Introduction
Lung cancer is a malignant lung tumor characterized
by uncontrolled lung cell proliferation (1). It is the most commonly diagnosed cancer
and the leading cause of cancer-associated mortality (2). Therefore, discovering an effective drug
with low toxicity for the treatment of lung cancer is necessary.
Human non-small cell lung cancer (NSCLC) cell line, including human
A549 lung adenocarcinoma epithelial cell line is widely used as a
lung cancer study model (3).
Cryptotanshinone is a natural quinoid diterpene
isolated from the roots of a traditional herb Salvia miltiorrhiza,
which is considered a potent anticancer agent with anticancer
activities (4). In our previous
study, it was reported that cryptotanshinone could inhibit the
growth of human lung cancer cells and induce apoptosis (5). Signal transducer and activator of
transcription 3 (STAT3) is a key transcription activator for cancer
cell proliferation. As a STAT3 inhibitor, cryptotanshinone responds
to STAT3-induced cytokines and growth factors (6,7);
however, to the best of our knowledge, whether STAT3 is the only
target of cryptotanshinone remains unclear.
MicroRNAs (miRNAs/miRs) are small non-coding RNA
molecules that are 22 nucleotides long and function in RNA
silencing and post-transcriptional gene regulation (8). miRNAs serve an important role in lung
cancer (9,10). The principal miRNAs involved in lung
cancer are members of the let-7 family, including miR-34, miR133,
miR-17 and miR-124 (11–13). The present study first investigated
if STAT3 was the only target of cryptotanshinone in lung cancer
cells. It was identified that even without regulation of STAT3
signaling, cryptotanshinone could inhibit the proliferation and
colony formation of lung cancer cells. Based on this result, the
effects of cryptotanshinone on miRNAs were investigated using
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). In addition, the expression levels of matrix
metalloproteinases (MMPs) were studied. Finally, the effects of
cryptotanshinone on lung cancer cell invasion were also detected.
In summary, the findings of the present study provides further
insight into the anticancer effects of cryptotanshinone.
Materials and methods
Chemical treatments and cytotoxic
effects assessment
The human A549 lung adenocarcinoma epithelial cell
line, one of the NSCLC cell lines, is the most common human lung
cancer cell line used for studies regarding lung cancer (1,3). Cell
culture was performed following our previous method (5). Briefly, A549 was propagated in
RPMI-1640 cell culture medium (Gibco; Thermo Fisher Scientific,
Inc.) with L-glutamine, 100 U/ml penicillin, 100 µg/ml
streptomycin, and 10% (v/v) fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.) at 37°C with 5% CO2. A549 cells
(5×103 cells/well) were seeded in a 96-well plate
(Corning, Inc.). Cells were treated with cryptotanshinone (LKT
Laboratories, Inc.) dissolved in 0.1% dissolved in dimethyl
sulfoxide (DMSO), defined as the CT group, at a final concentration
of 20 µM (4) for 24, 36 and 48 h.
The control group was treated with 0.1% DMSO, defined as the NC
group. Colivelin, a STAT3 activator, was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The final concentration of
colivelin (dissolved in DMSO) was 100 µM and cells treated, at
37°C, with colivelin were termed the CV group, while cells treated
with both 20 µM cryptotanshinone and 100 µM colivelin were termed
the CT+CV group. Activity of STAT3 in the NC, CT, CV and CT+CV
groups was detected by enzyme-linked immunosorbent assay (ELISA)
using a STAT3 (Phospho) [pY705] Multispecies ELISA kit (cat. no.
KHO0481; Thermo Fisher Scientific Inc.), according to the
manufacturer's protocol. Following treatment at 37°C for 24, 48 and
72 h the cytotoxic effects of cryptotanshinone and colivelin in the
NC, CT and CT+CV groups were detected using an MTT assay. The
optical density (OD) was measured using iMark™ Microplate
Absorbance Reader (Bio-Rad Laboratories, Inc.) at 450 nm,
representing the activity of STAT3. Colony formation was detected
as described previously (4).
Briefly, A549 cells (n=250) were seeded in 6-well Corning™ Costar™
Flat Bottom Cell Culture Plates (D=35 mm, Corning, Inc.) and
allowed to attach overnight prior to treatment with 20 µM
cryptotanshinone dissolved in DMSO or 0.1% DMSO (control), and were
incubated for 10 days. The colonies were stained with 0.5% crystal
violet in methanol/acetic acid (3:1) and those composed of >50
cells were counted. Experiments were performed three times in
duplicate.
RT-qPCR and western blot analysis
A549 cells were treated with 20 µM cryptotanshinone
or with 0.1% DMSO at 37°C for 6 h, followed by miRNA detection.
miRNA was isolated from the NC, CT and CT+CV groups using an
mirVana™ miRNA Isolation kit with phenol (Thermo Fisher Scientific
Inc.) and converted to complementary DNA using a TaqMan™ MicroRNA
Reverse Transcription kit (cat. no. 4366597; Thermo Fisher
Scientific Inc.). miRNA analysis was conducted using a miScript
miRNA PCR array (cat. no. MIHS-114Z; Qiagen GmbH, Hilden, Germany)
following its instruction. RT-qPCR was performed on Applied
Biosystems™ 7500 Fast Real-time PCR. PCR thermocycling conditions
were as follows: 50°C 2 min; 95°C 2 min; 95°C 15 sec; 60°C 1 min,
40 cycles. Melt curve stage: 95°C 15 sec; 60°C 1 min; 95°C 15
sec.
Western blot analysis
For western blot analysis, 20 µg protein was
extracted using Cell lysis buffer for Western and IP (cat no.
P0013; Beyotime Institute of Biotechnology), determined using BCA
kits (cat no. P0012; Beyotime Institute of Biotechnology) and
boiled with NuPAGE™ LDS Sample Buffer (4X) (Invitrogen; Thermo
Fisher Scientific, Inc.; cat no. NP0007) at 100°C for 5 min prior
to injection in a 12.5% SDS-PAGE. Following electrophoresis and
transfer, the polyvinylidene fluoride (PVDF) membrane (Invitrogen;
Thermo Fisher Scientific Inc.) was blocked in 5% non-fat milk for 1
h, incubated overnight at 4°C with primary antibodies as follows:
MMP14 (dilution, 1:800; cat. no. ab53712), MMP15 (dilution, 1:500;
cat. no. ab15475), MMP16 (dilution, 1:800; cat. no. ab53145), MMP24
(dilution, 1:400; cat. no. ab135564) and tissue inhibitor of
metalloproteinases 2 (TIMP2; dilution, 1:1,000; cat. no. ab199707).
All primary antibodies were purchased from Abcam (Cambridge, UK)
and incubated subsequently with secondary antibody Goat Anti-Rabbit
IgG H&L (HRP) (dilution, 1:5,000; cat. no. ab205718) for 1 h at
room temperature, washed and exposed. GAPDH (dilution, 1:10,000;
cat. no. ab181602) was used as loading control. Membranes were
incubated in Pierce™ ECL Western Blotting Substrate for 1 min at
room temperature (solution A:solution B=1:1; Thermo Scientific
Inc.; cat. no. 32106) and images were captured using LAS-3000
Imaging System (Fuji, Japan). The expression value (target
protein/GAPDH) in the control group was set as 1, while the
relative values for the cryptotanshinone group were calculated in
association with the control group using ImageJ (National
Institutes of Health, Bethesda). All experiments were performed
three times with a duplicate each time. Data are compared between
the NC and CT groups.
Cancer cell invasion assay
A cancer cell invasion assay was conducted using a
Corning® BioCoat™ Matrigel® Invasion Chamber
(Corning Inc., Corning, NY, USA), according to the manufacturer's
protocol at 37°C. The culture medium was Gibco DMEM, High Glucose,
Pyruvate (cat. no. 11-995-040; Gibco; Thermo Fisher Scientific
Inc.) with 10% FBS (cat. no. 10437010; Gibco; Thermo Fisher
Scientific Inc.) for lower chamber and 0.1% BSA (Gibco; Thermo
Fisher Scientific, Inc.; cat. no. 11021029) for upper chamber.
Briefly, the medium was subsequently rehydrated for 2 h, and fresh
culture medium containing 5×104 A549 cells/ml was plated
in the upper 24-well chambers with 0.1% DMSO (NC group) or 20 µM
cryptotanshinone (CT group). The cells were then incubated for 36 h
at 37°C with 5% CO2. Subsequently, the inserts were
transferred to 100% methanol for 2 min and then to 1% Toluidine
Blue in 1% borax for 2 min at room temperature. Three fields/view
were selected randomly and then analyzed. Images were obtained
under Nikon TE2000 light microscope (magnification, ×100; Nikon
Corporation, Japan).
Statistical analysis
Data are presented as the mean ± standard deviation.
A Mann Whitney U test was used for comparisons between two groups.
One-way analysis of variance followed by a post-hoc Tukey's test
was used to compare multiple groups. All statistical analysis was
performed using SPSS 19.0 (IBM Corp., Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Inhibition of STAT3 activity
As demonstrated in Fig.
1A, the OD value representing the STAT3 activity, was
significantly decreased following treatment with cryptotanshinone,
while treatment with colivelin significantly increased the OD 450
value compared with the control group. In the combined treatment
group, the OD value was significantly increased compared with NC
and CT groups, but remained lower than the CV group. These results
indicated that cryptotanshinone could serve as a STAT3 inhibitor
and colivelin as a STAT3 activator.
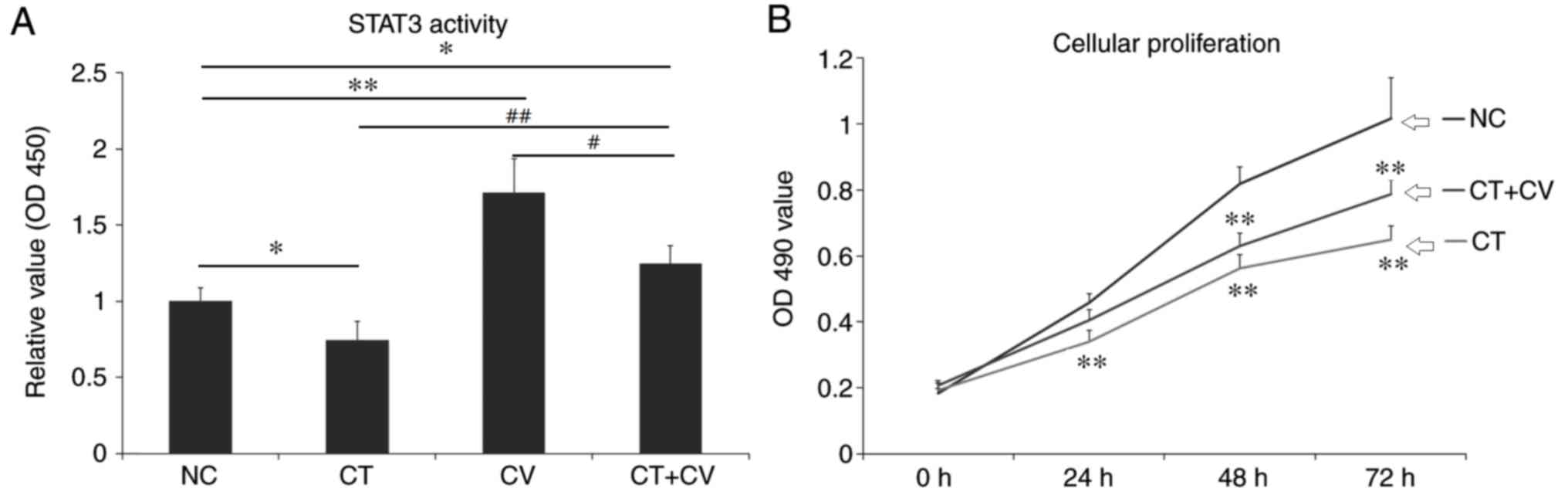 | Figure 1.STAT3 activity and cellular
proliferation of A549 cells. (A) OD 450 values of STAT3 activity in
the CT, CV, CT+CV and NC groups. STAT3 activity significantly
decreased following treatment with cryptotanshinone and
significantly increased following treatment with colivelin. The OD
450 value was higher in the combined treatment group compared with
the NC and CT groups. *P<0.05, **P<0.01 vs. NC group.
#P<0.05, ##P<0.01 vs. CT+CV combined
treatment group. (B) The OD 450 values represent the cellular
proliferation rate of A549 cells in the CT, CT+CV and NC groups.
Following treatment with cryptotanshinone, the cellular
proliferation rate was significantly decreased compared with the NC
group. Combined treatment with colivelin partly reduced the
cytotoxic effects of cryptotanshinone. Data are presented as the
mean ± standard deviation. **P<0.01 vs. NC group. STAT3, signal
transducer and activator of transcription 3; OD, optical density;
CT, cryptotanshinone-treated; CV, colivelin-treated; CT+CV,
cryptotanshinone and colivelin-treated; NC, negative control. |
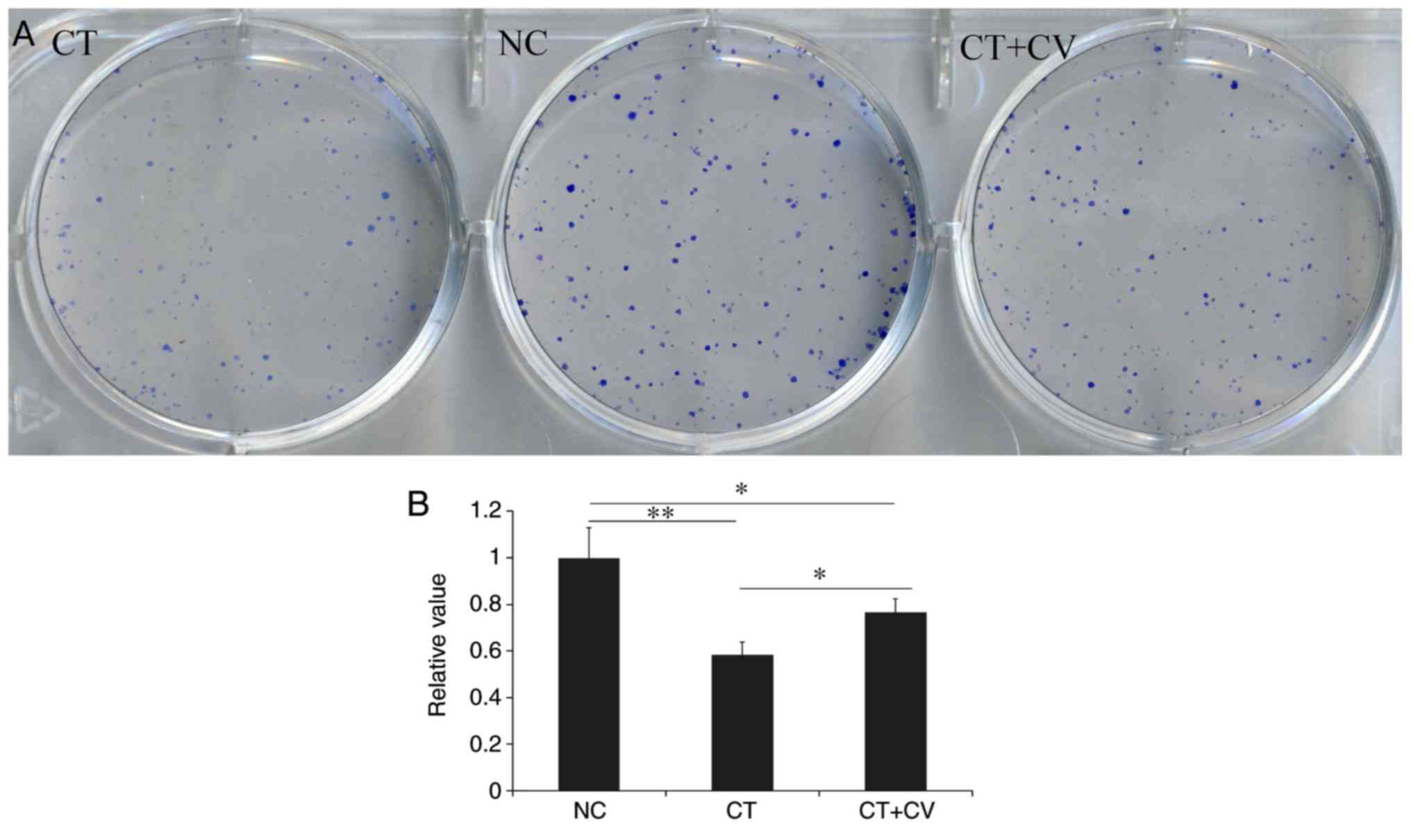
Cytotoxic effects of cryptotanshinone
and colivelin
As demonstrated in Fig.
1B, following treatment with cryptotanshinone for 24, 48 and 72
h the cellular proliferation of A549 cells significantly decreased
compared with the control. Combined treatment with the STAT3
activator colivelin, was revealed to reduce the cytotoxic effects
of cryptotanshinone, however, the cellular proliferation rate
remained lower compared with control group. As demonstrated in
Fig. 2, compared with NC group, the
number and size of colony formation was significantly decreased in
the CT group. Combined treatment with the STAT3 activator
colivelin, significantly increased the number and size of colony
formation compared with the CT group; however, colony formation was
notably reduced compared with the control group. These results
indicate that, in addition to the STAT3 pathway, there may be
another mechanism involved in the cytotoxic effects of
cryptotanshinone on A549 cells. The colony number in NC group was
set as 1 and relative values in other groups were calculated by
comparison. Data are presented in Fig.
2B.
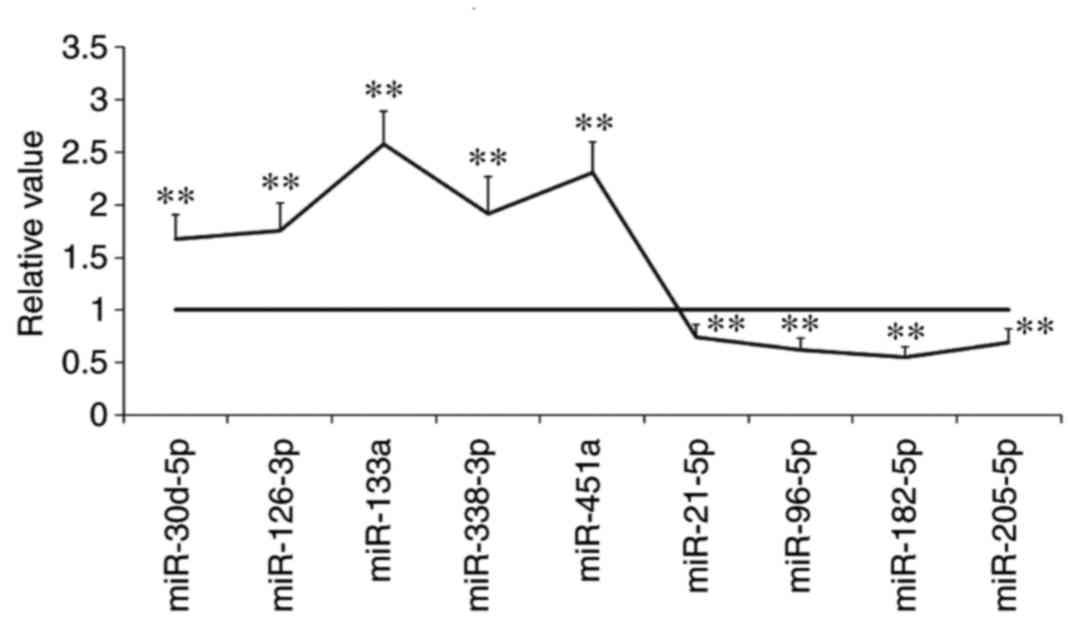
Upregulation of miR-133a following
treatment with cryptotanshinone
As demonstrated in Fig.
3, the following miRNAs were identified to be upregulated in
A549 cells following treatment with cryptotanshinone: miR-30d-5p,
miR-126-3p, miR-133a, miR-338-3p and miR-451a. By contrast, the
following miRNAs were revealed to be downregulated following
treatment with cryptotanshinone: miR-21-5p, miR-96-5p, miR-182-5p
and miR-205-5p. These miRNAs target anti-apoptotic or
pro-anti-apoptotic genes (14).
Among the microRNAs identified, miR-133a was the most significantly
upregulated.
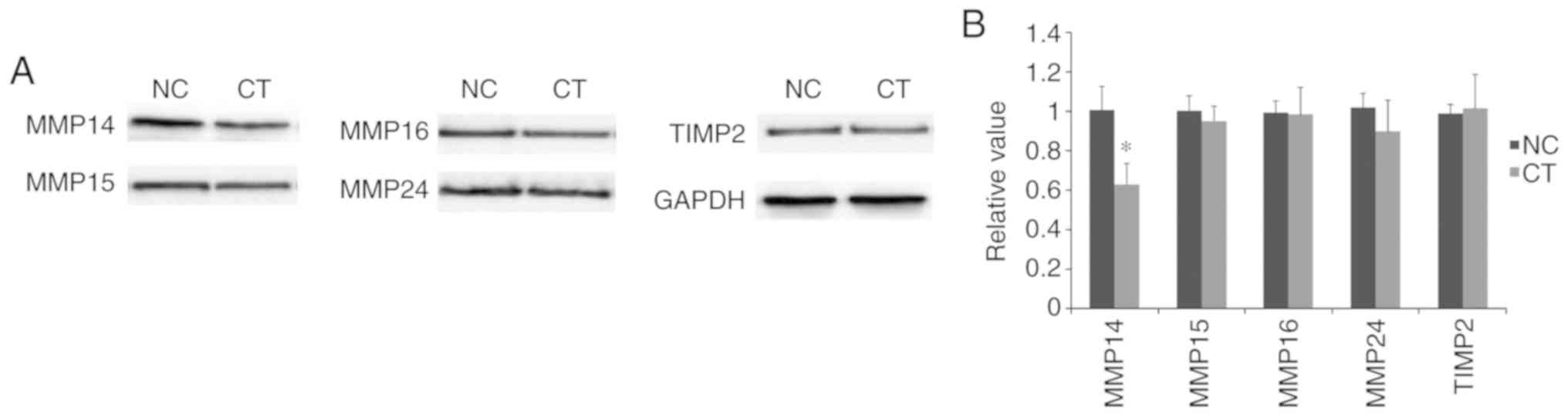
Downregulation of MMP14 following
treatment with cryptotanshinone
As demonstrated in Fig.
4, following treatment with cryptotanshinone, the expression
levels of MMP14 were significantly downregulated compared with the
control. By contrast, no significant differences were identified in
the expression levels of MMP15, MMP16 and MMP24. This suggested the
potential involvement of MMP14 in cryptotanshinone-mediated
cellular apoptosis. In addition, no significant difference was
revealed in the expression of TIMP2 following treatment with
cryptotanshinone. Based on these findings, MMP14 may be considered
as a potential target of miR-133a, which acts independently of
TIMP2 regulation.
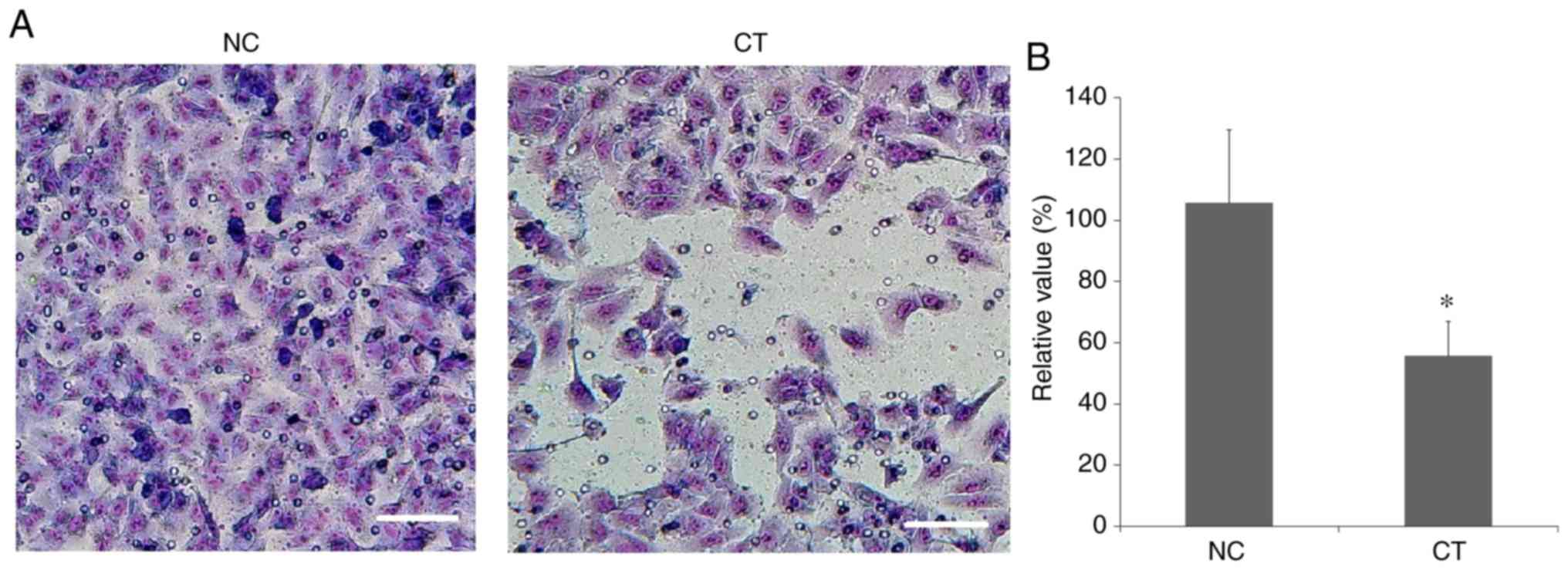
Inhibition of cancer cell
invasion
As demonstrated in Fig.
5, the number of invasive A549 cells was significantly reduced
following treatment with cryptotanshinone. This indicated that the
invasive capability of human lung adenocarcinoma epithelial A549
cells was significantly inhibited following treatment with
cryptotanshinone.
Discussion
As a transcription activator that is translocated to
the cell nucleus, STAT3 is activated following phosphorylation by
receptor-associated Janus kinases (15). STAT3 serves a key role in the growth
and apoptosis of cancer cells, including lung cancer cells
(16). Cryptotanshinone can regulate
mitochondrial function (17). As a
STAT3 inhibitor, cryptotanshinone was demonstrated to inhibit the
proliferation of lung cancer cells in the present study. Colivelin
is a neuroprotective peptide and activator of STAT3 that suppresses
neuronal death by activating STAT3 in vitro (18). The current study identified that even
following activation of STAT3 with colivelin, cryptotanshinone
could inhibit cancer cell growth and colony formation, and promote
cancer cell apoptosis. This suggests that cryptotanshinone may
serve a role independent of the STAT3 pathway.
miRNAs are small non-coding RNA molecules that are
22 nucleotides long, which exhibit functions in RNA silencing and
post-transcriptional regulation of gene expression by targeting
mRNAs. The human genome encodes >1,000 miRNAs in many cell
types, which target >60% of all genes (19). The current study investigated the
effects of cryptotanshinone on a number of miRNAs. miRNA expression
analysis was performed following 6 h of treatment, while protein
expression analysis was performed following 24 h of treatment as
alterations in miRNA expression levels have been reported to occur
at a faster rate compared with alterations in protein expression
levels (20). It was identified that
the expression levels of the following miRNAs were significantly
altered following treatment with cryptotanshinone: miR-30d-5p,
miR-126-3p, miR-133a, miR-338-3p, miR-451a, miR-21-5p, miR-96-5p,
miR-182-5p and miR-205-5p. Among these miRNAs, the expression
levels of miR-133a were the most significantly affected following
treatment with cryptotanshinone.
MMPs, also termed matrixins, are calcium-dependent
zinc-containing endopeptidases (21). MMPs are capable of degrading numerous
extracellular matrix proteins and are involved in the cleavage of
cell surface receptors, the release of apoptotic ligands and
chemokine/cytokine inactivation (22). In addition, MMPs serve key roles in
cell proliferation, migration, differentiation, angiogenesis,
apoptosis and host defense (23,24). The
present study identified that the expression levels of MMP14 were
significantly downregulated following treatment with
cryptotanshinone. Conversely, the expression levels of other type-I
transmembrane proteins, MMP15, MMP16 and MMP24, were not
significantly affected by cryptotanshinone treatment. Therefore, it
can be suggested that MMP14 is a potential target of miR-133a.
MMP14 can interact with TIMP2, an inhibitor of MMPs that is
critical for the maintenance of tissue homeostasis (25,26). The
current study investigated the expression of TIMP2 following
treatment with cryptotanshinone, however, no significant change in
expression level was detected compared with untreated control
cells. This suggests that miR-133a, instead of TIMP2, regulates
MMP14. A previous study has also demonstrated that miR-133a may
target and regulate the expression of MMP14 (27).
The tumor microenvironment is important in the
process of cancer metastasis and can be regulated by many natural
products of herbs (28). Therefore,
in future studies it may be beneficial to investigate the tumor
microenvironment following treatment with cryptotanshinone.
Furthermore, the metabolism of numerous chemicals is dependent on
cytochrome P450, therefore, it may be beneficial to study the
effects of cryptotanshinone on cytochrome P450 reductase, which
serves a key role in cellular proliferation, and astrocytosis in
particular (29).
Cancer metastasis is the leading cause of
cancer-associated mortality, and is defined as the spread of cancer
cells to neighboring tissues and organs beyond the initial tumor
location (30). Cell invasion is the
first and most important step of metastasis, which is a complex
process that involves invasive cancer cells infiltrating nearby
tissues and disseminating to secondary sites through the
extracellular matrix (31,32). To the best of our knowledge, it
remains unclear whether miR-133a directly induces a decrease in
MMP14 expression or indirectly regulates this process via other
factors. Providing that miR-133 is upregulated during this process,
it may be beneficial to use an miR-133 inhibitor in the presence or
absence of cryptotanshinone to determine the mechanism of
regulation in future studies.
In conclusion, the present study identified that
following treatment with cryptotanshinone, the metastatic
capabilities of lung cancer cells were significantly inhibited.
This indicates that cryptotanshinone may serve as a potential
therapeutic agent for the treatment of lung cancer. Further
research regarding this topic is currently being performed in our
lab.
Acknowledgements
Not applicable.
Funding
This study was supported by Science and Technology
Major Project of Gansu Province (grant no. 143FKDH002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YSZ made substantial contributions to the design of
the study. HW, YSZ, YGZ and WL performed the experiments. YGZ and
JW performed the data analysis. HW and YSZ wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Korrodi-Gregório L, Soto-Cerrato V,
Vitorino R, Fardilha M and Pérez-Tomás R: From Proteomic Analysis
to Potential Therapeutic Targets: Functional Profile of Two Lung
Cancer Cell Lines, A549 and SW900, Widely Studied in Pre-Clinical
Research. PLoS One. 11:e01659732016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maj E, Filip-Psurska B, Milczarek M,
Psurski M, Kutner A and Wietrzyk J: Vitamin D derivatives
potentiate the anticancer and anti-angiogenic activity of tyrosine
kinase inhibitors in combination with cytostatic drugs in an A549
non-small cell lung cancer model. Int J Oncol. 52:337–366.
2018.PubMed/NCBI
|
|
4
|
Chen W, Lu Y, Chen G and Huang S:
Molecular evidence of cryptotanshinone for treatment and prevention
of human cancer. Anticancer Agents Med Chem. 13:979–987. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen L, Wang HJ, Xie W, Yao Y, Zhang YS
and Wang H: Cryptotanshinone inhibits lung tumorigenesis and
induces apoptosis in cancer cells in vitro and in
vivo. Mol Med Rep. 9:2447–2452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu L, Zhang S, Li C, Zhou C, Li D, Liu P,
Huang M and Shen X: Cryptotanshinone inhibits human glioma cell
proliferation in vitro and in vivo through SHP-2-dependent
inhibition of STAT3 activation. Cell Death Dis. 8:e27672017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen L and Zhang G, Lou Z, Xu G and Zhang
G: Cryptotanshinone enhances the effect of Arsenic trioxide in
treating liver cancer cell by inducing apoptosis through
downregulating phosphorylated- STAT3 in vitro and in vivo. BMC
Complement Altern Med. 17:1062017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Macfarlane LA and Murphy PR: MicroRNA:
Biogenesis, Function and Role in Cancer. Curr Genomics. 11:537–561.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pratap P, Raza ST, Abbas S and Mahdi F:
MicroRNA-associated carcinogenesis in lung carcinoma. J Cancer Res
Ther. 14:249–254. 2018.PubMed/NCBI
|
|
10
|
Castro D, Moreira M, Gouveia AM, Pozza DH
and De Mello RA: MicroRNAs in lung cancer. Oncotarget.
8:81679–81685. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Osada H and Takahashi T: let-7 and
miR-17-92: small-sized major players in lung cancer development.
Cancer Sci. 102:9–17. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim YH, Lee WK, Lee EB, Son JW, Kim DS and
Park JY: Combined Effect of Metastasis-Related MicroRNA, miR-34 and
miR-124 Family, Methylation on Prognosis of Non-Small-Cell Lung
Cancer. Clin Lung Cancer. 18:e13–e20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou Y, Wu D, Tao J, Qu P, Zhou Z and Hou
J: MicroRNA-133 inhibits cell proliferation, migration and invasion
by targeting epidermal growth factor receptor and its downstream
effector proteins in bladder cancer. Scand J Urol. 47:423–432.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guttilla IK and White BA: Coordinate
regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast
cancer cells. J Biol Chem. 284:23204–23216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ortmann RA, Cheng T, Visconti R, Frucht DM
and O'Shea JJ: Janus kinases and signal transducers and activators
of transcription: Their roles in cytokine signaling, development
and immunoregulation. Arthritis Res. 2:16–32. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pastuszak-Lewandoska D,
Domańska-Senderowska D, Kordiak J, Antczak A, Czarnecka KH,
Migdalska-Sęk M, Nawrot E, Kiszałkiewicz JM and Brzeziańska-Lasota
E: Immunoexpression analysis of selected JAK/STAT pathway molecules
in patients with non- small-cell lung cancer. Pol Arch Intern Med.
127:758–764. 2017.PubMed/NCBI
|
|
17
|
Zhang Y, Chen L, Li F, Wang H, Yao Y, Shu
J and Ying MZ: Cryptotanshinone protects against adriamycin-induced
mitochondrial dysfunction in cardiomyocytes. Pharm Biol.
54:237–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chiba T, Nishimoto I, Aiso S and Matsuoka
M: Neuroprotection against neurodegenerative diseases: Development
of a novel hybrid neuroprotective peptide Colivelin. Mol Neurobiol.
35:55–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu W: MicroRNA, Noise, and Gene Expression
Regulation. Methods Mol Biol. 1699:91–96. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stankevicius V, Kuodyte K, Schveigert D,
Bulotiene D, Paulauskas T, Daniunaite K and Suziedelis K: Gene and
miRNA expression profiles of mouse Lewis lung carcinoma LLC1 cells
following single or fractionated dose irradiation. Oncol Lett.
13:4190–4200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tallant C, Marrero A and Gomis-Rüth FX:
Matrix metalloproteinases: Fold and function of their catalytic
domains. Biochim Biophys Acta. 1803:20–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li S, Lu J, Chen Y, Xiong N, Li L, Zhang
J, Yang H, Wu C, Zeng H and Liu Y: MCP-1-induced ERK/GSK-3β/Snail
signaling facilitates the epithelial-mesenchymal transition and
promotes the migration of MCF-7 human breast carcinoma cells. Cell
Mol Immunol. 14:621–630. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jabłońska-Trypuć A, Matejczyk M and
Rosochacki S: Matrix metalloproteinases (MMPs), the main
extracellular matrix (ECM) enzymes in collagen degradation, as a
target for anticancer drugs. J Enzyme Inhib Med Chem. 31 (Supp
1):177–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Q, Jin M, Yang F, Zhu J, Xiao Q and
Zhang L: Matrix metalloproteinases: Inflammatory regulators of cell
behaviors in vascular formation and remodeling. Mediators Inflamm.
2013:9283152013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu J, Liu XJ, Li L, Zhang SH, Li Y, Gao RJ
and Zhen YS: An engineered TIMP2-based and enediyne-integrated
fusion protein for targeting MMP-14 shows potent antitumor
efficacy. Oncotarget. 6:26322–26334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Prideaux M, Staines KA, Jones ER, Riley
GP, Pitsillides AA and Farquharson C: MMP and TIMP temporal gene
expression during osteocytogenesis. Gene Expr Patterns. 18:29–36.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu M and Wang YZ: miR 133a suppresses cell
proliferation, migration and invasion in human lung cancer by
targeting MMP 14. Oncol Rep. 30:1398–1404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Yao Y, Wang H, Guo Y, Zhang H and
Chen L: Effects of salidroside on glioma formation and growth
inhibition together with improvement of tumor microenvironment.
Chin J Cancer Res. 25:520–526. 2013.PubMed/NCBI
|
|
29
|
Yao Y, Liu S, Wang Y, Yuan W, Ding X,
Cheng T, Shen Q and Gu J: Suppression of cytochrome P450 reductase
expression promotes astrocytosis in subventricular zone of adult
mice. Neurosci Lett. 548:84–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guan X: Cancer metastases: Challenges and
opportunities. Acta Pharm Sin B. 5:402–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Perlikos F, Harrington KJ and Syrigos KN:
Key molecular mechanisms in lung cancer invasion and metastasis: A
comprehensive review. Crit Rev Oncol Hematol. 87:1–11. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pawelek JM and Chakraborty AK: The cancer
cell - leukocyte fusion theory of metastasis. Adv Cancer Res.
101:397–444. 2008. View Article : Google Scholar : PubMed/NCBI
|