Introduction
Breast cancer is the most frequently diagnosed
cancer in women, accounting for ~25% of the total number of cancer
cases, and is one of the leading causes of female mortalities
worldwide (1). Breast cancer is
categorized based on clinicopathological features and molecular
signatures. Estrogen receptor (ER), progesterone receptor (PR) and
human epidermal growth factor receptor 2 (HER2) serve as key
biomarkers in breast cancer, and their expression in tumor cells
forms the basis for endocrine therapy, targeted therapy and disease
prognosis.
Phosphatidylinositol-4,5-bisphosphate 3-kinase
catalytic subunit α (PIK3CA), which is located on chromosome
3q26.32, encodes the catalytic subunit p110α of class IA
phosphoinositide 3-kinase (PI3K). PIK3CA is one of the most
commonly mutated oncogenes in several types of human cancer,
including breast, colon and endometrial cancer (2). It serves a major role in downstream
signaling of receptor tyrosine kinases (RTKs), and is therefore
critical in the regulation of cell proliferation, growth,
differentiation, migration and survival (2,3). In
2004, somatic mutations in the PIK3CA gene were reported for
the first time in solid tumors (3).
Although the role of PIK3CA mutations in diagnosis and
disease progression has been studied extensively (4–15), there
is no consensus on the use of PIK3CA as a biomarker.
A previous study by Samuels et al (3) reported that the frequency of
PIK3CA mutations in breast cancer was 8%, while further
studies revealed that approximately 21–34% of breast cancer cases
presented PIK3CA mutations (2,16,17). It
has been reported that PIK3CA somatic mutations are
clustered within exons 9 and 20, which correspond to the helical
and kinase domains, respectively (2,18,19), and
are gain-of-function mutations with a transforming capacity
(20). In addition, mutations in
exon 9 (helical domain) are resistant to the inhibitory effect of
p85 through Src-homology 2 domain. A higher level of mutations in
the kinase domain (14.6%) compared with the helical domain was
reported in a large cohort with predominantly lymph node-positive
breast cancer (21).
Among Saudi women with cancer, the incidence of
breast cancer ranges between 18 and 34.8%, with the lowest
frequency observed in the southwestern region (18%) and the highest
frequency in the eastern region (34.8%). The highest incidence of
breast cancer was observed in women aged between 30–44 years
(22). Therefore, the objective of
the present study was to evaluate the PIK3CA hotspot
mutations in breast cancer patients in Saudi Arabia and in subsets
of breast cancer cases based on hormone receptor expression.
Materials and methods
Study population and
histopathology
This retrospective study included a total of 118
breast tumor samples from 118 women who were histopathologically
diagnosed with breast cancer (23)
at King Fahd Hospital of the University (Al-Khobar, Saudi Arabia).
The inclusion criteria were: i) Confirmed breast cancer diagnosis
by histopathology analysis; and ii) patient age, 18–80 years. The
tumor samples were collected between May 2005 and February 2014,
and the mean age of the patients was 50.26±11.36 years. All
patients were from the eastern province of Saudi Arabia. The
formalin-fixed paraffin-embedded (FFPE) tissue specimens were
subjected to hematoxylin and eosin staining to examine the tumor
cell content and reconfirm the diagnosis. FFPE specimens containing
>70% of tumor cells were selected for further analysis. The
clinicopathological parameters and expression pattern of ER, PR and
HER2 determined by immunohistochemistry were obtained from the
medical records of the patients. Patient outcome was estimated
according to the status of 12 parameters, including local
recurrence, absence of ER and PR expression, grade 3 tumor, stage
III and IV tumors, Ki67 expression (>50%), lymph node
positivity, visceral metastasis, lympho-vascular invasion, age
(<50 years), tumor size (>5 cm) and HER2 positivity. The
prognosis of patients exhibiting ≥8 of the aforementioned
characteristics was considered to be poor, while patients with
<8 of these characteristics were considered to have good
prognosis. Based on the ER, PR and HER2 expression pattern of the
tumor tissues the patient cohort was divided into 26 groups,
including the ER+, ER−, PR+,
PR−, HER2+, HER2−,
ER−PR−, ER+PR+,
ER−PR+, ER+PR−,
ER−HER2−, ER+HER2+,
ER−HER2+, ER+HER2−,
PR−HER2−, PR+HER2+,
PR−HER2+, PR+HER2−,
ER−PR−HER2−,
ER−PR+HER2−,
ER+PR+HER2−,
ER+PR+HER2+,
ER+PR−HER2+,
ER+PR−HER2−,
ER−PR−HER2+ and
ER−PR+HER2+ groups.
The current study was approved by the Institutional
Review Board of Imam Abdulrahman Bin Faisal University (approval
no. IRB-2014-01-007; Dammam, Saudi Arabia). Written informed
consent was obtained from patients for the use of FFPE specimens
for research purposes.
Detection of PIK3CA mutations by
direct sequencing
The FFPE tissue specimens were subjected to
microtomy to obtain 5-µm sections. Six sections from each specimen
were utilized for DNA isolation using the QIAmp DNA FFPE tissue kit
(Qiagen GmbH, Hilden, Germany) as per the manufacturer's protocol.
The purified DNA was checked for DNA quantity and quality by
Nanodrop spectrophotometer (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Mutations in exons 9 and 20 of PIK3CA
were detected by polymerase chain reaction (PCR) in a total volume
of 25.0 µl, containing 1.25 U GoTaq polymerase (Promega
Corporation, Madison, WI, USA), 0.2 mM of each dNTP, 1.5 mM
MgCl2, 1X Taq buffer, 20 pM of each primer (exon 9
forward, 5′-CCAGAGGGGAAAAATATGACA-3′; exon 9 reverse,
5′-CATTTTAGCACTTACCTGTGAC-3′; exon 20 forward,
5′-CATTTGCTCCAAACTGACCA-3′; and exon 20 reverse,
5′-TGAGCTTTCATTTTCTCAGTTATCTTTTC-3′) and 50 ng DNA. The
thermocycling conditions were as follows: 1 cycle of 5 min at 95°C;
40 cycles of 30 sec at 95°C, 30 sec at 54°C, and 30 sec at 72°C;
and 1 cycle of 5 min at 72°C. Subsequently, the PCR products were
analyzed on a 2% agarose gel, which indicated the expected amplicon
for exon 9 of 195 bp and for exon 20 of 338 bp (24). The sequence analysis was performed
using the CodonCode aligner software (CodonCode Corporation,
Centerville, MA, USA). The NG_012113.2 sequence (National Center
for Biotechnology Information, Bethesda, MD, USA) was used as a
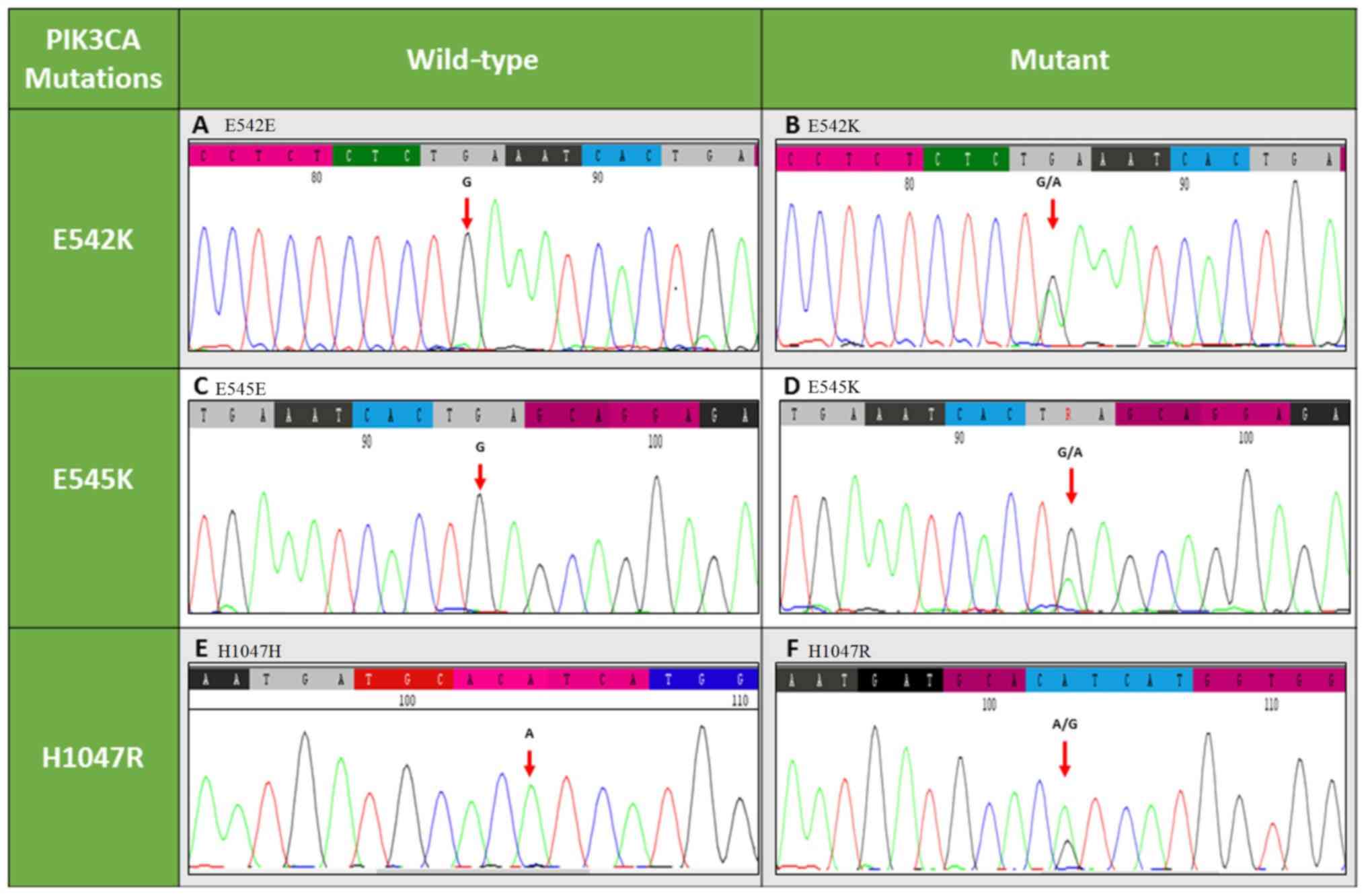
reference sequence. The helical and kinase mutations E542K, E545K
and H1047R are represented in Fig.
1.
Detection of PIK3CA mutations by
competitive allele-specific TaqMan (CAST) PCR
The mutation results were confirmed using CAST PCR
technology with the ABI 7500 Real-time PCR thermocycler (Thermo
Fisher Scientific, Inc.). A total of three TaqMan mutation
detection assays (Thermo Fisher Scientific, Inc.) for E542K (assay
ID, Hs00000822_mu; cat. no. 4465804), E545K (assay ID,
Hs00000824_mu; cat. no. 4465804) and H1047R (assay ID,
Hs00000831_mu; cat. no. 4465804) were performed using quantitative
PCR as per the manufacturer's protocol. Briefly, 1X TaqMan
genotyping master mix, locus specific TaqMan FAM dye labeled
mutation assay and 50 ng DNA were mixed, made up to 20 µl final
volume in a 96-well plate, and subjected to quantitative PCR on the
ABI7500 Real-time PCR system using the following thermocycling
conditions: 95°C for 10 min, followed by five cycles at 92°C for 15
sec and 58°C for 1 min, and forty cycles at 92°C for 15 sec and
60°C for 1 min. Simultaneously, reference PIK3CA was
amplified using a reference assay probe. Mutation Detector™
software (version 2.0; Thermo Fisher Scientific, Inc.) was used to
classify the mutation status. The tumor samples that did not
exhibit these three mutations were used as negative control samples
in CAST PCR.
Statistical analysis
The frequency of each mutation was confirmed by
direct counting. Fisher's exact test was used to determine the
significance of the association among the groups. A statistically
significant difference was determined if the P-value was <0.05.
SPSS version 19 (IBM Corp., Armonk, NY, USA) software was used to
perform the Fisher's exact test. Survival analysis was performed
using Kaplan-Meier curve analysis and log-rank test.
Results
Clinical and histopathological
characteristics
The mean age of the patient population was
50.26±11.36 years. Histopathological analysis revealed that the
majority of the patients presented with grade 2 and stage 2 breast
cancer, and the patient prognosis was ascertained based on 12
parameters, as described earlier (≥8 parameters indicated poor, and
<8 parameters indicated good prognosis; Table I). Ductal carcinoma was observed in
95.76% of the patients, whereas only 4.2% of the patients presented
with lobular carcinoma. Based on ER, PR and HER2 expression levels,
it was determined that 62.7% of the cases exhibited high ER
expression and 50% exhibited low PR expression, while 74.6% of
patients were HER2−. In the patient cohort, 45% of cases
were ER+/PR+, while 64.4% were
ER−/PR−. The lowest frequency of two marker
positive expression was seen in the PR+/HER2+
and ER+/HER2+ groups at 7.6 and 9.3%
respectively. When the patients were classified into the different
marker groups based on ER, PR and HER2 expression, the majority of
the cases were assigned to the ER+/PR+ and
HER2− group (39%), with the
ER−/PR+ and HER2+ group exhibiting
the lowest frequency (1.7%).
 | Table I.Association of baseline
characteristics with the PIK3CA helical and kinase mutation
status. |
Table I.
Association of baseline
characteristics with the PIK3CA helical and kinase mutation
status.
|
|
| All cases, n | PIK3CA
helical, n | PIK3CA
kinase, n |
|---|
|
|
|
|
|
|
|---|
| Category | Subjects, n
(%) | PIK3CA
mutation | No PIK3CA
mutation | P-value | Mutation | No mutation | P-value | Mutation | No mutation | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
≤50 | 66 (55.9) | 15 | 51 | 0.820 | 4 | 62 | >0.999 | 11 | 55 | 0.797 |
|
>50 | 52 (44.1) | 10 | 42 |
| 3 | 49 |
| 7 | 45 |
|
| Histological
grade |
|
|
|
|
|
|
|
|
|
|
| Grade
1 | 14 (11.86) | 1 | 13 |
| 0 | 14 |
| 1 | 13 |
|
| Grade
2 | 69 (58.47) | 15 | 54 | 0.285 | 4 | 65 | >0.999 | 11 | 58 | 0.680 |
| Grade
3 | 35 (29.66) | 9 | 26 | 0.243 | 3 | 32 | 0.547 | 6 | 29 | 0.656 |
| Histological
stage |
|
|
|
|
|
|
|
|
|
|
| Stage
1 | 14 (11.86) | 2 | 12 |
| 1 | 13 |
| 1 | 13 |
|
| Stage
2 | 58 (49.15) | 12 | 46 | 0.722 | 2 | 56 | 0.482 | 10 | 48 | 0.679 |
| Stage
3 | 34 (28.81) | 9 | 25 | 0.469 | 3 | 31 | >0.999 | 6 | 28 | 0.656 |
| Stage
4 | 12 (10.17) | 2 | 10 | >0.999 | 1 | 11 | >0.999 | 1 | 11 | 1 |
| Prognosis |
|
|
|
|
|
|
|
|
|
|
|
Good | 63 (53.39) | 8 | 55 | 0.023 | 2 | 61 | 0.248 | 6 | 57 | 0.076 |
|
Poor | 55 (46.61) | 17 | 38 |
| 5 | 50 |
| 12 | 43 |
|
PIK3CA helical and kinase domain
mutations
Three mutations, namely E542K and E545K within exon
9, and H1047R within exon 20, were observed in 25 of the 118
patients (21.2%; Table I). Among
these 25 patients, 3 carried the E542K helical domain mutation,
while 4 carried the E545K helical domain mutation (data not shown).
The remaining 18 patients carried the H1047R mutation of the kinase
domain (Table I). This indicated
that the frequency of the kinase domain mutation was significantly
greater when compared with the helical domain mutation (P=0.019;
data not shown). The patients were then classified into two groups
based on good prognosis (53%) or poor prognosis (46.6%).
Accordingly, it was observed that the PIK3CA mutation was
significantly associated with poor prognosis (P=0.023).
Furthermore, once the patient cohort was stratified based on the
expression of the three receptors (ER, PR and HER2), PIK3CA
mutations were associated with the ER+/PR+
group of tumors in contrast to the ER−/PR−
group (P=0.021).
The helical and kinase domain mutations were
independently analyzed to determine the association with
histopathological and prognostic indicators. This analysis revealed
that there was no independent association of the indicators,
whereas the PIK3CA mutation was overall associated with a
poor prognosis. A significant association was identified between
the ER+/PR+ subgroup and the H1047R kinase
domain mutation of PIK3CA (P=0.038); however, no association
with the helical domain mutations was identified (Table II).
 | Table II.Hormone receptor subsets, and the
PIK3CA helical and kinase mutation status. |
Table II.
Hormone receptor subsets, and the
PIK3CA helical and kinase mutation status.
|
| All cases, n | PIK3CA
helical, n | PIK3CA
kinase, n |
|
|
|
|
|
| Category | PIK3CA
mutation | No PIK3CA
mutation | P-value | Mutation | No mutation | P-value | Mutation | No mutation | P-value |
|---|
| ER+ | 17 | 57 |
| 4 | 70 |
| 13 | 61 |
|
| ER− | 8 | 36 | 0.644 | 3 | 41 | 0.711 | 5 | 39 | 0.435 |
| PR+ | 14 | 45 |
| 4 | 55 |
| 10 | 49 |
|
| PR− | 11 | 48 | 0.652 | 2 | 57 | 0.679 | 8 | 51 | 0.608 |
|
HER2+ | 6 | 24 |
| 3 | 27 |
| 3 | 27 |
|
|
HER2− | 19 | 69 | >0.999 | 4 | 84 | 0.368 | 15 | 73 | 0.556 |
|
ER−PR− | 6 | 70 |
| 2 | 74 |
| 4 | 72 |
|
|
ER+PR+ | 12 | 41 | 0.021 | 3 | 50 | 0.401 | 9 | 44 | 0.038 |
|
ER−PR+ | 6 | 32 | 0.210 | 2 | 36 | 0.599 | 4 | 34 | 0.437 |
|
ER+PR− | 5 | 16 | 0.056 | 1 | 20 | 0.523 | 4 | 17 | 0.064 |
|
ER−HER2− | 5 | 20 |
| 1 | 24 |
| 4 | 21 |
|
|
ER+HER2+ | 3 | 8 | 0.678 | 1 | 10 | 0.523 | 2 | 9 | >0.999 |
|
ER−HER2+ | 3 | 16 | >0.999 | 2 | 17 | 0.569 | 1 | 18 | 0.370 |
|
ER+HER2− | 14 | 49 | >0.999 | 3 | 60 | >0.999 | 11 | 52 | >0.999 |
|
PR−HER2− | 7 | 31 |
| 2 | 36 |
| 5 | 33 |
|
|
PR+HER2+ | 2 | 7 | >0.999 | 2 | 7 | 0.160 | 0 | 9 | 0.566 |
|
PR−HER2+ | 4 | 17 | >0.999 | 1 | 20 | >0.999 | 4 | 17 | 0.707 |
|
PR+HER2− | 12 | 38 | 0.607 | 2 | 48 | >0.999 | 10 | 40 | 0.568 |
|
ER−PR−HER2− | 4 | 17 |
| 1 | 20 |
| 3 | 18 |
|
|
ER−PR+HER2− | 1 | 3 | >0.999 | 0 | 4 | >0.999 | 1 | 3 | 0.526 |
|
ER+PR+HER2− | 11 | 35 | 0.760 | 2 | 44 | >0.999 | 9 | 37 | 0.739 |
|
ER+PR+HER2+ | 1 | 6 | >0.999 | 1 | 6 | 0.444 | 0 | 7 | 0.551 |
|
ER+PR−HER2+ | 2 | 2 | 0.234 | 0 | 4 | >0.999 | 2 | 2 | 0.166 |
|
ER+PR−HER2− | 3 | 14 | >0.999 | 1 | 16 | >0.999 | 2 | 15 | >0.999 |
|
ER−PR−HER2+ | 2 | 15 | 0.672 | 1 | 16 | >0.999 | 1 | 16 | 0.613 |
|
ER−PR+HER2+ | 1 | 1 | 0.395 | 1 | 1 | 0.170 | 0 | 2 | >0.999 |
Discussion
RTK signaling induces the activation of
Ras/mitogen-activated protein kinase and the Ras/PI3K/protein
kinase B (AKT) signaling pathways, resulting in increased
proliferation, survival and metastasis of cancer cells. Somatic
mutations in RTKs, Ras, B-Raf, PI3K and AKT are commonly observed
in various cancer types (25,26).
Thus, research has focused on the genetic alterations in the genes
involved in these pathways for use in targeted therapy. Samuels
et al (3) identified
PIK3CA gene mutations in a small study cohort and suggested
that these mutations may be involved in the development of cancer.
These mutations were primarily concentrated in the helical, kinase
and p85 binding domains. Subsequent studies in different population
groups revealed that the frequency of these mutations ranged
between 21.3 and 34.7% (2,16). A study by Karakas et al
(27) conducted on a central Saudi
Arabian population reported the frequency of these mutations to be
26%. The present study indicated a frequency of 21.2% for these
mutations in breast cancer patients in the eastern province of
Saudi Arabia, which was comparable with the findings of Karakas
et al (27). Furthermore, the
current study revealed that the frequency of mutations in exons 9
and 20 was 5.9 and 15.25%, respectively. However, none of the
patients included in the current study presented with these two
mutations simultaneously. These results are in line with previously
reported studies in other population groups (2,16,28,29).
According to the current study data, it can be
suggested that PIK3CA mutations are observed in similar
percentages worldwide. However, the higher frequency of kinase
domain mutations in the present study, particularly H1047R,
suggested the need for targeted therapy to inhibit PIK3CA
activity via the H1047R mutation. A number of studies have
investigated the association between these mutations and
clinicopathological parameters, including hormone receptor
expression, stage, grade, metastases and prognosis of breast cancer
(4–8). A previous meta-analysis comprising 32
different studies reported that ER and PR expression levels were
significantly correlated with PIK3CA mutations
(P<0.00001) (4).
HER2-overexpressing breast tumors were correlated with a high
PIK3CA mutation rate (5). A
study by Li et al (6)
confirmed that there was a correlation between PIK3CA
mutations, and the ER and PR overexpression in large tumors. The
present study results were in line with these aforementioned
studies, and reinforced the correlation of ER and PR overexpression
with the high frequency of PIK3CA mutations (P=0.021).
However, ER and PR overexpression were significantly correlated
with the kinase domain mutation (H1047R; P=0.038) rather than the
helical domain mutations (P=0.401). It has also been reported that
ER+ tumors were associated with a higher frequency of
PIK3CA mutations and that ER could be activated in the
absence of estrogen, which causes tamoxifen resistance (7). Furthermore, a study by Ellis et
al (8) demonstrated that tumors
with PIK3CA mutations did not respond to neoadjuvant
endocrine therapy as compared with tumors without PIK3CA
mutations.
Several retrospective and prospective studies have
evaluated the prognostic and predictive value of PIK3CA
mutations in breast cancer tumors. However, these studies reached
contradictory conclusions, with certain studies demonstrating the
association of PIK3CA mutations with poor survival (9,10), while
others indicating that PIK3CA mutations was associated with
a better survival rate (11,12). In addition, a study by Barbareschi
et al (13) reported that
only exon 9 mutations were associated with poor survival.
Furthermore, a meta-analysis comprising seven studies revealed that
PIK3CA mutations have no prognostic impact (4). In this previous meta-analysis, the
prognosis was defined by overall survival and progression-free
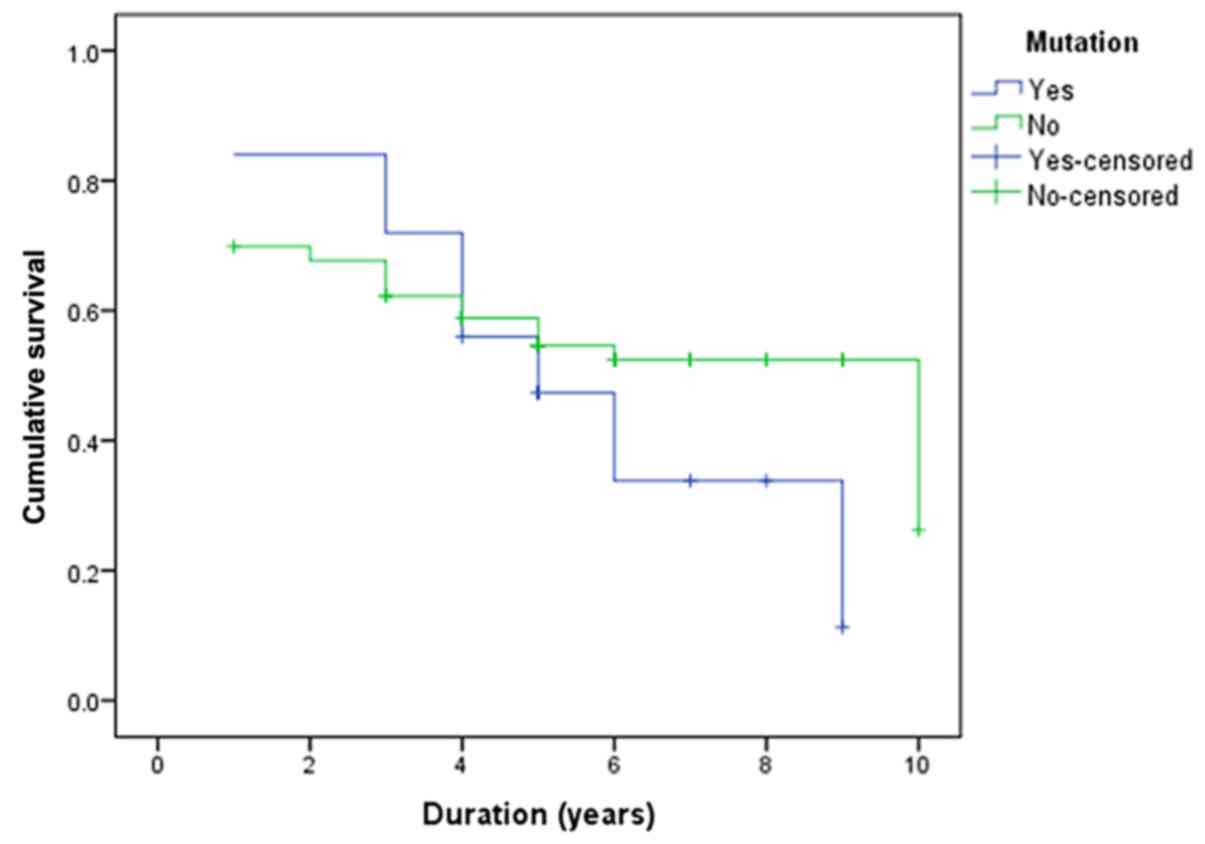
survival. Similarly, in the present study, the PIK3CA
mutations did not yield a significant impact on overall survival
(P=0.248; Fig. 2). However, sub-data
analysis based on 12 parameters indicated that the PIK3CA
mutations within exons 9 and 20 were associated with poor prognosis
(P=0.023). Several studies revealed variation in the significant
correlation between PIK3CA mutation and overall survival
(9–13), while the data of the present study
indicated no significant impact. The possible reasons may include
the different exonic mutations that may impact different
mechanisms, the breast cancer-specific effect of PIK3CA
mutations (4), and the impact of
treatment, which greatly varies subsequent to recurrence (17).
Recent clinical and experimental studies suggested
that PI3K pathway activation may negatively affect the response of
breast cancer patients to trastuzumab, a monoclonal therapy drug
(2). This conclusion emphasized the
need to assess the PIK3CA mutation status following
trastuzumab therapy for breast cancer in order to predict disease
progression (14,15). Currently, only one drug that targets
the PI3K pathway is available for breast cancer treatment, namely
everolimus, which is an mTOR inhibitor. PIK3CA mutations may
provide an opportunity to develop novel drugs that target altered
PIK3CA, or combination therapy based on the current regimen,
which may yield the maximum effect on breast tumors. Therefore,
determining the PIK3CA mutation status has valuable clinical
relevance in terms of disease prediction and prognosis.
Different detection methods exist to identify
mutations in a gene. The standard method is direct sequencing,
which identifies existing and de novo mutations, unlike
amplification-refractory mutation system PCR or quantitative
PCR-based methods (30). All these
methods have their respective sensitivity in detecting the mutation
status. The vast majority of studies have employed Sanger
sequencing to determine PIK3CA mutations (4). Based on the meta-analysis conducted by
Pang et al (4) on
PIK3CA mutations in breast cancer, 18 out of 26 studies
determined the PIK3CA sequence by Sanger sequencing. Another
study by Papaxoinis et al (21) reported a good concordance between
direct sequencing and next-generation sequencing methods. The
present study employed direct sequencing and a TaqMan mutation
detection assay by CAST PCR. Initially, all the samples were
subjected to direct sequencing, followed by further validation of
samples with PIK3CA mutations using CAST PCR. All
PIK3CA mutation positive samples and 10% of the
PIK3CA mutation negative samples assessed using CAST PCR
recorded 100% concordance with direct sequencing results. Thus,
both Sanger sequencing and CAST PCR assays can be used to detect
PIK3CA mutations. However, CAST PCR may be a preferred
method due to high detection sensitivity, low cost and time
efficiency.
In conclusion, the present study emphasized that
PIK3CA mutations may serve as important biomarkers for
breast cancer classification and for targeted therapies using
PIK3CA inhibitors.
Acknowledgements
The authors would like to thank Mr. Geoffrey James
Tam Moro, Mr. Florentino Mata Jr and Mr. Mohammed H. Al-Shamlan
(Imam Abdulrahman Bin Faisal University) for their technical
support.
Funding
The current study was supported by The Deanship of
Scientific Research, Imam Abdulrahman Bin Faisal University (grant
nos. 2014048 and 2016-090-IRMC).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CV and AMAA conceived and designed the study. AMAA,
AAl, AAh, MSA, NFA and HAW collected the samples and provided the
clinical data. CV, CC and SC conducted experiments. CV and NJ
performed statistical analysis. CV drafted the manuscript. AAZ and
AKAA conceived, designed the study and revised the manuscript for
important intellectual content. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Institutional
Review Board of Imam Abdulrahman Bin Faisal University, Dammam,
Saudi Arabia. Written informed consent was obtained from the
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ghoncheh M, Pournamdar Z and Salehiniya H:
Incidence and mortality and epidemiology of breast cancer in the
world. Asian Pac J Cancer Prev. 17:43–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cizkova M, Dujaric ME, Lehmann-Che J,
Scott V, Tembo O, Asselain B, Pierga JY, Marty M, de Cremoux P,
Spyratos F and Bieche I: Outcome impact of PIK3CA mutations in
HER2-positive breast cancer patients treated with trastuzumab. Br J
Cancer. 108:1807–1809. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Samuels Y, Wang Z, Bardelli A, Silliman N,
Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al:
High frequency of mutations of the PIK3CA gene in human cancers.
Science. 304:5542004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pang B, Cheng S, Sun SP, An C, Liu ZY,
Feng X and Liu GJ: Prognostic role of PIK3CA mutations and their
association with hormone receptor expression in breast cancer: A
meta-analysis. Sci Rep. 4:62552014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stemke-Hale K, Gonzalez-Angulo AM, Lluch
A, Neve RM, Kuo WL, Davies M, Carey M, Hu Z, Guan Y, Sahin A, et
al: An integrative genomic and proteomic analysis of PIK3CA, PTEN,
and AKT mutations in breast cancer. Cancer Res. 68:6084–6091. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li SY, Rong M, Grieu F and Iacopetta B:
PIK3CA mutations in breast cancer are associated with poor outcome.
Breast Cancer Res Treat. 96:91–95. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Campbell RA, Bhat-Nakshatri P, Patel NM,
Constantinidou D, Ali S and Nakshatri H: Phosphatidylinositol
3-kinase/AKT-mediated activation of estrogen receptor alpha: A new
model for anti-estrogen resistance. J Biol Chem. 276:9817–9824.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ellis MJ, Lin L, Crowder R, Tao Y, Hoog J,
Snider J, Davies S, DeSchryver K, Evans DB, Steinseifer J, et al:
Phosphatidyl-inositol-3-kinase alpha catalytic subunit mutation and
response to neoadjuvant endocrine therapy for estrogen receptor
positive breast cancer. Breast Cancer Res Treat. 119:379–390. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lai YL, Mau BL, Cheng WH, Chen HM, Chiu HH
and Tzen CY: PIK3CA exon 20 mutation is independently associated
with a poor prognosis in breast cancer patients. Ann Surg Oncol.
15:1064–1069. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lerma E, Catasus L, Gallardo A, Peiro G,
Alonso C, Aranda I, Barnadas A and Prat J: Exon 20 PIK3CA mutations
decreases survival in aggressive (HER-2 positive) breast
carcinomas. Virchows Arch. 453:133–139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maruyama N, Miyoshi Y, Taguchi T, Tamaki
Y, Monden M and Noguchi S: Clinicopathologic analysis of breast
cancers with PIK3CA mutations in Japanese women. Clin Cancer Res.
13:408–414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pérez-Tenorio G, Alkhori L, Olsson B,
Waltersson MA, Nordenskjöld B, Rutqvist LE, Skoog L and Stål O:
PIK3CA mutations and PTEN loss correlate with similar prognostic
factors and are not mutually exclusive in breast cancer. Clin
Cancer Res. 13:3577–3584. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barbareschi M, Buttitta F, Felicioni L,
Cotrupi S, Barassi F, Del Grammastro M, Ferro A, Dalla Palma P,
Galligioni E and Marchetti A: Different prognostic roles of
mutations in the helical and kinase domains of the PIK3CA gene in
breast carcinomas. Clin Cancer Res. 13:6064–6069. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ligresti G, Militello L, Steelman LS,
Cavallaro A, Basile F, Nicoletti F, Stivala F, McCubrey JA and
Libra M: PIK3CA mutations in human solid tumors: Role in
sensitivity to various therapeutic approaches. Cell Cycle.
8:1352–1358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jensen JD, Knoop A, Laenkholm AV,
Grauslund M, Jensen MB, Santoni-Rugiu E, Andersson M and Ewertz M:
PIK3CA mutations, PTEN, and pHER2 expression and impact on outcome
in HER2-positive early-stage breast cancer patients treated with
adjuvant chemotherapy and trastuzumab. Ann Oncol. 23:2034–2042.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hashimoto K, Tsuda H, Koizumi F, Shimizu
C, Yonemori K, Ando M, Kodaira M, Yunokawa M, Fujiwara Y and Tamura
K: Activated PI3K/AKT and MAPK pathways are potential good
prognostic markers in node-positive, triple-negative breast cancer.
Ann Oncol. 25:1973–1979. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mukohara T: PI3K mutations in breast
cancer: Prognostic and therapeutic implications. Breast Cancer
(Dove Med Press). 7:111–123. 2015.PubMed/NCBI
|
|
18
|
Saal LH, Holm K, Maurer M, Memeo L, Su T,
Wang X, Yu JS, Malmström PO, Mansukhani M, Enoksson J, et al:
PIK3CA mutations correlate with hormone receptors, node metastasis,
and ERBB2, and are mutually exclusive with PTEN loss in human
breast carcinoma. Cancer Res. 65:2554–2559. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mankoo PK, Sukumar S and Karchin R: PIK3CA
somatic mutations in breast cancer: Mechanistic insights from
Langevin dynamics simulations. Proteins. 75:499–508. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao JJ, Liu Z, Wang L, Shin E, Loda MF
and Roberts TM: The oncogenic properties of mutant p110alpha and
p110beta phosphatidylinositol 3-kinases in human mammary epithelial
cells. Proc Natl Acad Sci USA. 102:18443–18448. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Papaxoinis G, Kotoula V, Alexopoulou Z,
Kalogeras KT, Zagouri F, Timotheadou E, Gogas H, Pentheroudakis G,
Christodoulou C, Koutras A, et al: Significance of PIK3CA mutations
in patients with early breast cancer treated with adjuvant
chemotherapy: A Hellenic Cooperative Oncology Group (HeCOG) study.
PLoS One. 10:e01402932015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cancer Incidence report Saudi Arabia 2014,
. http://www.chs.gov.sa/En/HealthRecords/CancerRegistry/Pages/CancerRegistryRecords.aspx2014
|
|
23
|
Zhang BN, Cao XC, Chen JY, Chen J, Fu L,
Hu XC, Jiang ZF, Li HY, Liao N, Liu DG, et al: Guidelines on the
diagnosis and treatment of breast cancer (2011 edition). Gland
Surg. 1:39–61. 2012.PubMed/NCBI
|
|
24
|
Al-Amri AM, Vatte C, Cyrus C, Chathoth S,
Hashim TM, Mohamed YS, Al Ali R, Alsaid A and Al Ali A: Novel
mutations of PIK3CA gene in head and neck squamous cell carcinoma.
Cancer Biomark. 16:377–383. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Regad T: Targeting RTK signaling pathways
in cancer. Cancers (Basel). 7:1758–1784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vatte C, Al Amri AM, Cyrus C, Chathoth S,
Acharya S, Hashim TM, Al Ali Z, Alshreadah ST, Alsayyah A and
Al-Ali AK: Tyrosine kinase domain mutations of EGFR gene in head
and neck squamous cell carcinoma. Onco Targets Ther. 10:1527–1533.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Karakas B, Colak D, Kaya N, Ghebeh H,
Al-Qasem A, Hendrayani F, Toulimat M, Al-Tweigeri T, Park BH and
Aboussekhra A: Prevalence of PIK3CA mutations and the SNP
rs17849079 in Arab breast cancer patients. Cancer Biol Ther.
14:888–896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beelen K, Opdam M, Severson TM, Koornstra
RH, Vincent AD, Wesseling J, Muris JJ, Berns EM, Vermorken JB, van
Diest PJ and Linn SC: PIK3CA mutations, phosphatase and tensin
homolog, human epidermal growth factor receptor 2, and insulin-like
growth factor 1 receptor and adjuvant tamoxifen resistance in
postmenopausal breast cancer patients. Breast Cancer Res.
16:R132014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abramson VG, Cooper Lloyd M, Ballinger T,
Sanders ME, Du L, Lai D, Su Z, Mayer I, Levy M, LaFrance DR, et al:
Characterization of breast cancers with PI3K mutations in an
academic practice setting using SNaPshot profiling. Breast Cancer
Res Treat. 145:389–399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Diekstra A, Bosgoed E, Rikken A, van Lier
B, Kamsteeg EJ, Tychon M, Derks RC, van Soest RA, Mensenkamp AR,
Scheffer H, et al: Translating sanger-based routine DNA diagnostics
into generic massive parallel ion semiconductor sequencing. Clin
Chem. 61:154–162. 2015. View Article : Google Scholar : PubMed/NCBI
|