Introduction
Soft tissue sarcomas (STS) constitute a heterogenic
group of tumors that accounts for only 1% of the overall human
burden of malignant tumors, with an annual incidence of
approximately 4.5/100,000 in Europe (1). Around 70 to 80% of patients are
diagnosed in a local or locally advanced stage of the disease. The
median age at diagnosis is 58 years, and the STS-related death
around 65 years (2).
Extremities are the most frequent location for STS,
accounting for 21.7% of all STS. However, a lower incidence (25–30%
of STS of the extremities) and an earlier median age of diagnosis
(38 years, ranging from 4 to 77 years) have been reported for the
upper extremities compared to lower extremities' STS (1). Around 50% of STS of upper extremities
arise in the shoulder-upper arm region, 30 to 40% in the
elbow-forearm and only a 10 to 20% in the wrist-hand (3,4).
However, when the upper extremity is subdivided in proximal and
distal, a distribution of 50% in each site has been observed
(5) (Table I).
 | Table I.Description of studies reporting on
frequency and location of STS of the upper extremity. |
Table I.
Description of studies reporting on
frequency and location of STS of the upper extremity.
| Authors, year | Total | Shoulder-Arm, n
(%) | Elbow-Forearm, n
(%) | Wrist-Hand, n
(%) | (Refs.) |
|---|
| Gustafson and
Arner, 1999 | 108 | 50 (46.3) | 48 (44.4) | 10 (9.2) | (3) |
| Gerrand et
al, 2003 | 139 | 74 (53.2) | 41 (29.5) | 24 (17.3) | (4) |
| Müller et
al, 2016 | 195 | 98 (50.2) | 97 (49.8) |
| (5) |
| Total | 442 | Proximal: 222
(50.2) | Distal: 220
(49.8) | – |
Histological examination
Over 50 different histological and molecular
subtypes have been described, occurring ubiquitously throughout the
human body (6). As every subtype of
sarcoma has a particular biological behavior and response profile
to systemic therapy, histologic diagnosis is a crucial criterion
when selecting the appropriate therapy. Distribution of these
subtypes varies between registries, due to evolution of
classification of STS as a result of histological and molecular
biology advances (6). Potentially,
all histologic subtypes can arise on the upper extremities, but a
higher incidence of malignant
histiocytofibrosarcoma/undifferentiated pleomorphic sarcoma (UPS)
and synovial sarcoma (SS) has been reported, reaching 50–65%
(7). Of note is that epithelioid
sarcoma (ES) arises almost exclusively on the extremities, while
clear cell sarcoma (CCS) is considered a specific subtype of the
hand and the wrist (2).
Histological, immunohistochemical and cytogenetical characteristics
of the specific subtypes of STS of the upper extremities are
described in Table II.
 | Table II.Histological, immunohistochemical and
cytogenetical characteristics of the more frequent subtypes of STS
of the upper extremities. |
Table II.
Histological, immunohistochemical and
cytogenetical characteristics of the more frequent subtypes of STS
of the upper extremities.
| Sarcoma type | Histology | IHC | Cytogenetics |
|---|
| UPS | Cytological and
nuclear pleomorphism | Positivity for
antigens suggesting diverse lines of differentiation in the same
tumor. | Great number of
genetic alterations. Phenotypic spectrum of a single molecular
entity with myxoid fibrosarcoma. |
| SS | Biphasic: Spindle
cell component with an epithelial component. Monophasic: Entirely
compounded by the spindle component. | CK, EMA, S100, TLE1
positive. | Translocation
t(X;18)(p11;q11) (90% of cases); fusion gene SSX-SYT. |
| ES | Epithelial and
spindle cells that form nodules. | Vimentin, CK, EMA
positive. SMARCB/INI1 negative. | No conclusions
about the genetic aberrations can be drawn due to the low incidence
of this tumor. |
| CCS | Spindle or
polygonal cells with abundant cytoplasm disposed in nests with
fibrous tracts between them. | Vimentin, HMB-45,
S100, Melan-A positivity | Translocation
t(12;22)(q13;q12); fusion gene EWS-ATF1. |
Accurate pathologic characterization of STS requires
adequate and representative tumoral tissue, such as that harvested
by core needle biopsy (CNB), which attains a specificity of 70 to
98% (8). The most accepted grading
system for STS is the FNCLCC (Fédération Nationale des Centres
de Lutte Contre le Cancer) system, based on three scores:
Differentiation, mitotic count and necrosis. STS of the upper
extremities are categorized as high grade in 45–70% of cases, due
to the high score attributed to each of the four most frequent but
aggressive histologic subtypes (3,9).
Diagnostic approach
Most STS of the hand and upper extremities present
as a painless, slow growing and movable mass. In rare cases, the
mass may cause nerve compression and present clinically as a nerve
compression neuropathy. Thus, malignant lesions are ill-conceived
as benign tumors and treated inadequately (10). Although the most common soft tissue
histology of the upper extremities is lipoma, superficial soft
tissue tumors larger than 5 cm or deep-seated tumors are associated
with a higher risk of malignancy. Within the 3–5 cm size category,
akin to a golf ball (4.3 cm), the risk of malignancy is influenced
by increasing age (11).
Acral myxoinflammatory fibroblastic sarcoma, ES and
CCS are frequently located in the hand and often present as
painless slow growing nodules, commonly confused with wrist
ganglions and erroneously treated accordingly. As these tumors are
solid and tend to extend along tendon sheaths, narrow resections
may result in high recurrence rates. Contrary to other subtypes,
CCS and ES have a high rate of regional node involvement (12,13).
Magnetic resonance imaging (MRI) is the method of
choice for the radiological evaluation of suspicious lumps,
informing on the anatomical relations with the surrounding tissues
for optimal surgical planning (14).
Despite established imaging criteria, precise diagnosis can be made
on the basis of MRI in only 24% of cases (15). Gadolinium contrast administration
provides important information on tumor heterogeneity, guiding
biopsy to the most vascular, non-necrotic part of the lesion
(16).
A percutaneous core 14G-needle biopsy is frequently
performed under local anesthesia. Through a skin stub 3–4 tissue
cylinders are harvested. This procedure allows to harvest adequate
tissue volume to make the diagnosis in over 90% of cases, with a
sensitivity of 95% for malignancy and 88% for grade (17). Nevertheless, major diagnostic errors
associated with the use of CNB can be drawn due to tumor
heterogeneity and low cellularity in cases of lipomatous,
hemorrhagic or myxoid tumors. More specifically, in
dedifferentiated sarcomas, low-grade and high-grade components
coexist in the same mass and a biopsy taken from the low-grade part
may therefore result in underestimation of the true tumor grade. In
order to increase the harvesting of representative tissue, the
careful consideration of the MRI features and the accomplishment of
CNB under CT or U/S guidance are recommended.
When CNB is repeatedly non-diagnostic, an open
biopsy should be performed, as it has a diagnostic accuracy of
94–100%. Open biopsies should be carefully planned and performed.
The incision line should be part of the final surgical approach. We
avoid transverse incisions as they usually create a soft tissue
defect difficult to reconstruct after final resection of the tumor
with the biopsy tract. The surgeon should be aware of the complex
nerve and vessel anatomy of the upper extremity. The biopsy tract
must not violate more than one anatomic compartment and avoid
exposure of the neurovascular bundles to the tumor cells. The
pseudocapsule of the tumor should be closed with sutures after
tissue harvesting. Adequate hemostasis should be performed in order
to avoid hematoma formation and when a draining tube is placed it
should be in line and close to the incision.
Despite its higher diagnostic value, open biopsy is
kept as the last resource as it is expensive, carry a complication
rate of up to 16% and may cause contamination of the incisional
path. At the final surgery for tumor resection, the surgical path
of the biopsy (including 1–3 cm of the skin around the incision and
subcutaneous tissues) should be excised en block with the final
tumor specimen (18).
In case of small superficial lesions, well planned
excisional biopsies with negative margins can be performed
(19). An absolute prerequisite to
decide for an excisional biopsy is the ability to resect the mass
with negative histology margins. The surgeon performing an
excisional biopsy should have measured in detail on pre-op MRI the
size of the lesion and the relation to surrounding tissue. An
exception to this concept, is the benign giant cell tumor of tendon
sheath located in the fingers, where frequently a marginal
resection is performed that may result in a higher local recurrence
rate (20). In contrast to
well-planned excisional biopsy, the ‘unplanned excision’ is defined
as the gross removal of tumor without pre-operative staging or
consideration for the need for removal of normal tissue around the
tumor (21). Unplanned excisional
biopsies of the upper extremity, frequently leave microscopic
residual disease requiring a more aggressive and debilitating
subsequent treatment in up to 45% of cases (22).
Once the diagnosis of malignancy is suspected or
established, staging is preferably performed by computed tomography
(CT) of the thorax and abdomen whereas positron-emission tomography
(PET) is reserved for selected cases (23).
General considerations on therapeutic
approach
Compared to tumors of the lower limb, upper
extremity tumors tend to be smaller, more often superficial
(3) and more likely to undergo
unplanned excision (24).
Oncological surgery represents a major challenge in most cases. The
complex and intimate surgical anatomy of tendons, vessels and
nerves jeopardizes both the success of appropriate surgical margins
and the postoperative loss of function (25). An unplanned excision of upper-limb
tumors tends to have a higher rate of positive surgical margins,
along with a significant higher rate of local recurrence when
compared to lower-limb tumors (3).
The addition of adjuvant chemotherapy (26) and radiotherapy (27) has been reported to improve the
outcomes of surgery in unselected patients with STS. As a result,
limb-sparing surgery is performed in around 90% of patients with
local recurrence rates similar to those observed following
amputation (28). Centralized,
multidisciplinary diagnosis and treatment in dedicated, high-volume
centers is directly related to significantly better survival rates
and quality of care for patients, underlying the importance of
sarcoma centers of excellence (29).
Treatment of localized disease
Surgery
Until late 1970s, amputation was regularly performed
for localized STS of the extremities, on account of higher rates of
local recurrence (30). Enneking
et al (31), described four
types of resection margins: Intralesional, marginal within the
reactive zone, wide with a cuff of normal tissue and radical
resection involving excision of the entire anatomical compartment.
Through decades, we moved from the surgical principle of resection
of the involved anatomic compartment, to tumor free resection
margins as a minimum of acceptable resection. Although a wide soft
tissue envelop in all directions around the tumor is desirable, the
feasible goal is resection to negative margins (1 mm from the inked
resection margin) (32). Still, for
STS of the hand, amputation of a digit may be necessary to obtain
clear margins.
Currently more than 90% of STS can be treated with
local resection and limb salvage. However, a primary amputation
should be considered when the tumor cannot be excised to clear
margins, based on the pre-op imaging. Extensive soft tissue
infiltration and/or involvement of a major neurovascular bundle
frequently result in amputation. A primary amputation should be
considered when tumor resection results in significant loss of soft
tissue with severe function impairment, that cannot be
reconstructed with available surgical techniques, or the expected
complication rate will be high.
Suboptimal biopsies and positive resection margins
are associated with local and distant disease recurrence in
patients with hand STS (22).
Pradhan et al (33), reported
on 63 patients with hand STS. Six patients underwent below elbow
amputation and 12 patients had partial amputation. All the
amputated patients had clear margins, while 42% out of patients
with local tumor excision had involved margins (33). Single ray amputations (excluding
thumb) for hand tumors have a low local recurrence rate and high
functional scores (34). However,
ray transposition should not be performed with ray amputation for
tumor excision. In order to achieve negative margins, wider
resection may be needed. Double ray amputation results in worse
functional outcome than single ray. Good key, tip and tripod pinch
can nonetheless be maintained when the deep motor branch of the
ulnar nerve is preserved, and this hand can still assist in
bimanual hand activities (35).
Preoperative radiation therapy (RT) is useful in
cases where the tumor mass is in contact to nerves and vessels, as
it may facilitate negative margin resection by inciting a thicker
reactive fibrous tumor pseudocapsule, which can be dissected from
the neurovascular bundle (36).
Clarkson et al (37),
concluded that meticulous sharp epineural dissection of the ischial
nerve combined with RT is a safe technique and nerve preservation
can be attempted when the tumor does not encase the nerve trunk.
Although there are no randomized studies available, RT and
epineural dissection is the common practice for STS of the upper
extremity abutting on major nerves.
When the tumor mass surrounds important vessels,
limb-sparing surgery can be performed as an en bloc
resection of the sarcoma and vessels with vascular reconstruction.
For large diameter vessel reconstruction, the great saphenous vein
is usually harvested, reversed and anastomosed proximally and
distally to restore anatomic continuity and circulation (38).
Contact of the mass with the bone is a common
finding in MRI of large, deep STS and invasion of the bone cortex
can be found in cases of SS and UPS. Cortical and medullary signal
intensity changes and cortical destruction observed on T1 and
T2-weighted MR images are highly sensitive and specific signs of
osseous invasion by STS (39). A
study from Mount Sinai reported that true bone invasion occurs in a
5.5% and it is associated with increased metastatic disease at
presentation and decreased overall survival (OS) (40). Preoperative RT enables resection of
the mass with periosteum serving as the deep margin, without
expecting increased recurrence rate (41). For STS invading the bone, or when
negative margins cannot be achieved using periosteum as a deep
margin, en bloc resection of the soft tissue mass with the
affected bone should be performed. For segmental bone defects,
reconstruction can be done with either avascular bone autograft,
allograft, a vascularized fibula graft or a hybrid reconstruction
of an allograft combined with a free vascularized fibula graft.
Flap reconstruction is an essential part of STS
surgical treatment (42).
Tensionless primary wound closure is important to avoid wound
healing complications, especially if preoperative RT has been
administered. For small size soft tissue defects, wound closure can
be achieved either by simple sutures or muscle approximation and
split thickness skin grafting. Flap coverage is essential in the
case of exposed vessels, nerves or bone. Flap usage is also
important in the prevention or management of wound healing
complications (43). Frequently used
flaps are the lateral arm flap, the radial forearm,
anterior-lateral thigh and latissimus dorsi flap (42). Vasileios et al (16) reported on 57 patients with soft
tissue malignant fibrous histiocytoma. A rotational or free flap
was eventually needed in 28 patients. A major wound complication
occurred in 17% of patients. All complications were related to
preoperative RT and 90% involved the lower limbs. Wound breakdown
was associated with infection in 50% of cases (16).
Prognostic factors
Metastatic relapse after complete surgery occurs in
around 40% of patients, leading to death from the disease within
the first 8 years after initial diagnosis (44). Several prognostic factors have been
identified to assess the probability of recurrence after surgery.
High histological grade, size >5 cm, deep location and positive
surgical margin status have been characterized as the most
important poor prognostic factors (45). Of them, histological grade has been
pointed out as the factor with the heaviest prognostic impact for
systemic control after surgery (46), while surgical margin status has been
described as the most important factor for local control (47). In order to reduce the high
probability of relapse, complementary radiation therapy and
chemotherapy may be applied.
Radiation therapy
Radiation therapy (RT) is a crucial adjunct to
surgery for STS of the extremities. The most important outcome by
the use of RT is the local control of the disease, but this is not
associated with a significant reduction in distant metastasis or
improvement in disease-specific survival (48). There are several RT modalities
applied such as external beam radiation therapy (EBRT),
intensity-modulated radiation therapy (IMRT), intraoperative
radiation therapy (IORT) and brachytherapy.
External beam radiation therapy
The administration of preoperative or adjuvant EBRT
in order to avoid amputation is supported by evidence reported from
several clinical trials. Selected randomized prospective clinical
trials are shown in Table III. The
addition of EBRT after LSS attains similar results as amputation
and significantly reduces the local-recurrence rate of LSS alone
(27). The benefit of adjuvant EBRT
seems higher for STS with poor prognostic factors, and data suggest
that it might be omitted in patients with completely resected
low-risk STS (49,50). Significant differences in toxicity
have been reported with the use of postoperative EBRT compared to
LSS alone with respect to edema, limb strength and range of motion.
Preoperative EBRT, is significantly related to greater acute
toxicity and major wound complications than postoperative EBRT,
without differences in local-relapse rates and long-term OS
(51).
 | Table III.Safety and efficacy of selected
randomized clinical trials of external-beam radiation therapy in
STS of the upper extremities. |
Table III.
Safety and efficacy of selected
randomized clinical trials of external-beam radiation therapy in
STS of the upper extremities.
| Authors, year | Methods | N | Efficacy
outcomes | Safety
outcomes | (Refs.) |
|---|
| Rosenberg et
al, 1978 | Amputation vs.
LSS/EBRT | 41 | 5y DFS: 78% vs. 71%
(P=0.75); 5y OS: 88% vs. 83% | No improvement in
quality of life. | (27) |
| Yang et al,
1998 | LSS followed by
EBRT vs. no adjuvant treatment | 91 | HGSTS: 10y LRR, 0%
vs. 19% (p=0.003); 10y OS: 75% vs. 74%. | Persistent
reduction in joint motion. Transient increase in edema and limb
weakness. | (49) |
|
|
|
| LGSTS: 10y LRR,
3.8% vs. 33.3% (p=0.016); 10y OS, 3.8% vs 8.3% |
|
|
| O'Sullivan et
al, 2002 | Preoperative EBRT
vs. postoperative EBRT | 190 | 5y LRR 93% vs. 92%
(P=0.79); 5y DFS 58% vs. 59% (P=0.83); 5y OS 73% vs. 67%
(P=0.48) | Major wound
complications: 35% vs. 17% (P=0.01). | (100) |
As complex trade-off issues are involved in the
sequencing of LSS and RT for patients with localized STS of the
extremities, it seems important to define subsets of patients who
might be adequately treated by surgery alone and the optimal
sequence of surgery and EBRT for patients who require both types of
local therapy (52). An attempt has
been made to develop a nomogram to quantify the 3- and 5-year risk
of local recurrence after LSS without postoperative EBRT that
includes age, size, margin status, grade of tumor and histology
(53).
Intensity-modulated radiation
therapy
The main advantage of IMRT is its ability to deliver
high dose RT to the tumor minimizing the dose of RT to the
surrounding normal structures. Such a tight margin might compromise
tumor coverage and result in a higher rate of local recurrences. A
retrospective analysis from the Memorial Sloan Kettering Cancer
including 41 patients with poor prognostic factors (34 patients
with high grade STS, 21 patients with infiltrated/close <1 mm
surgical margins), treated with preoperative (7 patients) or
postoperative IMRT (34 patients), reported encouraging data on
acute and late toxicity. The 5-year local control rate was 94%,
which compares favorably with that of historical controls ranging
from 82% in negative margins to 51% in positive margins (54).
A retrospective comparative study including 319
patients with STS of the extremities treated with postoperative
EBRT (154 patients) or IMRT (165 patients) with similar dosing
schedules indicated that IMRT was associated with significantly
reduced local recurrence compared with conventional EBRT (55).
A following prospective phase II study included 80
patients with localized STS of extremities (51 patients) and trunk
wall (29 patients). After treatment with function-conserving
surgery and postoperative IMRT, an excellent local control was
assessed, with low IMRT-associated toxicity such as edema and joint
stiffness (56). As a result, IMRT
is a promising RT approach in STS of the extremity as it provides
excellent local control in a group of patients with high-risk
features with a beneficial effect in sparing the surrounding normal
tissue.
Intraoperative radiation therapy
Intraoperative RT consists of a single large dose of
RT, administered during LSS after tumor removal and prior to wound
suturing. As a result, the tumor bed can be irradiated directly
sparing the surrounding normal tissue. IORT is usually combined
with postoperative RT, but special hospital infrastructures are
required. IORT used as a boost to EBRT seems to provide excellent
local control with only mild acute side effects, as indicated by a
retrospective study of 17 patients with STS of upper or lower
extremities (57).
A more recent retrospective analysis of 61 patients
with upper-extremity STS treated with surgery, IORT (12.50 Gy) and
EBRT (45–50 Gy), associated this strategy with excellent local
control, limb preservation and survival, even in patients with
positive margins. Only 4 patients developed RT-associated toxicity
(58).
Brachytherapy
Brachytherapy is the direct application of
radioactive sources into the tumor bed through catheters, which
allows a high dose of RT to the tumor in a more conformal way
compared to EBRT. This is translated in shorter treatment periods,
fewer side effects and a faster recovery. It permits evaluation at
the time of surgery and complications can be avoided by sparing the
surrounding tissues (59).
A prospective trial randomized 164 patients to
receive either adjuvant brachytherapy or no further RT after
complete resection of STS of the extremity (60). The RT was administered by iridium-192
implants, which delivered 42 to 45 Gy over 4 to 6 days. The results
indicated that in patients with high-grade histologies
brachytherapy provides convenient means to complete RT within a
short period with no long-term functional sequelae and with a local
control benefit comparable to that obtained with more protracted
courses of EBRT. These data suggest that brachytherapy is an
effective alternative to EBRT.
Adjuvant and preoperative
chemotherapy
Chemotherapy for resectable STS of the extremities
has been evaluated in both the adjuvant and the preoperative
settings.
Adjuvant chemotherapy
The data of nearly twenty clinical trials, carried
out from 1980's to 2008, have been gathered in two different
meta-analyses (61,62). Tierney et al (61) showed that adjuvant chemotherapy for
unselected patients results in an absolute benefit at 10 years of
4%. When subgroup analysis was carried out, sarcoma of the
extremities had an absolute benefit at 10 years of 7% (p=029)
(61). In a more recent
meta-analysis, Pervaiz et al (62) evaluated data from 1,953 patients
receiving postoperative chemotherapy. According to this
meta-analysis, administration of adjuvant anthracyclines and
ifosfamide leads to a significant reduction in the risk of
recurrence and death of 10 and 11%, respectively (62).
Conversely, the largest, phase III, placebo
controlled, clinical trial for adjuvant chemotherapy in STS, which
randomized 351 patients to receive or not 5 cycles of adriamycin
and ifosfamide after surgery, found no significant differences,
either in relapse free survival or in OS. However, a clear
advantage of chemotherapy can be inferred from the forest plot for
extremities, grade 3 and greater size (63).
Preoperative chemotherapy
The role of preoperative chemotherapy was first
evaluated retrospectively, showing a positive effect mainly for
patients with deep, high-grade tumors over 10 cm (64). Prospectively, a large phase III
clinical trial randomized 328 high-risk patients to receive
neoadjuvant epirubicin 120 mg/m2 and ifosfamide 9
g/m2 for 3 or 5 cycles (65). It was confirmed that 3 cycles do not
worsen survival rates (5-year OS of 68% with 3 cycles versus 70%
with 5 cycles), and they are comparable to those seen with the same
combination in the adjuvant setting (66). The addition of RT was permitted and
subgroup analysis of patients with affected surgical margins showed
a local relapse rate of 17% if RT was administered after surgery
versus 0% when it was performed before (67).
The unselected populations of the previously
mentioned clinical trials constitutes a major hindrance when
deciding the best therapeutic option for sarcoma patients. Evidence
indicates that chemotherapy is more useful for sarcomas of the
extremities and the trunk wall, but no differences between upper
and lower limbs have been reported. In the absence of clinical
trials with selected populations, chemotherapy for STS of the upper
limb should be administered for chemosensitive histologic subtypes
when poor prognostic factors are present.
Despite the preoperative treatments described
hitherto, some cases are not amenable but with amputation of the
extremity. These cases led to the exploration of other methods in
an attempt to improve the LSS. The isolated limb perfusion (ILP)
consists in the administration of chemotherapy after separating the
circulation of a limb from that of the rest of the organism. The
introduction of TNF-α in combination with melphalan after some
frustrating results of ILP with doxorubicin (68) allowed the LSS in 76% of patients who,
otherwise, would have required amputation (69).
Follow-up and recurrence
As for STS of other locations, follow-up of STS of
the upper extremities has the objective of controlling the sequelae
from the administered treatments as well as the detection of local
or metastatic relapse. Thus, follow-up is also an important part of
the multidisciplinary approach, since early and late complications
from surgery, radiotherapy and chemotherapy have to be diagnosed
and treated promptly.
Surveillance for uncovering local and metastatic
relapse is a controversial subject. Local recurrence of the upper
extremity is easily detected by physical examination and even by
self-examination, which calls into question the routine use of
image tests of the limb (70).
Surveillance periodicity varies between clinical guidelines and it
depends on several factors, such as histologic grade and subtype or
even the experience of every sarcoma center. Despite the tendency
to use high-definition image tests, it seems that chest CT does not
add a benefit to the use of simple X-ray in the detection of
resectable pulmonary metastases (71). However, no prospective trials have
determined to date the best surveillance strategy for the detection
of both local and metastatic relapse of STS.
Local relapse of a previously treated STS of the
upper extremities may not be amenable to re-excision, but the
option of radiation and even re-radiation could be possible in
selected cases (72).
Similarly, the therapeutic strategy for metastatic
disease depends on the number and the site of metastases,
potentially managed with local treatments, and on the histologic
subtype, potentially sensitive to systemic therapy. The lung is the
most common site of metastases, as up to 80% of metastatic STS
present with lung metastases (73).
Although prospective and randomized studies are lacking, pulmonary
metastasectomy with complete resection of all disease burden may be
considered for selected patients. According to different reported
series, pulmonary metastasectomy attains a 5-year survival rate of
15 to 50.9% (74). The complete
resection of all metastases is the most important prognostic
factor, as it duplicates the survival compared to that of
incomplete resection (75). The
number of metastases has also been indicated as an important
prognostic factor, though the maximum number of metastases
contraindicating the surgery has not been established (74).
Chemotherapy and targeted therapy for
metastatic STS of the upper extremities
After multidisciplinary curative treatment, the risk
of metastatic relapse within the next 2 years after surgery is as
high as 46% (29). Thus, improvement
in the treatment of metastatic disease is imperative.
If not amenable to salvage surgical procedures,
treatment of metastatic sarcoma is still based primarily on
chemotherapy. Despite the intrinsic heterogeneity, clinical trials
have often been designed for all histological subtypes taken
together. Since the decade of the 80 s, anthracyclines have been
the drug class of choice for the first line treatment, attaining a
limited response rate of under 25% at conventional dose of 60–75
mg/m2 (76). In order to
optimize the effectiveness of treatment, combinations of
doxorubicin and ifosfamide have been investigated at different
doses. Effectiveness of the combination has been reported superior
at conventional doses (doxorubicin 60–75 mg/m2 and
ifosfamide up to 9 g/m2) at the expense of a greater toxicity,
without significant improvement in OS (77,78).
High doses of doxorubicin and ifosfamide have also been tested,
resulting in a better clinical benefit and progression-free
survival (PFS) with the counterpoint of a mainly hematological
greater toxicity (79).
Combination of doxorubicin and olaratumab, showed a
significant improvement in OS that could not be confirmed in the
recently reported, phase III clinical trial ANNOUNCE (NCT02451943),
which did not meet its primary endpoint of OS (80).
Following failure on preoperative, adjuvant or
first-line chemotherapy with anthracyclines, therapeutic options
for STS include other cytotoxic agents, tyrosine-kinase inhibitors
(TKI) and immunotherapy (81).
Monotherapy with trabectedin at 1, 5 mg/m2 in a
continuous 24-hour infusion every 3 weeks is an approved second
line option after anthracyclines, achieving a median PFS of 4.2
months (82). Combinations of the
antimetabolite gemcitabine, with docetaxel (83) or dacarbazine (84) achieve a median PFS of 6.2 and 4.2
months, respectively. The TKI pazopanib, targeting the vascular
endothelial growth factors (VEGF) 1–3, the PDGFR A and B and
mast/stem cell growth factor receptor KIT, was tested in a
randomized phase III clinical trial and a median PFS of 4.6 months
was found (85).
As several reports have indicated differential
responses to the available drugs, the choice of specific
therapeutics may vary according to histologic subtypes (Table IV) and toxicity (Table V).
 | Table IV.First and second line options for the
predominant histological subtypes of STS of the upper
extremities. |
Table IV.
First and second line options for the
predominant histological subtypes of STS of the upper
extremities.
| Sarcoma type | First line | Second and further
lines | Drugs under
investigation |
|---|
| UPS | Doxorubicin ±
Ifosfamidea |
Gemcitabine-Docetaxelb; Ifosfamidec; Trabectedind; Pazopanibe |
Pembrolizumabf |
| SS | Doxorubicin ±
Ifosfamidea |
Ifosfamidec; Trabectedind; Pazopanibe | Tazemetostat |
| ES | Doxorubicin ±
Ifosfamidea |
Gemcitabine-Docetaxelb; Pazopanibe; Trabectedind | Tazemetostat |
| CCS | – | – |
Caffeine-potentiated doxorubicin;
Sorafenib; Sunitinib; Tinvatinib |
 | Table V.Selection of the toxicities of
principal available drugs for soft tissue sarcomas. |
Table V.
Selection of the toxicities of
principal available drugs for soft tissue sarcomas.
|
| Frequency of
toxicities |
|---|
|
|
|
|---|
| Drug | Very common and
common | Uncommon | Rare and very
rare |
|---|
| Doxorubicin | Myelosuppression,
Cardiotoxicity | Dehydration | Tissue
necrosis |
| Ifosfamide | Myelosuppression,
Hepatotoxicity, Hemorrhagic cystitis, Acute renal failure | Peripheral
neuropathy, Stomatitis | CNS toxicity,
Dermatitis |
| Gemcitabine | Myelosuppression,
Elevation of liver transaminases, Allergic skin rash,
Influenza-like symptoms | Interstitial
pneumonitis | Anaphylactoid
reaction, PRES, Capillary leak syndrome |
| Docetaxel | Myelosuppression,
Hypersensitivity, Peripheral neuropathy, Fluid retention | Arthralgia,
Elevation of liver transaminases | Cardiotoxicity |
| Trabectedin | Myelosuppression,
Elevation of liver transaminases, PPEDS | Capillary leak
syndrome, Pulmonary edema | Hepatic
failure |
| Pazopanib | Hypothyroidism,
Hypertension, Hair color change, Elevation of liver transaminases,
Diarrhea | Hypomagnesaemia,
Retinal detachment, Cardiotoxicity, Intestine perforation | Thrombotic
microangiopathy, Posterior reversible encephalopathy,
Pneumonitis |
Undifferentiated pleomorphic
sarcoma
The UPS subtype has been included in almost all
clinical trials that led to the approval of second line therapies.
Anthracyclines, ifosfamide, gemcitabine and trabectedin are active
drugs in this histology but UPS is primarily sensitive to the
combination of doxorubicin and ifosfamide (86). Based on a phase II study, the
combination of gemcitabine with docetaxel was considered to be
active in UPS (83), but later a
larger phase III study confirmed that the combination of epirubicin
with ifosfamide was more effective (87). Despite the meager research of
immunotherapy in STS, a small phase II study testing the anti-PD1
antibody pembrolizumab in UPS showed 40% objective response rate
(88), placing pembrolizumab a
potential therapeutic option for the future.
Synovial sarcoma
Although SS is particular sensitive to ifosfamide
(89), high dose of ifosfamide is
not superior to the combination of anthracycline and ifosfamide in
the first line (87). After
first-line chemotherapy, trabectedin monotherapy showed better
responses for SS compared to other STS subtypes (90), as well as the TKI pazopanib (85).
Research on new targeted agents has led to the
exploration of potential molecular targets. In SS, the specific
gene fusion SYT-SXX leads to the hyperexpression of intracellular
pathways involved in survival and metastases, highlighting a number
of potential targets (91). The
oncogenic fusion SYT-SXX results in SMARCB1/INI1 proteolytic
degradation, boosting the action of EZH2 on heterochromatin
(92). Tazemetostat, an
EZH2-inhibitor, has shown activity in preliminary results of a
phase 1/2 clinical trial (NCT02601950) (93) and a phase 2 trial (NCT02601950) is
currently ongoing.
Epithelioid sarcoma
Due to its rarity, prospective data on effectiveness
of chemotherapeutic agents in ES are scarce. Retrospective analyses
showed that gemcitabine-based and anthracycline-based chemotherapy
regimens are active in metastatic ES (94). The combination gemcitabine/docetaxel
is thus a second-line option for these patients. Trabectedin has
been reported ineffective in ES (95), and pazopanib showed an inferior PFS
and OS when compared to anthracyclines and to gemcitabine in a
retrospective study (81).
ES is marked by SMARCB1/INI1 deficiency in a 90% of
cases, and patients with this histology are included in the
previously detailed clinical trials with tazemetostat (NCT02601950;
NCT02601950).
Clear cell sarcoma
The CCS is a rare entity without prospective
clinical trials and it is considered a primarily chemo-resistant
sarcoma (81).
No objective responses have been reported with
pazopanib, and only a small case series reporting partial responses
with sorafenib and sunitinib has been reported (96).
Tinvatinib, a MET-inhibitor, has been tested in a
phase II trial with 11 cases where a clinical benefit rate of 36%
and a median PFS of 1.9 months were documented (97). The MET/ALK-inhibitor crizotinib and
several immunotherapeutic options have also been tested in CSS
patients without objective responses (98,99).
Discussion
STS of the upper extremity represent less than 10%
of all STS and affect young patients, with a mean age at diagnosis
of 38 years. Surgery remains the cornerstone of the treatment of
STS of the upper extremities, which tend to be small and
superficial, leading to a higher number of unplanned resections.
This may be the cause of a higher rate of relapse of STS of this
location. Besides, the anatomical particularities represent a
surgical challenge, as wide excision may worsen the functional
outcomes and debilitate this young patient subgroup. Although
amputation could still be an obligatory option for selected cases,
preservation of as many anatomical structures as possible
performing a limb-sparing surgery (LSS) is desirable and represents
the standard of care, using different reconstruction techniques to
safeguard member's functionality.
However, about half of the patients, most commonly
those with poor prognostic factors, who undergo surgery will
relapse within the first 5 years following the intervention.
Adjuvant external-beam radiation therapy (RT)
achieves similar outcomes as amputation when combined with LSS.
Nowadays, different RT methods are available with less toxicity and
similar efficacy but restricted to centers with expertise in the
field. Whether administration of RT is preferable before or after
surgery is an unanswered question. There are no great differences
in terms of effectiveness and preoperative RT is associated with a
higher rate of acute toxicity, but it may be preferred when
downsizing of the tumor is required for increasing the
probabilities of a successful LSS.
Adjuvant chemotherapy improves the survival of
selected patients with STS of the extremities. However, clinical
trials for adjuvant chemotherapy lack of specificity as their
design have not considered the intrinsic heterogeneity of the
disease and the prognostic factors due to the histological rarity
and molecular heterogeneity of the subtypes. Given the greater
incidence of high-grade STS in the upper extremities, it is
expected that most patients with this diagnosis will undergo
adjuvant chemotherapy.
Metastatic disease must be radically treated with
surgery or radiotherapy whenever it is possible, as this practice
may achieve an improvement in overall survival. Systemic therapy
improves survival when radical therapy cannot be accomplished. The
understanding of the underlying biology of each subtype of sarcoma
is essential for the selection of one or another drug in each line
of treatment.
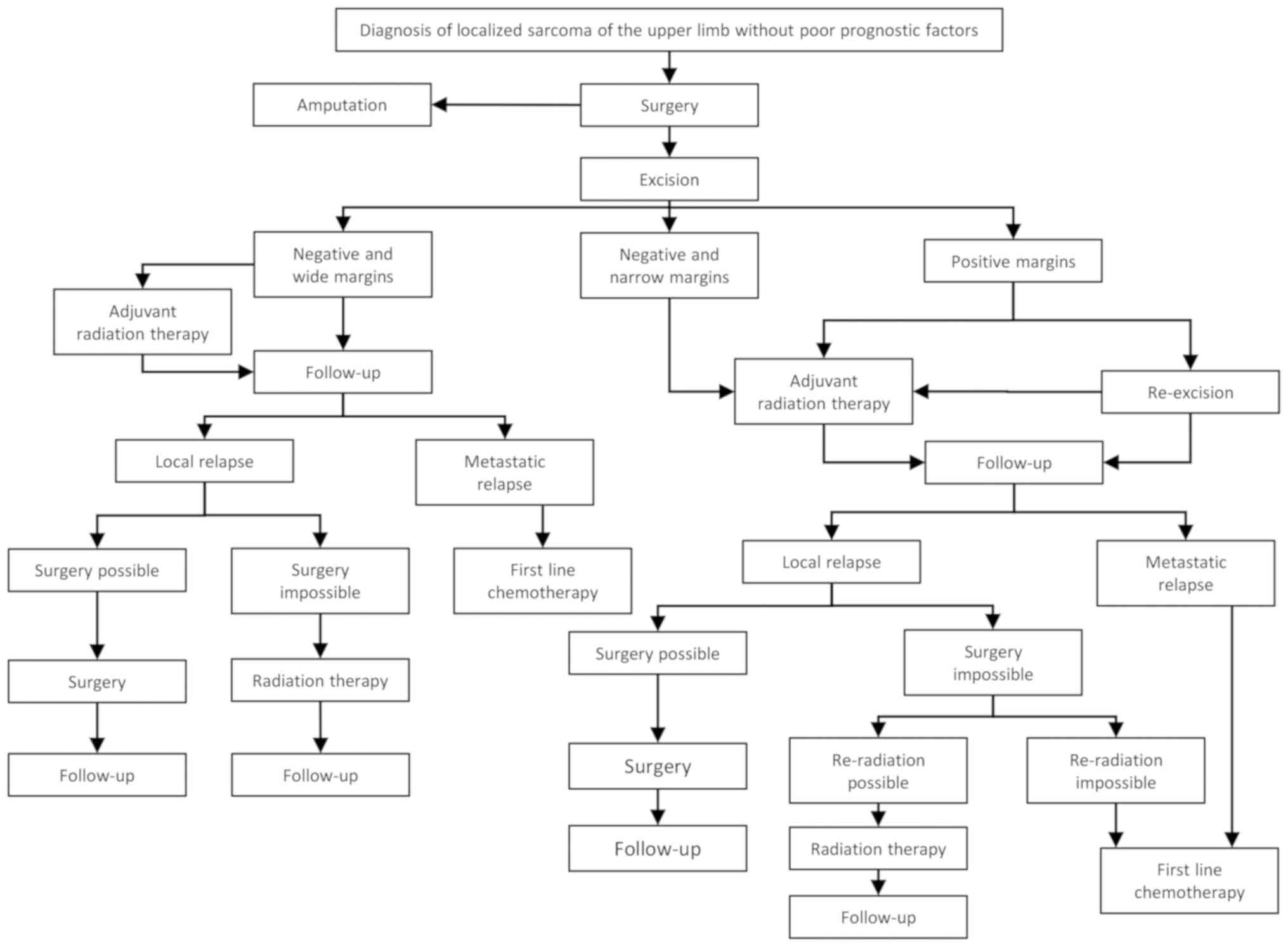
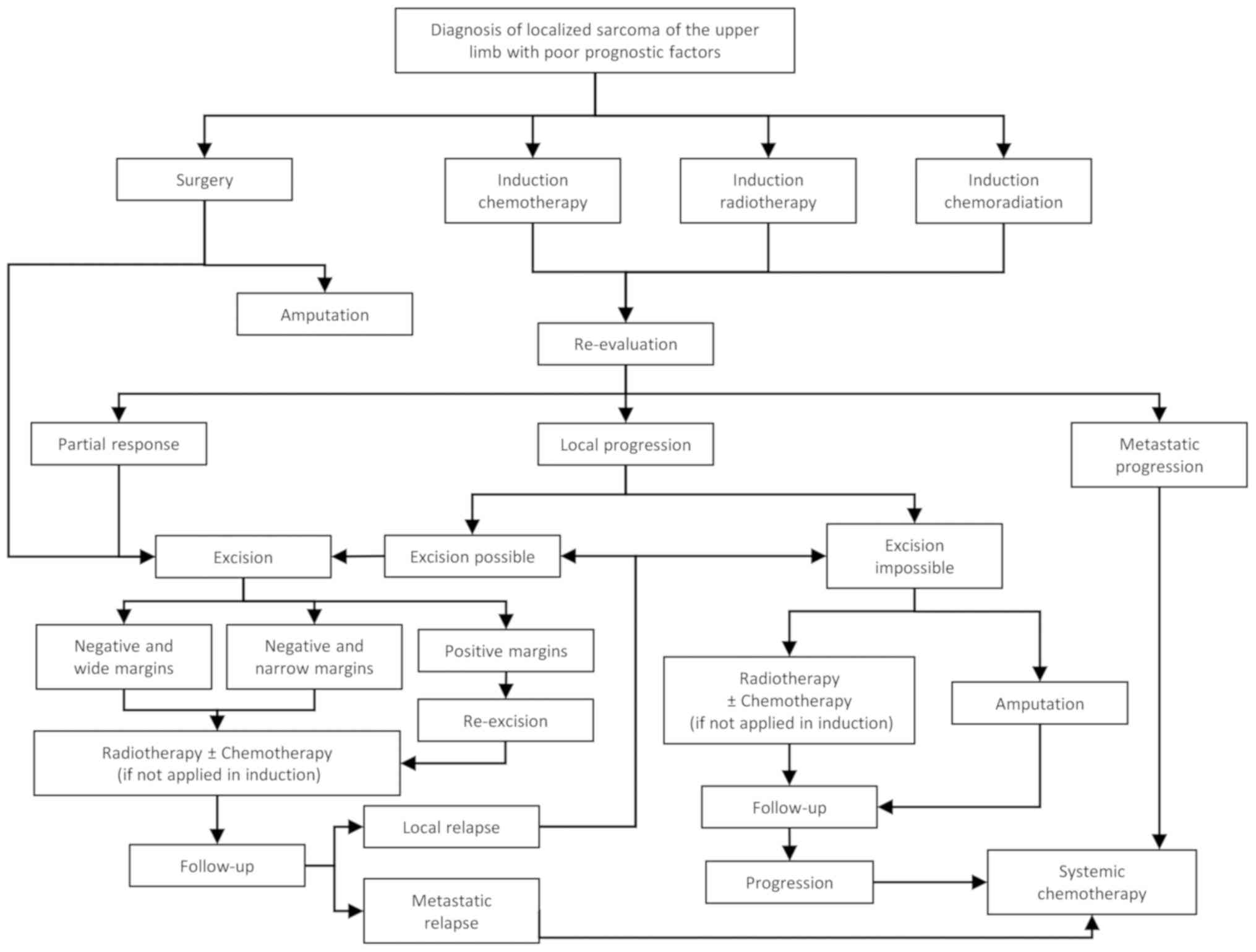
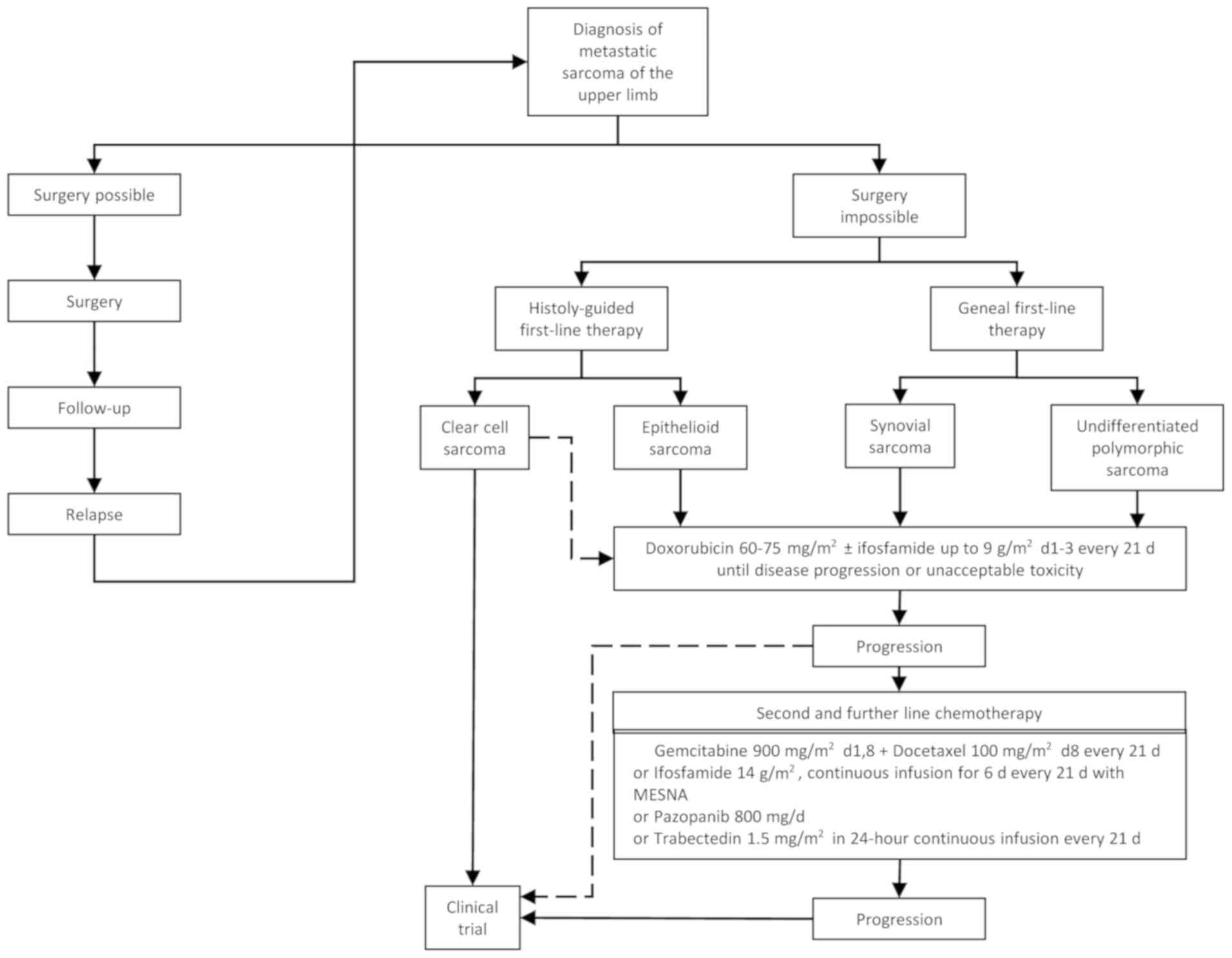
With the information gathered above, the authors
propose a therapeutic algorithm for the treatment of STS of the
upper extremities according to the absence (Fig. 1) or presence of poor prognostic
factors (Fig. 2) in the early
disease stage or metastatic disease (Fig. 3). However, each individual patient
should be discussed in the context of a specialized
multidisciplinary meeting at the earliest possible stage of
diagnosis, in order to establish a radical therapeutic plan,
maximizing the profitability of the procedures and shortening the
time between interventions. Indeed, the presence of a dedicated
tumor board has been associated with an improvement of about 5% in
the 2-year disease-free survival, and its absence has been defined
as a new poor-prognostic factor for STS (29).
STS of the upper extremities represent a challenge
due to anatomical and histopathological particularities, as well as
to their low incidence. Although multidisciplinary treatments have
increased the functional outcomes and the survival of these
patients, a need of improvement of treatment for metastatic disease
is of outmost importance, urging for multinational cooperation for
the recruitment of patients in multicenter clinical trials.
Acknowledgements
Not applicable.
Funding
Funding was received from the Hellenic Study group
of psychoneuroimmunology in cancer.
Availability of data and materials
Not applicable.
Authors' contributions
AK and VK conceived and designed the review. JDM
performed the literature review. All authors were involved in the
preparation and revision of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stiller CA, Trama A, Serraino D, Rossi S,
Navarro C, Chirlaque MD and Casali PG; RARECARE Working Group, :
Descriptive epidemiology of sarcomas in Europe: Report from the
RARECARE project. Eur J Cancer. 49:684–695. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burningham Z, Hashibe M, Spector L and
Schiffman JD: The epidemiology of Sarcoma. Clin Sarcoma Res.
2:142012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gustafson P and Arner M: Soft tissue
sarcoma of the upper extremity: Descriptive data and outcome in a
population-based series of 108 adult patients. J Hand Surg Am.
24:668–674. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gerrand CH, Wunder JS, Kandel RA,
O'Sullivan B, Catton CN, Bell RS, Bell RS, Griffin AM and Davis AM:
The influence of anatomic location on functional outcome in
lower-extremity soft-tissue sarcoma. Ann Surg Oncol. 11:476–482.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Müller DA, Beltrami G, Scoccianti G,
Frenos F and Capanna R: Combining limb-sparing surgery with
radiation therapy in high-grade soft tissue sarcoma of
extremities-is it effective? Eur J Surg Oncol. 42:1057–1063. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fletcher CDM, Unni KK and Mertens F: World
Health Organization Classification of Tumours. Pathology and
genetics of tumours of soft tissue and bone, IARC Press. pp.
Lyon2002
|
|
7
|
Lazerges C: Soft tissue sarcomas of the
forearm, wrist and hand. Hand Surg Rehabil. 36:233–243. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strauss DC, Qureshi YA, Hayes AJ, Thway K,
Fisher C and Thomas JM: The role of core needle biopsy in the
diagnosis of suspected soft tissue tumours. J Surg Oncol.
102:523–529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Korah MP, Deyrup AT, Monson DK, Oskouei
SV, Weiss SW, Landry J and Godette KD: Anatomic tumor location
influences the success of contemporary limb-sparing surgery and
radiation among adults with soft tissue sarcomas of the
extremities. Int J Radiat Oncol Biol Phys. 82:933–939. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dailiana ZH, Bougioukli S, Varitimidis S,
Kontogeorgakos V, Togia E, Vlychou M and Malizos KN: Tumors and
tumor-like lesions mimicking carpal tunnel syndrome. Arch Orthop
Trauma Surg. 134:139–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nandra R, Forsberg J and Grimer R: If your
lump is bigger than a golf ball and growing, think Sarcoma. Eur J
Surg Oncol. 41:1400–1405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Callister MD, Ballo MT, Pisters PW, Patel
SR, Feig BW, Pollock RE, Benjamin RS and Zagars GK: Epithelioid
sarcoma: Results of conservative surgery and radiotherapy. Int J
Radiat Oncol Biol Phys. 51:384–391. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawai A, Hosono A, Nakayama R, Matsumine
A, Matsumoto S, Ueda T, Tsuchiya H, Beppu Y, Morioka H and Yabe H;
Japanese Musculoskeletal Oncology Group, : Clear cell sarcoma of
tendons and aponeuroses: A study of 75 patients. Cancer.
109:109–116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Walker EA, Salesky JS, Fenton ME and
Murphey MD: Magnetic resonance imaging of malignant soft tissue
neoplasms in the adult. Radiol Clin North Am. 491219–1234.
(vi)2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kransdorf MJ, Bancroft LW, Peterson JJ,
Murphey MD, Foster WC and Temple HT: Imaging of fatty tumors:
Distinction of lipoma and well-differentiated liposarcoma.
Radiology. 224:99–104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vasileios KA, Eward WC and Brigman BE:
Surgical treatment and prognosis in patients with high-grade soft
tissue malignant fibrous histiocytoma of the extremities. Arch
Orthop Trauma Surg. 132:955–961. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heslin MJ, Lewis JJ, Woodruff JM and
Brennan MF: Core needle biopsy for diagnosis of extremity soft
tissue sarcoma. Ann Surg Oncol. 4:425–431. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Errani C, Traina F, Perna F, Calamelli C
and Faldini C: Current concepts in the biopsy of musculoskeletal
tumors. ScientificWorldJournal. 013:5381522013.
|
|
19
|
Khoo M, Pressney I, Hargunani R and
Saifuddin A: Small, superficial, indeterminate soft-tissue lesions
as suspected sarcomas: Is primary excision biopsy suitable?
Skeletal Radiol. 46:919–924. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van der Heijden L, Gibbons CL, Hassan AB,
Kroep JR, Gelderblom H, van Rijswijk CS, Nout RA, Bradley KM,
Athanasou NA, Dijkstra PD, et al: A multidisciplinary approach to
giant cell tumors of tendon sheath and synovium-a critical
appraisal of literature and treatment proposal. J Surg Oncol.
107:433–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giuliano AE and Eilber FR: The rationale
for planned reoperation after unplanned total excision of
soft-tissue sarcomas. J Clin Oncol. 3:1344–1348. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Puhaindran ME, Rothrock CP and Athanasian
EA: Surgical management for malignant tumors of the thumb. Hand
(NY). 6:373–377. 2011. View Article : Google Scholar
|
|
23
|
Casali PG, Abecassis N, Aro HT, Bauer S,
Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG,
Brodowicz T, et al: Soft tissue and visceral sarcomas: ESMO-EURACAN
clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 29 (Suppl 4):iv268–iv269. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Serpell JW, Ball AB, Robinson MH, Fryatt
I, Fisher C and Thomas JM: Factors influencing local recurrence and
survival in patients with soft tissue sarcoma of the upper limb. Br
J Surg. 78:1368–1372. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin PP, Guzel VB, Pisters PWT, Zagars GK,
Weber KL, Feig BW, Pollock RE and Yasko AW: Surgical management of
soft tissue sarcomas of the hand and foot. Cancer. 95:852–861.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Perloff M and Holland JF: Surgical
adjuvant chemotherapy. Annu Rev Med. 28:475–488. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rosenberg SA, Kent H, Costa J, Webber BL,
Young R, Chabner B, Baker AR, Brennan MF, Chretien PB, Cohen MH, et
al: Prospective randomized evaluation of the role of limb-sparing
surgery, radiation therapy, and adjuvant chemoimmunotherapy in the
treatment of adult soft-tissue sarcomas. Surgery. 84:62–69.
1978.PubMed/NCBI
|
|
28
|
Alamanda VK, Crosby SN, Archer KR, Song Y,
Schwartz HS and Holt GE: Amputation for extremity soft tissue
sarcoma does not increase overall survival: A retrospective cohort
study. Eur J Surg Oncol. 38:1178–1183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Blay JY, Soibinet P, Penel N, Bompas E,
Duffaud F, Stoeckle E, Mir O, Adam J, Chevreau C, Bonvalot S, et
al: Improved survival using specialized multidisciplinary board in
sarcoma patients. Ann Oncol. 28:2852–2859. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cantin J, McNeer GP, Chu FC and Booher RJ:
The problem of local recurrence after treatment of soft tissue
sarcoma. Ann Surg. 168:47–53. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Enneking WF, Spanier SS and Goodman MA: A
system for the surgical staging of musculoskeletal sarcoma. Clin
Orthop Relat Res. 106–120. 1980.PubMed/NCBI
|
|
32
|
Harati K, Goertz O, Pieper A, Daigeler A,
Joneidi-Jafari H, Niggemann H, Stricker I and Lehnhardt M: Soft
Tissue sarcomas of the extremities: Surgical margins can be close
as long as the resected tumor has no ink on it. Oncologist.
22:1400–1410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pradhan A, Cheung YC, Grimer RJ, Peake D,
Al-Muderis OA, Thomas JM and Smith M: Soft-tissue sarcomas of the
hand: Oncological outcome and prognostic factors. J Bone Joint Surg
Br. 90:209–214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Puhaindran ME, Healey JH and Athanasian
EA: Single ray amputation for tumors of the hand. Clin Orthop Relat
Res. 468:1390–1395. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Puhaindran ME and Athanasian EA: Double
ray amputation for tumors of the hand. Clin Orthop Relat Res.
468:2976–2979. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsukushi S, Nishida Y, Urakawa H, Arai E,
Kozawa E and Ishiguro N: Planned preservation surgery for soft
tissue sarcomas adjacent to critical structures. Arch Orthop Trauma
Surg. 133:481–486. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Clarkson PW, Griffin AM, Cotton CN,
O'Sullivan B, Ferguson PC, Wunder JS and Bell RS: Epineural
dissection is a safe technique that facilitates limb salvage
surgery. Clin Orthop Relat Res. 438:92–96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Radaelli S, Fiore M, Colombo C, Ford S,
Palassini E, Sanfilippo R, Stacchiotti S, Sangalli C, Morosi C,
Casali PG and Gronchi A: Vascular resection en-bloc with tumor
removal and graft reconstruction is safe and effective in soft
tissue sarcoma (STS) of the extremities and retroperitoneum. Surg
Oncol. 25:125–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Elias DA, White LM, Simpson DJ, Kandel RA,
Tomlinson G, Bell RS and Wunder JS: Osseous invasion by soft-tissue
sarcoma: Assessment with MR imaging. Radiology. 229:145–152. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ferguson PC, Griffin AM, O'Sullivan B,
Catton CN, Davis AM, Murji A, Bell RS and Wunder JS: Bone invasion
in extremity soft-tissue sarcoma: Impact on disease outcomes.
Cancer. 106:2692–2700. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin PP, Pino ED, Normand AN, Deavers MT,
Cannon CP, Ballo MT, Pisters PW, Pollock RE, Lewis VO, Zagars GK
and Yasko AW: Periosteal margin in soft-tissue sarcoma. Cancer.
109:598–602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Megerle K and Sauerbier M: Reconstructive
treatment of soft tissue sarcoma of the upper extremity. J Hand
Surg Am. 36:1241–1247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tseng JF, Ballo MT, Langstein HN, Wayne
JD, Cormier JN, Hunt KK, Feig BW, Yasko AW, Lewis VO, Lin PP, et
al: The effect of preoperative radiotherapy and reconstructive
surgery on wound complications after resection of extremity
soft-tissue sarcomas. Ann Surg Oncol. 13:1209–1215. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Weitz J, Antonescu CR and Brennan MF:
Localized extremity soft tissue sarcoma: Improved knowledge with
unchanged survival over time. J Clin Oncol. 21:2719–2725. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stojadinovic A, Leung DHY, Allen P, Lewis
JJ, Jaques DP and Brennan MF: Primary adult soft tissue sarcoma:
Time-dependent influence of prognostic variables. J Clin Oncol.
20:4344–4352. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Italiano A, Delva F, Mathoulin-Pelissier
S, Le Cesne A, Bonvalot S, Terrier P, Trassard M, Michels JJ, Blay
JY, Coindre JM and Bui B: Effect of adjuvant chemotherapy on
survival in FNCLCC grade 3 soft tissue sarcomas: A multivariate
analysis of the French sarcoma group database. Ann Oncol.
21:2436–2441. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gronchi A, Casali PG, Mariani L, Miceli R,
Fiore M, Lo Vullo S, Bertulli R, Collini P, Lozza L, Olmi P and
Rosai J: Status of surgical margins and prognosis in adult soft
tissue sarcomas of the extremities: A series of patients treated at
a single institution. J Clin Oncol. 23:96–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jebsen NL, Trovik CS, Bauer HC, Rydholm A,
Monge OR, Hall KS, Alvegård T and Bruland OS: Radiotherapy to
improve local control regardless of surgical margin and malignancy
grade in extremity and trunk wall soft tissue sarcoma: A
Scandinavian sarcoma group study. Int J Radiat Oncol Biol Phys.
71:1196–1203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang JC, Chang AE, Baker AR, Sindelar WF,
Danforth DN, Topalian SL, DeLaney T, Glatstein E, Steinberg SM,
Merino MJ and Rosenberg SA: Randomized prospective study of the
benefit of adjuvant radiation therapy in the treatment of soft
tissue sarcomas of the extremity. J Clin Oncol. 16:197–203. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Koshy M, Rich SE and Mohiuddin MM:
Improved survival with radiation therapy in high-grade soft tissue
sarcomas of the extremities: A SEER Analysis. Int J Radiat Oncol
Biol Phys. 77:203–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Davis AM, O'Sullivan B, Bell RS, Turcotte
R, Catton CN, Wunder JS, Chabot P, Hammond A, Benk V, Isler M, et
al: Function and health status outcomes in a randomized trial
comparing preoperative and postoperative radiotherapy in extremity
soft tissue sarcoma. J Clin Oncol. 20:4472–4477. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pisters PWT, Pollock RE, Lewis VO, Yasko
AW, Cormier JN, Respondek PM, Feig BW, Hunt KK, Lin PP, Zagars G,
et al: Long-term results of prospective trial of surgery alone with
selective use of radiation for patients with T1 extremity and trunk
soft tissue sarcomas. Ann Surg. 246:675–682. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cahlon O, Brennan MF, Jia X, Qin LX,
Singer S and Alektiar KM: A postoperative nomogram for local
recurrence risk in extremity soft tissue sarcomas after
limb-sparing surgery without adjuvant radiation. Ann Surg.
255:343–347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Alektiar KM, Brennan MF, Healey JH and
Singer S: Impact of intensity-modulated radiation therapy on local
control in primary soft-tissue sarcoma of the extremity. J Clin
Oncol. 26:3440–3444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Folkert MR, Singer S, Brennan MF, Kuk D,
Qin LX, Kobayashi WK, Crago AM and Alektiar KM: Comparison of local
recurrence with conventional and intensity-modulated radiation
therapy for primary soft-tissue sarcomas of the extremity. J Clin
Oncol. 32:3236–3241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang J, Wang S, Song Y, Liu X, Jin J, Wang
W, Yu Z, Liu Y and Li Y: Postoperative intensity-modulated
radiation therapy provides favorable local control and low
toxicities in patients with soft tissue sarcomas in the extremities
and trunk wall. Onco Targets Ther. 8:2843–2847. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tran QNH, Kim AC, Gottschalk AR, Wara WM,
Phillips TL, O'Donnell RJ, Weinberg V and Haas-Kogan DA: Clinical
outcomes of intraoperative radiation therapy for extremity
sarcomas. Sarcoma. 2006:916712006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Call JA, Stafford SL, Petersen IA and
Haddock MG: Use of intraoperative radiotherapy for upper-extremity
soft-tissue sarcomas: Analysis of disease outcomes and toxicity. Am
J Clin Oncol. 37:81–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pellizzon ACA: Evidence and clinical
outcomes of adult soft tissue sarcomas of the extremities treated
with adjuvant high-dose-rate brachytherapy-a literature review. J
Contemp Brachytherapy. 6:318–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pisters PW, Harrison LB, Leung DH,
Woodruff JM, Casper ES and Brennan MF: Long-term results of a
prospective randomized trial of adjuvant brachytherapy in soft
tissue sarcoma. J Clin Oncol. 14:859–868. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tierney JF, Mosseri V, Stewart LA, Souhami
RL and Parmar MK: Adjuvant chemotherapy for soft-tissue sarcoma:
Review and meta-analysis of the published results of randomised
clinical trials. Br J Cancer. 72:469–475. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Pervaiz N, Colterjohn N, Farrokhyar F,
Tozer R, Figueredo A and Ghert M: A systematic meta-analysis of
randomized controlled trials of adjuvant chemotherapy for localized
resectable soft-tissue sarcoma. Cancer. 113:573–581. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Woll PJ, Reichardt P, Le Cesne A, Bonvalot
S, Azzarelli A, Hoekstra HJ, Leahy M, Van Coevorden F, Verweij J,
Hogendoorn PC, et al: Adjuvant chemotherapy with doxorubicin,
ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC
62931): A multicentre randomised controlled trial. Lancet Oncol.
13:1045–1054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Grobmyer SR, Maki RG, Demetri GD, Mazumdar
M, Riedel E, Brennan MF and Singer S: Neo-adjuvant chemotherapy for
primary high-grade extremity soft tissue sarcoma. Ann Oncol.
15:1667–1672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gronchi A, Frustaci S, Mercuri M, Martin
J, Lopez-Pousa A, Verderio P, Mariani L, Valagussa P, Miceli R,
Stacchiotti S, et al: Short, full-dose adjuvant chemotherapy in
high-risk adult soft tissue sarcomas: A randomized clinical trial
from the Italian Sarcoma Group and the Spanish Sarcoma Group. J
Clin Oncol. 30:850–856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Frustaci S, Gherlinzoni F, De Paoli A,
Bonetti M, Azzarelli A, Comandone A, Olmi P, Buonadonna A, Pignatti
G, Barbieri E, et al: Adjuvant chemotherapy for adult soft tissue
sarcomas of the extremities and girdles: Results of the Italian
randomized cooperative trial. J Clin Oncol. 19:1238–1247. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gronchi A, Verderio P, De Paoli A, Ferraro
A, Tendero O, Majó J, Martin J, Comandone A, Grignani G,
Pizzamiglio S, et al: Quality of surgery and neoadjuvant combined
therapy in the ISG-GEIS trial on soft tissue sarcomas of limbs and
trunk wall. Ann Oncol. 24:817–823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wray CJ, Benjamin RS, Hunt KK, Cormier JN,
Ross MI and Feig BW: Isolated limb perfusion for unresectable
extremity sarcoma: Results of 2 single-institution phase 2 trials.
Cancer. 117:3235–3241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Eggermont AM and ten Hagen TL: Isolated
limb perfusion for extremity soft-tissue sarcomas, in-transit
metastases, and other unresectable tumors: Credits, debits, and
future perspectives. Curr Oncol Rep. 3:359–367. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Cheney MD, Giraud C, Goldberg SI,
Rosenthal DI, Hornicek FJ, Choy E, Mullen JT, Chen YL and Delaney
TF: MRI surveillance following treatment of extremity soft tissue
sarcoma. J Surg Oncol. 109:593–596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Rothermundt C, Whelan JS, Dileo P, Strauss
SJ, Coleman J, Briggs TW, Haile SR and Seddon BM: What is the role
of routine follow-up for localised limb soft tissue sarcomas? A
retrospective analysis of 174 patients. Br J Cancer. 110:2420–2426.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Abatzoglou S, Turcotte RE, Adoubali A,
Isler MH and Roberge D: Local recurrence after initial
multidisciplinary management of soft tissue sarcoma: Is there a way
out? Clin Orthop Relat Res. 468:3012–3018. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Potter DA, Glenn J, Kinsella T, Glatstein
E, Lack EE, Restrepo C, White DE, Seipp CA, Wesley R and Rosenberg
SA: Patterns of recurrence in patients with high-grade soft-tissue
sarcomas. J Clin Oncol. 3:353–366. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Marulli G, Mammana M, Comacchio G and Rea
F: Survival and prognostic factors following pulmonary
metastasectomy for sarcoma. J Thorac Dis. 9 (Suppl 12):S1305–S1315.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Smith R, Pak Y, Kraybill W and Kane JM
III: Factors associated with actual long-term survival following
soft tissue sarcoma pulmonary metastasectomy. Eur J Surg Oncol.
35:356–3561. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Borden EC, Amato DA, Rosenbaum C,
Enterline HT, Shiraki MJ, Creech RH, Lerner HJ and Carbone PP:
Randomized comparison of three adriamycin regimens for metastatic
soft tissue sarcomas. J Clin Oncol. 5:840–850. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Edmonson JH, Ryan LM, Blum RH, Brooks JS,
Shiraki M, Frytak S, Frytak S and Parkinson DR: Randomized
comparison of doxorubicin alone versus ifosfamide plus doxorubicin
or mitomycin, doxorubicin, and cisplatin against advanced soft
tissue sarcomas. J Clin Oncol. 11:1269–1275. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Antman K, Crowley J, Balcerzak SP, Rivkin
SE, Weiss GR, Elias A, Natale RB, Cooper RM, Barlogie B, Trump DL,
et al: An intergroup phase III randomized study of doxorubicin and
dacarbazine with or without ifosfamide and mesna in advanced soft
tissue and bone sarcomas. J Clin Oncol. 11:1276–1285. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Judson I, Verweij J, Gelderblom H,
Hartmann JT, Schöffski P, Blay JY, Kerst JM, Sufliarsky J, Whelan
J, Hohenberger P, et al: Doxorubicin alone versus intensified
doxorubicin plus ifosfamide for first-line treatment of advanced or
metastatic soft-tissue sarcoma: A randomised controlled phase 3
trial. Lancet Oncol. 15:415–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lilly Reports Results of Phase 3 Soft
Tissue Sarcoma Study of LARTRUVO®, . https://lilly.mediaroom.com/index.php?s=9042&item=137861January
18–2019
|
|
81
|
Frezza AM, Stacchiotti S and Gronchi A:
Systemic treatment in advanced soft tissue sarcoma: What is
standard, what is new. BMC Med. 15:1092017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Demetri GD, Von Mehren M, Jones RL,
Hensley ML, Schuetze SM, Staddon A, Milhem M, Elias A, Ganjoo K,
Tawbi H, et al: Efficacy and safety of trabectedin or dacarbazine
for metastatic liposarcoma or leiomyosarcoma after failure of
conventional chemotherapy: Results of a phase III randomized
multicenter clinical trial. J Clin Oncol. 34:786–793. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Maki RG, Wathen JK, Patel SR, Priebat DA,
Okuno SH, Samuels B, Fanucchi M, Harmon DC, Schuetze SM, Reinke D,
et al: Randomized phase II study of gemcitabine and docetaxel
compared with gemcitabine alone in patients with metastatic soft
tissue sarcomas: Results of sarcoma alliance for research through
collaboration study 002 [corrected]. J Clin Oncol. 25:2755–2763.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
García-del-Muro X, López-Pousa A, Maurel
J, Martín J, Martínez-Trufero J, Casado A, Gómez-España A, Fra J,
Cruz J, Poveda A, et al: Randomized phase II study comparing
gemcitabine plus dacarbazine versus dacarbazine alone in patients
with previously treated soft tissue sarcoma: A Spanish group for
research on sarcomas study. J Clin Oncol. 29:2528–2533. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Van Der Graaf WT, Blay JY, Chawla SP, Kim
DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP,
Beppu Y, et al: Pazopanib for metastatic soft-tissue sarcoma
(PALETTE): A randomised, double-blind, placebo-controlled phase 3
trial. Lancet. 379:1879–1886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Young RJ, Litière S, Lia M, Hogendoorn
PCW, Fisher C, Mechtersheimer G, Daugaard S, Sciot R, Collin F,
Messiou C, et al: Predictive and prognostic factors associated with
soft tissue sarcoma response to chemotherapy: A subgroup analysis
of the European Organisation for research and treatment of cancer
62012 study. Acta Oncol. 56:1013–1020. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Gronchi A, Ferrari S, Quagliuolo V, Broto
JM, Pousa AL, Grignani G, Basso U, Blay JY, Tendero O, Beveridge
RD, et al: Histotype-tailored neoadjuvant chemotherapy versus
standard chemotherapy in patients with high-risk soft-tissue
sarcomas (ISG-STS 1001): An international, open-label, randomised,
controlled, phase 3, multicentre trial. Lancet Oncol. 18:812–822.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Tawbi HA, Burgess M, Bolejack V, Van Tine
BA, Schuetze SM, Hu J, D'Angelo S, Attia S, Riedel RF, Priebat DA,
et al: Pembrolizumab in advanced soft-tissue sarcoma and bone
sarcoma (SARC028): A multicentre, two-cohort, single-arm,
open-label, phase 2 trial. Lancet Oncol. 18:1493–1501. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lee SH, Chang MH, Baek KK, Han B, Lim T,
Lee J and Park JO: High-dose ifosfamide as second- or third-line
chemotherapy in refractory bone and soft tissue sarcoma patients.
Oncology. 80:257–261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kawai A, Araki N, Sugiura H, Ueda T,
Yonemoto T, Takahashi M, Morioka H, Hiraga H, Hiruma T, Kunisada T,
et al: Trabectedin monotherapy after standard chemotherapy versus
best supportive care in patients with advanced,
translocation-related sarcoma: A randomised, open-label, phase 2
study. Lancet Oncol. 16:406–416. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
de Necochea-Campion R, Zuckerman LM,
Mirshahidi HR, Khosrowpour S, Chen CS and Mirshahidi S: Metastatic
biomarkers in synovial sarcoma. Biomark Res. 5:42017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kadoch C and Crabtree GR: Reversible
disruption of mSWI/SNF (BAF) complexes by the SS18-SSX oncogenic
fusion in synovial sarcoma. Cell. 153:71–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Agulnik M, Tannir NM, Pressey JG, Gounder
MM, Cote GM, Roche M, Doleman S, Blakemore SJ, Clawson A, Daigle S,
et al: A phase II, multicenter study of the EZH2 inhibitor
tazemetostat in adult subjects with INI1-negative tumors or
relapsed/refractory synovial sarcoma. J Clin Oncol. 34:110712016.
View Article : Google Scholar
|
|
94
|
Pink D, Richter S, Gerdes S, Andreou D,
Tunn PU, Busemann C, Ehninger G, Reichardt P and Schuler MK:
Gemcitabine and docetaxel for epithelioid sarcoma: Results from a
retrospective, multi-institutional analysis. Oncology. 87:95–103.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Grivas A, Trafalis DT, Thanopoulou E,
Ziras NG and Athanasiou AE: Treatment with trabectedin: Should be
indicated to all soft tissue sarcoma histotypes? J BUON.
15:791–793. 2010.PubMed/NCBI
|
|
96
|
Cornillie J, van Cann T, Wozniak A, Hompes
D and Schöffski P: Biology and management of clear cell sarcoma:
State of the art and future perspectives. Expert Rev Anticancer
Ther. 16:839–845. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wagner AJ, Goldberg JM, Dubois SG, Choy E,
Lee R, Pappo A, Geller J, Judson I, Hogg D, Senzer N, et al:
Tivantinib (ARQ 197), a selective inhibitor of MET, in patients
with microphthalmia transcription factor-associated tumors: Results
of a multicenter phase 2 trial. Cancer. 118:5894–5902. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Schöffski P, Adkins D, Blay JY, Gil T,
Elias AD, Rutkowski P, Pennock GK, Youssoufian H, Gelderblom H,
Willey R and Grebennik DO: An open-label, phase 2 study evaluating
the efficacy and safety of the anti-IGF-1R antibody cixutumumab in
patients with previously treated advanced or metastatic soft-tissue
sarcoma or Ewing family of tumours. Eur J Cancer. 49:3219–3228.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Goldberg JM, Fisher DE, Demetri GD,
Neuberg D, Allsop SA, Fonseca C, Nakazaki Y, Nemer D, Raut CP,
George S, et al: Biologic activity of autologous,
granulocyte-macrophage colony-stimulating factor secreting alveolar
soft-part sarcoma and clear cell sarcoma vaccines. Clin Cancer Res.
21:3178–3186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
O'Sullivan B, Davis AM, Turcotte R, Bell
R, Catton C, Chabot P, Wunder J, Kandel R, Goddard K, Sadura A, et
al: Preoperative versus postoperative radiotherapy in soft-tissue
sarcoma of the limbs: A randomised trial. Lancet. 359:2235–2241.
2002. View Article : Google Scholar : PubMed/NCBI
|