Introduction
Thyroid cancer (TC) is one of the most common
malignant tumours of the endocrine system (1). In the USA, 53,990 new cases were
estimated in 2018 (2). Similarly,
the number of newly diagnosed cases and TC-associated mortalities
in China were estimated at ~90,000 and 6,800, respectively
(3). TC has been predicted to
surpass colorectal cancer and become the fourth most common cancer
in 2030 worldwide (4,5). Papillary thyroid cancer (PTC), which
comprises diverse biological subtypes, accounts for ~80% of TC
cases (6). The incidence of PTC is
high, but it has positive clinical outcomes and a high 5-year
survival rate compared with other malignant tumours (7). Following surgery or radioactive iodine
therapy, patients with PTC exhibit satisfactory prognoses, as well
as an overall 10-year survival rate of 90% (8).
Although PTC is associated with relatively good
prognosis and a high cure rate, metastasis and recurrence are
common following routine treatment; of all patients with PTC,
10–15% experience recurrence and distant metastasis (9). In addition, previous studies have
reported that gene mutations, such as the stimulation of oncogenes
and the silencing of tumour suppressor genes, are the most common
initiators of tumourigenesis and progression of PTC (10,11).
Therefore, it is important to identify the molecular markers and
potential therapeutic targets for PTC.
Genomics is a useful tool for the exploration of
gene mutations. Over the past two decades, several studies have
reported that certain gene mutations result in tumourigenesis and
TC progression. For instance, B-type Raf kinase V600E is a common
gene mutation that can accelerate thyroid tumourigenesis by
abnormally triggering mitogen-activated protein kinase (12). In addition, Ret proto-oncogene (RET)
somatic rearrangements RET/PTC1 and RET/PTC3 can sustain the
activation of the RET tyrosine kinase domain; stimulation of the
RET tyrosine kinase domain induces changes in PTC cell cytoplasm
(13,14). Mutations in other genes, such as PTEN
(15), p53 (16) and telomerase reverse transcriptase
(17), have been demonstrated to
serve significant roles in tumourigenesis and progression of TC.
Despite the progress in thyroid cancer research over the past
decades, the exact mechanisms of PTC development and progression
remain unknown.
To explore the occurrence and progression of TC,
whole-transcriptome resequencing of 19 pairs of primary PTC and
adjacent healthy tissue samples was performed in our previous
unpublished study. A series of comprehensive analyses revealed that
the PKHD1L1 gene was highly associated with PTC, especially during
tumourigenesis. PKHD1L1 was initially identified as a mouse gene
(18), but the function of the gene
was not well understood. A further study reported that PKHD1L1
encodes fibrocystin-L, which serves a crucial role in cellular
immunity (19). In addition, a
PKHD1L1 mutation may cause autosomal recessive polycystic kidney
disease (ARPKD) (20). Erdman et
al demonstrated that the PKHD1L1 genotype may be a relevant
factor in male longevity (21).
Although the gene has been discovered for a long time the exact
functions of PKHD1L1 in humans remain poorly understood.
Materials and methods
Patients and samples
For the validated cohort, 52 fresh PTC tissue
samples and paired adjacent (~10 mm away from the tumour) normal
tissue samples were collected surgically from patients with PTC
treated at The First Affiliated Hospital of Wenzhou Medical
University between January 2014 and January 2018. None of the
patients underwent chemotherapy or radiotherapy prior to the
surgery. The mean age of the patients with PTC was 46.9 years
(range, 22–75 years). 85% of the patients were female. Following
tissue sample were collection, liquid nitrogen was used to
immediately freeze the samples, and all samples were stored at
−80°C for further experiments. The histological diagnoses were
confirmed by retrospective review of the cases, and the tumour
samples were assessed by two senior pathologists.
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA was isolated from the tissue samples using
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. For assessment of RNA
quality and quantity, the A260/A280 ratio (1.8–2.0) and
spectrophotometry values were used, respectively, as detected using
a NanoDrop™ 2000 (Thermo Fisher Scientific, Inc.). The total RNA
was reverse transcribed into cDNA using the ReverTra
Ace® qPCR RT kit (Toyobo Life Science) at 16°C for 5
min, 42°C for 30 min and 98°C for 5 min. The resulting cDNA samples
were stored at −80°C. The relative expression levels of PKHD1L1
were detected using the ABI PRISM 7500 Sequence Detection system
(Thermo Fisher Scientific, Inc.) with One Step SYBR®
PrimeScript™ PLUS and the RTRNA PCR kit (Takara Bio, Inc.)
according to the manufacturer's protocol. RT-qPCR was performed in
triplicate. The thermocycling conditions were: 95°C for 2 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 60 sec, and a
final step of 72°C for 5 min. The relative expression of mRNA was
calculated using the 2−ΔΔCq method (22). GAPDH expression was used as an
internal control. The primer sequences were as follows: PKHD1L1
forward, 5′-TGTGAAGTGAGTGTGGTTAA-3′; and PKHD1L1 reverse,
5′-GCAGTGATGAGTGGAGTC-3′; GAPDH forward,
5′-GGTCGGAGTCAACGGATTTG-3′; and GAPDH reverse,
5′-ATGAGCCCCAGCCTTCTCCAT-3′.
The Cancer Genome Atlas (TCGA)
data
To explore the prognostic value of PKHD1L1 in TC,
PTC RNA-seq data for PKHD1L1 and corresponding patient clinical
data, comprising the PKHD1L1 expression data and follow-up
information, were obtained from TCGA database (https://tcgadata.nci.nih.gov/tcga). In total,
data from 502 PTC tissue samples and 58 normal thyroid tissue
samples were analysed.
Cell lines and cell culture
TPC-1 and BCPAP TC cell lines were kindly supplied
by Professor Ming-Zhao Xing of Johns Hopkins University School of
Medicine. KTC-1 and HTORI3 cell lines were purchased from the Stem
Cell Bank of the Chinese Academy of Sciences. The use of the cell
lines was approved by the Ethics Committee of The First Affiliated
Hospital of Wenzhou Medical University. No cell line authentication
was performed by the authors prior to the study. The cells were
cultured in RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 10% foetal bovine serum (FBS; Invitrogen;
Thermo Fisher Scientific, Inc.) in a 37°C humidified incubator with
5% CO2.
RNA interference
PKHD1L1 small interfering (si)RNA and negative
control siRNA (si-NC) were provided by Shanghai GenePharma Co.,
Ltd. A total of three siRNAs were designed to investigate the
function of PKHD1L1; however, no statistically significant
differences were observed between them in the subsequent
experiments. The effective sequence for the PKHD1L1 siRNA was as
follows: Sense, GCUGAUGGCAUAAACAUAATT; antisense,
UUAUGUUUAUGCCAUCAGCTTTPC-1 (4.5×105 cells/well), KTC1
(8×105 cells/well) and BCPAP cells (7×105
cells/well) were digested with trypsin-EDTA (0.05%; Thermo Fisher
Scientific, Inc.) at 37°C for 2 min, plated onto 6-well plates, and
incubated for at least 24 h in RPMI-1640 medium. Following
incubation, siRNA transfection was performed using
Lipofectamine® RNAiMAX (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol (at 37°C
for 6–8 h). The final siRNA concentration was 100 nM for TPC-1, 50
nM for BCPAP and 75 nM for KTC-1. The analysis of RNA expression
was performed 48 h later. The experiments were conducted in
triplicate.
Cell proliferation assay
Cell Counting Kit-8 (CCK-8; Beyotime Institute of
Biotechnology) was used according to the manufacturer's protocol to
observe the proliferative ability of the cells. Following
transfection, TPC-1, KTC1 and BCPAP cells were plated into 96-well
plates at a density of 1.5×103 cells/well. On days 1, 2,
3 and 4, CCK-8 solution (10 µl) was added to each well and
incubated for 2.5 h at 37°C. Absorbance levels were detected at 450
nm (OD450) using a SpectraMax M5 (Molecular Devices, LLC). For
every group, data from five wells were collected for analysis. All
the assays were performed in triplicate.
Colony-formation assay
The three cell lines were seeded into six-well
plates at a density of 1.5×103 cells/well for TPC-1 and
KTC-1 and 2×103 cells/well for BCPAP. Cells were
incubated in 5% CO2 at 37°C for 8–14 days. When 50–70
cells in one colony-forming unit were observed, via an inverted
light microscopy (magnification, ×10), 4% paraformaldehyde
(Sigma-Aldrich; Merck KGaA) was used to fix cells at 27°C for 30
min. Subsequently, cells were stained with 0.01% crystal violet at
27°C for 30 min. All assays were performed in triplicate.
Migration and invasion assays
For the migration assay, Transwell chambers (Costar;
Corning, Inc.) were used. Transfected TC cells (3×105
cells/0.3 ml medium for TPC-1 and KTC-1 cells and
3.5×105 cells/0.3 ml medium for BCPAP cells) were seeded
into the upper chamber. A total of 0.6 ml RPMI-1640 medium
supplemented with 20% FBS was put in the lower chamber. Following
24-h incubation in 5% CO2 at 37°C, a cotton swab was
used to remove the cells that had not migrated to the lower surface
of the well. The cells on the lower surface were fixed with 4%
paraformaldehyde and stained with 0.4% crystal violet for (both
27°C, for 15 min). Images were captured at five random fields of
view under a light microscope (mgnification, ×40) for further
analysis.
BioCoat™ Matrigel Invasion Chambers (Corning, Inc.)
were left to equilibrate to room temperature for 30 min, and were
used for the invasion assay following the protocol described for
the migration assay.
Statistical analysis
Statistical data analyses were performed using SPSS
23.0 software (IBM Corp.). GraphPad Prism version 6.01 (GraphPad
Software, Inc.) was used to plot the data. Data with a normal
distribution were expressed as the mean ± standard deviation. In
the validated cohort, the differences between the tumour and normal
groups were estimated by paired Student's t-test (two-tailed). The
differences in characteristics between three groups were examined
by ANOVA, and the least significant difference test was used for
multiple comparisons. The differences between the treated and
untreated cells were analysed by unpaired Student's t-test
(two-tailed). In the TCGA cohort and validated cohort, the
associations between PKHD1L1 expression and clinicopathological
features were analysed by the χ2 test. In the TCGA
cohort, the association between PKHD1L1 expression and lymph node
metastasis (LNM) was analysed by univariate and multivariate
logistic regression analyses. P<0.05 was considered to indicate
a statistically significant difference.
Results
PKHD1L1 is significantly downregulated
in PTC
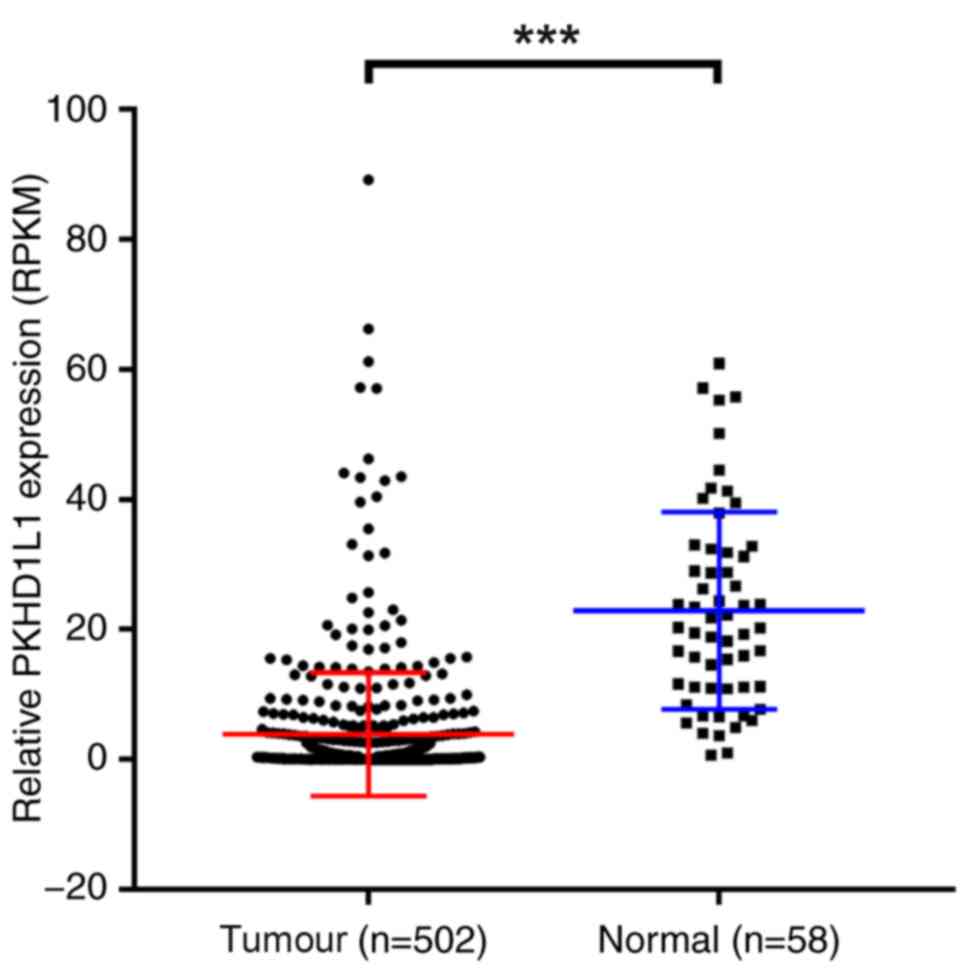
To investigate the function of PKHD1L1 in PTC, TCGA
data were used to confirm the expression level of PKHD1L1. PKHD1L1
was significantly downregulated in TCGA cohort (P<0.001;
Fig. 1). Subsequently, PKHD1L1 mRNA
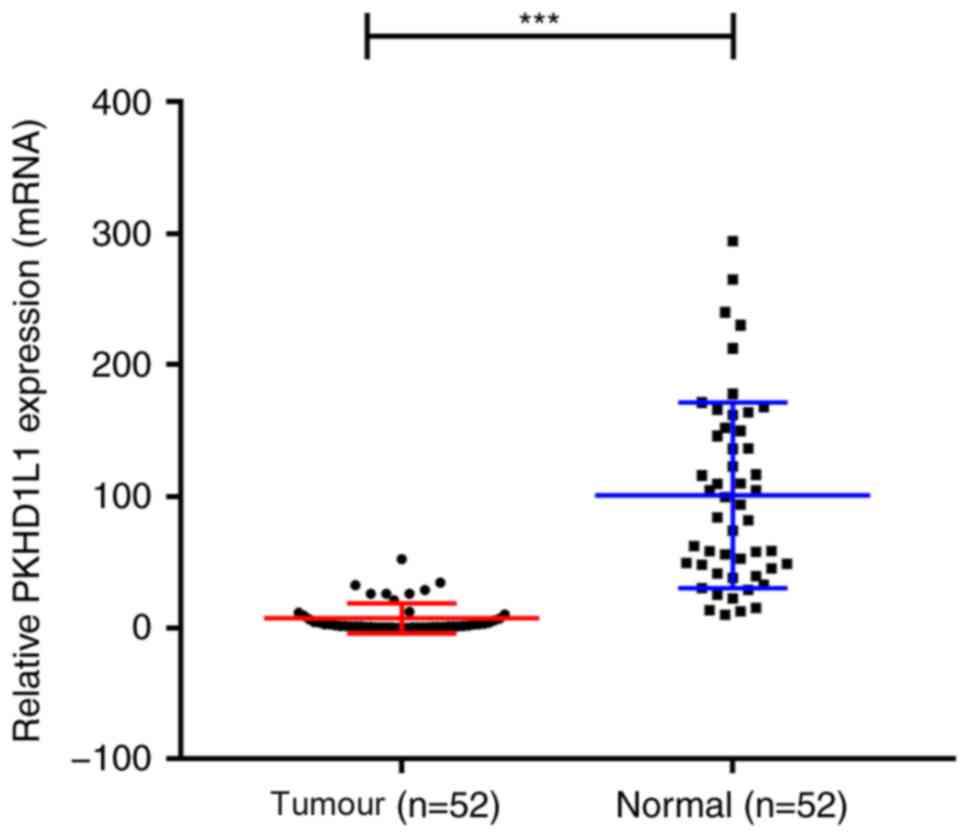
expression levels were analysed in the validated PTC cohort by
RT-qPCR. The results demonstrated that PKHD1L1 was significantly
downregulated in the tumour samples compared with adjacent normal
tissues (P<0.001; Fig. 2).
Therefore, these data suggest that PKHD1L1 may act as an
anti-tumour gene in PTC.
PKHD1L1 expression is associated with
the clinicopathological features of patients with PTC
To facilitate in-depth investigation of PKHD1L1 in
PTC, the association between PKHD1L1 and clinical features of
patients with PTC was analysed. Based on TCGA cohort, 502 patients
were divided into low and high PKHD1L1 expression groups based on
the median reads per kilobase of transcript per million value of
0.4238 (n=251). The results demonstrated that low PKHD1L1
expression was associated with LNM (P<0.001), tumour size
(P=0.003), disease stage (P<0.001) [based on the 7th edition of
the American Joint Committee on Cancer Staging Manual (23)] and distant metastasis (P=0.018)
(Table I). No significant
associations were identified between PKHD1L1 expression and age,
sex, histological type, or multifocality. In the validated cohort,
low PKHD1L1 expression was associated with LNM (P=0.035) and tumour
size (P=0.039) but not with disease stage (P=0.614), as
demonstrated in Table II.
 | Table I.Association between PKHD1L1 expression
and clinicopathological features of patients with papillary thyroid
carcinoma in The Cancer Genome Atlas cohort. |
Table I.
Association between PKHD1L1 expression
and clinicopathological features of patients with papillary thyroid
carcinoma in The Cancer Genome Atlas cohort.
| Clinicopathological
feature | Low expression
(n=251) | High expression
(n=251) | χ2 | P-value |
|---|
| Sex |
|
| 0.253 | 0.615 |
|
Female | 186 | 181 |
|
|
| Male | 65 | 70 |
|
|
| Age, years |
48.0±16.2a |
46.7±15.5a | 0.800 | 0.371 |
| ≤45 | 113 | 123 |
|
|
|
>45 | 138 | 128 |
|
|
| Histological
type |
|
| 2.473 | 0.116 |
|
Classical | 186 | 170 |
|
|
| Other
types | 65 | 81 |
|
|
|
Multi-nodularity |
|
| 0.392 | 0.531 |
|
Yes | 110 | 116 |
|
|
| No | 137 | 129 |
|
|
| Tumour size,
mm |
|
| 8.565 | 0.003b |
|
≥20 | 57 | 86 |
|
|
|
<20 | 194 | 163 |
|
|
| Lymph-node
metastasis |
|
| 14.369 |
<0.001b |
|
Yes | 136 | 87 |
|
|
| No | 99 | 130 |
|
|
| Distant
metastasis |
|
| 5.556 | 0.018b |
|
Yes | 12 | 3 |
|
|
| No | 239 | 248 |
|
|
| Disease
stagec |
|
| 12.309 |
<0.001b |
|
I+II | 148 | 185 |
|
|
|
III+IV | 102 | 65 |
|
|
 | Table II.Association between PKHD1L1
expression and clinicopathological features of patients with
papillary thyroid carcinoma in the validated cohort. |
Table II.
Association between PKHD1L1
expression and clinicopathological features of patients with
papillary thyroid carcinoma in the validated cohort.
| Clinicopathological
features | Low expression
(N=26) | High expression
(N=26) | χ2 | P-value |
|---|
| Sex |
|
|
| 1.000 |
|
Female | 22 | 22 |
|
|
|
Male | 4 | 4 |
|
|
| Age, years |
47.5±14.2a |
46.4±14.6a | 0.310 | 0.578 |
|
≤45 | 13 | 11 |
|
|
|
>45 | 13 | 15 |
|
|
| Histological
type |
|
|
| 1.000 |
|
Classical | 21 | 22 |
|
|
| Other
types | 5 | 4 |
|
|
|
Multi-nodularity |
|
| 0.077 | 0.781 |
|
Yes | 14 | 13 |
|
|
| No | 12 | 13 |
|
|
| Tumour size,
mm |
|
| 4.282 | 0.039b |
|
≥20 | 21 | 14 |
|
|
|
<20 | 5 | 12 |
|
|
| Lymph-node
metastasis |
|
| 4.475 | 0.035b |
|
Yes | 24 | 18 |
|
|
| No | 2 | 8 |
|
|
| Distant
metastasis |
|
|
| 1.000 |
|
Yes | 2 | 1 |
|
|
| No | 24 | 25 |
|
|
| Disease
stagec |
|
|
| 0.614 |
|
I+II | 22 | 20 |
|
|
|
III+IV | 3 | 1 |
|
|
Low PKHD1L1 expression increases the
risk of LNM in patients with PTC
Logistic regression analysis was used to further
assess the association between PKHD1L1 expression and LNM. In TCGA
cohort, PKHD1L1 expression [odds ratio (OR), 0.487; 95% confidence
interval (CI), 0.335–0.709; P<0.001], age (OR, 0.62; 95% CI,
0.427–0.899; P=0.012), histological subtype (OR, 2.383; 95% CI,
1.544–3.680; P<0.001), tumour size (OR, 2.525; 95% CI,
1.625–3.858; P<0.001) and sex (OR, 1.551; 95% CI, 1.022–2.353;
P=0.039) were significant predictors of LNM (Table III). Multivariate logistic
regression analysis confirmed that PKHD1L1 expression (OR, 0.555;
95% CI, 0.356–0.866; P=0.009), histological subtype (OR, 2.84; 95%
CI, 1.72–4.692; P<0.001) and age (OR, 0.03; 95% CI, 0.009–0.1;
P<0.001) were significant predictors of LNM (Table IV). Therefore, low expression of
PKHD1L1 may increase LNM risk in patients with PTC.
 | Table III.Univariate logistic regression
analysis for the risk factors of lymph node metastasis in patients
with papillary thyroid carcinoma. |
Table III.
Univariate logistic regression
analysis for the risk factors of lymph node metastasis in patients
with papillary thyroid carcinoma.
| Factor | OR | 95% CI | P-value |
|---|
| PKHD1L1 expression
(high vs. low) | 0.487 | 0.335–0.709 |
<0.001a |
| Histological
type | 2.383 | 1.544–3.680 |
<0.001a |
| Tumour size, mm
(≥20 vs. <20) | 2.525 | 1.652–3.858 |
<0.001a |
|
Multi-nodularity | 1.446 | 0.994–2.103 |
0.054 |
| Age, years (≤45 vs.
>45) | 0.62 | 0.427–0.899 |
0.012a |
| Sex (male vs.
female) | 1.551 | 1.022–2.353 |
0.039a |
| Disease
stageb | 3.493 | 2.316–5.258 |
<0.001a |
 | Table IV.Multivariate logistic regression
analysis for risk factors of lymph node metastasis in patients with
papillary thyroid carcinoma. |
Table IV.
Multivariate logistic regression
analysis for risk factors of lymph node metastasis in patients with
papillary thyroid carcinoma.
| Factor | OR | 95% CI | P-value |
|---|
| PKHD1L1 expression
(high vs. low) | 0.555 | 0.356–0.866 | 0.009a |
| Histological
type | 2.84 | 1.72–4.692 |
<0.001a |
| Tumour size, mm
(≥20 vs. <20) | 1.299 | 0.762–2.213 | 0.336 |
| Age, years (≤45 vs.
>45) | 0.03 | 0.009–0.1 |
<0.001a |
| Sex (male vs.
female) | 1.518 | 0.916–2.514 | 0.105 |
| Disease
stageb | 55.634 | 16.371–189.066 |
<0.001a |
Knockdown of PKHD1L1 promotes TC cell
proliferation
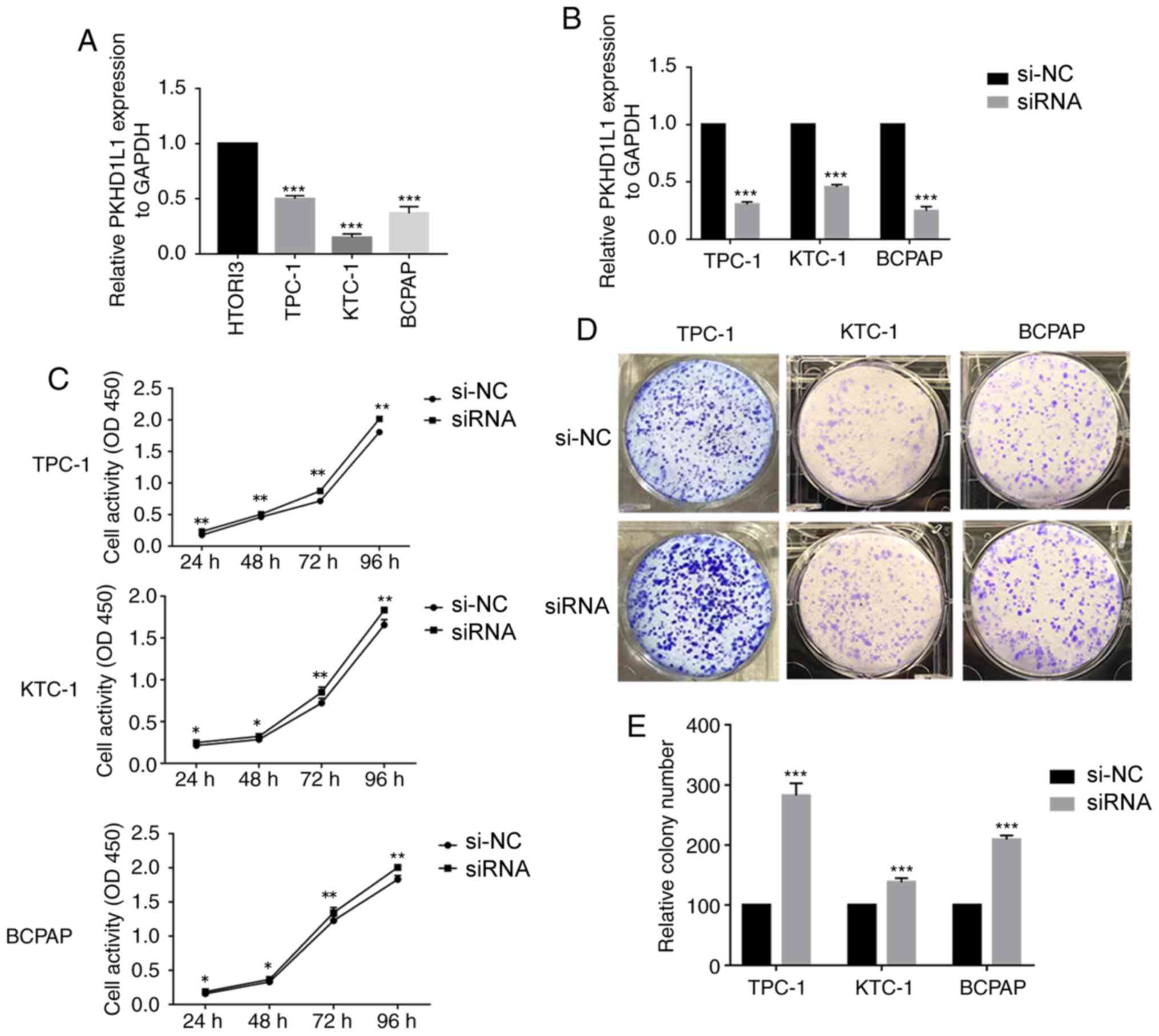
To confirm the function of PKHD1L1 in TC, PKHD1L1
expression levels were assessed in three TC cell lines (TPC-1, KTC1
and BCPAP) and normal thyroid cells (HTORI3) by RT-qPCR. Expression
of PKHD1L1 was higher in HTORI3 compared with that in TPC-1, KTC-1
and BCPAP (Fig. 3A). As PKHD1L1
expression is commonly downregulated in PTC, this gene may serve an
important function in tumourigenesis and progression of PTC.
A total of three siRNAs were designed to investigate
the function of PKHD1L1, and one effective siRNA was selected to
downregulate the expression level of PKHD1L1 in the TC cell lines.
RT-qPCR was used to detect PKHD1L1 expression levels in transfected
cells (Fig. 3B). CCK-8 assay
demonstrated that knockdown of PKHD1L1 promoted cell proliferation
in the three TC cell lines compared with the respective negative
controls (P<0.01; Fig. 3C). Cells
transfected with PKHD1L1 siRNA exhibited increased proliferative
capacity, which approached statistical significance at 3 or 4 days
of cell culture. In addition, PKHD1L1 knockdown promoted TC cell
colony formation compared with the control group (P<0.001;
Fig. 3D).
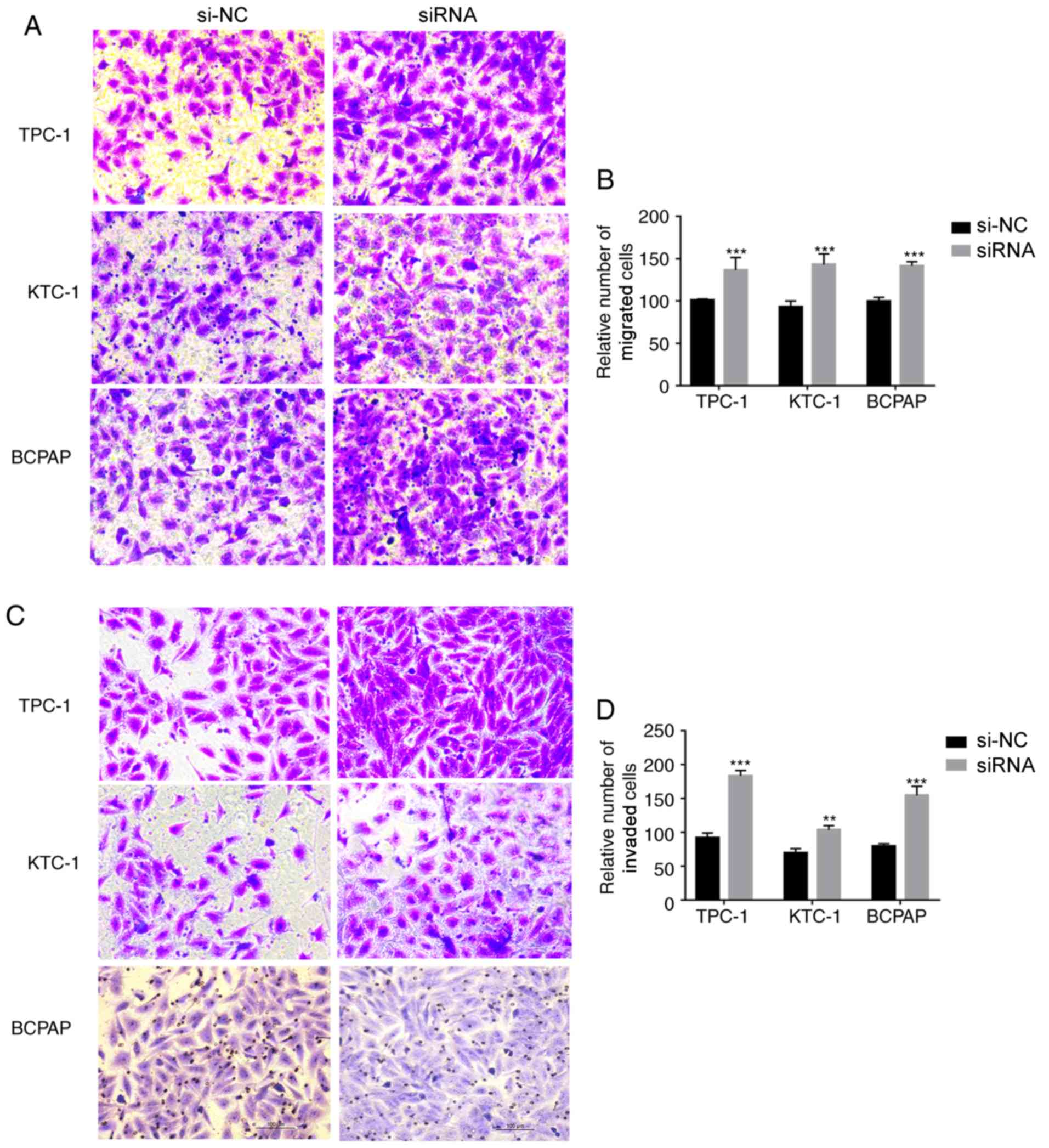
Knockdown of PKHD1L1 regulates the
migration and invasion of TC cell lines in vitro
Based on the results of the clinicopathological
feature analysis and cell proliferation and colony formation
experiments, the role of PKHD1L1 in the migratory and invasive
capacities of the three types of TC cell lines was further
investigated. The migratory ability of PKHD1L1-knockdown cells was
increased in the TC cell lines compared with that in the control
cells. The invasive capacity of the PKHD1L1-knockdown cells was
significantly higher compared with that of the corresponding
control cells (Fig. 4). Thus,
PKHD1L1 may serve a crucial role in the migratory and invasive
capacities of the TC cell lines.
Discussion
In recent decades, thyroid cancer (TC) has become
one of the most common endocrine malignancies, as the number of
annual TC cases has increased by 4% globally since 2010 (5). Although PTC has a positive prognosis
(8), capsular invasion, LNM and
distant metastasis in some patients with PTC are a non-negligible
problem. A large study has demonstrated that the incidence of
capsular invasion and LNM is 20–50% (24,25).
These events may also lead to disease recurrence (26) and cancer-specific mortality (27). In addition, 20–50% of patients
diagnosed with PTC experience LNM (28), and more than one-third of them have
clinically detected lymph node involvement on initial presentation
(29). Despite the advances in
medical science, the potential mechanism of such pathogenesis is
still not sufficiently confirmed, which indicates a major research
deficiency in TC. To avoid unnecessary lymph node dissection and to
further understand LNM in PTC, molecular markers that effectively
predict TC progression and are potential PTC targets need to be
identified.
A high-throughput sequencing aiming to explore
potential molecular biomarkers that could predict PTC progression
or assess the risk for PTC was conducted in our previous
unpublished study. A total of 19 pairs of PTC tumour samples and
adjacent normal tissue samples were subjected to this technique,
and the results demonstrated that PKHD1L1 expression levels were
lower in PTC tumour tissue compared with adjacent normal thyroid
tissue. This result suggested that PKHD1L1 may be a tumour
suppressor in PTC.
PKHD1L1 was initially reported as a mouse gene
(18,19), but it was subsequently demonstrated
that it encodes a putative receptor protein, and that its mutation
may cause ARPKD (19). Low PKHD1L1
expression is detected in many primary immune cell subtypes, but it
is specifically upregulated and serves as an initiator of
activation signals in T lymphocytes (20). In a Hispanic population, the PKHD1L1
gene is also associated with the pathophysiology of childhood
obesity (30). A previous study has
demonstrated that the PKHD1L1*I/D genotype is a predictive factor
in male longevity (21). Although
the gene has been discovered for a long time, the precise mechanism
of PKHD1L1 in human cancer remains poorly understood.
In a previous study (31), analysis of 19 matched pairs of
thyroid carcinoma tissues and nearby noncancerous tissue revealed
that PKHD1L1 expression was downregulated in tumour tissues. The
present study aimed to investigate the role of PKHD1L1 in TC. The
results demonstrated that the expression levels of PKHD1L1 were
significantly downregulated in PTC tumour tissue. In addition, low
PKHD1L1 expression in the TCGA cohort was associated with certain
clinicopathological features, such as tumour size, distant
metastasis, LNM and disease stage. In the validated cohort, tumour
size and LNM were associated with low expression of PKHD1L1, which
was consistent with TCGA data. Furthermore, PKHD1L1 was identified
as a significant predictor of LNM. Loss of function in PKHD1L1
in vitro promoted TC cell proliferation and colony formation
compared with control cells. Additionally, PKHD1L1-knockdown TC
cells exhibited compromised migratory and invasive capacity
compared with control cells.
Certain limitations to the present study should be
considered. Firstly, the association between PKHD1L1 and the
prognosis of PTC needs to be investigated in large samples.
Secondly, rescue experiments such as overexpression of PKHD1L1
in vitro need to be performed. The exact mechanism of the
tumourigenic role of PKHD1L1 in PTC needs to be further
studied.
In conclusion, the present study investigated the
association between PKHD1L1 and PTC LNM, as well as the function of
PKHD1L1 in TC in vitro. The results suggested that PKHD1L1
may be a suppressor gene associated with PTC and may be a potential
molecular biomarker in the future.
Acknowledgements
The authors would like to thank Professor Ming-Zhao
Xing of Johns Hopkins University School of Medicine (Baltimore, MD,
USA) for supplying the cell lines.
Funding
This study was funded by the National Natural
Science Foundation of China (grant no. 81572291), the Natural
Science Foundation of Zhejiang province (grant nos. LY17H160053,
LGF18H160031, GF18H160071 and LGF18H160032), the Medical and Health
Technology Projects of Zhejiang province (grant no. 2017187475) and
the Science and Technology Project of Wenzhou (grant no.
Y20170030).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CZ wrote the manuscript. RQ performed the
experiments. CZ, AB and EJX collected and processed the data. XZ
designed the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Ethical approval for this study was obtained from
the Ethics Committee of The First Affiliated Hospital of Wenzhou
Medical University, Wenzhou, China. Patients signed informed
consent forms, and the research protocols for the use of tissues
were approved by and conducted in accordance with the ethical
standards of the Institutional Review Board of The First Affiliated
Hospital of Wenzhou Medical University (approval no. 2012-57).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Burns WR and Zeiger MA: Differentiated
thyroid cancer. Semin Oncol. 37:557–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pellegriti G, Frasca F, Regalbuto C,
Squatrito S and Vigneri R: Worldwide increasing incidence of
thyroid cancer: Update on epidemiology and risk factors. J Cancer
Epidemiol. 2013:9652122013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morris LG, Tuttle RM and Davies L:
Changing trends in the incidence of thyroid cancer in the United
States. JAMA Otolaryngol Head Neck Surg. 142:709–711. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
La Vecchia C, Malvezzi M, Bosetti C,
Garavello W, Bertuccio P, Levi F and Negri E: Thyroid cancer
mortality and incidence: A global overview. Int J Cancer.
136:2187–2195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leboulleux S, Rubino C, Baudin E, Caillou
B, Hartl DM, Bidart JM, Travagli JP and Schlumberger M: Prognostic
factors for persistent or recurrent disease of papillary thyroid
carcinoma with neck lymph node metastases and/or tumor extension
beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol
Metab. 90:5723–5729. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parangi S and Suh H: The role of genetic
markers in the evaluation and management of thyroid nodules. Surg
Clin North Am. 94:515–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xing M: Genetic alterations in the
phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer.
Thyroid. 20:697–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xing M: BRAF mutation in thyroid cancer.
Endocr Relat Cancer. 12:245–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Elisei R, Viola D, Torregrossa L, Giannini
R, Romei C, Ugolini C, Molinaro E, Agate L, Biagini A, Lupi C, et
al: The BRAF(V600E) mutation is an independent, poor prognostic
factor for the outcome of patients with low-risk intrathyroid
papillary thyroid carcinoma: Single-institution results from a
large cohort study. J Clin Endocrinol Metab. 97:4390–4398. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tavares C, Melo M, Cameselle-Teijeiro JM,
Soares P and Sobrinho-Simões M: ENDOCRINE TUMOURS: Genetic
predictors of thyroid cancer outcome. Eur J Endocrinol.
174:R117–R126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gustafson S, Zbuk KM, Scacheri C and Eng
C: Cowden syndrome. Semin Oncol. 34:428–434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morita N, Ikeda Y and Takami H: Clinical
significance of p53 protein expression in papillary thyroid
carcinoma. World J Surg. 32:2617–2622. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, Bishop J, Shan Y, Pai S, Liu D,
Murugan AK, Sun H, El-Naggar AK and Xing M: Highly prevalent TERT
promoter mutations in aggressive thyroid cancers. Endocr Relat
Cancer. 20:603–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strausberg RL, Feingold EA, Grouse LH,
Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler
GD, Altschul SF, et al: Generation and initial analysis of more
than 15,000 full-length human and mouse cDNA sequences. Proc Natl
Acad Sci USA. 99:16899–16903. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lian PW, Fu YL, Li A, Dai BZ, Ding ZW, Li
L and Wu GQ: Preparation and characterization of a polyclonal
antibody against human Fibrocystin-L. Xi Bao Yu Fen Zi Mian Yi Xue
Za Zhi. 27:78–81. 2011.(In Chinese). PubMed/NCBI
|
|
20
|
Hogan MC, Griffin MD, Rossetti S, Torres
VE, Ward CJ and Harris PC: PKHDL1, a homolog of the autosomal
recessive polycystic kidney disease gene, encodes a receptor with
inducible T lymphocyte expression. Hum Mol Genet. 12:685–698. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Erdman VV, Karimov DD, Nasibullin TR,
Timasheva IR, Tuktarova IA and Mustafina OE: Role of PLAT, PKHD1L1,
STK38L and TEAD1 genes Alu-polymorphism for longevity. Adv
Gerontol. 29:709–716. 2016.(In Russian). PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Edge SB and Compton CC: The American Joint
Committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mustafa M, Kuwert T, Weber K, Knesewitsch
P, Negele T, Haug A, Linke R, Bartenstein P and Schmidt D: Regional
lymph node involvement in T1 papillary thyroid carcinoma: A
bicentric prospective SPECT/CT study. Eur J Nucl Med Mol Imaging.
37:1462–1466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schneider DF and Chen H: New developments
in the diagnosis and treatment of thyroid cancer. CA Cancer J Clin.
63:374–394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cho SY, Lee TH, Ku YH, Kim HI, Lee GH and
Kim MJ: Central lymph node metastasis in papillary thyroid
microcarcinoma can be stratified according to the number, the size
of metastatic foci, and the presence of desmoplasia. Surgery.
157:111–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ito Y, Tomoda C, Uruno T, Takamura Y, Miya
A, Kobayashi K, Matsuzuka F, Kuma K and Miyauchi A:
Ultrasonographically and anatomopathologically detectable node
metastases in the lateral compartment as indicators of worse
relapse-free survival in patients with papillary thyroid carcinoma.
World J Surg. 29:917–920. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee YM, Sung TY, Kim WB, Chung KW, Yoon JH
and Hong SJ: Risk factors for recurrence in patients with papillary
thyroid carcinoma undergoing modified radical neck dissection. Br J
Surg. 103:1020–1025. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sancho JJ, Lennard TW, Paunovic I,
Triponez F and Sitges-Serra A: Prophylactic central neck disection
in papillary thyroid cancer: A consensus report of the European
society of endocrine surgeons (ESES). Langenbecks Arch Surg.
399:155–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Comuzzie AG, Cole SA, Laston SL, Voruganti
VS, Haack K, Gibbs RA and Butte NF: Novel genetic loci identified
for the pathophysiology of childhood obesity in the Hispanic
population. PLoS One. 7:e519542012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang QX, Chen ED, Cai YF, Li Q, Jin YX,
Jin WX, Wang YH, Zheng ZC, Xue L, Wang OC and Zhang XH: A panel of
four genes accurately differentiates benign from malignant thyroid
nodules. J Exp Clin Cancer Res. 35:1692016. View Article : Google Scholar : PubMed/NCBI
|