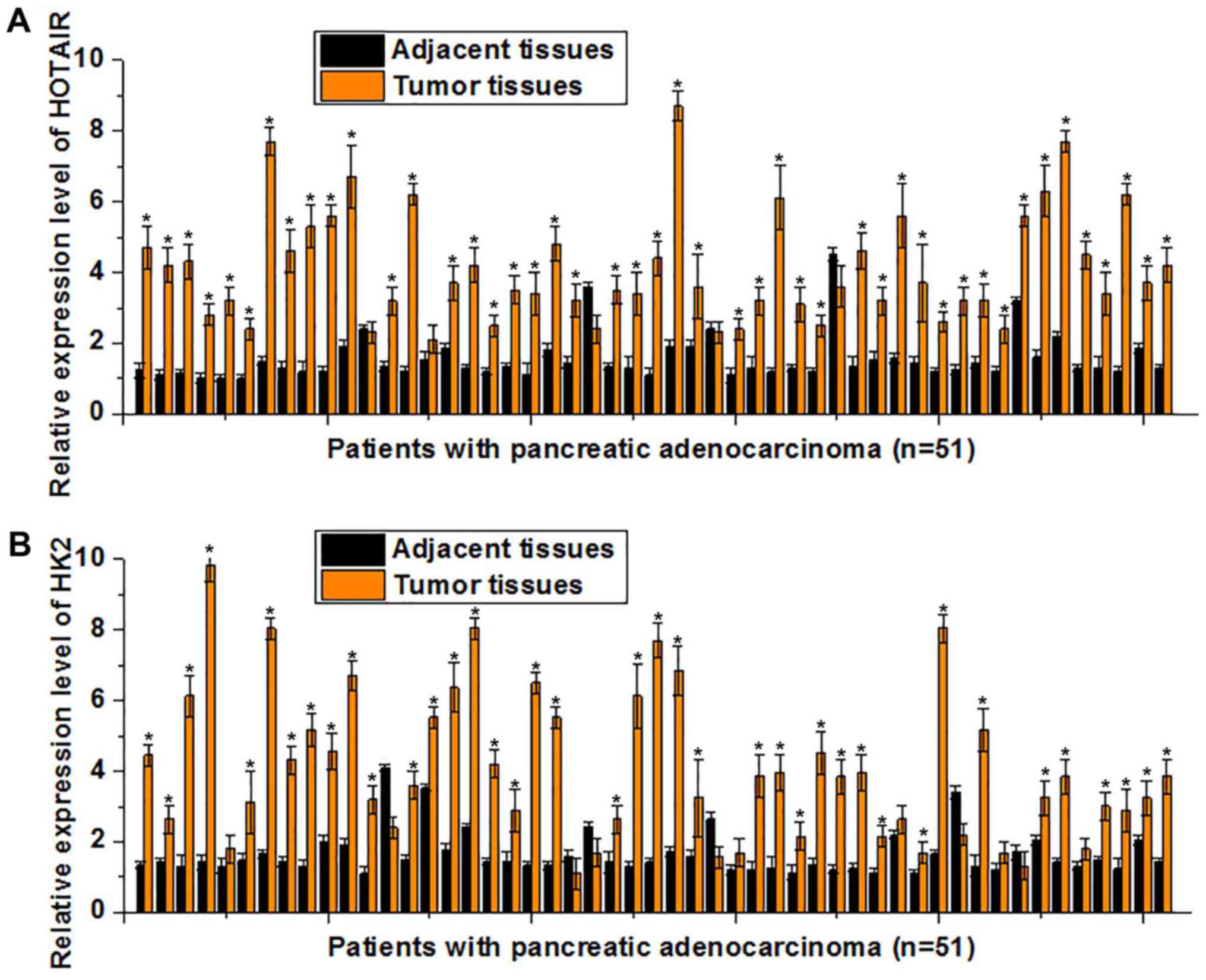
by RT-qPCR. As indicated in Fig. 1A,
the expression level of HOTAIR was significantly upregulated in
tumor tissues compared with adjacent healthy tissues (P<0.05) in
46/51 patients. Decreased HOTAIR expression levels in tumor tissues
compared with those in the adjacent healthy tissues (P<0.05)
were observed in only 2/51 patients, and no significant difference
was indicated in 4/51 patients. Similarly, the mRNA levels of HK2
were significantly upregulated in tumor tissues compared with
adjacent healthy tissues (P<0.05) in 41/51 patients. Decreased
HK2 levels of in tumor tissues compared with the adjacent healthy
tissues (P<0.05) were only observed in 4/51 cases, and no
significant difference was observed in 6/51 cases (Fig. 1B). These results suggest that the
upregulation of HOTAIR and HK2 is involved in the pathogenesis of
pancreatic adenocarcinoma.
Serum levels of HOTAIR and HK2 in
healthy controls and patients with different stages of pancreatic
adenocarcinoma
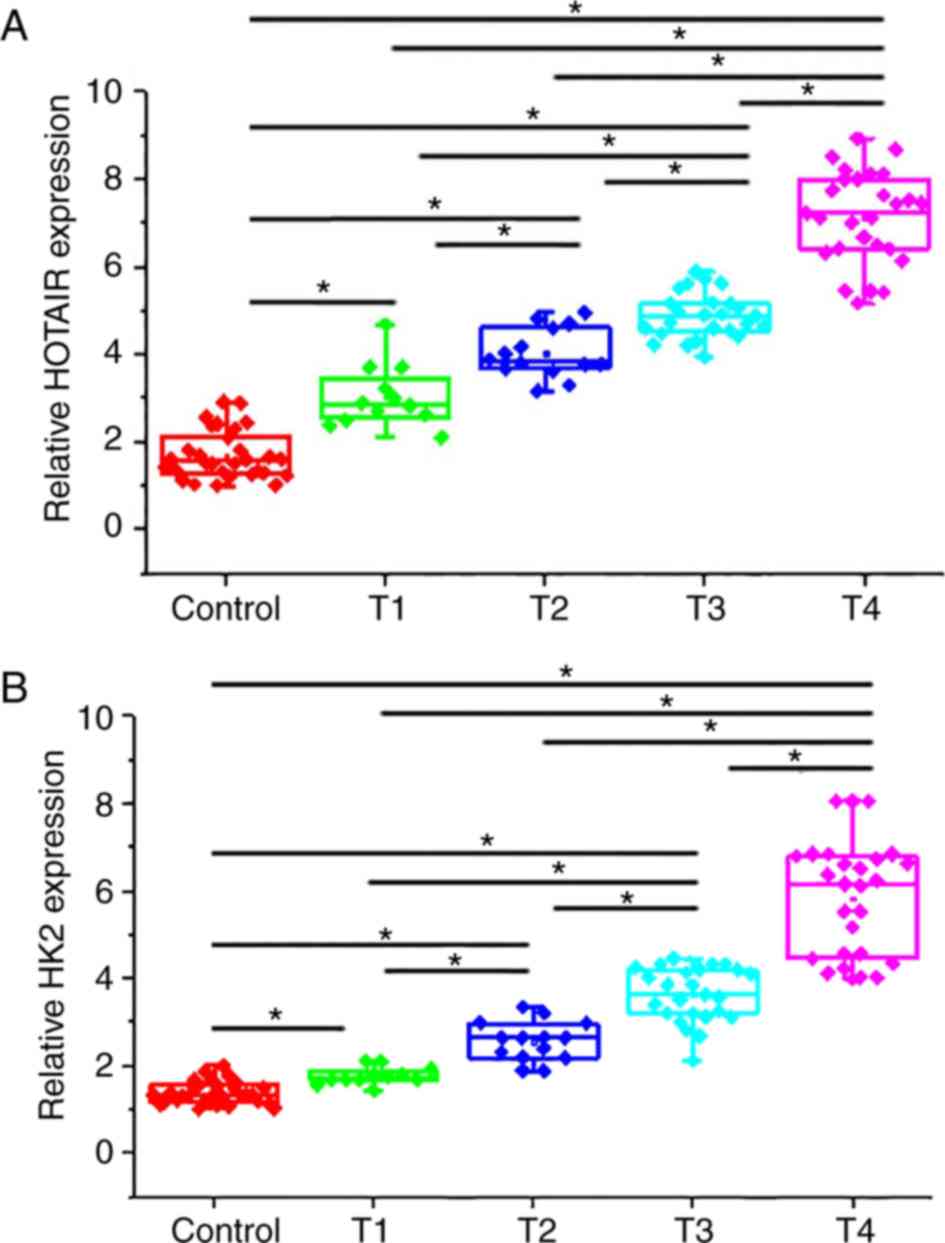
As presented in Fig.
2A, the expression levels of HOTAIR were significantly lower in
the serum of healthy controls compared with patients with different
stages of pancreatic adenocarcinoma (P<0.05). In addition, the
expression levels of HOTAIR were further increased at more advanced
stages of primary tumor. Similarly, serum levels of HK2 were
significantly higher in patients with different stages of
pancreatic adenocarcinoma compared with healthy controls (Fig. 2B; P<0.05). In addition, expression
levels of HK2 were increased with the later stages of primary
tumors.
Diagnostic and prognostic value of
HOTAIR and HK2 in pancreatic adenocarcinoma
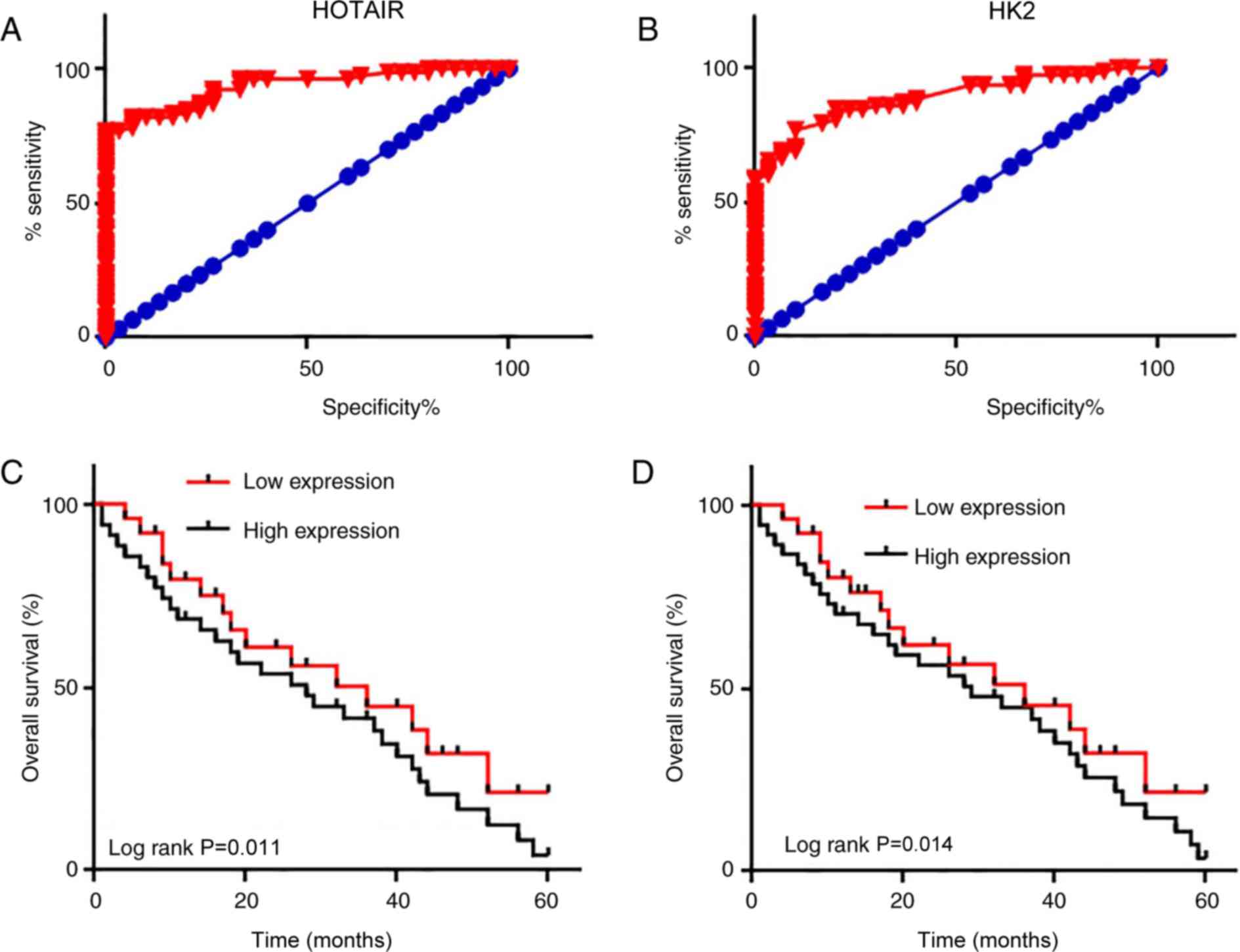
ROC curve analysis was performed in order to
evaluate the diagnostic value of serum HOTAIR and HK2 levels in
pancreatic adenocarcinoma. The area under the curve for serum
HOTAIR levels was 0.9329 [95% confidence interval (CI),
0.8888–0.9770; P<0.0001; Fig.
3A], and for serum HK2 it was 0.8893 (95% CI, 0.8295–0.9492;
P<0.0001; Fig. 3B). These data
suggest that serum HOTAIR and HK2 levels may serve as potential
biomarkers in the diagnosis of pancreatic adenocarcinoma. Patients
were divided into high- and low-expression groups, according to the
median serum levels of HOTAIR and HK2. Survival curves were plotted
using the Kaplan-Meier method and were compared using a log rank
test. As presented in Fig. 3C and D,
the overall survival rate of patients with high expression levels
of HOTAIR or HK2 was significantly lower compared with that of
patients with low expression levels (P=0.011 and P=0.014,
respectively). These data suggest that serum HOTAIR and HK2 may
serve as promising diagnostic and prognostic biomarkers for
pancreatic adenocarcinoma.
HOTAIR and HK2 overexpression promotes
the proliferation of pancreatic adenocarcinoma
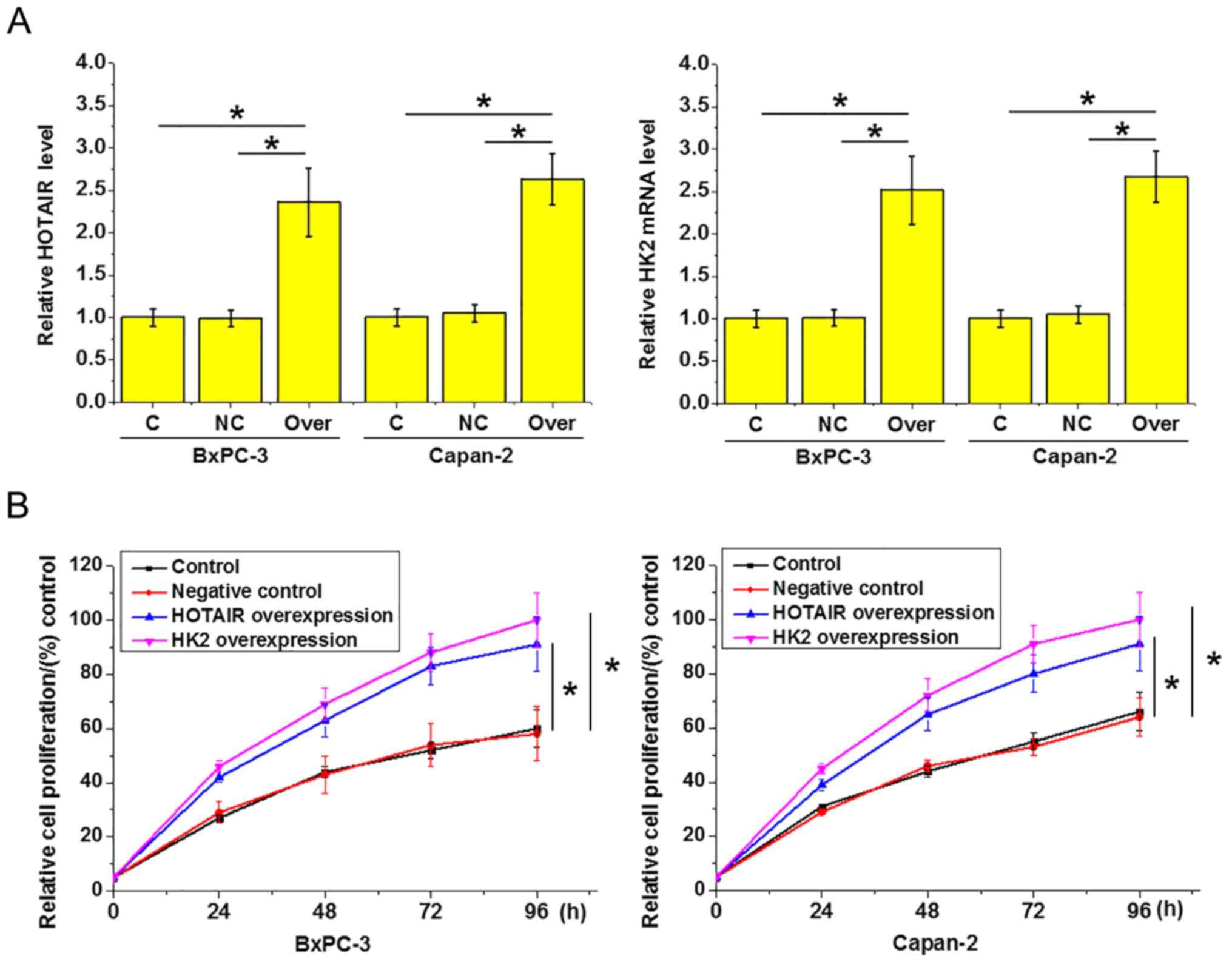
HOTAIR and HK2 overexpression in pancreatic
adenocarcinoma BxPC-3 and Capan-2 cells following transfection was
confirmed by RT-qPCR (Fig. 4A;
P<0.05). As indicated in Fig. 4B,
compared with the equivalent control cells, HOTAIR and HK2
overexpression appeared to promote the proliferation of the two
pancreatic adenocarcinoma cell types (P<0.05), and the enhancing
effect of HK2 overexpression on the proliferation rate was stronger
than that of HOTAIR overexpression, but the differences were not
statistically significant. These findings indicate that HOTAIR and
HK2 overexpression can promote the proliferation of pancreatic
adenocarcinoma cells.
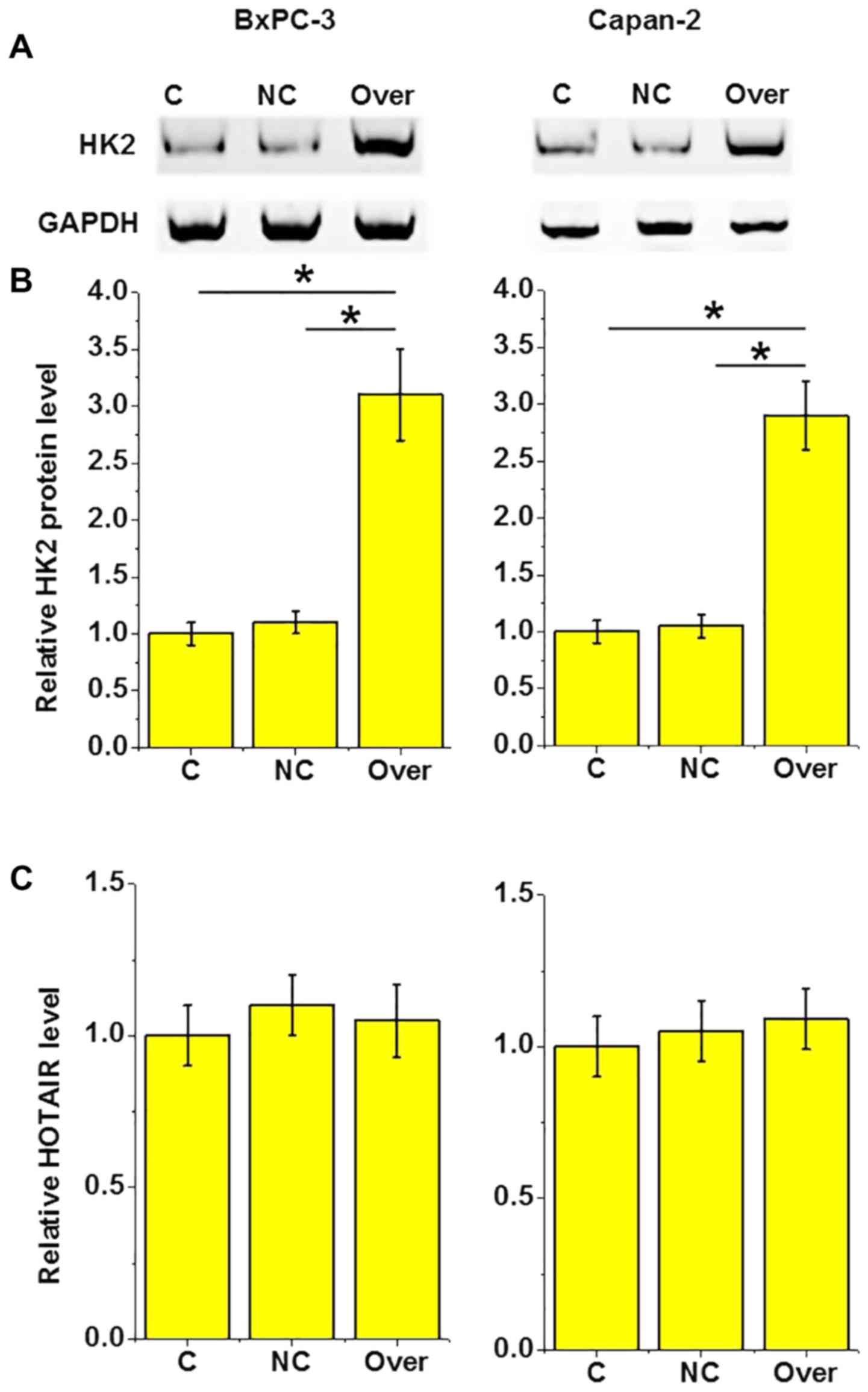
HOTAIR is an upstream regulator of HK2
in pancreatic adenocarcinoma
A recent study reported that HOTAIR can interact
with HK2 to achieve its biological functions (12). In the present study, HOTAIR
overexpression was revealed to lead to a significant increase the
level of HK2 protein in the two tested pancreatic adenocarcinoma
cell lines (P<0.05; Fig. 5A and
B). By contrast, HK2 overexpression led to no observed
significant effects on the expression of HOTAIR (P>0.05;
Fig. 5C). These results suggest that
HOTAIR is an upstream regulator of HK2 in these cells.
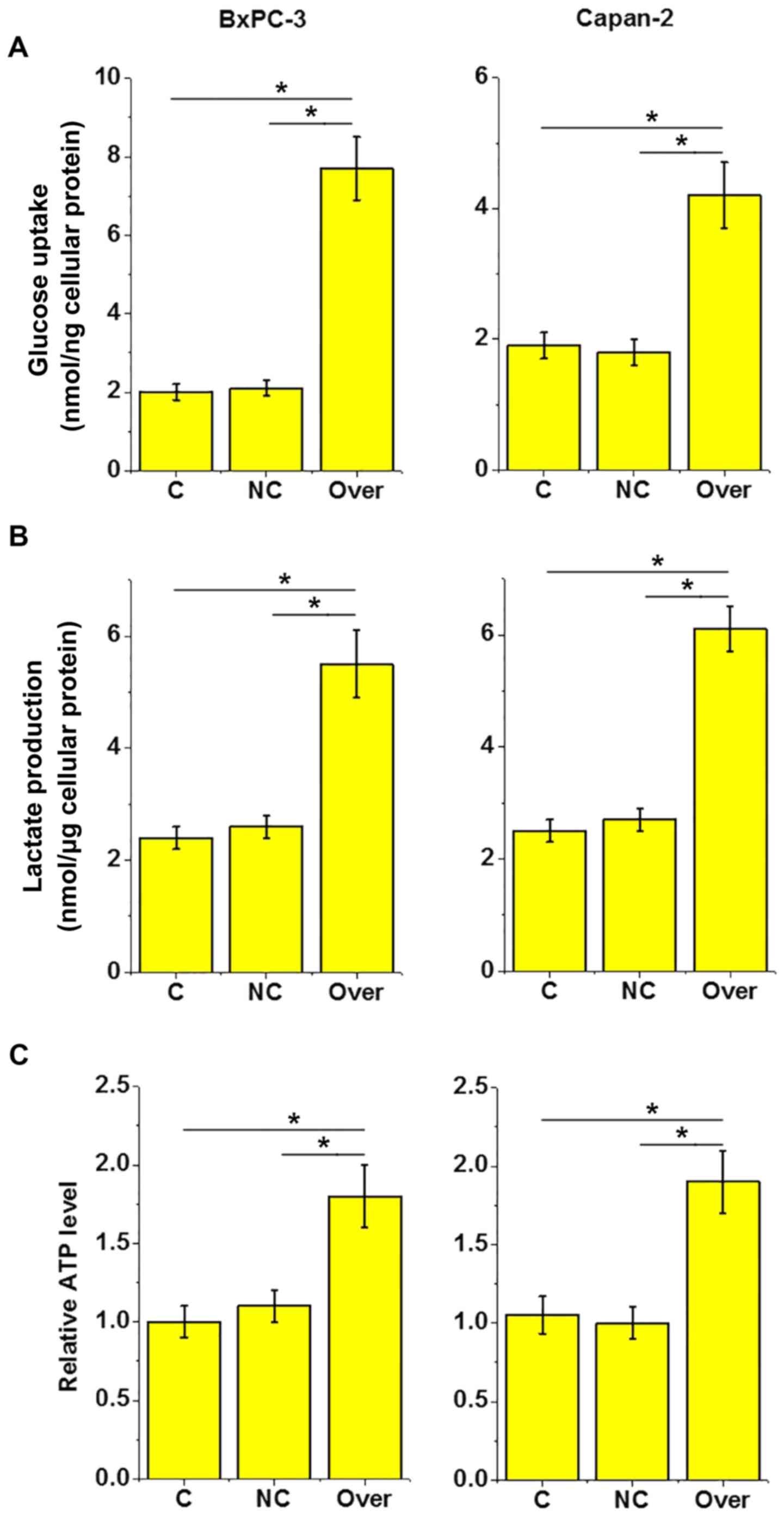
HOTAIR overexpression increases
lactate production, glucose uptake and ATP production in pancreatic
adenocarcinoma cells
As indicated in Fig.
6A, compared with the equivalent control cells, glucose uptake
was significantly increased in the cells of both pancreatic
adenocarcinoma cell lines following HOTAIR overexpression
(P<0.05). Similarly, lactate production (Fig. 6B) and ATP production (Fig. 6C) were significantly increased
following HOTAIR overexpression. This suggests that lncRNA HOTAIR
may promote cancer cell energy metabolism in pancreatic
adenocarcinoma.
Discussion
Pancreatic cancer, predominantly referred to as
pancreatic adenocarcinoma, is thought to be the most lethal
type of cancer, with a 1.5–2-fold higher incidence rate in males
compared with females (13). In the
present study, a total of 78 patients with pancreatic
adenocarcinoma with a male to female ratio of 1.6:1 were enrolled.
The occurrence, development and progression of pancreatic
adenocarcinoma involve various genetic factors, including lncRNAs
(14). In a recent study, Jiang
et al (15) reported that
lncRNA HOTAIR was upregulated in pancreatic ductal adenocarcinoma
tissue samples compared with adjacent healthy tissues. However, in
another study, epigenetic silencing of HOTAIR was proved to be at
least partially responsible for the metastasis of pancreatic cancer
(16). The present results revealed
that the expression of HOTAIR was significantly upregulated in
tumor tissues compared with adjacent healthy tissues in 46 out of
51 patients, whereas downregulation was observed in only 2 cases.
Altered metabolic processes are usually observed in cancer cells,
and the characteristic changes may include enhanced rates of
glutaminolysis and fatty acid synthesis, as well as increased
uptake of glucose, which can support the proliferation and survival
of tumor cells (17). HK2 is the
major isozyme in aerobic glycolysis that is upregulated in various
types of cancer, including liver and gastric cancer (18). In the present study, HK2 was also
revealed to be upregulated in tumor tissues compared with adjacent
healthy tissues in the majority of patients with pancreatic
adenocarcinoma. In addition, the expression levels of HOTAIR and
HK2 were increased as the primary tumor stage advanced. These
findings suggest that the upregulation of HOTAIR and HK2 is
involved in the development and progression of pancreatic
adenocarcinoma.
At the present time, surgical resection remains the
only radical treatment for pancreatic adenocarcinoma (13). However, the majority of pancreatic
adenocarcinoma cases are diagnosed at advanced stages due to lack
of obvious symptoms earlier on, making surgical resection no longer
an option for these patients (5).
Therefore, early diagnosis and treatment remain key for the
management of this disease. In the present study, ROC curve
analyses indicated that HOTAIR and HK2 could be employed to
accurately detect pancreatic adenocarcinoma. In addition, the
overall survival rate of patients with high expression levels of
HOTAIR or HK2 was significantly lower than that of patients with
low expression. These data suggest that HOTAIR and HK2 may serve as
diagnostic and prognostic biomarkers of pancreatic adenocarcinoma.
However, HOTAIR and HK2 have also been reported to be of diagnostic
and prognostic value in numerous other types of malignancy,
inevitably affecting their specificity (15,16,18).
Therefore, the combination of multiple biomarkers may improve the
diagnosis and prognosis of pancreatic adenocarcinoma.
HOTAIR and HK2 have been reported to serve a
critical role in the proliferation of cancer cells and development
of the disease (19,20). In the present study, compared with
their equivalent controls, HOTAIR and HK2 overexpression
significantly promoted the proliferation of the two tested
pancreatic adenocarcinoma cell lines, BxPC-3 and Capan-2. Despite
the fact that the function of HOTAIR has been studied in various
types of cancer, including pancreatic cancer (15,16), its
involvement in cancer cell energy metabolism, which is critical for
cell growth and proliferation, remains unclear. Until now, a number
of lncRNAs have been demonstrated to participate in glucose
metabolism in cancer (20), and Cao
et al (21) proposed an
electron-transfer equilibrium with a small reorganization energy in
aerobic glycolysis by using synthetic model systems. In the present
study, HOTAIR overexpression significantly increased lactate
production, glucose uptake and ATP production in pancreatic
adenocarcinoma cells, indicating that it can promote energy
metabolism in these cells. HK2 serves a key role in aerobic
glycolysis by regulating lactate production, glucose uptake and ATP
production (17), and aerobic
glycolysis is regulated by HOTAIR in the pathogenesis of certain
human diseases, including esophageal squamous cell carcinoma
(12). The present findings
demonstrated that HOTAIR overexpression led to a significant
increase in the expression of HK2 in BxPC-3 and Capan-2 cells,
whereas HK2 overexpression revealed no significant effects on
HOTAIR levels. These results suggest that HOTAIR is an upstream
regulator of HK2 in pancreatic adenocarcinoma.
In conclusion, HOTAIR and HK2 expression levels were
increased in tumor tissues compared with adjacent healthy tissues
in the majority of patients with pancreatic adenocarcinoma. Serum
levels of these two factors were higher in patients with this
cancer than in healthy controls, and increased with tumor
progression. Serum HOTAIR and HK2 may be used to accurately detect
pancreatic adenocarcinoma and predict its prognosis. Overexpression
of these two molecules promoted tumor cell proliferation, and
HOTAIR upregulation led to increased lactate production, glucose
uptake and ATP production in pancreatic adenocarcinoma cells.
HOTAIR overexpression promoted HK2 expression, however, HK2
upregulation exhibited no significant effects on HOTAIR. Therefore,
it can be concluded that lncRNA HOTAIR may promote cancer cell
energy metabolism in pancreatic adenocarcinoma by serving as a
positive upstream regulator of HK2.
Acknowledgements
Not applicable.
Funding
The present study was funded by Postgraduate
Research and Practice Innovation Program of Jiangsu Province (grant
no. KYCX17_0183).
Availability of data and materials
The datasets generated and/or analyzed during the
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM and PH designed experiments. YM, MH, LZ performed
experiments. SL, YL and BK analyzed data and assisted the
experiments. PH drafted the manuscript. All authors approved this
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Affiliated Hospital of School of Medicine, Southeast
University.
Patient consent for publication
Patients provided consent for publication of their
information.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stewart BW and Wild CP: World cancer
report 2014. Health. 2017.
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Zuo T, Zeng H, Zhang S
and He J: National cancer incidence and mortality in China, 2012.
Chin J Cancer Res. 28:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:1039–1049. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharma C, Eltawil KM, Renfrew PD, Walsh MJ
and Molinari M: Advances in diagnosis, treatment and palliation of
pancreatic carcinoma: 1990–2010. World J Gastroenterol. 17:867–897.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mattick JS and Makunin IV: Non-coding RNA.
Hum Mol Genet 15 (Spec No 1). R17–R29. 2006. View Article : Google Scholar
|
|
8
|
Cui Z, Ren S, Lu J, Wang F, Xu W, Sun Y,
Wei M, Chen J, Gao X, Xu C, et al: The prostate cancer-up-regulated
long noncoding RNA PlncRNA-1 modulates apoptosis and proliferation
through reciprocal regulation of androgen receptor. Urol Oncol.
31:1117–1123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai H, Yao J, An Y, Chen X, Chen W, Wu D,
Luo B, Yang Y, Jiang Y, Sun D and He X: LncRNA HOTAIR acts as
competing endogenous RNA to control the expression of Notch3 via
sponging miR-613 in pancreatic cancer. Oncotarget. 8:32905–32917.
2017.PubMed/NCBI
|
|
11
|
Kamarajah SK, Burns WR, Frankel TL, Cho CS
and Nathan H: Validation of the American Joint Commission on Cancer
(AJCC) 8th edition staging system for patients with pancreatic
adenocarcinoma: A Surveillance, Epidemiology and End Results (SEER)
Analysis. Ann Surg Oncol. 24:2023–2030. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pang EJ, Yang R, Fu XB and Liu YF:
Overexpression of long non-coding RNA MALAT1 is correlated with
clinical progression and unfavorable prognosis in pancreatic
cancer. Tumour Biol. 36:2403–2407. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang Y, Li Z, Zheng S, Chen H, Zhao X,
Gao W, Bi Z, You K, Wang Y, Li W, et al: The long non-coding RNA
HOTAIR affects the radiosensitivity of pancreatic ductal
adenocarcinoma by regulating the expression of Wnt inhibitory
factor 1. Tumour Biol. 37:3957–3967. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nie H, Zhang Y, Xing R, Li M and Mou Y:
Epigenetic silence of HOTAIR contributes to the metastasis of
pancreatic cancer via targeting miR-138. Oncol Res. Dec
21–2017.(Epub ahead of print). View Article : Google Scholar
|
|
17
|
Fadaka A, Ajiboye B, Ojo O, Adewale O and
Olayide I: Biology of glucose metabolization in cancer cells. J
Oncol Sci. 3:45–51. 2017.
|
|
18
|
Mathupala SP, Ko YH and Pedersen PL:
Hexokinase-2 bound to mitochondria: Cancer's stygian link to the
‘Warburg Effect’ and a pivotal target for effective therapy. Semin
Cancer Biol. 19:17–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katagiri M, Karasawa H, Takagi K, Nakayama
S, Yabuuchi S, Fujishima F, Naitoh T, Watanabe M, Suzuki T, Unno M
and Sasano H: Hexokinase 2 in colorectal cancer: A potent
prognostic factor associated with glycolysis, proliferation and
migration. Histol Histopathol. 32:351–360. 2017.PubMed/NCBI
|
|
20
|
Ono H, Motoi N, Nagano H, Miyauchi E,
Ushijima M, Matsuura M, Okumura S, Nishio M, Hirose T, Inase N and
Ishikawa Y: Long noncoding RNA HOTAIR is relevant to cellular
proliferation, invasiveness, and clinical relapse in small-cell
lung cancer. Cancer Med. 3:632–642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao R, Saracini C, Ginsbach JW,
Kieber-Emmons MT, Siegler MA, Solomon EI, Fukuzumi S and Karlin KD:
Peroxo and superoxo moieties bound to copper ion: Electron-transfer
equilibrium with a small reorganization energy. J Am Chem Soc.
138:7055–7066. 2016. View Article : Google Scholar : PubMed/NCBI
|