Introduction
Cervical cancer is a malignant tumor and its
incidence is second only to breast cancer. There is a gradual trend
for younger age, seriously threatening life and health of females
around the world and causing serious economic burden on families
and society (1,2). There are 528,000 new patients with
cervical cancer worldwide each year with 266,000 deaths, exceeding
any other gynecologic tumor (3,4).
According to reports in the literature, the mortality of cervical
cancer is the eighth in malignant tumors in China. Compared to the
1970s, the mortality has decreased in the past 10 years, but the
incidence in young females has increased. In addition, the
mortality is still high in rural areas (5).
Hypoxia-inducible factor-1α (HIF-1α), a subunit of
HIF-1 that regulates angiogenesis, and the growth, metastasis and
apoptosis of tumors, promotes angiogenesis by regulating vascular
endothelial growth factor (VEGF). It also improves oxygen carrying
capacity, and maintains cell oxygen stability in hypoxic tissues
and tolerance to hypoxia (6,7). The high expression of HIF-1α regulates
cellular energy metabolism and promotes tumor angiogenesis
(8). VEGF is by far the most potent
pro-angiogenic factor, which is synthesized and secreted by various
tumor cells (9). Stimulating the
growth of endothelial cells to enhance vascular permeability, it
promotes the expression of various cathepsins, which degrades
extracellular matrix and promotes tumor angiogenesis (10,11).
At present, advanced cervical cancer is mainly
treated by operation, radiotherapy and chemotherapy in clinical
practice (12). Patients with stage
II B or above are mainly comprehensively treated based on
radiotherapy, with a five-year disease-free survival rate of
approximately 67% and greater side effects (13,14).
Even if radiotherapy technology and equipment are constantly
updated and improved, the growth of primary tumors still cannot be
effectively controlled (15).
Therefore, it is of great significance to seek targeted drugs for
treating cervical cancer. Astragalus membranaceus is a
traditional Chinese medicine with invigorating qi and
diuretic efficacy, detoxification and myogenic efficacy (16). As a phytoestrogen, one of main
components of astragalus membranaceus, formononetin has the
effect of regulating estrogen, metabolism, inflammation, and
lowering blood pressure (17–19).
Currently, studies have shown that it can inhibit bladder cancer
and breast cancer (20,21).
In this study, the effects and significance of
formononetin on the expression levels of HIF-1α and VEGF in mouse
cervical cancer tissue were investigated, to provide references for
clinical use.
Materials and methods
Subjects, grouping and modeling
A total of 45 healthy, female, Balb/c nude mice were
selected, aged 6–8 weeks and weighing 15–20 g. They were purchased
from Changzhou Carvins Laboratory Animal Co., Ltd. (Changzhou,
China) with an animal certificate number of SCXK (Su) 2011–0003.
They were reared in cages at room temperature between 23 and 25°C
with a humidity of 55–62%, 12/12 light cycles, free access to food
and drink ad libitum. Modeling experiments were performed
after 1 week of adaptive feeding in all mice.
This study was approved by the Ethics Committee of
Wuxi People's Hospital Affiliated to Nanjing Medical University
(Wuxi, China). Patients who participated in this research had
complete clinical data. Signed informed consents were obtained from
the patients or the guardians.
Cervical cancer HeLa cells (item no. C015; Nanjing
Laifusai Biotechnology Co., Ltd., Nanjing, China) were digested
with trypsin (item no. EB04590; Shanghai Shifeng Biotechnology Co.,
Ltd., Shanghai, China) and prepared into single cell bacterial
suspension with a serum-free medium, at a concentration of
1×108/ml. Then, 0.3 ml of HeLa cell suspension was
inoculated into the subcutaneous position of the left side of the
armpit of the nude mouse near the back, and the tumor growth was
recorded. Tumor volume = (longest diameter of the tumor) ×
(shortest diameter)2 × 0.5 (22). On the 6th day of inoculation, 40 nude
mice were tumorigenic. The tumor diameter exceeding 2.0 mm
indicated successful modeling. Among the remaining 5 nude mice, 1
mouse was not tumorigenic, and the remaining 4 mice had tumor
diameters <2.0 mm.
The 40 mice successfully modeled were randomly
divided in formononetin group (n=15), cisplatin group (n=15) and
positive control group (n=10). Mice in positive control group were
administered with 0.1 ml of 0.9% saline (item no. PB180353; Wuhan
Bafeier Biotechnology Service Co., Ltd., Wuhan, China) once a day.
Mice in cisplatin group were intraperitoneally administered (3
mg/kg cisplatin dissolved in 0.9% saline) with 0.1 ml of cisplatin
(item no. RB187; Shanghai Guangrui Biotechnology Co., Ltd.,
Shanghai, China) solution, once every 7 days after modeling. On the
other days, 0.1 ml of 0.9% saline was intraperitoneally injected
every day. Mice in formononetin group were intragastrically
administered (10 mg/kg formononetin dissolved in 0.9% saline) with
0.1 ml of formononetin (item no. JKM0063; Shanghai Jingke Chemical
Technology Co., Ltd., Shanghai, China) solution once a day. The
mice were observed during the medication intervention. All the mice
were sacrificed on the 31st day after the administration, and their
tumors were excised and weighed to calculate the tumor inhibition
rate. Tumor inhibition rate = (average tumor weight of mice in
positive control group - that in medication intervention
groups)/that in positive control group × 100%. At the same time,
their cancer tissues were obtained. The mRNA and protein expression
levels of HIF-1α and VEGF in tissues were detected.
RT-qPCR detection of mRNA expression levels of
HIF-1α and VEGF. First 100 mg of cervical cancer tissue was
obtained from mice, ground and pulverized, then 1 ml of TRIzol
lysate (Shanghai Pufei Biotechnology Co., Ltd., Shanghai, China)
was added to isolate total RNA from the tissues. After extraction,
1.5% agarose gel electrophoresis was used for analyzing RNA
integrity, a micronucleic acid determinator (Beijing Meilin
Hengtong Instrument Co., Ltd., Beijing, China) for detecting the
purity and concentration of the extracted RNA. A260/A280 value was
considered to meet experimental requirements between 1.8 and 2.0.
Then, 2 µg of the total RNA was taken, and a reverse transcription
kit (ReverAid TM First Strand cDNA synthesis kit, #k1622; Promega
Corporation, Madison, WI, USA) was used to synthesize cDNA.
Reaction system: 5ΧPrimerScript Buffer 2 μl, PrimerScript RT
enzyme mix 0.5 μl, Random 6 mers (100 µM) 0.5 μl,
Oligo dT Primer (50 µM) 0.5 μl, total RNA 2 μg, dd
H2O was added to 10 μl. Reaction conditions: 25°C
for 5 min, 42°C for 60 min, 70°C for 5 min. Products of 2 µl was
subjected to PCR cycle with SYBR-Green PCCR kit (Beyotime,
Shanghai, China), after pre-denaturation at 94°C for 3 min,
denaturation at 94°C for 45 sec, renaturation at 58°C for 30 sec,
extension at 72°C for 45 sec, for a total of 35 cycles, and then
extension at 72°C for 10 min after the cycles. β-actin was used as
a reaction internal reference. All the samples were determined 3
times. 2−ΔCq was used to calculate the mRNA expression
levels of HIF-1α and VEGF in normal tissues of mice in the control
group and in cancer tissues of mice in the formononetin, the
cisplatin and the positive control groups (23). Primer sequences are shown in Table I.
 | Table I.Primers for HIF-1α mRNA and VEGF mRNA
and internal reference sequences. |
Table I.
Primers for HIF-1α mRNA and VEGF mRNA
and internal reference sequences.
| Genes | Upstream primers | Downstream
primers |
|---|
| HIF-1α |
5′-TCACGAGGGGTTCCCGCCTCGCA-3′ |
5′-TGCGAGGCGGGAAACCCCTCGTGA-3′ |
| VEGF |
5′-GGATCCATGAACTTTCTGCT-3′ |
5′-GAATCCACCGCCTCGGCTTGTC-3′ |
| β-actin |
5′-CCAGCCTTCCTTCTTGGGTAT-3′ |
5′-TTGGCATAGAGGTCTTTACGG-3′ |
Western blotting for the detection of
protein expression levels of HIF-1α and VEGF
Cervical cancer tissue (100 mg) was obtained from
mice, ground and pulverized. An appropriate amount of RIPA lysate
(item no. EX6010-100 ml; Jinkelong Biotechnology Co., Ltd.,
Beijing, China) containing 100 mM PMSF was added, and shaken on ice
for 30 min to fully lyse the cells to extract total protein. Then
8% SDS-PAGE (item no. LM0014A; Shanghai Lianmai Bioengineering Co.,
Ltd., Shanghai, China) was used for electrophoresis separation.
PVDF (item no. 28416245; Suzhou Ruinuode Biotechnology Co., Ltd.,
Suzhou, China) was left at room temperature for 2 h after
transmembrane. Rabbit anti mice HIF-1α monoclonal antibody (cat.
no. 36169; dil, 1:700) and VEGF primary antibody (cat. no. 2479;
dil, 1:1,000) both from Cell Signaling Technology, Inc., (Danvers,
MA, USA) were respectively added, shaken for 30 min and incubated
at 4°C overnight. After the membrane was washed with TBST (item no.
P0233; Beyotime Institute of Biotechnology, Shanghai, China) 3
times, HRP-labeled secondary antibody dilution (cat. no. SA00001-2;
dil, 1:2,000; Wuhan Sanying Biotechnology, Wuhan, China) was added,
incubated at room temperature for 120 min and then exposed in the
dark. β-actin (cat. no. 8457; dil, 1:1,000; Cell Signaling
Technology, Inc.) was used as an internal reference. A
chemiluminescence imaging system (GelView 6000 Pro; Guangzhou
Yunxing Scientific Instrument Co., Ltd.) was used for gray-light
scanning, and photoshop software for analyzing the relative
expression of the protein, repeated 3 times.
Statistical analysis
SPSS17.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for analysis. Measurement data were expressed as
mean ± standard deviation, and tested by the t-test. One-way ANOVA
was used for comparison among groups, paired t-test for comparison
in the group. P<0.05 was considered to indicate a statistically
significant difference.
Results
Inhibition rates of formononetin and
cisplatin on cervical cancer tumors
During the medication intervention, mice in the
formononetin group had no obvious adverse reactions, and were in
good condition, whereas mice in the cisplatin group had poor
appetite, drooping spirits and decreased activity. There were
statistically significant differences in the tumor mass and volume
of mice among the cisplatin, the formononetin and the positive
control groups (P<0.001). Mice in the cisplatin and the
formononetin groups had significantly lower tumor mass and tumor
volume than those in the positive control group, with statistically
significant differences (P<0.05), but there was no significant
difference in those between the formononetin and the cisplatin
groups (P>0.05). The tumor inhibition rate of mice was 56.24% in
the cisplatin group, and 50.17% in the formononetin group (Table II).
 | Table II.Inhibition rates of formononetin and
cisplatin on cervical cancer. |
Table II.
Inhibition rates of formononetin and
cisplatin on cervical cancer.
| Groups | n | Tumor mass (g) | Tumor volume
(cm3) | Tumor inhibition rate
(%) |
|---|
| Positive control | 10 | 8.73±2.15 | 10.91±4.58 | 0.00 |
| Formononetin | 15 |
4.35±0.86a |
6.22±1.61a | 50.17 |
| Cisplatin | 15 |
3.82±0.73a |
5.76±1.26a | 56.24 |
| F |
| 51.040 | 13.700 |
|
| P-value |
| <0.001 | <0.001 |
|
Effects of formononetin and cisplatin
on mRNA expression levels of HIF-1α and VEGF in cervical cancer
tissue
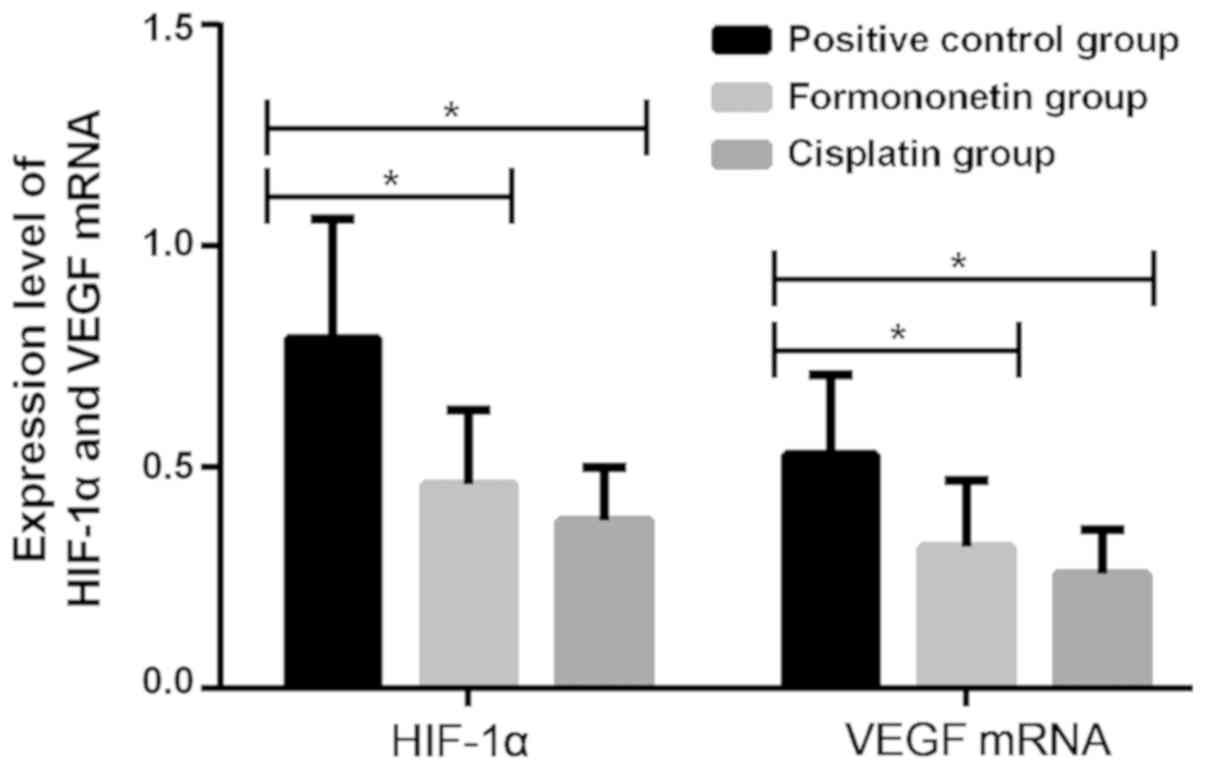
There were statistically significant differences in
the mRNA expression levels of HIF-1α and VEGF in mouse tissue among
the cisplatin, the formononetin and the positive control groups
(P<0.001). Mice with cervical cancer in the formononetin and the
cisplatin groups had significantly lower mRNA expression levels of
HIF-1α and VEGF in tissues than those in the positive control
group, with statistically significant differences (P<0.05), but
there was no significant difference in those between the
formononetin and the cisplatin groups (P>0.05; Fig. 1 and Table III).
 | Table III.Effects of formononetin and cisplatin
on mRNA expression levels of HIF-1α and VEGF in cervical cancer
tissue. |
Table III.
Effects of formononetin and cisplatin
on mRNA expression levels of HIF-1α and VEGF in cervical cancer
tissue.
| Groups | n | HIF-1α | VEGF |
|---|
| Positive
control | 10 | 0.79±0.27 | 0.53±0.18 |
| Formononetin | 15 |
0.46±0.17a |
0.32±0.15a |
| Cisplatin | 15 |
0.38±0.12a |
0.26±0.10a |
| F |
| 15.750 | 11.370 |
| P-value |
| <0.001 | <0.001 |
Effects of formononetin and cisplatin
on protein expression levels of HIF-1α and VEGF in tissues of
cervical cancer
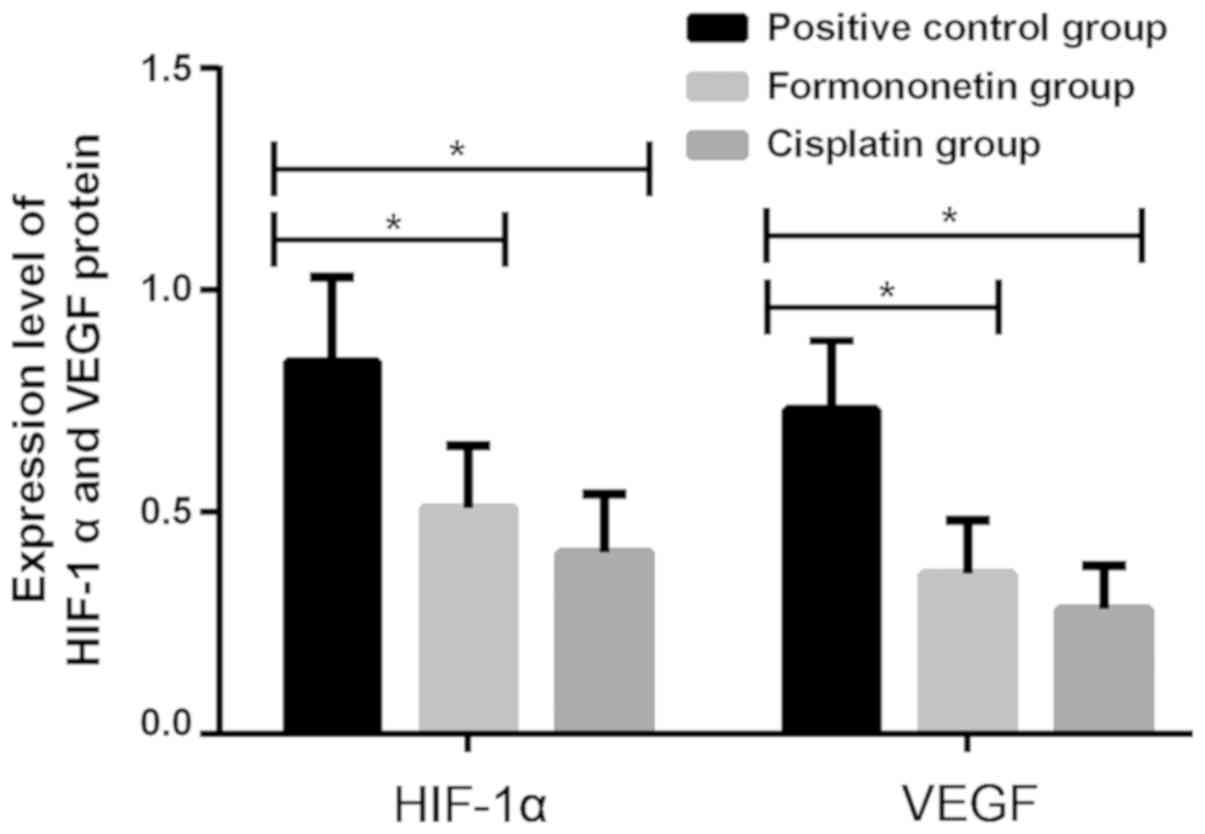
There were statistically significant differences in
the protein expression levels of HIF-1α and VEGF in tissues of mice
among the cisplatin, the formononetin and the positive control
groups (P<0.001). Mice with cervical cancer in the formononetin
and the cisplatin group had significantly lower protein expression
levels of HIF-1α and VEGF in tissues than those in the positive
control group, with statistically significant differences
(P<0.05), but there was no significant difference in those
between the formononetin and the cisplatin groups (P>0.05;
Fig. 2 and Table IV).
 | Table IV.Effects of formononetin and cisplatin
on protein expression levels of HIF-1α and VEGF in cervical cancer
tissue. |
Table IV.
Effects of formononetin and cisplatin
on protein expression levels of HIF-1α and VEGF in cervical cancer
tissue.
| Groups | n | HIF-1α | VEGF |
|---|
| Positive
control | 10 | 0.84±0.19 | 0.73±0.16 |
| Formononetin | 15 |
0.51±0.14a |
0.36±0.12a |
| Cisplatin | 15 |
0.41±0.13a |
0.28±0.10a |
| F |
| 25.630 | 42.330 |
| P-value |
| <0.001 | <0.001 |
Discussion
Cervical cancer, a common malignant tumor in female,
is caused by complex changes in multi-gene and multi-factor
interactions (24). Its pathogenesis
in tumor angiogenesis is particularly important in the hypoxic
condition (25–27). HIF-1α promotes the proliferation of
cancer cells, and VEGF induces the division of vascular endothelial
cells to promote tumor growth (28).
In addition to killing cancer cells, radiotherapy also causes
damage to patients' immune function (29). Chemotherapeutics affect patients'
quality of life, with greater toxic and side effects (30). Therefore, medical workers are
increasingly concerned about new bio-targeted therapies. According
to reports in the literature, formononetin can inhibit the
proliferation of osteosarcoma cell line U2OS (31), colorectal cancer cell line HCT-116,
DU-145, HeLa and gastric cancer cell line SGC-7901 (32), as well as promote apoptosis.
This study showed that during the medication
intervention, mice in the formononetin group had no obvious adverse
reactions, and were in good condition, but mice in the cisplatin
group had poor appetite, drooping spirits and decreased activity.
Mice in the cisplatin and the formononetin groups had significantly
lower tumor mass and tumor volume than those in the positive
control group, with statistically significant differences
(P<0.05), but there was no significant difference in those
between the formononetin and the cisplatin groups (P>0.05). The
tumor inhibition rate of mice was 56.24% in the cisplatin group,
and 50.17% in the formononetin group. Therefore, in this
experiment, formononetin had an anti-tumor effect on treating mice
with cervical cancer similar to cisplatin, but the former has no
significant adverse reactions with more mildly effects. This may be
due to the fact that as a phytoestrogen with mild nature, small
toxic and side effects, and diverse biological activities,
formononetin has multiple effects such as anti-tumor, immune
regulation, anti-oxidation, lowering blood lipid and cholesterol
(33). According to research by Kim
et al (34), the high
expression of HIF-1α and VEGF in cervical cancer tissues is
correlated with clinical stage, pathological grade and lymph node
metastasis. In order to verify the correlation between the
inhibition of tumor growth by formononetin and the expression
levels of HIF-1α and VEGF, RT-qPCR and western blotting were
performed. The results showed that mice with cervical cancer in the
formononetin and the cisplatin group had significantly lower mRNA
expression levels of HIF-1α and VEGF in tissues than those in the
positive control group, with statistically significant differences
(P<0.05), but there was no significant difference in those
between the formononetin and the cisplatin groups (P>0.05). Mice
with cervical cancer in the formononetin and the cisplatin groups
had significantly lower protein expression levels of HIF-1α and
VEGF in tissues than those in the positive control group, with
statistically significant differences (P<0.05), but there was no
significant difference in those between the formononetin and the
cisplatin groups (P>0.05). In the study by Bachtiary et
al (35), the results of
immunohistochemistry show that patients with HIF-1α expression in
cervical cancer tissues account for 72.1%. The HIF-1α expression
occurred in the early stage of tumor formation, and it is
speculated that HIF-1α may play an important role in the occurrence
and development of tumors. The study by Birner et al
(36) found that patients with high
expression of HIF-1α have significantly lower overall survival time
than patients with moderate or no expression of HIF-1α, and the
high expression of HIF-1α is an important prognostic indicator of
early cervical cancer. The study by Chen et al (37) shows that the expression of HIF-1α and
VEGF are closely related to tumor angiogenesis, and HIF-1α may play
an important role in the invasion and tumor angiogenesis of gastric
cancer. Highly expressed HIF-1α is closely correlated with tumor
recurrence and distant metastasis. The finding of Jin et al
(38) are consistent with ours.
Their results show that formononetin promotes apoptosis of cervical
cancer HeLa cells, and has an anti-tumor effect. Our experiments
have confirmed that formononetin can inhibit cervical cancer. It is
speculated that it may inhibit cancer by inhibiting the expression
levels of HIF-1α and VEGF. However, the specific mechanism is still
unclear, which requires more in-depth research.
In summary, formononetin can inhibit the growth of
cervical cancer tumors and reduce the mRNA and protein expression
levels of HIF-1α and VEGF in mouse tissue with cervical cancer.
Formononetin has an inhibitory effect on cervical cancer tumors
similar to cisplatin, but the former has smaller side effects, and
can provide useful data for clinical application.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ wrote the manuscript. YZ and JZ contributed to
PCR and western blotting. CC and JZ were responsible for the model
construction. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Wuxi People's Hospital Affiliated to Nanjing Medical University
(Wuxi, China). Patients who participated in this research had
complete clinical data. Signed informed consents were obtained from
the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li X, Zheng R, Li X, Shan H, Wu Q, Wang Y
and Chen W: Trends of incidence rate and age at diagnosis for
cervical cancer in China, from 2000 to 2014. Chin J Cancer Res.
29:477–486. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao S, Liu W, Li F, Zhao W and Qin C:
Decreased expression of lncRNA GAS5 predicts a poor prognosis in
cervical cancer. Int J Clin Exp Pathol. 7:6776–6783.
2014.PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cancer Genome Atlas Research Network,
Albert Einstein College of Medicine, Analytical Biological
Services, Barretos Cancer Hospital, Baylor College of Medicine,
Beckman Research Institute of City of Hope, Buck Institute for
Research on Aging, Canada's Michael Smith Genome Sciences Centre,
Harvard Medical School, Helen F. Graham Cancer Center &Research
Institute at Christiana Care Health Services, ; et al: Integrated
genomic and molecular characterization of cervical cancer. Nature.
543:378–384. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
WHO Guidelines Approved by the Guidelines
Review Committee, . Comprehensive cervical cancer control: a guide
to essential practice. 2nd. World Health Organization; Geneva:
2014
|
|
6
|
Du PL, Wu KS, Fang JY, Zeng Y, Xu ZX, Tang
WR, Xu XL and Lin K: Cervical cancer mortality trends in China,
1991–2013, and predictions for the future. Asian Pac J Cancer Prev.
16:6391–6396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Li G, Wang Y, Tang S, Sun X, Feng
X, Li Y, Bao G, Li P, Mao X, et al: Suppression of tumor
angiogenesis by metformin treatment via a mechanism linked to
targeting of HER2/HIF-1α/VEGF secretion axis. Oncotarget.
6:44579–44592. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Noman MZ, Desantis G, Janji B, Hasmim M,
Karray S, Dessen P, Bronte V and Chouaib S: PD-L1 is a novel direct
target of HIF-1α, and its blockade under hypoxia enhanced
MDSC-mediated T cell activation. J Exp Med. 211:781–790. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wigerup C, Påhlman S and Bexell D:
Therapeutic targeting of hypoxia and hypoxia-inducible factors in
cancer. Pharmacol Ther. 164:152–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jussila L and Alitalo K: Vascular growth
factors and lymphangiogenesis. Physiol Rev. 82:673–700. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rocha SF, Schiller M, Jing D, Li H, Butz
S, Vestweber D, Biljes D, Drexler HC, Nieminen-Kelhä M, Vajkoczy P,
et al: Esm1 modulates endothelial tip cell behavior and vascular
permeability by enhancing VEGF bioavailability. Circ Res.
115:581–590. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao K, Song X, Huang Y, Yao J, Zhou M, Li
Z, You Q, Guo Q and Lu N: Wogonin inhibits LPS-induced tumor
angiogenesis via suppressing PI3K/Akt/NF-κB signaling. Eur J
Pharmacol. 737:57–69. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hosaka M, Watari H, Takeda M, Moriwaki M,
Hara Y, Todo Y, Ebina Y and Sakuragi N: Treatment of cervical
cancer with adjuvant chemotherapy versus adjuvant radiotherapy
after radical hysterectomy and systematic lymphadenectomy. J Obstet
Gynaecol Res. 34:552–556. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siva S, Herschtal A, Thomas JM, Bernshaw
DM, Gill S, Hicks RJ and Narayan K: Impact of post-therapy positron
emission tomography on prognostic stratification and surveillance
after chemoradiotherapy for cervical cancer. Cancer. 117:3981–3988.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee HJ, Kim YS, Shin SS, Nam JH, Kim YT,
Han S and Choi EK: Long-term outcomes of concomitant
chemoradiotherapy incorporating high-dose-rate brachytherapy to
treat locally advanced cervical cancer. Tumori. 98:615–621. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Casagrande N, De Paoli M, Celegato M,
Borghese C, Mongiat M, Colombatti A and Aldinucci D: Preclinical
evaluation of a new liposomal formulation of cisplatin, lipoplatin,
to treat cisplatin-resistant cervical cancer. Gynecol Oncol.
131:744–752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Agyemang K, Han L, Liu E, Zhang Y, Wang T
and Gao X: Recent advances in astragalus membranaceus anti-diabetic
research: pharmacological effects of its phytochemical
constituents. Evid Based Complement Alternat Med. 2013:6546432013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun T, Wang J, Huang LH and Cao YX:
Antihypertensive effect of formononetin through regulating the
expressions of eNOS, 5-HT2A/1B receptors and α1-adrenoceptors in
spontaneously rat arteries. Eur J Pharmacol. 699:241–249. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ha H, Lee HY, Lee JH, Jung D, Choi J, Song
KY, Jung HJ, Choi JS, Chang SI and Kim C: Formononetin prevents
ovariectomy-induced bone loss in rats. Arch Pharm Res. 33:625–632.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huh JE, Nam DW, Baek YH, Kang JW, Park DS,
Choi DY and Lee JD: Formononetin accelerates wound repair by the
regulation of early growth response factor-1 transcription factor
through the phosphorylation of the ERK and p38 MAPK pathways. Int
Immunopharmacol. 11:46–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu Y, Zhang X, Li Z, Yan H, Qin J and Li
T: Formononetin inhibits human bladder cancer cell proliferation
and invasiveness via regulation of miR-21 and PTEN. Food Funct.
8:1061–1066. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou R, Xu L, Ye M, Liao M, Du H and Chen
H: Formononetin inhibits migration and invasion of MDA-MB-231 and
4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through
PI3K/AKT signaling pathways. Horm Metab Res. 46:753–760. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 [-Delta Delta C(T)] method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lachenmayer A, Toffanin S, Cabellos L,
Alsinet C, Hoshida Y, Villanueva A, Minguez B, Tsai HW, Ward SC,
Thung S, et al: Combination therapy for hepatocellular carcinoma:
additive preclinical efficacy of the HDAC inhibitor panobinostat
with sorafenib. J Hepatol. 56:1343–1350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Waggoner SE: Cervical cancer. Lancet.
361:2217–2225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mahasiripanth T, Hokputsa S, Niruthisard
S, Bhattarakosol P and Patumraj S: Effects of Acanthus ebracteatus
Vahl on tumor angiogenesis and on tumor growth in nude mice
implanted with cervical cancer. Cancer Manag Res. 4:269–279.
2012.PubMed/NCBI
|
|
27
|
Eckert AW, Lautner MH, Schütze A, Taubert
H, Schubert J and Bilkenroth U: Coexpression of hypoxia-inducible
factor-1α and glucose transporter-1 is associated with poor
prognosis in oral squamous cell carcinoma patients. Histopathology.
58:1136–1147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi D, Guo W, Chen W, Fu L, Wang J, Tian
Y, Xiao X, Kang T, Huang W and Deng W: Nicotine promotes
proliferation of human nasopharyngeal carcinoma cells by regulating
α7AChR, ERK, HIF-1α and VEGF/PEDF signaling. PLoS One.
7:e438982012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y, Liu BL, Shang B, Chen AS, Liu SQ,
Sun W, Yin HZ, Yin JQ and Su Q: Nutrition support in surgical
patients with colorectal cancer. World J Gastroenterol.
17:1779–1786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kayl AE and Meyers CA: Side-effects of
chemotherapy and quality of life in ovarian and breast cancer
patients. Curr Opin Obstet Gynecol. 18:24–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu W and Xiao Z: Formononetin induces
apoptosis of human osteosarcoma cell line U2OS by regulating the
expression of Bcl-2, Bax and MiR-375 in vitro and in vivo. Cell
Physiol Biochem. 37:933–939. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ren J, Xu HJ, Cheng H, Xin WQ, Chen X and
Hu K: Synthesis and antitumor activity of formononetin nitrogen
mustard derivatives. Eur J Med Chem. 54:175–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen J and Sun L: Formononetin-induced
apoptosis by activation of Ras/p38 mitogen-activated protein kinase
in estrogen receptor-positive human breast cancer cells. Horm Metab
Res. 44:943–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim NS, Kang YJ, Jo JO, Kim HY, Oh YR, Kim
YO, Jung MH, Ock MS and Cha HJ: Elevated expression of thymosin β4,
vascular endothelial growth factor (VEGF), and hypoxia inducible
factor (HIF)-1α in early-stage cervical cancers. Pathol Oncol Res.
17:493–502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bachtiary B, Schindl M, Pötter R, Dreier
B, Knocke TH, Hainfellner JA, Horvat R and Birner P: Overexpression
of hypoxia-inducible factor 1alpha indicates diminished response to
radiotherapy and unfavorable prognosis in patients receiving
radical radiotherapy for cervical cancer. Clin Cancer Res.
9:2234–2240. 2003.PubMed/NCBI
|
|
36
|
Birner P, Schindl M, Obermair A, Plank C,
Breitenecker G and Oberhuber G: Overexpression of hypoxia-inducible
factor 1alpha is a marker for an unfavorable prognosis in
early-stage invasive cervical cancer. Cancer Res. 60:4693–4696.
2000.PubMed/NCBI
|
|
37
|
Chen WT, Huang CJ, Wu MT, Yang SF, Su YC
and Chai CY: Hypoxia-inducible factor-1alpha is associated with
risk of aggressive behavior and tumor angiogenesis in
gastrointestinal stromal tumor. Jpn J Clin Oncol. 35:207–213. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jin YM, Xu TM, Zhao YH, Wang YC and Cui
MH: In vitro and in vivo anti-cancer activity of formononetin on
human cervical cancer cell line HeLa. Tumour Biol. 35:2279–2284.
2014. View Article : Google Scholar : PubMed/NCBI
|