Introduction
Metastatic bone tumor, a tumor that is metastasized
from other parts of the body to the bone through blood or lymphatic
pathway (1), occurs in children with
malignant tumors (2,3). During the clinical diagnosis and
treatment, metastatic bone tumors are often confused with primary
bone tumors due to the lack of symptoms of the primary bone tumors
(4). The most common malignant
tumors in children with bone metastasis are neuroblastoma and
nephroblastoma (5,6). Pathological fractures at the metastatic
bone often occurs when tumor cells metastasize into the bone,
accompanied by severe pain (7), and
in some cases, the unbearable pain becomes the main aspect in
survival of patients with bone metastases (8). To give safe and effective analgesic
treatment in children with bone metastasis has become the top
priority in improving the quality of life of children with bone
metastases.
High-intensity focused ultrasound (HIFU) [an
emerging technology that focuses ultrasound beams emitted from
outside the body at target lesions in the body to produce local
hyperthermia to kill tumor cells (9)], has been proven by studies to be able
to greatly relieve severe pain in patients with advanced pancreatic
cancer that cannot be treated surgically (10), and to give good efficacy in the
treatment of prostate cancer and breast cancer (11,12).
However, the incapability of the HIFU method to perform real-time
monitoring on the temperature and the treatment sites brings
potential risk that the displaced ultrasound beams and the
excessively high temperature may damage the tissues around the
lesions and bring other unexpected damage (13). MR-guided focused ultrasound surgery
(MRgFUS), with the help of MR imaging technology, can perform
temperature monitoring and dynamic localization to guide the
ultrasonic scalpel for rapid ablation of target lesions (14), which has achieved good efficacy in
treating bone tumors (15). Also,
MRgFUS can burn the nerve with focusing heat for the analgesia of
severe pain in patients with advanced bone metastasis of malignant
tumors (16), thus successfully
relieving the pain in patients, improving their quality of life and
shortening the treatment time. The analgesic effect of MRgFUS on
children with metastatic bone tumors was studied and the efficacy
and safety evaluated.
Patients and methods
General information
Thirty children with bone metastases from the
Oncology Department of Jinan Maternity and Child Care Hospital
(Jinan, China) from March 2015 to March 2018 were collected. There
were, 12 children were with neuroblastoma, 7 children with acute
leukemia, 6 children with nephroblastoma, and 5 children with
lymphoma. Metastatic lesions: 13 cases to ribs, 11 cases to ilium,
4 cases to long bones of the extremities, and 2 cases to sacral
vertebrae. There were 20 males and 10 females, aged from 3 to 14
years, with an average age of 4.27±0.83 years. Twenty three
children were at preschool age and 7 children at school age. Basic
information of the patients is shown in Table I. The study was approved by the
Ethics Committee of Jinan Maternity and Child Care Hospital. The
patients and their guardians were informed, and the informed
consent was signed by the parents or guardians.
 | Table I.General clinical data (mean ± SD) [n
(%)]. |
Table I.
General clinical data (mean ± SD) [n
(%)].
| Clinical factors | Number of the case
(%) |
|---|
| Sex |
|
| Male | 20 (66.67) |
|
Female | 10 (33.33) |
| Age |
|
| ≤7 | 23 (76.67) |
|
>7 | 7 (23.33) |
| Weight | 18.73±10.34 |
| Height | 98.24±8.83 |
| Primary tumors |
|
|
Neuroblastoma | 12 (40.00) |
| Acute
leukemia | 7 (23.33) |
|
Nephroblastoma | 6 (20.00) |
|
Lymphoma | 5 (16.67) |
| Other diseases | 2 (6.67) |
| Received treatments 3
months before the surgery |
|
|
Radiotherapy | 9 (30.00) |
|
Chemotherapy | 11 (36.67) |
|
Operation | 6 (20.00) |
| Other
treatments | 4 (13.33) |
| Location of
lesions |
|
| Rib | 13 (43.33) |
|
Ilium | 11 (36.67) |
| Long bone
of limbs | 4 (13.33) |
| Sacral
vertebrae | 2 (6.67) |
| Nature of the
lesions |
|
|
Osteoblastic | 14 (46.67) |
|
Osteolytic | 5 (16.67) |
| Mixed
type | 11 (36.67) |
| Size of the
lesions |
|
|
Anteroposterior
diameter/mm | 13.49±8.34 |
|
Transverse diameter/mm | 23.37±10.47 |
|
Vertical diameter/mm | 25.62±9.35 |
| Volume/ml |
932.45±6,142.36 |
Inclusion criteria
i) Patients who were confirmed by pathology and
imaging examination as bone metastases, aged ≤16 years; ii) bone
metastasis target lesions were located in the trunk bones of the
extremities, bone joints, pelvis and the posterior part of the
spine bone under the fourth lumbar vertebra and were clearly imaged
on MRI; and iii) pain sites were fixed, and Numerical Rating Scale
(NRS) score (17) ≥4 points, with
stable degree of pain and the same type of painkiller 1 week before
treatment.
Exclusion criteria
i) Patients who recently received radiotherapy and
chemotherapy for target lesions; ii) patients with more than 6
target lesions and an estimated survival time of no more than three
months; iii) patients complicated with heart, brain, liver and
kidney dysfunction or severe infection; iv) patients who could not
be examined by MRI due to built-in metal objects or other reasons;
and v) patients who obtained less than 60 points for the Karnofsky
Performance Status (KPS) (18)
scores.
Experimental methods
Therapeutic instruments and drugs
Magnetic resonance imaging system (Shanghai Ge
Medical Instrument Co., Ltd., 3460244); MRgFUS (Exablate 2100;
InSightec, Ltd.); Gd-DTPA (MR-00P10; Shanghai Sunr Biotech Co.,
Ltd.); ketamine hydrochloride injection (H32022820; Jiangsu Hengrui
Medicine Co., Ltd.); atropine sulfate injection (H41021272; Jiaozuo
Furuitang Pharmaceutical Co., Ltd.).
Treatment methods
After finishing all routine clinical examinations
and completing the basic information, all patients were required to
perform the preoperative preparations such as fasting and water
deprivation for 6 h before surgery, preoperative hair removal, to
establish venous access and indwell the catheter. To reduce
movement, patients were given intramuscular injection of sedative
(ketamine, 0.5 mg/kg + atropine 0.02 mg/kg), then they were
escorted to the operating room by the anesthesiologist and family
members 10 min after the injection when the blood concentration
reached its peak. The treatment bed was adjusted according to the
location of the specific target lesion so that the ultrasonic
scalpel beam could directly reach the painful part of the target
lesion, and a soft pad was used for fixing the position of patients
to make the long axis of the bone where the target lesions were
located parallel to the treatment axis. Real-time
electrocardiographic monitoring was performed together with
artificial monitoring by the anesthesiologist who closely checked
the patient's complexion and the chest expansion and stopped the
treatment if any abnormality occurred. Fat-suppression T1W1 and
T2W1 scans were performed on the sagittal and cross sections of the
target lesion before treatment (the layer thickness was 4–7 mm,
spacing 1 mm per layer. Gd-DTPA was injected through the cubital
vein during the enhanced scan, 0.15 ml/kg). The contour of the
target lesion area and the protective area of the key structure
were manually outlined on the fat-suppression T2W1 image on the
cross-section and then entered into the MRgFUS system to make
relevant treatment plan with the help of artificial adjustment on
the actual number of target lesions and the energy required by the
target lesions. The focus of the ultrasonic beam was continuously
moved, and the temperature of the target lesion area was maintained
at 60–85°C for 20–30 sec each time. The MRI imaging system was used
during the whole process of treatment to monitor the real-time
temperature and shape of target lesions and their surrounding
area.
Observation indicators
NRS score, visual analog scale (VAS) (19), and European Organization for Research
and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)
score (20) before surgery and 1
week, 1, 2, and 3 months after surgery, as well as the KPS scores
before surgery and 1 week, 1 and 3 months after surgery were used
to assess the analgesic efficacy of the children. Among them, the
items of the QLQ-C30 scores were classified as ‘completely absent,
mild, severe and deeply severe’, with a score of 1 to 4 points. The
analgesic drugs before and after treatment were recorded, and the
adverse reactions such as treatment-related pathological fractures,
regional tissue edema, and abnormal feelings in the treatment area
were closely observed.
Statistical analysis
Experimental data were statistically analyzed using
SPSS software 19.0 (SPSS Inc.). Measurement data were expressed as
(mean ± SD), enumeration data were expressed as cases (percentage)
[n (%)]. Repeated measures ANOVA was used to compare the data of
multiple time-points, and the Bonferroni test was used for
comparison between two different points in the group. Statistical
significance was recognized at P<0.05.
Results
Treatment outcome
MRgFUS treatment was successfully completed in 30
children with metastatic bone tumors, with an operation time of
123±21 min per patient. The average ultrasound pulse for each
target lesion was 13±8 times. Eight of the 30 children with
metastatic bone tumors received a second treatment over 3 weeks
after the first treatment. All patients underwent a 3-month
follow-up.
Score of the analgesia efficacy after
surgery
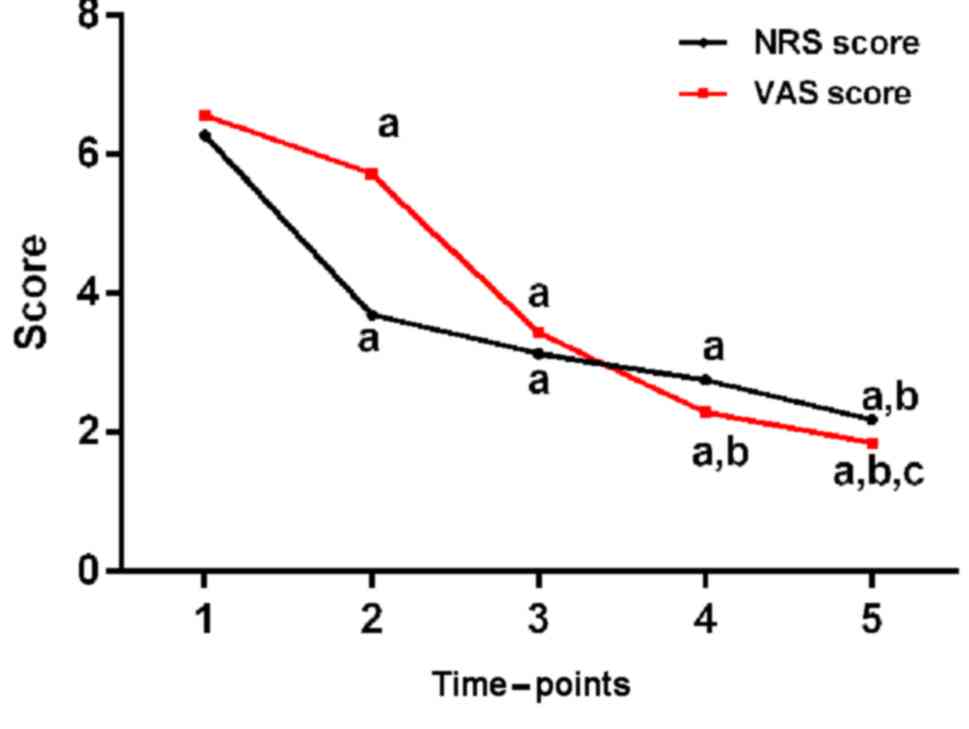
NRS scores and VAS scores of all different
observation time-points after surgery were statistically lower than
those before surgery (P<0.05), and the NRS and VAS scores 3
months after surgery were lower than those 1 week after surgery
(P<0.05). The analgesic effect was relatively sustained. The
specific data are shown in Table II
and Fig. 1.
 | Table II.Score of the analgesia efficacy after
surgery (mean ± SD). |
Table II.
Score of the analgesia efficacy after
surgery (mean ± SD).
|
|
| After surgery |
|
|
|---|
|
|
|
|
|
|
|---|
| Score | Before surgery | 1 week | 1 month | 2 months | 3 months | F-value | P-value |
|---|
| NRS | 6.27±1.53 |
3.69±1.71a |
3.13±1.87a |
2.76±1.53a |
2.18±1.04a,b | 31.020 | <0.001 |
| VAS | 6.56±2.38 |
4.72±2.34a |
3.43±2.16a |
2.29±1.15a,b |
1.85±0.96a–c | 30.880 | <0.001 |
QLQ-C30 scores before and after
treatment
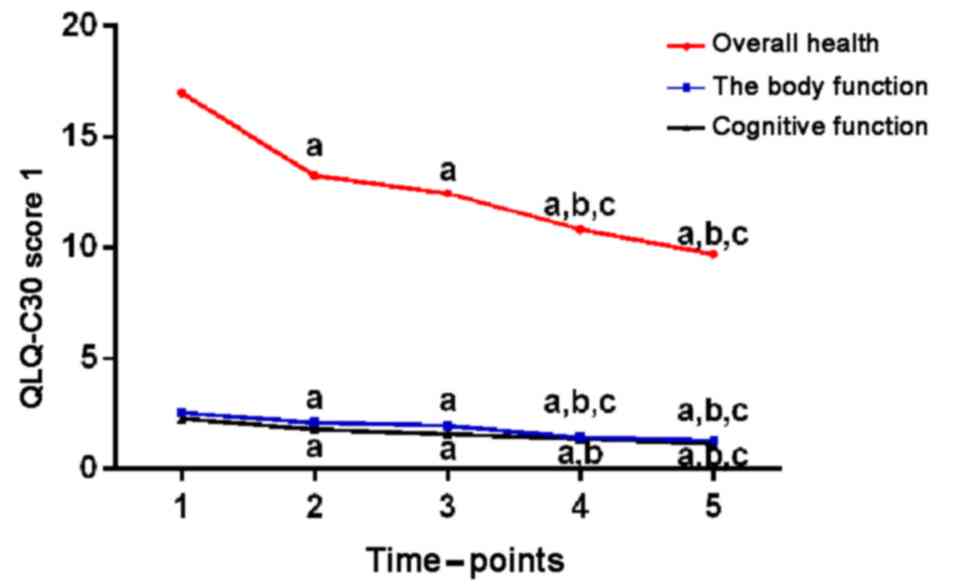
Compared with the preoperative conditions, QLQ-C30
scores of physical function, cognitive function, nausea and
vomiting, and degree of pain 1 week, 1, 2, and 3 months after
surgery were decreased, and the clinical symptoms of the children
were relieved (P<0.05). There was a statistical difference
between total QLQ-C30 scores 3 months after operation and total
QLQ-C30 scores before operation (P<0.05). The specific data are
shown in Table III, Figs. 2 and 3.
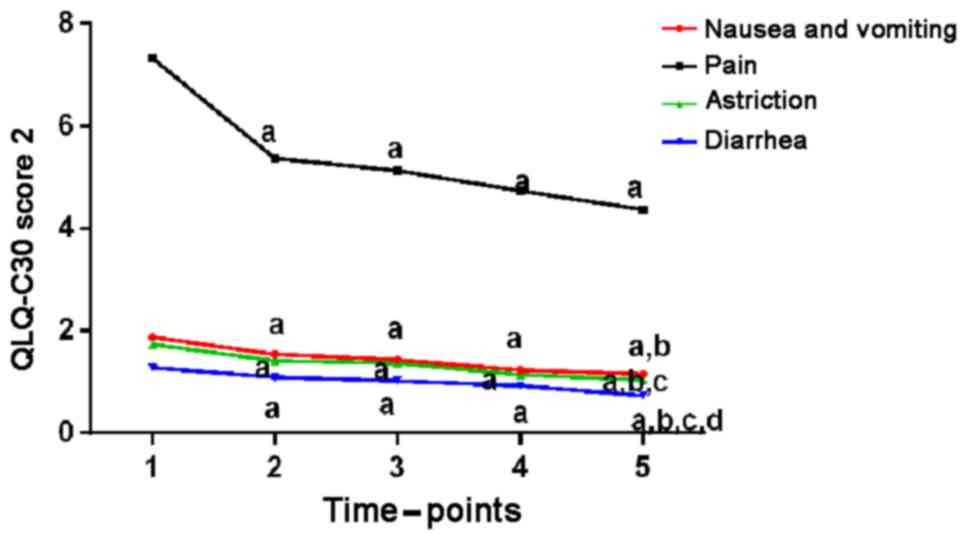 | Figure 3.QLQ-C30 scores before and after
treatment (2). Compared with the
preoperative conditions, QLQ-C30 scores such as nausea, vomiting,
feeling painful, constipation, and diarrhea 1 week, 1, 2, and 3
months after surgery were lower, and clinical symptoms of children
were all relieved to different extent (P<0.05).
aP<0.05, when compared with QLQ-C30 scores before
surgery; bP<0.05, when compared with QLQ-C30 scores 1
week after surgery; cP<0.05, when compared with
QLQ-C30 scores 1 month after surgery; dP<0.05, when
compared with QLQ-C30 scores 2 months after surgery. QLQ, Quality
of Life Questionnaire. |
 | Table III.QLQ-C30 scores before and after the
treatment of the patients (mean ± SD). |
Table III.
QLQ-C30 scores before and after the
treatment of the patients (mean ± SD).
| Time | Total score | Physical
function | Cognitive
function | Nausea and
vomiting | Degree of pain | Astriction | Diarrhea |
|---|
| Before surgery | 16.98±5.38 | 2.52±0.64 | 2.26±0.42 | 1.87±0.62 | 7.32±2.45 | 1.73±0.78 | 1.28±0.47 |
| After surgery |
| 1
week |
13.26±3.89a |
2.07±0.68a |
1.78±0.58a |
1.54±0.52a |
5.37±1.56a |
1.41±0.32a |
1.09±0.23a |
| 1
month |
12.44±3.20a |
1.94±0.68a |
1.56±0.35a |
1.43±0.33a |
5.13±1.45a |
1.36±0.28a |
1.02±0.11a |
| 2
months |
10.80±3.44a–c |
1.41±0.72a–c |
1.36±0.47a,b |
1.23±0.32a |
4.74±1.36a |
1.14±0.37a |
0.92±0.20a |
| 3
months |
9.70±2.98a–c |
1.27±0.36a–c |
1.14±0.63a–c |
1.15±0.34a,b |
4.37±1.26a |
1.04±0.25a–c |
0.73±0.14a–d |
| F-value | 93.830 | 19.580 | 22.050 | 12.340 | 14.100 | 10.900 | 18.000 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
KPS scores before and after treatment
in patients
As shown in Table
IV, the KPS scores of children with metastatic tumors of all
different observation time-points after surgery were slightly lower
than those before surgery, but the difference was not statistically
significant (P>0.05), indicating that the patients' condition
was stable during the treatment period without deterioration.
 | Table IV.KPS scores before and after the
treatment of the patients (mean ± SD). |
Table IV.
KPS scores before and after the
treatment of the patients (mean ± SD).
|
|
| After
treatment |
|
|
|---|
|
|
|
|
|
|
|---|
| Score | Before
treatment | 1 week | 3 months | F-value | P-value |
|---|
| KPS | 86.37±7.19 | 84.78±7.38 | 83.15±7.86 | 1.389 | 0.255 |
Medication
Thirteen patients took fixed analgesics with stable
dosage before surgery, among whom six patients stopped taking drug
after treatment, 4 patients stopped taking drug 2 months after
surgery, 2 patients reduced the dosage of analgesics after surgery
and 1 patient took the same dosage as that before surgery. The
other 17 patients had no analgesic medication before operation,
among whom only 1 patient was in need for analgesics due to failed
analgesic effect on unbearable pain, 2 patients had to take
analgesics 3 months after surgery because of the pain aroused by
metastatic bone tumor at other sites, and the other 14 patients
still did not required any analgesics after surgery.
Adverse reactions
During the treatment of the 30 children with
metastatic bone tumors, 2 patients had I degree burns which were
relieved 3 days after symptomatic treatment; 3 patients were
troubled by local swelling and numbness in the treatment area which
was caused by compression of peripheral nerves due to local
swelling according to the MRI examination, and felt better after
symptomatic treatment. After the treatment 3 patients suffered from
low fever which disappeared within 1 month after surgery. No
serious complications such as deep vein thrombosis of the lower
limbs or death of patients occurred during the treatment.
Discussion
As a group of diseases with high risk among various
pediatric diseases, malignant tumors in children are characterized
by fast condition change, since children are growing (21), and many tumors at their early stage
are hard to diagnose due to children's poor ability to express
feelings and the inapparent early symptoms (22), contributing to the easily occurring
metastasis of advanced tumors among which the bone metastasis is
one of the most common ones (23).
The pain of bone and joint caused by bone metastases of malignant
tumors is often an important factor in damaging patients' physical
and mental health (24), causing
great suffering to children and their families (25).
Analgesic drugs combined with chemotherapy, often
applied in the treatment of advanced malignant tumors after bone
metastasis, bring great toxic side effects on children and cause
much damage to the growth and development of children, and the
abuse of painkillers can easily lead to addiction (26,27).
Currently MRgFUS has achieved good results in the analgesic of
metastatic bone tumors in adults (28) as the targeted therapy of MRgFUS on
local target lesions performed by the thermal ablation technique
can achieve satisfactory analgesia by burning nerve endings
attached to the periosteum around the target lesions and killing
tumor cells at the same time (29).
Despite the safety and good efficacy of this MRgFUS technology, it
is seldom applied to the treatment of metastatic bone tumors in
children, so this study was designed to investigate the analgesic
effect and safety of MRgFUS in children with metastatic bone
tumors.
According to the results of this study, the NRS
score and VAS score of the children at different time-points after
surgery were significantly lower than those before surgery. The
application of analgesics was reduced in most children, suggesting
that the MRgFUS treatment could effectively relieve the pain in
children aroused by metastatic bone tumors, working as a substitute
for some analgesics. The study of Joo et al (30) showed that the NRS score could even be
reduced from 7 to 0.3 on children with metastatic bone tumors one
year after the MRgFUS treatment. However, the present study focused
on the short-term efficacy of MRgFUS, and performed a double
evaluation of the NRS and the VAS score, and showed that the MRgFUS
treatment could also achieve good analgesic effect on children with
metastatic bone tumors, since the pain of children in this study
was relieved after treatment. Compared with the preoperative
conditions, the QLQ-C30 scores of physical function, cognitive
function, nausea and vomiting, and degree of pain 1 week, 1, 2 and
3 months after surgery were decreased, and the clinical symptoms of
children were relieved at different degrees. There was a
statistical difference between the total QLQ-C30 scores 3 months
after operation and the total QLQ-C30 scores before operation,
showing good improvement in life treatment and clinical symptoms
and sustained efficacy in children with metastatic bone tumors
after the MRgFUS treatment. Gu et al (31) found that the EORTC QLQ score after
MRgFUS treatment was significantly decreased, and the quality of
life of patients was significantly improved, which is consistent
with the results of our study. In addition, we conducted a more
specific analysis and comparison of the quality of life and
clinical symptoms of children with metastatic bone tumors in
multiple aspects and multiple periods, providing a more convincing
result that the MRgFUS treatment may achieve good and continuous
efficacy in children with metastatic bone tumors. The KPS scores of
children with metastatic tumors of all different observation
time-points after surgery were slightly lower than those before
surgery, but the difference was not statistically significant.
Previous studies have shown that poor KPS scores may be the
prognostic risk factors of many malignant tumors (32,33).
During the treatment, the KPS score was relatively stable, and the
children did not have serious adverse reactions related to
treatment, indicating that the treatment of MRgFUS in children with
metastatic bone tumors had good safety. As to the 2 cases of I
degree burns during the treatment, a possible reason was the
temperature of the thermal ablation which was relatively too high
to children with more delicate skin than adults. After taking this
into consideration in the later stage of the experiment, no other
reports of burning occurred.
However, there are some limitations in this
study
The children were generally too young, so most of
the evaluation of the analgesic efficacy scores was assisted by the
family members of the children, which might have some impacts on
the results of the study. No control group was set up due to the
limited experimental conditions. The sample size was small in this
study. Multi-center and multi-regional samples need to be included,
and the study period needs to be extended in future studies.
In summary, the MRgFUS technology is worthy of
further research since it was proved to achieve a good analgesic
effect on children with metastatic bone tumors, and to improve the
quality of life and clinical symptoms to a certain extent, with
fewer adverse reactions and higher safety.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BW was responsible for the writing of the manuscript
and treatment of patients. JL and XW analyzed observation
indicators and revised the manuscript. All the authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Jinan Maternity and Child Care Hospital (Jinan, China). Patients
who participated in this research had complete clinical data. The
signed informed consents were obtained from the parents of the
child patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Krzeszinski JY and Wan Y: New therapeutic
targets for cancer bone metastasis. Trends Pharmacol Sci.
36:360–373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Drubach LA: Nuclear medicine techniques in
pediatric bone imaging. Semin Nucl Med. 47:190–203. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yi JL, Galgano MA, Tovar-Spinoza Z and
Deshaies EM: Coil embolization of an intracranial aneurysm in an
infant with tuberous sclerosis complex: A case report and
literature review. Surg Neurol Int. 3:1292012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reufsteck C, Lifshitz-Shovali R, Zepp M,
Bäuerle T, Kübler D, Golomb G and Berger MR: Silencing of skeletal
metastasis-associated genes impairs migration of breast cancer
cells and reduces osteolytic bone lesions. Clin Exp Metastasis.
29:441–456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishiguchi H, Ito S, Kato K, Sakurai Y,
Kawai H, Fujita N, Abe S, Narita A, Nishio N, Muramatsu H, et al:
Diagnostic performance of 18F-FDG PET/CT and whole-body
diffusion-weighted imaging with background body suppression (DWIBS)
in detection of lymph node and bone metastases from pediatric
neuroblastoma. Ann Nucl Med. 32:348–362. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chung EM, Graeber AR and Conran RM: Renal
tumors of childhood: Radiologic-pathologic correlation Part 1. The
1st decade: From the radiologic pathology archives. Radiographics.
36:499–522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim LD, Bueno FT, Yonamine ES, Próspero JD
and Pozzan G: Bone metastasis as the first symptom of tumors: Role
of an immunohistochemistry study in establishing primary tumor. Rev
Bras Ortop. 53:467–471. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
von Moos R, Body JJ, Egerdie B, Stopeck A,
Brown J, Fallowfield L, Patrick DL, Cleeland C, Damyanov D, Palazzo
FS, et al: Pain and analgesic use associated with skeletal-related
events in patients with advanced cancer and bone metastases.
Support Care Cancer. 24:1327–1337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trimboli P, Bini F, Marinozzi F, Baek JH
and Giovanella L: High-intensity focused ultrasound (HIFU) therapy
for benign thyroid nodules without anesthesia or sedation.
Endocrine. 61:210–215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marinova M, Strunk HM, Rauch M, Henseler
J, Clarens T, Brüx L, Dolscheid-Pommerich R, Conrad R, Cuhls H,
Radbruch L, et al: High-intensity focused ultrasound (HIFU) for
tumor pain relief in inoperable pancreatic cancer: Evaluation with
the pain sensation scale (SES). Schmerz. 31:31–39. 2017.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ganzer R: High intensity focused
ultrasound (HIFU): Importance in the treatment of prostate cancer.
Radiologe. 57:659–664. 2017.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Merckel LG, Knuttel FM, Deckers R, van
Dalen T, Schubert G, Peters NH, Weits T, van Diest PJ, Mali WP,
Vaessen PH, et al: First clinical experience with a dedicated
MRI-guided high-intensity focused ultrasound system for breast
cancer ablation. Eur Radiol. 26:4037–4046. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sanghvi NT, Chen WH, Carlson R, Weis C,
Seip R, Uchida T and Marberger M: Clinical validation of real-time
tissue change monitoring during prostate tissue ablation with high
intensity focused ultrasound. J Ther Ultrasound. 5:242017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salomir R, Vimeux FC, de Zwart JA, Grenier
N and Moonen CT: Hyperthermia by MR-guided focused ultrasound:
Accurate temperature control based on fast MRI and a physical model
of local energy deposition and heat conduction. Magn Reson Med.
43:342–347. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ringe KI, Panzica M and von Falck C:
Thermoablation of bone tumors. RoFo. 188:539–550. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hurwitz MD, Ghanouni P, Kanaev SV, Iozeffi
D, Gianfelice D, Fennessy FM, Kuten A, Meyer JE, LeBlang SD,
Roberts A, et al: Magnetic resonance-guided focused ultrasound for
patients with painful bone metastases: Phase III trial results. J
Natl Cancer Inst. 106:1062014. View Article : Google Scholar
|
|
17
|
Løhre ET, Klepstad P, Bennett MI, Brunelli
C, Caraceni A, Fainsinger RL, Knudsen AK, Mercadante S, Sjøgren P
and Kaasa S; European association for palliative care research
network, : From ‘Breakthrough’ to ‘Episodic’ cancer pain? A
European association for palliative care research network expert
Delphi survey toward a common terminology and classification of
transient cancer pain exacerbations. J Pain Symptom Manage.
51:1013–1019. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chambless LB, Kistka HM, Parker SL,
Hassam-Malani L, McGirt MJ and Thompson RC: The relative value of
postoperative versus preoperative Karnofsky Performance Scale
scores as a predictor of survival after surgical resection of
glioblastoma multiforme. J Neurooncol. 121:359–364. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JJ, Lee MK, Kim JE, Kim HZ, Park SH,
Tae JH and Choi SS: Pain relief scale is more highly correlated
with numerical rating scale than with visual analogue scale in
chronic pain patients. Pain Physician. 18:E195–E200.
2015.PubMed/NCBI
|
|
20
|
Raman S, Ding K, Chow E, Meyer RM, van der
Linden YM, Roos D, Hartsell WF, Hoskin P, Wu JSY, Nabid A, et al:
Minimal clinically important differences in the EORTC QLQ-C30 and
brief pain inventory in patients undergoing re-irradiation for
painful bone metastases. Qual Life Res. 27:1089–1098. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lozier AM, Rich ME, Grawe AP, Peck AS,
Zhao P, Chang ATT, Bond JP and Sholler GS: Targeting ornithine
decarboxylase reverses the LIN28/Let-7 axis and inhibits glycolytic
metabolism in neuroblastoma. Oncotarget. 6:196–206. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Merchant TE, Hua CH, Shukla H, Ying X,
Nill S and Oelfke U: Proton versus photon radiotherapy for common
pediatric brain tumors: Comparison of models of dose
characteristics and their relationship to cognitive function.
Pediatr Blood Cancer. 51:110–117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang X, Li C, Xu C, Hao X, Yu X and Li Q:
Correlation of CT signs with lymphatic metastasis and pathology of
neuroblastoma in children. Oncol Lett. 16:2439–2443.
2018.PubMed/NCBI
|
|
24
|
Wu Z, Yang H, Weng D and Ding Y: Rapid
recurrence and bilateral lungs, multiple bone metastasis of
malignant solitary fibrous tumor of the right occipital lobe:
Report of a case and review. Diagn Pathol. 10:912015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Olagunju AT, Sarimiye FO, Olagunju TO,
Habeebu MY and Aina OF: Child's symptom burden and depressive
symptoms among caregivers of children with cancers: An argument for
early integration of pediatric palliative care. Ann Palliat Med.
5:157–165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moroe NF and Hughes K: Parents are aware
of the ototoxic effects of chemotherapy in paediatrics undergoing
cancer treatment - Professional versus parental views: A pilot
study. S Afr J Commun Disord. 64:e1–e10. 2017.PubMed/NCBI
|
|
27
|
Zelikowsky M and Fanselow MS: Opioid
regulation of Pavlovian overshadowing in fear conditioning. Behav
Neurosci. 124:510–519. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Catane R, Beck A, Inbar Y, Rabin T,
Shabshin N, Hengst S, Pfeffer RM, Hanannel A, Dogadkin O, Liberman
B, et al: MR-guided focused ultrasound surgery (MRgFUS) for the
palliation of pain in patients with bone metastases - preliminary
clinical experience. Ann Oncol. 18:163–167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dababou S, Marrocchio C, Scipione R,
Erasmus HP, Ghanouni P, Anzidei M, Catalano C and Napoli A:
High-intensity focused ultrasound for pain management in patients
with cancer. Radiographics. 38:603–623. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Joo B, Park MS, Lee SH, Choi HJ, Lim ST,
Rha SY, Rachmilevitch I, Lee YH and Suh JS: Pain palliation in
patients with bone metastases using magnetic resonance-guided
focused ultrasound with conformal bone system: A preliminary
report. Yonsei Med J. 56:503–509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gu J, Wang H, Tang N, Hua Y, Yang H, Qiu
Y, Ge R, Zhou Y, Wang W and Zhang G: Magnetic resonance guided
focused ultrasound surgery for pain palliation of bone metastases:
Early experience of clinical application in China. Zhonghua Yi Xue
Za Zhi. 95:3328–3332. 2015.(In Chinese). PubMed/NCBI
|
|
32
|
Geng M, Xu H, Ren R, Qu Q, Shangguan C, Wu
J, Jiang J, Li H and Cao W: Prognostic value of clinicopathological
characteristics in patients with pancreatic cancer. Chin J Cancer
Res. 27:509–515. 2015.PubMed/NCBI
|
|
33
|
Ventriglia J, Petrillo A, Huerta Alváro M,
Laterza MM, Savastano B, Gambardella V, Tirino G, Pompella L, Diana
A, Iovino F, et al: Neutrophil to lymphocyte ratio as a predictor
of poor prognosis in metastatic pancreatic cancer patients treated
with Nab-Paclitaxel Plus Gemcitabine: A propensity score analysis.
Gastroenterol Res Pract. 2018:23738682018. View Article : Google Scholar : PubMed/NCBI
|