Introduction
Malignant tumors are a major disease, and pose
serious threat to human life and health (1). There are currently >100 known types
of malignancy that affect humans (2). In recent years, the overall incidence
of malignant tumors has exhibited an upward trend (3). Malignant tumors are the main cause of
death, and the mortality rate of patients with malignant tumors is
slightly lower compared with that due to heart disease,
particularly in the majority of developed countries (4). In China, malignant tumors are the
second major cause of mortalities in cities and rural areas
(5). Patients who live in developed
countries, including Europe and the United States have a five-year
standardized survival rate of 60–70%; however, a survival rate of
30.9% has been reported for China, while that of rural areas is
half of that in cities (6).
Therefore, it is imperative to identify more effective novel
anti-tumor drugs.
The fermentation products of Actinomycetes are an
important natural source of anti-tumor drugs and have various
chemical structures (7). The
majority of them have innovative mechanisms and are easy to mass
produce. Siomycin A is a type of thiopeptide antibiotic that is
isolated from the fermentation products of an endophytic
actinomycin, which is derived from the medicinal plant
Acanthopanax senticosus (8).
At present, the inhibitory effect of siomycin A on tumor cells has
only been demonstrated in a limited number of cell lines and the
mechanism of siomycin A in tumor cells is not yet clear (9,10). In
the present study, a number of human tumor cell lines were selected
to investigate whether siomycin A has antitumor effects in
vitro.
Materials and methods
Cell lines, media and chemical
compounds
Siomycin A was isolated and identified by Liu et
al (11). In the present study,
siomycin A at a final concentration of 0, 0.625, 1.25, 2.5, 5 or 10
µmol/l; medium containing 0.5% dimethyl sulfoxide or 0 µmol/l
siomycin A served as the controls. The K562 human leukemia cell
line, MCF7 human breast cancer cell line and MiaPaCa-2 human
pancreatic cancer cell line were purchased from the Cell Center,
Peking Union Medical College (Beijing, China). Dulbecco's Modified
Eagle's medium (DMEM) was obtained from Corning Inc., and fetal
bovine serum (FBS) was purchased from Bovogen Biologicals Pty Ltd.
Triton X-100, dimethyl sulfoxide and DAPI were acquired from
Sigma-Aldrich (Merck KGaA). Transwell chambers were obtained from
BD Pharmingen (BD Biosciences). α-tubulin and β-actin antibodies
were purchased from Antibody Revolution company, and matrix
metalloproteinase (MMP)-2 and MMP-9 antibodies were acquired from
Affinity Biosciences. Peroxidase-labeled antibody to mouse
immunoglobulin G (IgG) was purchased from KPL Inc.
Cell culture
The K562 cell line was cultured with RPMI-1640
medium (Invitrogen; Thermo Fisher Scientific, Inc.). The MCF7 and
MiaPaCa-2 cell lines were grown in DMEM medium (Corning Inc.). Both
types of media were supplemented with 10% FBS and 1%
penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
The cell lines were maintained at 37°C in 5% CO2.
Viability assay
The viability assay was performed according to the
manufacturer's protocols of the CCK-8 kit (Beijing Zoman
Biotechnology Co., Ltd.). Cell suspensions (100 µl;
3×104 cells/ml) with 10% FBS medium were added to a
96-well plate and the plate was incubated for 6 h at 37°C.
Subsequently, 10 µl CCK-8 reagent was added to each well and then
the cells were incubated for 2 h at 37°C. The optical density at a
wavelength of 450 nm was measured using an ELx800 microplate reader
(BioTek Instruments, Inc.). Similar assays were performed after
incubation for 24, 48 and 72 h and the experiments were performed
in triplicate. Cell viability was expressed as the half maximum
inhibitory concentration (IC50) value and data were
analyzed with the software Microsoft Office Excel 2010 (Microsoft
Corporation).
Morphological changes
Briefly, 3×105 MiaPaCa-2 cells were
incubated at 37°C for 24 h with or without siomycin A at
concentrations of 0.625, 1.25, 2.5, 5 and 10 µmol/l in 60-mm
diameter tissue culture dishes. Subsequently, the medium was
discarded and the cells were washed once with PBS. The
morphological changes of the apoptotic cells were observed using an
inverted phase contrast microscope (CX31; Olympus Corporation,
Tokyo, Japan) at ×100 magnification.
Transwell migration assay
The assays were performed using a Corning Transwell
permeable support system with 8.0 µm pore size. A total of
1×104 cells were suspended in serum-free DMEM and were
placed in the upper chamber of a Transwell plate. Siomycin A at a
final concentration of 0, 0.625, 1.25, 2.5, 5 or 10 µmol/l was
added to the lower chamber, which DMEM containing 10% FBS. After
incubation for 20 h at 37°C, the cells on the upper surface of the
filter were removed using a cotton swab, 2 drops of the Rapid Gram
Stain (Zhuhai Baso Biotechnology Co., Ltd.) solution were added to
the cells that had migrated to the membrane at room temperature for
30 sec and were washed with water. Migration was observed in six
randomly selected fields with a light Olympus CX31 microscope.
Cells in each image were counted with the ImageJ 1.52a software
(National Institutes of Health, USA).
Cytoskeleton assay
MiaPaCa-2 cells were inoculated into laser confocal
culture dishes at a density of 6×104 cells/well and
cultured for 24 h. The cells were treated and incubated with
various concentrations of Siomycin A. After rinsing three times
with Hank's Balanced Salt Solution (HBSS; Beijing Solarbio Science
& Technology Co., Ltd.), cells were incubated with 200 µl
murine anti-human α-tubulin (1:200; cat. no. ARH4207; Antibody
Revolution Inc.) overnight at 4°C in a wet box. Cells were then
washed with HBSS three times for 10 min and incubated with 200 µl
goat anti-mouse IgG-fluorescein isothiocyanate (FITC) (1:50; cat.
no. 074-1506; SeraCare Life Sciences) at room temperature for 30
min in the dark. Cells were washed three times with HBSS for 10 min
and incubated with 10 µl DAPI nuclear stain for 5 min at room
temperature in the dark. The unbound nuclear stain was washed with
HBSS. The dishes were placed under a laser scanning confocal
microscope (TCS SP8 STED 3X; Leica Microsystems GmbH;
magnification, ×600) and scanned at 488 nm (excitation) and 543 nm
(detection) for the analysis of green fluorescence. A total of five
fields were randomly scanned for each group to obtain fluorescence
images and analyzed with ImageJ 1.6.0_24 software (National
Institutes of Health), in order to observe the cytoskeleton of the
cells.
Apoptosis detection by flow
cytometry
Cells that have reached ~80% confluence were
detached using 0.25% trypsin without EDTA (Beijing Solarbio Science
& Technology Co., Ltd.) at 37°C. The control and treated cells
were stained using an Annexin V-FITC/propidium iodide (PI)
apoptosis detection kit (BD Biosciences), according to the
manufacturer's protocols. After double staining with
FITC-conjugated Annexin V and PI, the cells were analyzed by flow
cytometry (Leica TCS SP8 STED; Leica Microsystems GmbH). The
experiments were performed in triplicate (SPSS 19.0; IBM
Corp.).
Western blot analysis
All of the treated cell groups were harvested and
lysed with cell lysis buffer (cat. no. BB-3201-1; BestBio) for
western blot analysis. The protein concentrations were determined
by a NanoDrop 2000 spectrophotometer (NanoDrop Technologies; Thermo
Fisher Scientific, Inc.). Proteins (5–10 µl, 10–30 µg) were
separated using 8–10% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (EMD Millipore). The membranes were blocked in
TBST containing 5% non-fat skim milk at room temperature for 2 h.
After washing with TBST in triplicate, the membranes were incubated
with primary antibodies against MMP-2 (1:500; cat. no. AF0577;
Affinity Biosciences), MMP-9 (1:1,500; cat. no. AF5228; Affinity
Biosciences), α-tubulin (1:5,000; cat. no. ARH4207; Antibody
Revolution Co., Ltd.) and β-actin (1:5,000; cat. no. ARH4149;
Antibody Revolution Co., Ltd.) for 2 h at 37°C. Subsequently, the
membranes were washed and incubated with an appropriate horseradish
peroxidase-linked secondary antibody (1:5,000; cat. no. 074-1506;
KPL Inc.) for 20 min at 37°C. Bands were developed with an enhanced
chemiluminescence blot detection system (UVP BioSpectrum Imaging
System; Analytik Jena AG). The data were analyzed via densitometry
using ImageJ 1.52a software (National Institutes of Health).
Statistical analysis
Statistical comparisons were performed with SPSS
19.0 software (IBM Corp.). The results are presented as the mean ±
standard deviation. One-way analysis of variance was used for
multiple comparisons followed by a Student-Newman-Keuls post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Siomycin A inhibits the proliferation
of human tumor cell lines
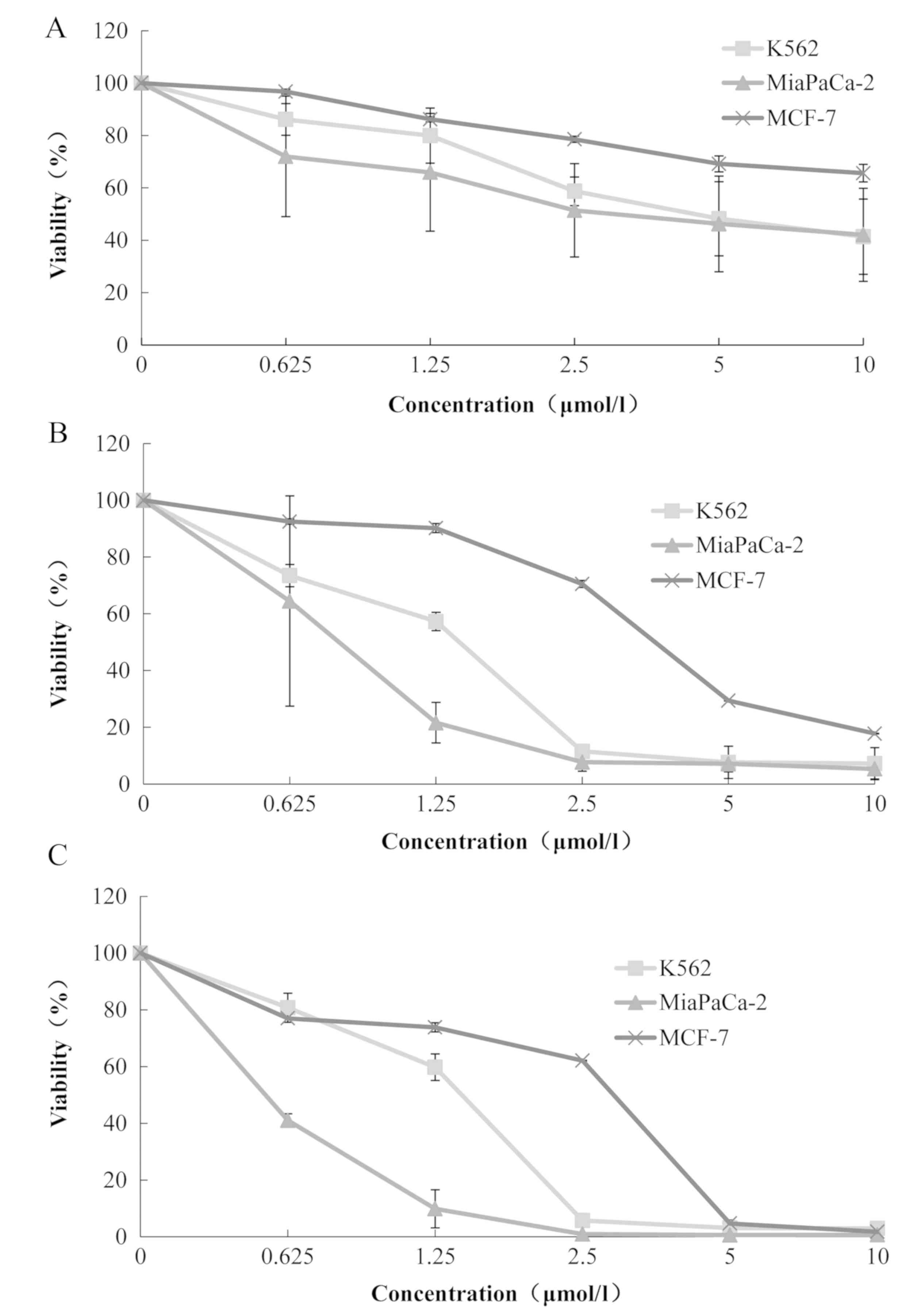
Siomycin A inhibited the proliferation of a variety
of human tumor cell lines. Cell viability was reduced with
increases in drug concentration, which demonstrates a
dose-dependent effect. Furthermore, cell viability markedly
decreased as the drug treatment period increased, which
demonstrates a time-dependent association (Fig. 1). Among the three cell lines, the
IC50 of the human leukemia K562 cells was the lowest at
6.25±3.60 µmol/l at 24 h, while that for the human pancreatic
cancer MiaPaCa-2 cells was 6.38±5.73 µmol/l. However, the
IC50 of the human pancreatic cancer MiaPaCa-2 cells at
48 and 72 h were the lowest of the three cell lines, which were
0.76±0.51 and 0.54±0.02 µmol/l, respectively (Table I). Therefore, MiaPaCa-2 cells were
selected for the subsequent experiments.
 | Table I.IC50 of three tumor cell
lines at different time points. |
Table I.
IC50 of three tumor cell
lines at different time points.
|
| IC50
(µmol/l) |
|---|
|
|
|
|---|
| Cell line | 24 h | 48 h | 72 h |
|---|
| K562 | 6.25±3.60 | 1.18±0.04 | 1.24±0.12 |
| MiaPaCa-2 | 6.38±5.73 | 0.76±0.51 | 0.54±0.02 |
| MCF-7 | 19.61±7.28 | 2.97±0.02 | 1.98±0.03 |
Siomycin A changes the morphology and
inhibits the migration of MiaPaCa-2 cells
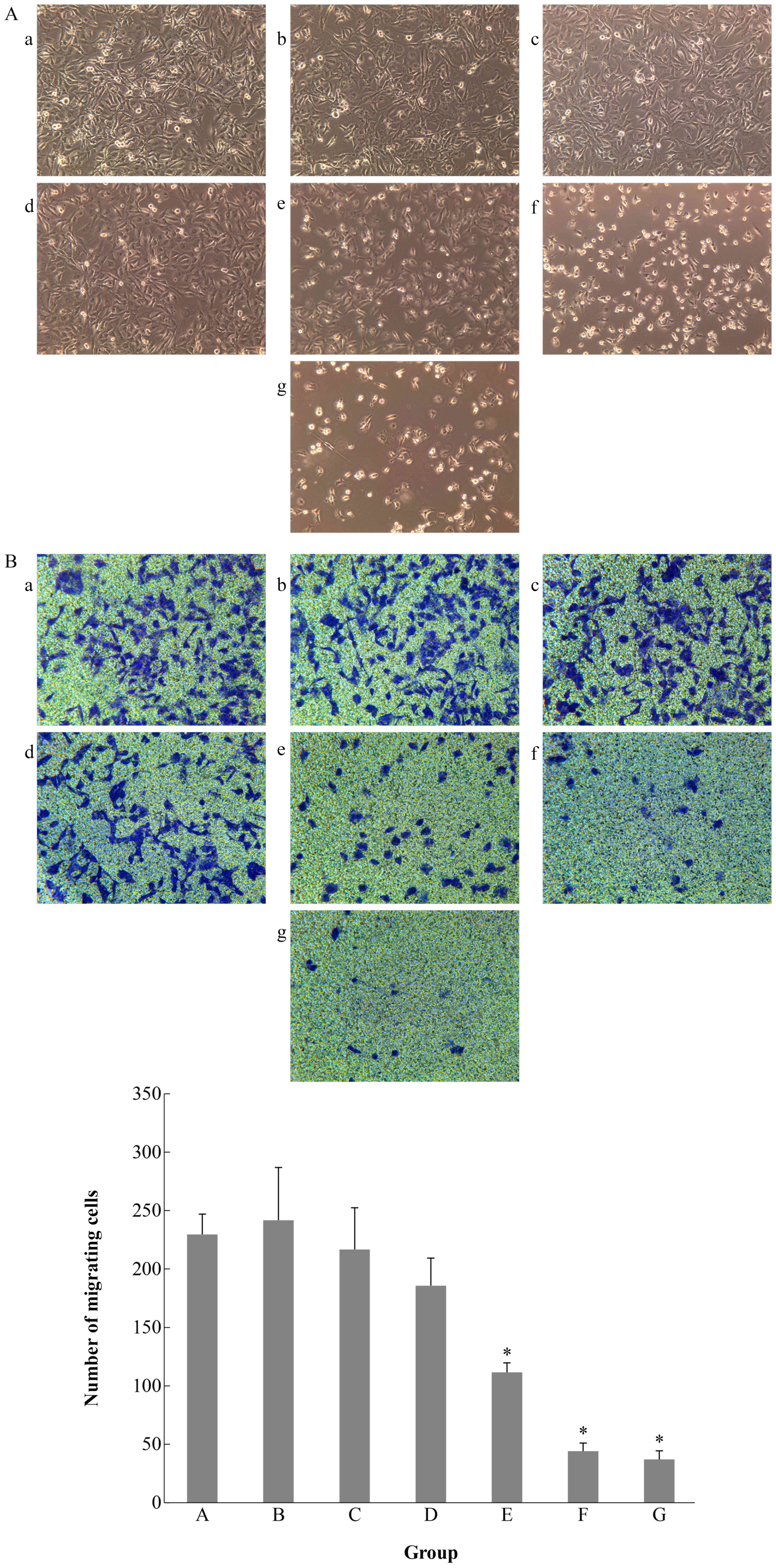
The MiaPaCa-2 cells were treated with different
concentrations of siomycin A for 24 h and then changes in cell
morphology were observed under an inverted phase contrast
microscope. The cells in the solvent, and 0, 0.625 and 1.25 µmol/l
groups had a high cell density and a better growth state.
Furthermore, there was no significant difference in the cell
morphology of these groups. When the concentration of siomycin A
was 2.5 µmol/l, the cell density began to decrease and the cell
size was reduced, but the cells remained spindle-shaped. In the 5
and 10 µmol/l groups, a large number of cells had shrunk into a
spherical shape and exhibited many small bright spots; the cell
density was notably reduced (Fig.
2A). Subsequently, the effect of siomycin A on the migration of
the MiaPaCa-2 cells was detected. After treatment with various
concentrations of siomycin A for 24 h, the number of cells
penetrating into the Transwell chamber was reduced with increases
in drug concentration (Fig. 2B).
Compared with the 0 µmol/l group, cell mobility was significantly
reduced in the 2.5, 5 and 10 µmol/l groups (P<0.05).
Effects of siomycin A on the
cytoskeleton of MiaPaCa-2 cells
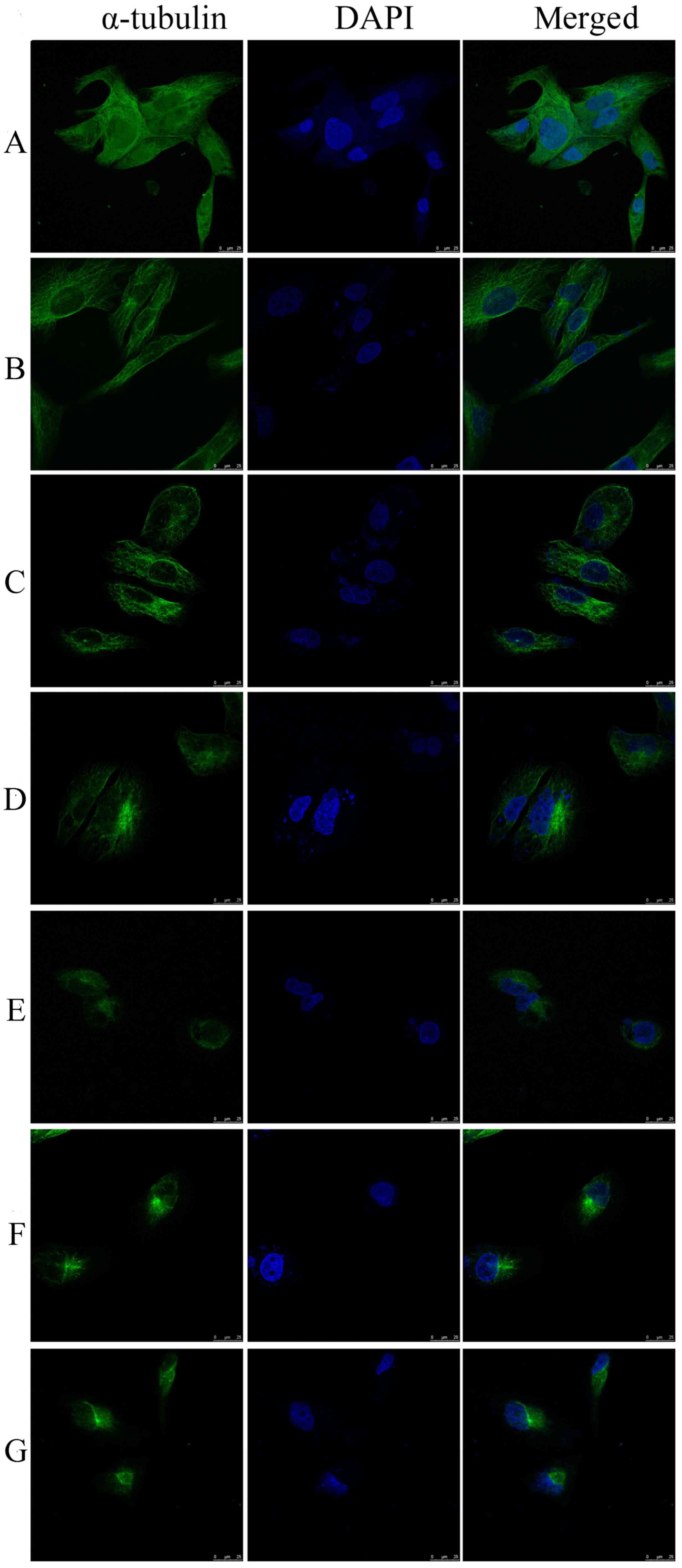
The MiaPaCa-2 cells were treated with various
concentrations of siomycin A for 24 h, and then the changes in the
cytoskeletons were detected using a laser confocal microscope. In
the 0 µmol/l group, the distribution of green filamentous
microtubules in the cytoplasm of the MiaPaCa-2 cells was regular
and they were arranged radially from the nucleus to the
surroundings. With the increase in drug concentration, the cell
began to shrink, the number of microtubules reduced or had
disappeared, the fluorescence distribution was clustered and
partially contracted to the nucleus, and the fluorescence intensity
was notably enhanced. There were slight changes in the nuclei of
the cells in the 2.5, 5 and 10 µmol/l groups (Fig. 3). The images were processed according
to the method of a previous study (12). The distribution of the microtubule
skeleton was analyzed with ImageJ software fractal box counting
tool. The software automatically maps the abscissa log (box size)
and ordinate log (count) values, and the resulting slope is the
fractal dimension D. The results are presented in Table II. The fractal dimensions of the
2.5, 5 and 10 µmol/l groups were significantly reduced compared
with the 0 µmol/l group (P<0.05). The results demonstrated that
the complexity of the cytoskeleton was reduced and the
morphological differences in the cytoskeleton were increased in the
MiaPaCa-2 cells that were treated with siomycin A.
 | Table II.Fractal dimension of MiaPaCa-2 cells
treated with different concentrations of siomycin A. |
Table II.
Fractal dimension of MiaPaCa-2 cells
treated with different concentrations of siomycin A.
| Concentration
(µmol/l) | Cell dimension |
|---|
| Control | 1.67±0.03 |
| 0 | 1.65±0.05 |
| 0.625 | 1.63±0.11 |
| 1.25 | 1.67±0.04 |
| 2.5 |
1.47±0.14a |
| 5 |
1.37±0.17a |
| 10 |
1.31±0.13a |
Effects of siomycin A on the apoptosis
of MiaPaCa-2 cells
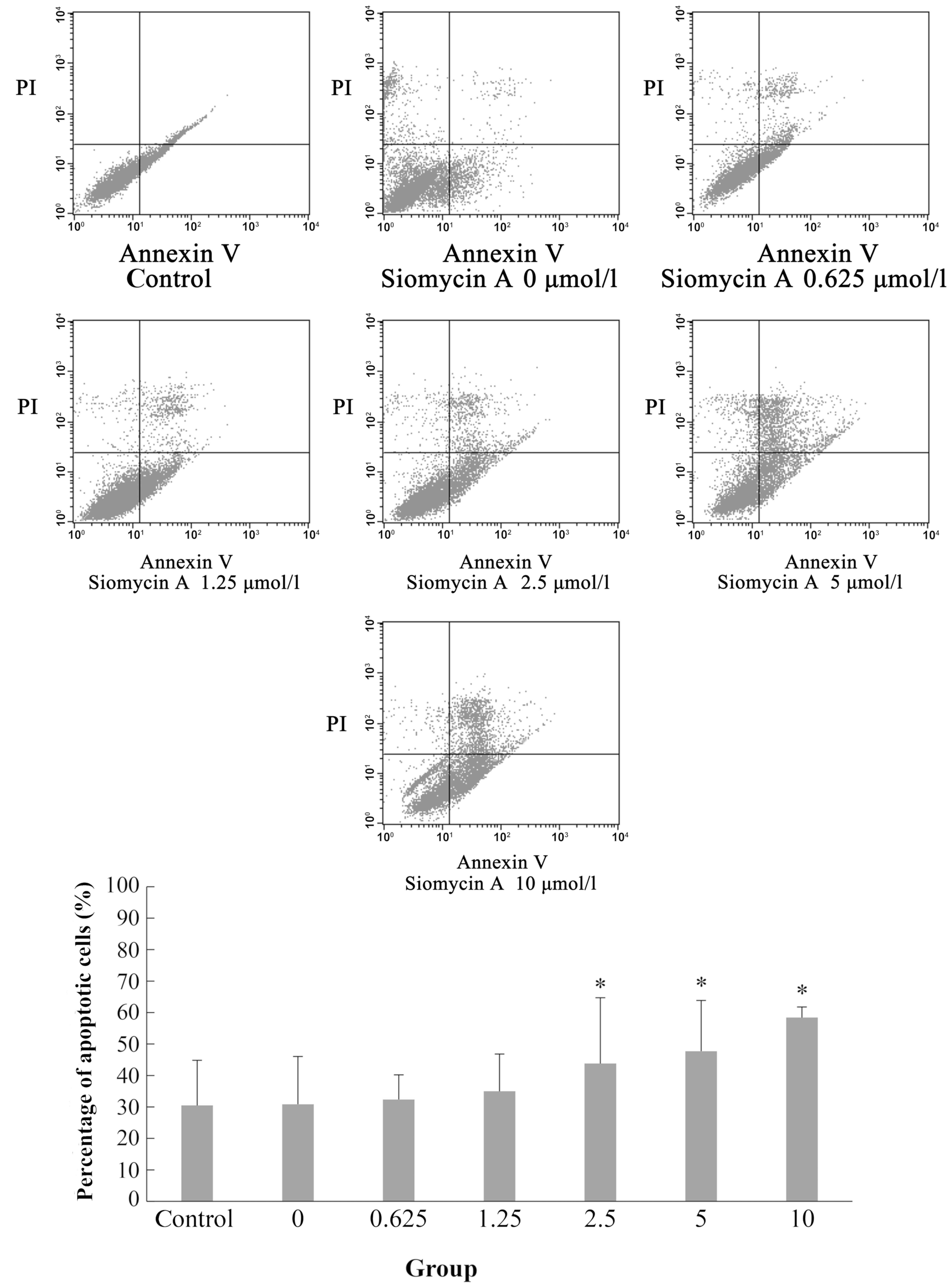
Using Annexin V-FITC/PI double staining, analysis of
the apoptosis of the MiaPaCa-2 cells treated with different
concentrations of siomycin A demonstrated the potent proapoptotic
effect of siomycin A. Compared with the 0 µmol/l group, the
percentage of apoptotic cells was significantly increased in the
2.5, 5 and 10 µmol/l groups (P<0.05; Fig. 4).
Effects of siomycin A on the protein
levels of MMP-2, MMP-9 and α-tubulin in MiaPaCa-2 cells
In order to investigate the mechanism of siomycin A
on inhibiting migration and affecting the cytoskeleton of MiaPaCa-2
cells, the protein levels of MMP-2, MMP-9 and α-tubulin were
evaluated. The results of the western blot analysis demonstrated
that the expression levels of MMP-2 and MMP-9 protein in the
MiaPaCa-2 cells were significantly reduced in the 2.5, 5 and 10
µmol/l groups. The expression of α-tubulin protein was
significantly decreased in the 1.25, 2.5, 5 and 10 µmol/l groups
compared with the 0 µmol/l group (Fig.
5).
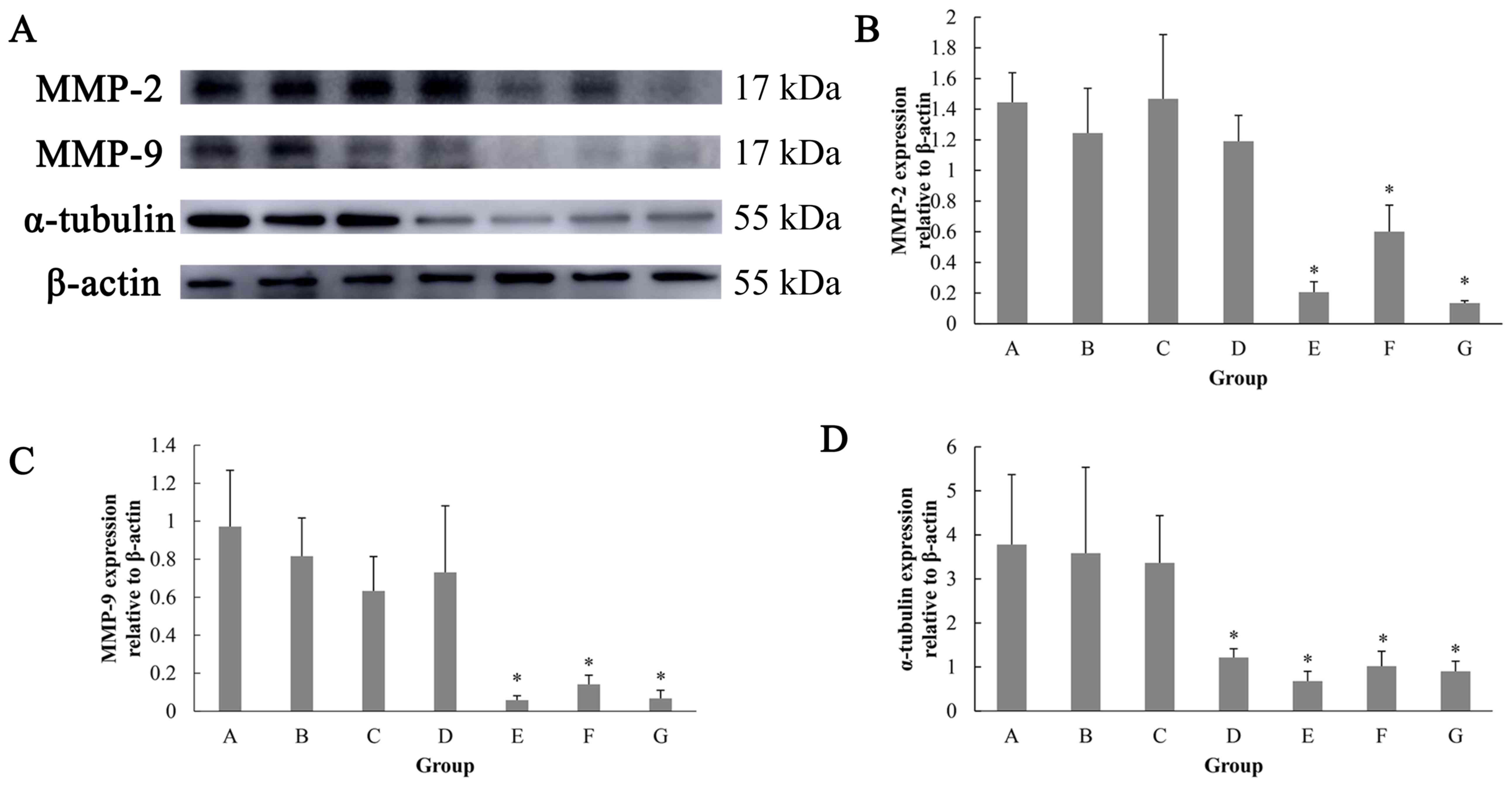 | Figure 5.Effects of siomycin A on the protein
levels of MMP-2, MMP-9 and α-tubulin in MiaPaCa-2 cells. (A)
Detection of MMP-2, MMP-9 and α-tubulin protein expression by
western blot. (B) Expression of MMP-2 relative to β-actin protein
under different concentrations of siomycin A. (C) Expression of
MMP-9 relative to β-actin protein under different concentrations of
siomycin A. (D) Expression of α-tubulin relative to β-actin protein
under different concentrations of siomycin A. The cells were
treated with solvent control, or 0, 0.625, 1.25, 2.5, 5 and 10
µmol/l siomycin A for 24 h. The protein levels were determined
using western blot analysis. *P<0.05 vs. the 0 µmol/l group.
MMP, matrix metalloproteinase. For each panel, A-G represents
treatments with increasing concentrations of siomycin A (control
and 0, 0.625, 1.25, 2.5, 5 and 10 µmol/l). |
Discussion
The increasing incidence of malignant tumors
necessitates the identification of novel therapeutic compounds. The
majority of novel compounds identified originate from microbes
(13). Siomycin A is a thiopeptide
antibiotic that was isolated from the fermentation product of
Streptomyces sioyaensis by Nishimura in 1959 (14). Using a high-throughput drug screening
system, Radhakrishnan et al (9) demonstrated that siomycin A specifically
inhibits the transcription and expression of Forkhead box family
(Fox)M1 without affecting other members of the Forkhead box family.
FoxM1 is a transcription factor of the Forkhead family (15) that is overexpressed in a variety of
tumor cell types, including liver cancer, pancreatic cancer, breast
cancer and lung adenocarcinoma cells (16–21).
A previous study demonstrated that siomycin A
effectively reduces the expression of maternal embryonic leucine
zipper kinase and inhibits tumor growth in mice (10). Another study found that siomycin A
inhibits tumor cell growth and survival by downregulating the
expression of BUB1 mitotic checkpoint serine/threonine kinase B
protein (22). In addition, siomycin
A can inhibit the invasive ability of laryngeal carcinoma HEp-2
cells and reduce the levels of MMP-2 and MMP-9 protein in them
(23).
The present study demonstrated that siomycin A has
an inhibitory effect on three human tumor cell lines in a time- and
a dose-dependent manner. Based on the comprehensive evaluation of
the IC50, the human pancreatic cancer MiaPaCa-2 cell
line was selected as a model to analyze the inhibitory effect of
siomycin A on tumor cells and investigate the underying
mechanism.
The density of the human pancreatic cancer MiaPaCa-2
cells treated with siomycin A for 24 h was observed using an
inverted phase contrast microscope. The cell density was reduced in
response to siomycin A at concentration of 2.5 µmol/l. With the
increase in the drug concentration, shrunken cells that were
rounded with small bright spots were observed. Under a light
microscope, the common feature of apoptosis is cell shrinkage, and
nuclear alterations (24). Apoptotic
cells and bodies are clear under phase contrast microscopy
(25). In the present study, the
cells were notably shrunken and rounded, which is consistent with
the morphological changes of apoptosis. The percentage of apoptotic
MiaPaCa-2 cells was significantly increased with increases in drug
concentration. When the drug concentration was 10 µmol/l, the
percentage of apoptotic cells reached 58.40±3.35% (Fig. 4), which demonstrates a dose-dependent
effect. This result is consistent with the results of a previous
study, which showed that siomycin A induces apoptosis of human
laryngeal carcinoma HEp-2 cells (23).
In order to investigate the effect of siomycin A on
the migration of tumor cells, a Transwell migration assay was used
to detect changes in cell migration. Compared with the 0 µmol/l
group, the number of migrated cells was significantly lower in the
2.5–10 µmol/l groups (P<0.05). The results suggest that siomycin
A inhibited the migration of tumor cells; the aforementioned
results are consistent with that of Jiang et al (26), who demonstrated that siomycin A
inhibits the migration and invasion of human nasopharyngeal
carcinoma C666-1 cells. Furthermore, the expression levels of MMP-2
and MMP-9 were detected using western blot analysis. The results
demonstrated that the expression levels of MMP-2 and MMP-9 in the
2.5–10 µmol/l groups were significantly lower than in the 0 µmol/l
group (P<0.05). Therefore, the mechanism of the inhibitory
effect of siomycin A on human pancreatic cancer cell migration may
be associated with downregulation of MMP-2 and MMP-9 protein
expression. The results are similar to those of Nakano et al
(10), who treated polymorphic
glioblasts with siomycin A, and demonstrated that the expression
levels of MMP-2 and MMP-9 were reduced. Previous studies have shown
the association of FoxM1 with MMP-2 and MMP-9 in various types of
cancer, including clear cell renal cell carcinoma, glioma cells and
papillary thyroid carcinoma, leading to the increased invasiveness
and migratory ability of cancer cells causing metastasis, And
targeting FoxM1 has been shown to inhibit these cellular properties
via MMP-2 and MMP-9 downregulation (27–29).
In the initial morphological observation in the
present study, tumor cells appeared to be shrunk and rounded after
administration with siomycin A, which suggests that the skeletal
structure of the tumor cells may change. The cytoskeleton serves a
role in diverse cellular functions, including maintenance of cell
shape, cell movement, cell division and intracellular organization
and trafficking (30). One of the
main structures of the cytoskeleton is the microtubules, which
serve an important role in maintaining cell morphology, organelle
composition, cell division, intracellular transport of substances
and signal transduction (31).
Therefore, the expression of α-tubulin was analyzed in the present
study using laser confocal microscopy and western blot analysis.
The results demonstrated that the microtubules in the cytoplasm
reduced gradually or disappeared with increasing concentration of
siomycin A. The microtubule contraction and the fluorescence around
the nucleus were markedly enhanced, which indicated that the
tubulin was depolymerized and rearranged. The measure of complex
irregularity is reflected by the fractal dimension, which
represents the validity of complex space. The analysis of the cell
fractal dimension with ImageJ software identified that it was
significantly different in the 2.5–10 µmol/l groups compared to the
0 µmol/l group (P<0.05). The results indicated that the
cytoskeleton complexity was reduced by treatment with siomycin A,
which is consistent with the morphological changes in the cells.
The expression of α-tubulin protein was detected by western blot
analysis and the results demonstrated that the expression levels of
α-tubulin protein in the 1.25–10 µmol/l groups were significantly
lower than those in the 0 µmol/l group (P<0.05). These results
are consistent with that of the laser confocal microscopy, which
were consistent with the trend of the cytoskeletal fractal
dimension. It was observed that α-tubulin protein expression
decreased with the increase of siomycin A and the mechanism of
siomycin A on the cytoskeleton of human tumor cells may be
associated with the downregulation of α-tubulin protein expression.
However, regardless of this finding, it is still unclear whether
the function of α-tubulin in tumor cells depends on siomycin A, or
not. Therefore, further examination on this subject is
required.
In conclusion, the present study proposed that
siomycin A inhibits the proliferation and migration of cancer
cells, and demonstrated that siomycin A also induces cell
apoptosis. The inhibitory effects of siomycin A on tumor cells may
be associated with downregulation of α-tubulin skeletal protein
expression, which affects the cytoskeleton of tumor cells.
Experiments of the present study were limited to investigations at
the cellular level and these results are not sufficient to
elucidate the anti-tumor mechanism of siomycin A. Further animal
experiments will be conducted to elucidate the mechanism of
siomycin A action, as well as the role of α-tubulin skeletal
protein.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Science
Foundation of Hebei Province (grant nos. C2014209137 and
H2013209040).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BW, JC and LY designed experiments. BW, WW and HM
performed experiments. BW analyzed the data. BW, JC and LY wrote
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ji-Jun D, Ya-Qiong Y, Nian-Nian Y, Jing Z,
Rong-Shou Z and Si-Wei Z: International comparison analysis of
china's cancer incidence and mortality. Chin J Frontiers of Med Sci
(Electronic Version). 2016.(In Chinese).
|
|
2
|
Hu XQ, Zhou GQ and Wang SX: Application of
combined detection of T lymphocyte and DNT cells in early diagnosis
of malignant tumor. Labeled Immunoassays Clin Med. 23:139–142.
2016.(In Chinese).
|
|
3
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zaorsky NG, Churilla TM, Egleston BL,
Fisher SG, Ridge JA, Horwitz EM and Meyer JE: Causes of death among
cancer patients. Ann Oncol. 28:400–407. 2017.PubMed/NCBI
|
|
5
|
Shengshou HU, Runlin GAO, Lisheng LIU,
Manlu ZHU, Wen WANG, Yongjun WANG, Zhaosu WU, Huijun LI, Dongfeng
GU, Yuejin YANG, et al: Summary of the 2018 report on
cardiovascular diseases in China. Chin Circulation J.
34:2092019.(In Chinese).
|
|
6
|
Chen W, Zheng R, Zhang S, Zhao P, Zeng H
and Zou X: Report of cancer incidence and mortality in China, 2010.
Ann Transl Med. 2:612014.PubMed/NCBI
|
|
7
|
Sharma M, Dangi P and Choudhary M:
Actinomycetes: Source, identification, and their applications. Int
J Curr Microbiol App Sci. 3:801–832. 2014.
|
|
8
|
Li L, Ai-Hua L, Hao R, Ning H, Ru-Xian C
and Li-Jie Y: Bioactive secondary metabolite study and strain
identification of planomonospora sp. 12 from acanthopanax
senticosus. Chin J Antibiotics. 40:321–324, 349. 2019.
|
|
9
|
Radhakrishnan SK, Bhat UG, Hughes DE, Wang
IC, Costa RH and Gartel AL: Identification of a chemical inhibitor
of the oncogenic transcription factor forkhead box M1. Cancer Res.
66:9731–9735. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakano I, Joshi K, Visnyei K, Hu B,
Watanabe M, Lam D, Wexler E, Saigusa K, Nakamura Y, Laks DR, et al:
Siomycin A targets brain tumor stem cells partially through a
MELK-mediated pathway. Neuro Oncol. 13:622–634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu L, Liu AH and Ren H: Bioactive
secondary metabolite study and strain identification of
Planomonospora sp.12 from Acanthopanax senticosus.
Chin J Antibiotics. 40:321–324, 349. 2015.
|
|
12
|
Qian AR, Li D, Han J, Gao X, Di SM, Zhang
W and Shang P: Fractal dimension as a measure of altered actin
cytoskeleton in MC3T3-E1 cells under simulated microgravity using
3-D/2-D clinostats. IEEE Trans Biomed Eng. 59:1374–1380. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sethi S, Kumar R and Gupta S: Antibiotic
production by microbes isolated from soil. Int J Pharmaceutical Sci
Res. 4:29672013.
|
|
14
|
Kuniko M, Mhionogi E and Hideo O: Studies
on siomycin. III Structural features of siomycin A. J Antibiotics.
22:434–441. 1969. View Article : Google Scholar
|
|
15
|
Myatt SS and Lam EW: The emerging roles of
forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 7:847–859.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okabe H, Satoh S, Kato T, Kitahara O,
Yanagawa R, Yamaoka Y and Nakamura Y: Genome-wide analysis of gene
expression in human hepatocellular carcinomas using cDNA
microarray: Identification of genes involved in viral
carcinogenesis and tumor progression. Cancer Res. 61:2129–2137.
2001.PubMed/NCBI
|
|
17
|
Nakamura T, Furukawa Y, Nakagawa H,
Tsunoda T, Ohigashi H, Murata K, Ishikawa O, Ohgaki K, Kashimura N,
Miyamoto M, et al: Genome-wide cDNA microarray analysis of gene
expression profiles in pancreatic cancers using populations of
tumor cells and normal ductal epithelial cells selected for purity
by laser microdissection. Oncogene. 23:2385–2400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bektas N, Ten Haaf A, Veeck J, Wild PJ,
Lüscher-Firzlaff J, Hartmann A and Dahl E: Tight correlation
between expression of the Forkhead transcription factor FOXM1 and
HER2 in human breast cancer. BMC Cancer. 8:422008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garber ME, Troyanskaya OG, Schluens K,
Petersen S, Thaesler Z, Pacyna-Gengelbach M, van de Rijn M, Rosen
GD, Perou CM, Whyte RI, et al: Diversity of gene expression in
adenocarcinoma of the lung. Proc Natl Acad Sci USA. 98:13784–13789.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van den Boom J, Wolter M, Kuick R, Misek
DE, Youkilis AS, Wechsler DS, Sommer C, Reifenberger G and Hanash
SM: Characterization of gene expression profiles associated with
glioma progression using oligonucleotide-based microarray analysis
and real-time reverse transcription-polymerase chain reaction. Am J
Pathol. 163:1033–1043. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Obama K, Ura K, Li M, Katagiri T, Tsunoda
T, Nomura A, Satoh S, Nakamura Y and Furukawa Y: Genome-wide
analysis of gene expression in human intrahepatic
cholangiocarcinoma. Hepatology. 41:1339–1348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wan X, Choh Y, Kim SY, Dolan JG, Ngo VN,
Burkett S, Khan J, Staudt LM and Helman LJ: Identification of the
FoxM1/Bub1b signaling pathway as a required component for growth
and survival of rhabdomyosarcoma. Cancer Res. 72:5889–5899. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang LZ, Liu YN, Wen TY and Chen HY:
Down-regulation of forkhead box protein M1 by siomycin A can
inhibit the malignant behaviors of laryngeal carcinoma cells.
Academic J Second Military Med Univ. 37:963–968. 2016.
|
|
24
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Henry CM, Hollville E and Martin SJ:
Measuring apoptosis by microscopy and flow cytometry. Methods.
61:90–97. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang L, Wang P and Chen H: Overexpression
of FOXM1 is associated with metastases of nasopharyngeal carcinoma.
Ups J Med Sci. 119:324–332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xue YJ, Xiao RH, Long DZ, Zou XF, Wang XN,
Zhang GX, Yuan YH, Wu GQ, Yang J, Wu YT, et al: Overexpression of
FoxM1 is associated with tumor progression in patients with clear
cell renal cell carcinoma. J Transl Med. 10:2002012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Zhang N, Dai B, Liu M, Sawaya R,
Xie K and Huang S: FoxM1B transcriptionally regulates vascular
endothelial growth factor expression and promotes the angiogenesis
and growth of glioma cells. Cancer Res. 68:8733–8742. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahmed M, Uddin S, Hussain AR, Alyan A,
Jehan Z, Al-Dayel F, Al-Sobhi S, Amin T, Bavi P and Al-Kuraya KS:
FoxM1 and its association with matrix metalloproteinases (MMP)
signaling pathway in papillary thyroid carcinoma. J Clin Endocrinol
Metab. 97:E1–E13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ghosh S, Kaplan KJ, Schrum LW and
Bonkovsky HL: Cytoskeletal proteins: Shaping progression of
hepatitis c virus-induced liver disease. Int Rev Cell Mol Biol.
302:279–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Malhotra SK and Shnitka TK: Chapter 1 the
cytoskeleton- microtubules and microfilaments: A biological
perspective. Principles Med Biol. 4:1–41. 1996. View Article : Google Scholar
|