Introduction
Non-small-cell lung cancer (NSCLC) is a
heterogeneous class of lung cancer, and in 2015 ranked as the most
common cause of cancer-associated mortality worldwide (1). The majority of patients with NSCLC are
diagnosed during the metastatic period, and the median survival
time following diagnosis is 1 year (2). Although surgical techniques and
biological treatments have been developed, the overall 5-year
survival rate is 18% (3). In
addition, patients diagnosed at the advanced stage are not suitable
for surgery (3). Thus, the
identification of novel targeted agents may produce effective
methods for treating patients with advanced NSCLC (4). However, the underlying molecular
mechanisms of NSCLC remain poorly defined.
MicroRNAs (miRNAs/miRs) are a class of endogenous,
non-coding RNAs, which are 18–25 nucleotides in length and can
regulate gene expression by inhibiting translation and/or
degradation of mRNA (5). The
regulation of gene expression by miRNAs is mediated via base
pairing with 3′-untranslated regions (3′-UTRs) in mRNA molecules
(6,7). miRNAs are commonly dysregulated in
cancer, which affects tumorigenesis processes and the expression of
specific genes (7). miR-296 is
located at the chromosome 20q13.32 genomic locus. It functions as a
tumor suppressor in cervical, pancreatic and colorectal cancer by
regulating specific targets (8–10).
miR-296-3p is derived from the 3′ arm of mature miR-296, and
miR-296-5p is derived from the 5′ arm (11). In NSCLC, miR-296-5p has been
demonstrated to suppress tumor progression by targeting polo like
kinase 1 (12). In addition,
miR-296-3p has been reported to inhibit NSCLC cell proliferation,
promote cell apoptosis, and enhance resistance to cisplatin and
paclitaxel (13). However, to the
best of our knowledge, whether miR-296-3p is involved in the
migration and invasion of NSCLC has not been investigated.
The aim of the current study was to investigate the
biological and functional role of miR-296-3p in NSCLC. The effects
of miR-296-3p on NSCLC cell migration and invasion in vitro
were investigated, which indicated that miR-296-3p may exhibit
tumor-suppressive functions. Understanding the molecular mechanism
of miR-296-3p may provide novel therapeutic targets for the
treatment of NSCLC.
Materials and methods
Clinical tissue specimens
NSCLC tissues and adjacent normal tissues (the
distance between tumor tissues and normal tissues was 5 cm) were
collected from 50 patients with NSCLC, including 29 male and 21
female patients, with a median age of 59.92 years (range, 45–75
years) that received surgery at Xi'an High-Tech Hospital (Xi'an,
China) and Shaanxi Provincial People's Hospital (Xi'an, China)
between September 2015 and December 2016. All specimens were
immediately frozen in liquid nitrogen and stored at −80°C prior to
further use. The present study was approved by the Ethics
Committees of Xi'an High-Tech Hospital and Shaanxi Provincial
People's Hospital. Written informed consent was obtained from all
patients.
Cell culture
A549 cells were purchased from the American Type
Culture Collection (Manassas, VA, USA). Cells were cultured in
RPMI-1640 medium (GE Healthcare Life Sciences) with 10% fetal
bovine serum (FBS; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and 1% (w/v) penicillin/streptomycin (Invitrogen; Thermo
Fisher Scientific, Inc.). The cells were maintained at 37°C with 5%
CO2.
Cell transfection
miR-296-3p mimics and corresponding negative control
(NC) vectors were purchased from Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). A549 cells were transfected with 30 nM
miR-296-3p mimics (5′-GAGGGUUGGGUGGAGGCUCUCC-3′) and miR-NC
(5′-UUCUCCGAACGUGUCACGU-3′) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from tissues and cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Subsequently, RNA
was reverse transcribed to cDNA using the PrimeScript™ RT reagent
kit (Takara Biotechnology Co., Ltd., Dalian, China). RT conditions
were as follows: 37°C for 15 min and 85°C for 5 sec. RT-qPCR was
performed using SYBR® Premix Ex Taq™ kit (Takara
Biotechnology Co., Ltd.) using an ABI 7500 real-time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). qPCR reaction
conditions were as follows: 95°C for 10 min, followed by 95°C for
30 sec, 60°C for 30 sec and 72°C for 30 sec (40 cycles). U6 was
used as the internal control for miR-296-3p, and GAPDH was used as
the internal control for apurinic/apyrimidinic
endodeoxyribonuclease 1 (APEX1), phosphoinositide-3-kinase (PI3K),
AKT serine/threonine kinase (AKT), mammalian target of rapamycin
(mTOR), matrix metallopeptidase 2 (MMP2) and SRY-box 4 (SOX4). All
samples were normalized to internal controls and the fold change
was calculated using the 2−ΔΔCq method (14). The sequences of the primers used were
as follows: miR-296-3p forward, 5′-GAGGGTTGGGTGGAGGCTCTCC-3′; U6
forward, 5′-ATGACACGCAAATTCGTGAAGC-3′; the reverse primers of
miR-296-3p and U6 used universal primers. APEX1 forward,
5′-TGAAGCCTTTCGCAAGTTCCT-3′ and reverse,
5′-TGAGGTCTCCACACAGCACAA-3′; PI3K forward,
5′-CATCACTTCCTCCTGCTCTAT-3′ and reverse,
5′-CAGTTGTTGGCAATCTTCTTC-3′; AKT forward,
5′-GGACAACCGCCATCCAGACT-3′ and reverse, 5′-GCCAGGGACACCTCCATCTC-3′;
mTOR forward, 5′-ATTTGATCAGGTGTGCCAGT-3′ and reverse,
5′-GCTTAGGACATGGTTCATGG-3′; MMP2 forward,
5′-GACCTTGACCAGAACACCATCG-3′ and reverse,
5′-GCTGTATTCCCGACCGTTGAAC-3′; SOX4 forward,
5′-GTGAGCGAGATGATCTCGGG-3′ and reverse,
5′-CAGGTTGGAGATGCTGGACTC-3′; and GAPDH forward,
5′-TGCCAAATATGATGACATCAAGAA-3′ and reverse
5′-GGAGTGGGTGTCGCTGTTG-3′.
Western blot analysis
Proteins were extracted from tissues and cells using
radioimmunoprecipitation assay lysis buffer, and the concentration
of each sample was measured using a bicinchoninic acid protein
assay kit (Pierce; Thermo Fisher Scientific, Inc.). The proteins
(30 µg) were separated by 10% SDS-PAGE and transferred to
polyvinylidene difluoride membranes. The membranes were blocked
with 5% not-fat milk in 0.05% TBS-Tween-20 (TBST) for 1 h at room
temperature. The membranes were incubated with primary antibodies
against APEX1 (cat. no. ab137708; 1:2,000), PI3K (cat. no.
Ab191606; 1:1,000), phospho (p)-PI3K (cat. no. ab192651; 1:1,000),
AKT (cat. no. Ab179463; 1:10,000), p-AKT (cat. no. ab131443;
1:1,000), mTOR (cat. no. Ab2732; 1:2,000), p-mTOR (p-S2448; cat.
no. ab109268; 1:1,000), MMP2 (cat. no. ab92536; 1:1,000), SOX4
(cat. no. ab80261; 1:500) and GAPDH (cat. no. ab181602; 1:1,000)
overnight at 4°C. All primary antibodies were purchased from Abcam
(Cambridge, MA, USA). Subsequently, the membranes were washed in
TBST and then incubated with secondary antibody (goat anti-rabbit
IgG horseradish peroxidase-conjugated antibody; cat. no. ab205718;
1:2,000; Abcam) at room temperature for 1 h. Protein expression
signals were detected using enhanced chemiluminescence reagents (GE
Healthcare, Chicago, IL, USA) with GAPDH used as the loading
control. Data was analyzed by Image-Pro Plus 6.0 software (Media
Cybernetics, Inc.).
Cell viability assay
Cell viability was analyzed using the Cell Counting
Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). Cells (4×103 cells/well) were seeded
into 96-well plates and cultured at 37°C with 5% CO2 for
0, 12, 24 and 48 h. CCK-8 reagent (10 µl) was added into each well.
Subsequently, cells were cultured at 37°C for 2 h. The optical
density value was measured at a wavelength of 450 nm using a
microplate reader.
Wound healing assay
Cells (4×105) were seeded in 6-well
plates. When confluence reached >90%, a 10-µl pipette tip was
used to scratch two parallel wounds in the cell monolayer. The cell
debris was washed twice with PBS and cells were incubated at 37°C
with 5% CO2 in serum-free RPMI-1640 medium. Following 24
h of incubation at 37°C, images of the cells were captured using a
light microscope (magnification, ×200) and the width of the wound
was calculated using a standard caliper.
Matrigel assays
A549 cells (2×105 cells/ml) were seeded
in the upper chamber of Transwell filled with serum-free RPIM-1640
medium inserts coated with 100 µl Matrigel (BD Biosciences, San
Jose, CA, USA) at 37°C for 4 h. The bottom chamber was filled with
RPMI-1640 medium containing 10% FBS. Following incubation for 24 h,
the cells that had invaded into the lower chambers were fixed with
4% paraformaldehyde for 10 min and stained with 0.1% crystal violet
for 15 min at room temperature. Cells were imaged and quantified
from 5 randomly-selected fields using a light microscope
(magnification, ×200).
Prediction of target genes and
dual-luciferase reporter assay
The potential targets of miR-296-3p were predicted
using the TargetScan online tool (targetscan.org/). The DNA regions coding wild-type
(WT) APEX1 3′-untranslated region (3′-UTR; miR-296-3p binding
sites) and a mutant sequence (miR-296-3p binding site deletion)
were inserted into a pmirGLO vector (Promega Corporation).
miR-296-3p mimics and miR-NC were co-transfected into A549 cells
with WT and mutant vectors using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). After transfection
for 48 h, firefly luciferase activity was normalized to
Renilla luciferase activity using the dual-luciferase
reporter assay system (Promega Corporation, Madison, WI, USA),
according to the manufacturer's protocol.
Statistical analysis
All experiments were repeated three times. All data
are analyzed by GraphPad Prism 7 software (GraphPad, Inc.)
presented as the mean ± standard deviation. Student's t-test was
used to analyze two groups, except for the comparison between tumor
tissues and normal tissues where a paired Student's t-test was
used. One-way analysis of variance followed by the Newman-Keuls
test was used to compare the differences among multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Downregulated expression of miR-296-3p
in NSCLC tissues
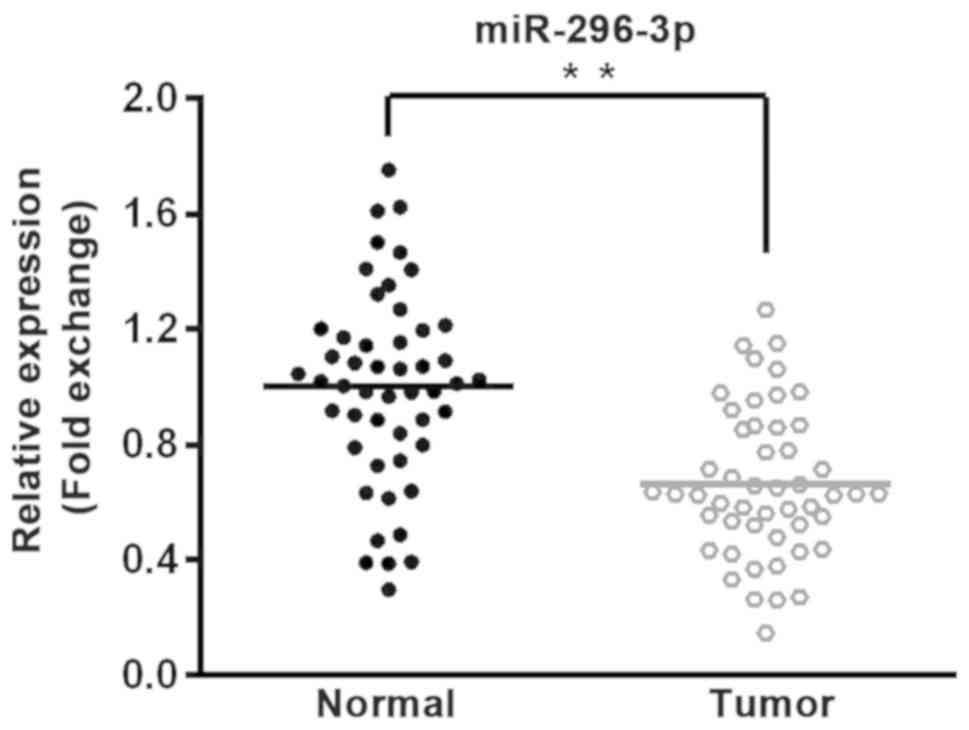
In order to determine the role of miR-296-3p in
NSCLC, 50 pairs of tumor tissues and adjacent normal tissues were
collected from patients with NSCLC. miR-296-3p expression was
detected by RT-qPCR, revealing that miR-296-3p expression was
significantly lower in tumor tissues compared with corresponding
normal tissues (P<0.01; Fig.
1).
miR-296-3p inhibits NSCLC cell
viability, migration and invasion in vitro
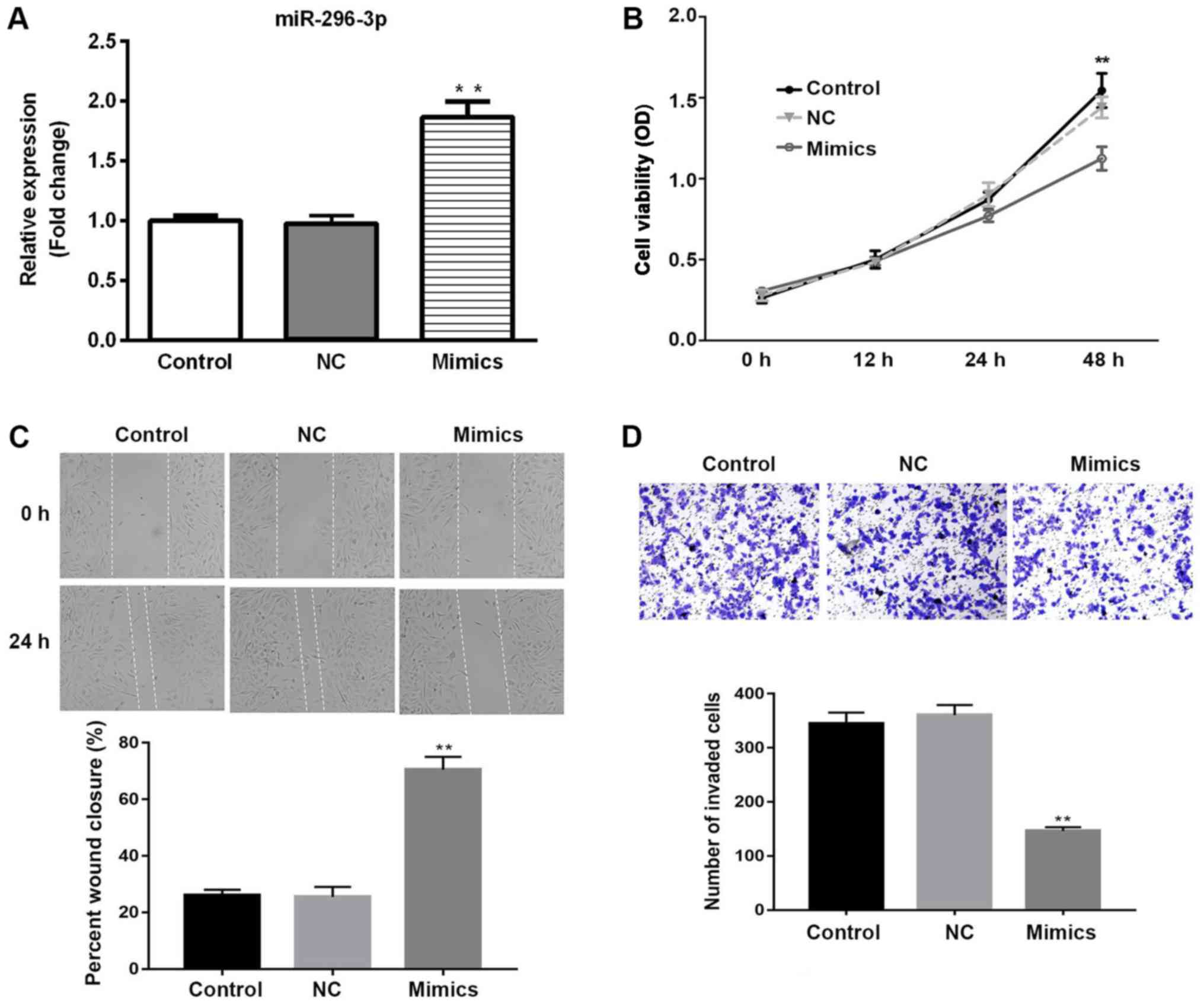
Transfection of miR-296-3p mimics into NSCLC cells
was used to investigate the functional role of miR-296-3p.
Initially, the transfection efficiency of miR-296-3p was determined
by RT-qPCR. miR-296-3p expression was significantly upregulated in
A549 cells transfected with miR-296-3p mimic compared with the
miR-NC group (P<0.01; Fig. 2A).
The effects of miR-296-3p overexpression on cell viability,
migration and invasion were determined by CCK-8, wound healing and
Matrigel assays, respectively. Cell viability was significantly
downregulated in the miR-296-3p mimics group compared with the
miR-NC-transfected cells at 48 h (P<0.01), with similar
viability rates in the miR-NC and untransfected control group
(Fig. 2B). As presented in Fig. 2C, miR-296-3p mimic inhibited the
migration of cells at 24 h, compared with the miR-NC group
(P<0.01). Similarly, overexpression of miR-296-3p significantly
reduced the number of invaded cells, suggesting that miR-296-3p
suppressed cell invasion in vitro (P<0.01; Fig. 2D). These findings indicate that
overexpression of miR-296-3p exhibits tumor suppressive effects via
reduced cell viability, migration and invasion.
APEX1 is a potential target of
miR-296-3p
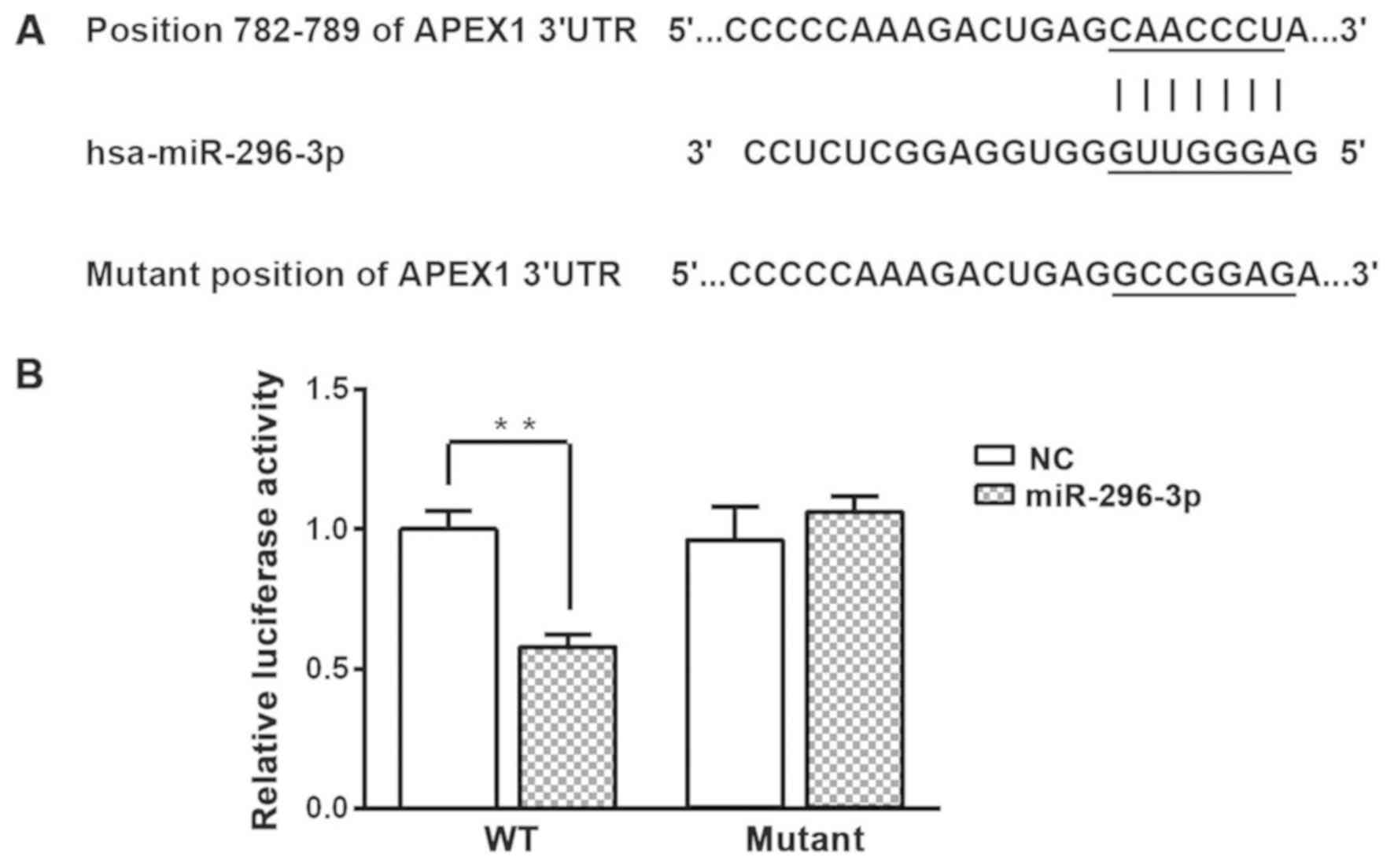
To determine how miR-296-3p mediates the
aforementioned effects in NSCLC cells, bioinformatics analysis was
used to predict the potential targets of miR-296-3p. The results
indicated that miR-296-3p directly binds to position 782–789 of the
APEX1 mRNA 3′-UTR (Fig. 3A). A
dual-luciferase reporter assay was used to validate this
prediction. Overexpression of miR-296-3p significantly decreased
luciferase activity in A549 cells transfected with WT APEX1 3′-UTR
compared with the miR-NC group (P<0.01; Fig. 3B). However, overexpression of
miR-296-3p did not effect the luciferase activity in A549 cells
transfected with the mutant APEX1 3′-UTR.
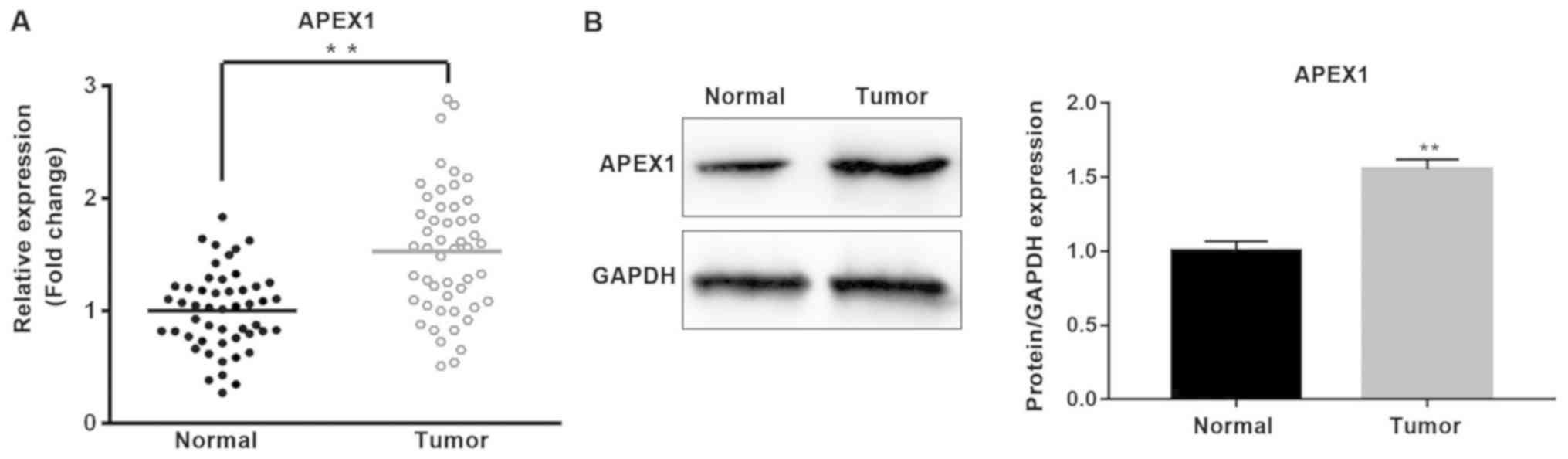
Furthermore, the expression of APEX1 was analyzed in
NSCLC tissues and cells. The mRNA and protein expression levels of
APEX1 were significantly higher in tumor tissues compared with
normal tissues (P<0.01; Fig. 4A and
B). The expression levels of APEX1 mRNA and protein were
significantly downregulated in A549 cells transfected with
miR-296-3p mimics compared with the miR-NC group (P<0.05;
Fig. 5A, I and J).
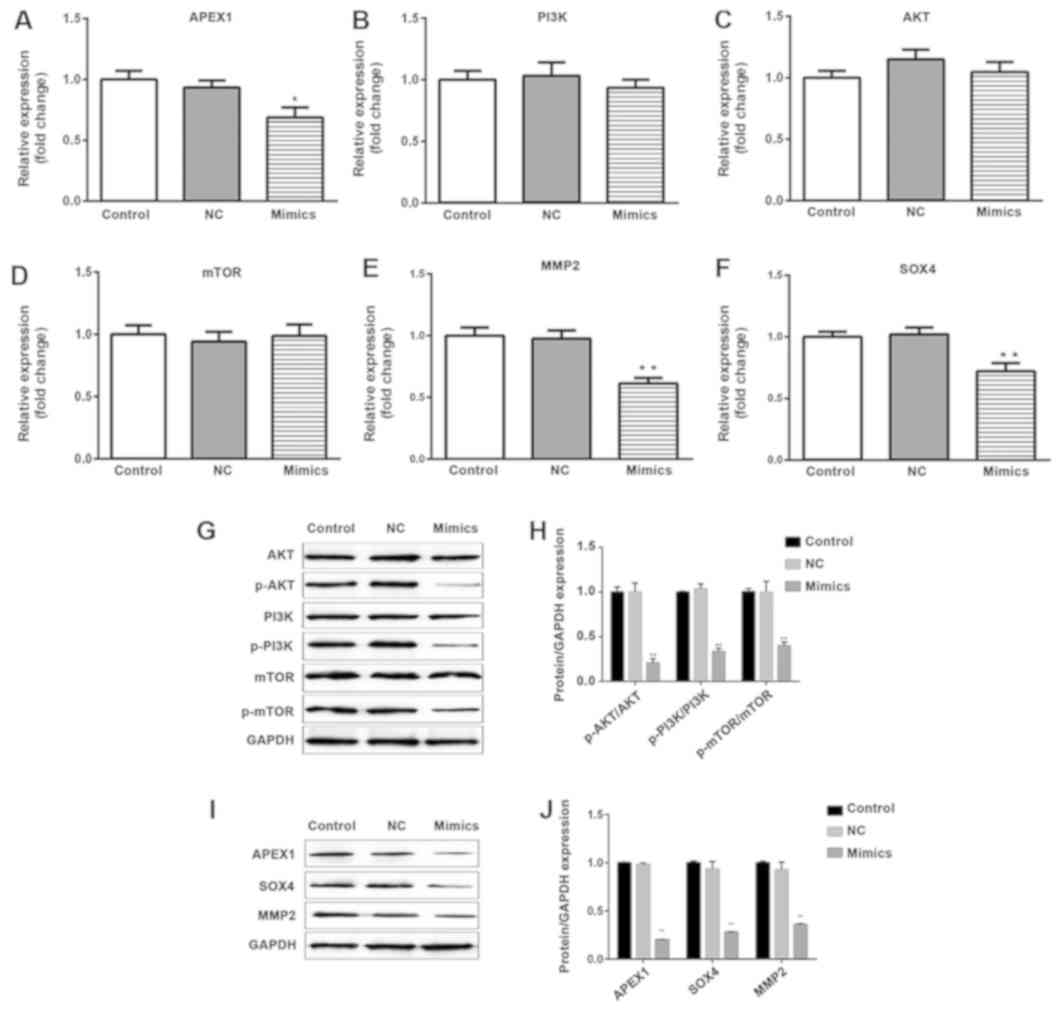 | Figure 5.Overexpression of miR-296-3p does not
affect the mRNA and protein expression levels of PI3K, AKT and
mTOR; however, it does reduce the phosphorylation of PI3K, AKT and
mTOR. mRNA and protein levels of APEX1, MMP2 and SOX4 were
decreased following transfection of A549 cells with miR-296-3p
mimics. The mRNA expression levels of (A) APEX1, (B) PI3K, (C) AKT,
(D) mTOR, (E) MMP2 and (F) SOX4 were detected using reverse
transcription-quantitative polymerase chain reaction. (G) Protein
levels of total PI3K, p-PI3K, total AKT, p-AKT, total mTOR and
p-mTOR were examined using western blot analysis. GAPDH was used as
a normalization control. (H) Quantification of the ratios of
p-AKT/AKT, p-PI3K/PI3K and p-mTOR/mTOR. (I) Protein levels of
APEX1, MMP2 and SOX4 were measured by western blot analysis. (J)
The fold change of proteins/GAPDH according to the APEX1, MMP2 and
SOX4 protein levels. Data are presented as the mean ± standard
deviation. *P<0.05, **P<0.01 vs. NC. miR, microRNA; PI3K,
phosphoinositide-3-kinase; AKT, AKT serine/threonine kinase; mTOR,
mammalian target of rapamycin; MMP2, matrix metallopeptidase 2;
SOX4, SRY-box 4; p-, phosphorylated-; NC, negative control. |
miR-296-3p regulates the PI3K/AKT/mTOR
signaling pathway and the expression of MMP2 and SOX4
In order to investigate how miR-296-3p regulates the
signaling associated with cellular processes, RT-qPCR was used to
evaluate the mRNA expression levels of PI3K, AKT, mTOR, MMP2 and
SOX4. As presented in Fig. 5B-F,
overexpression of miR-296-3p did not induce a change in PI3K, AKT
and mTOR expression at the mRNA level (P>0.05); however, it did
significantly decrease the mRNA and protein levels of MMP2 and SOX4
(P<0.01), compared with the miR-NC group (Fig. 5E, F, I and J). The protein expression
levels of total PI3K, AKT and mTOR were not affected by miR-296-3p;
however, the phosphorylation levels of PI3K, AKT and mTOR were
significantly reduced in A549 cells transfected with miR-296-3p
mimic (P<0.01; Fig. 5G and
H).
Discussion
In the present study, the molecular mechanism of
miR-296-3p in NSCLC was investigated, and the results revealed that
miR-296-3p acted as a tumor suppressor in NSCLC. miR-296-3p was
demonstrated to inhibit NSCLC cell viability, migration and
invasion in vitro, and was revealed to directly target APEX1
mRNA.
Previous studies have examined the expression and
role of miR-296-3p in human cancer. For example, miR-296-3p was
demonstrated to be upregulated in prostate cancer, which increased
tumor cell resistance to natural killer cells by targeting
intercellular adhesion molecule-1 (15). By contrast, miR-296-3p has been
reported to suppress tumor cell proliferation, migration and
invasion in several types of malignancy. In choroidal malignant
melanoma, overexpression of miR-296-3p suppressed tumor cell
proliferation, migration and invasion (16). Additionally, miR-296-3p was
downregulated in glioblastoma cells and decreased cell
proliferation and invasion by inhibition of potassium voltage-gated
channel subfamily H member 1 (17).
The current study revealed that miR-296-3p expression was lower in
NSCLC tissues compared with para-cancerous tissues, which was
consistent with a previous study by Luo et al (13). Furthermore, overexpression of
miR-296-3p inhibited tumor cell viability, migration and invasion
in vitro. These results indicate that miR-296-3p exerts
antitumor effects in NSCLC. However, the underlying molecular
mechanism of how miR-296-3p mediates these effects in NSCLC
remained unclear.
Bioinformatics analysis was used to predict the
potential mRNA targets of miR-296-3p. The results indicated that
miR-296-3p may bind to the 3′-UTR of APEX1 at nucleotides 782–789.
A dual-luciferase reporter assay revealed that APEX1 was a direct
target of miR-142-3p. APEX1 is a DNA repair enzyme that recognizes
and cleaves apurinic/apyrimidinic sites in damaged DNA (18). APEX1 is commonly upregulated in human
cancer, including in prostate cancer (19), osteosarcoma (20) and human melanoma (21). Furthermore, high expression of APEX1
is associated with unfavorable prognosis in breast cancer and
osteosarcoma (20,22). APEX1 is involved in regulating
biological behavior in human cancer. For example, APEX1 has been
demonstrated to promote colon cancer tumorigenicity and progression
in vitro and in vivo (23). Furthermore, overexpression of APEX1
is an independent predictor of osteosarcoma local recurrence and
metastasis (24). Additionally,
APEX1 was established as a prognostic indicator in NSCLC, and
cytoplasmic expression of APEX1 was associated with poor survival
rates (25). However, the expression
and functional roles of APEX1 in NSCLC have remained unclear;
therefore, the expression of APEX1 was detected in the present
study. The results demonstrated that APEX1 was significantly
upregulated in NSCLC tissues at the mRNA and protein levels
compared with para-cancerous tissues, which was consistent with
previous studies in other cancer types, such as prostate cancer,
osteosarcoma and human melanoma (19–21).
Additionally, the expression of APEX1 was significantly
downregulated by transfection with miR-296-3p mimic in NSCLC cells;
this suggested that overexpression of miR-296-3p may inhibit NSCLC
cell migration and invasion by targeting APEX1.
The PI3K signaling pathway is commonly activated in
human cancer (26). AKT and mTOR are
major effectors that act downstream of PI3K (27). PI3K is activated by various
extracellular cytokines via auto-phosphorylation of cell surface
receptors (27). Once activated,
PI3K can phosphorylate downstream kinases, including AKT, and
activated AKT phosphorylates other downstream targets to regulate
numerous cellular processes (28–30). The
PI3K/AKT/mTOR signaling pathway is an important mediator of tumor
cell proliferation, apoptosis, migration, invasion, metabolism and
angiogenesis (28,31). MMP2 and SOX4 are also involved in
regulating cell migration and invasion (32,33). To
the best of our knowledge, no previous studies have investigated
the association between APEX1, the PI3K/AKT/mTOR pathway, and MMP2
and SOX4. In the current study, overexpression of miR-296-3p
reduced the phosphorylation of PI3K, AKT and mTOR, but did not
affect their mRNA expression level. These results suggest that
miR-296-3p may have an inhibitory effect on the PI3K/AKT/mTOR
signaling pathway, although this action potentially occurs at the
translation/post-translation level, rather than by affecting mRNA
stability. Additionally, miR-296-3p reduced the mRNA and protein
levels of MMP2 and SOX4. These findings suggest that miR-296-3p may
inactivate the PI3K/AKT/mTOR signaling pathway and suppress the
expression of MMP2 and SOX4 by targeting APEX1, resulting in
reduced cell migration and invasion.
In conclusion, the present study demonstrated that
miR-296-3p was downregulated in NSCLC tissues compared with
para-cancerous tissues. Additionally, miR-296-3p inhibited NSCLC
cell migration and invasion, reduced PI3K/AKT/mTOR signaling, and
reduced the expression of MMP2 and SOX4, potentially by targeting
APEX1. APEX1 was validated as a novel target of miR-296-3p, and
these insights may be useful for identifying novel therapeutic
targets for the clinical treatment of patients with NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data genarated or analyzed during the present
study are included in this published article.
Authors' contributions
LW and YZ contributed to study design. LW and RC
performed experiments and data analysis. LW was a major contributor
in writing the manuscript. All author have read and approved the
final manuscript.
Ethics approval and consent to
participate
The human sample collection was approved by the
Ethics Committees of Xi'an High-Tech Hospital and Shaanxi
Provincial People's Hospital. All patients provided written
informed consent.
Patient consent for publication
Patients provided consent for the publication of the
present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gridelli C, Rossi A, Carbone DP, Guarize
J, Karachaliou N, Mok T, Petrella F, Spaggiari L and Rosell R:
Non-small-cell lung cancer. Nat Rev Dis Primers. 1:150092015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosell R, Bivona TG and Karachaliou N:
Genetics and biomarkers in personalisation of lung cancer
treatment. Lancet. 382:720–731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Skjefstad K, Johannessen C, Grindstad T,
Kilvaer T, Paulsen EE, Pedersen M, Donnem T, Andersen S, Bremnes R,
Richardsen E, et al: A gender specific improved survival related to
stromal miR-143 and miR-145 expression in non-small cell lung
cancer. Sci Rep. 8:85492018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Custodio A, Méndez M and Provencio M:
Targeted therapies for advanced non-small-cell lung cancer: Current
status and future implications. Cancer Treat Rev. 38:36–53. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Selbach M, Schwanhäusser B, Thierfelder N,
Fang Z, Khanin R and Rajewsky N: Widespread changes in protein
synthesis induced by microRNAs. Nature. 455:58–63. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doench JG and Sharp PA: Specificity of
microRNA target selection in translational repression. Genes Dev.
18:504–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farazi TA, Spitzer JI, Morozov P and
Tuschl T: miRNAs in human cancer. J Pathol. 223:102–115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lv L and Wang X: MicroRNA-296 targets
specificity protein 1 to suppress cell proliferation and invasion
in cervical cancer. Oncol Res. 26:775–783. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Li J, Shi B and Chen F: MicroRNA-296
targets AKT2 in pancreatic cancer and functions as a potential
tumor suppressor. Mol Med Rep. 16:466–472. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He Z, Yu L, Luo S, Li M, Li J, Li Q, Sun Y
and Wang C: miR-296 inhibits the metastasis and
epithelial-mesenchymal transition of colorectal cancer by targeting
S100A4. BMC Cancer. 17:1402017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li H, Ouyang XP, Jiang T, Zheng XL, He PP
and Zhao GJ: MicroRNA-296: A promising target in the pathogenesis
of atherosclerosis? Mol Med. 24:122018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu C, Li S, Chen T, Hu H, Ding C, Xu Z,
Chen J, Liu Z, Lei Z, Zhang HT, et al: MiR-296-5p suppresses cell
viability by directly targeting PLK1 in non-small cell lung cancer.
Oncol Rep. 35:497–503. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo W, Lin Y, Meng S, Guo Y, Zhang J and
Zhang W: miRNA-296-3p modulates chemosensitivity of lung cancer
cells by targeting CX3CR1. Am J Transl Res. 8:1848–1856.
2016.PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Chen Q, Yan J, Wang Y, Zhu C, Chen
C, Zhao X, Xu M, Sun Q, Deng R, et al: MiRNA-296-3p-ICAM-1 axis
promotes metastasis of prostate cancer by possible enhancing
survival of natural killer cell-resistant circulating tumour cells.
Cell Death Dis. 4:e9282013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Hu Y, Cui J, Zhou Y and Chen L:
Coordinated targeting of MMP-2/MMP-9 by miR-296-3p/FOXCUT exerts
tumor-suppressing effects in choroidal malignant melanoma. Mol Cell
Biochem. 445:25–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ba Y, Liao H, Liu T, Zeng X, Xiao F, Luo
L, Guo H and Guo L: MiR-296-3p regulates cell growth and multi-drug
resistance of human glioblastoma by targeting ether-à-go-go (EAG1).
Eur J Cancer. 49:710–724. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li H, Liu G, Xia L, Zhou Q, Xiong J, Xian
J, Du M, Zhang L, Liao L, Su X, et al: A polymorphism in the DNA
repair domain of APEX1 is associated with the radiation-induced
pneumonitis risk among lung cancer patients after radiotherapy. Br
J Radiol. 87:201400932014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kelley MR, Cheng L, Foster R, Tritt R,
Jiang J, Broshears J and Koch M: Elevated and altered expression of
the multifunctional DNA base excision repair and redox enzyme
Ape1/ref-1 in prostate cancer. Clin Cancer Res. 7:824–830.
2001.PubMed/NCBI
|
|
20
|
Wang D, Luo M and Kelley MR: Human
apurinic endonuclease 1 (APE1) expression and prognostic
significance in osteosarcoma: Enhanced sensitivity of osteosarcoma
to DNA damaging agents using silencing RNA APE1 expression
inhibition. Mol Cancer Ther. 3:679–686. 2004.PubMed/NCBI
|
|
21
|
Yang S, Irani K, Heffron SE, Jurnak F and
Meyskens FL Jr: Alterations in the expression of the
apurinic/apyrimidinic endonuclease-1/redox factor-1 (APE/Ref-1) in
human melanoma and identification of the therapeutic potential of
resveratrol as an APE/Ref-1 inhibitor. Mol Cancer Ther.
4:1923–1935. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Woo J, Park H, Sung SH, Moon BI, Suh H and
Lim W: Prognostic value of human apurinic/apyrimidinic endonuclease
1 (APE1) expression in breast cancer. PLoS One. 9:e995282014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim MH, Kim HB, Yoon SP, Lim SC, Cha MJ,
Jeon YJ, Park SG, Chang IY and You HJ: Colon cancer progression is
driven by APEX1-mediated upregulation of Jagged. J Clin Invest:.
(pii): 655212013.PubMed/NCBI
|
|
24
|
Yang J, Yang D, Cogdell D, Du X, Li H,
Pang Y, Sun Y, Hu L, Sun B, Trent J, et al: APEX1 gene
amplification and its protein overexpression in osteosarcoma:
Correlation with recurrence, metastasis, and survival. Technol
Cancer Res Treat. 9:161–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Puglisi F, Aprile G, Minisini AM, Barbone
F, Cataldi P, Tell G, Kelley MR, Damante G, Beltrami CA and Di
Loreto C: Prognostic significance of Ape1/ref-1 subcellular
localization in non-small cell lung carcinomas. Anticancer Res.
21:4041–4049. 2001.PubMed/NCBI
|
|
26
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lv X, Li CY, Han P and Xu XY:
MicroRNA-520a-3p inhibits cell growth and metastasis of non-small
cell lung cancer through PI3K/AKT/mTOR signaling pathway. Eur Rev
Med Pharmacol Sci. 22:2321–2327. 2018.PubMed/NCBI
|
|
29
|
Tsurutani J, Fukuoka J, Tsurutani H, Shih
JH, Hewitt SM, Travis WD, Jen J and Dennis PA: Evaluation of two
phosphorylation sites improves the prognostic significance of Akt
activation in non-small-cell lung cancer tumors. J Clin Oncol.
24:306–314. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Testa JR and Bellacosa A: AKT plays a
central role in tumorigenesis. Proc Natl Acad Sci USA.
98:10983–10985. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bruhn MA, Pearson RB, Hannan RD and
Sheppard KE: AKT-independent PI3-K signaling in cancer-emerging
role for SGK3. Cancer Manag Res. 5:281–292. 2013.PubMed/NCBI
|
|
32
|
Wang XX, Cheng Q, Zhang SN, Qian HY, Wu
JX, Tian H, Pei DS and Zheng JN: PAK5-Egr1-MMP2 signaling controls
the migration and invasion in breast cancer cell. Tumour Biol.
34:2721–2729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Du Q, Liu J, Zhang X, Zhang X, Zhu H, Wei
M and Wang S: Propofol inhibits proliferation, migration, and
invasion but promotes apoptosis by regulation of Sox4 in
endometrial cancer cells. Braz J Med Bio Res. 51:e68032018.
|