Introduction
Affecting >2.1 million females each year, breast
cancer is the primary cause of cancer-associated mortality among
females and remains an increasing health threat in developed and
developing countries. Despite of this serious situation, the
overall survival rate has significantly increased due to screening
programs and improved treatment. Various screening and follow-up
examinations, including annual mammograms, ultrasounds, computed
tomography and magnetic resonance imaging (MRI) scans, are
recommended by various guidelines (1). Among them, MRI has demonstrated a
relatively increased sensitivity (2)
in breast cancer detection compared with mammograms and
ultrasounds, which have reported overall sensitivities of 30–60 and
40–80%, respectively (3). In
addition, considering the factors of radiation safety and image
quality, MRI has its own unique advantages compared with
mammography. However, conventional MRI technologies also have
limitations, including relatively low specificity (2), which may lead to difficulties in
distinguishing malignant tumors from benign ones, and are therefore
unable to determine overall prognosis; as a result, a proportion of
patients undergo unnecessary biopsies. In comparison,
diffusion-weighted imaging (DWI) and its derived measurements,
including the apparent diffusion coefficient (ADC), allow for
improved characterization of the biological properties of tissues,
and this technique has therefore been recognized for its superior
oncology applications.
Different from conventional dynamic
contrast-enhanced (DCE) MRI, DWI requires no administration of
contrast agents and quantifies tissue cellularity by measuring the
Brownian motion of water molecules (4). Previous studies have repeatedly
demonstrated decreased water diffusion, which appears brighter on
DWI (5,6) and darker on ADC maps, and higher
cellularity in malignant breast lesions compared with normal
fibroglandular tissue. ADC has also been widely used to distinguish
malignant breast cancer from benign lesions. Min et al
(7) revealed that benign lesions
exhibited an increased mean ADC value compared with their malignant
counterparts regardless of b-values. They also demonstrated that
DWI achieved a sensitivity of 82.8% and specificity of 90%, with a
cutoff ADC value of 1.23×10−3 mm2/sec. With a
promising differential diagnostic value in clinical practice, DWI
and ADC are now integrated into routine MRI breast examination
protocols, primarily to distinguish between benign and malignant
lesions (8,9). However, only a limited number of
studies have examined the association between ADC and clinical
prognostic factors.
Traditional prognostic factors for breast cancer
include histology, staging (size and axillary node involvement),
tumor grading, heredity, obesity, smoking and molecular markers
(10). Among them, molecular markers
including estrogen receptor (ER), progesterone receptor (PR) and
human epidermal growth factor 2 receptor (HER-2) status are
currently widely used as indicators to guide adjuvant therapy and
predict long-term outcomes (11). As
MRI examinations are less expensive and more available in
developing countries compared with Oncotype DX, the development of
MRI-derived biomarkers for breast cancer has been a focus of
numerous studies. Although the potential of ADC in assessing
malignancy of breast lesions was demonstrated by previous studies
(6,12,13) and
the ADC exhibited a correlation with certain prognostic factors,
these results have not been verified in large Chinese populations.
Therefore, considering the fact that pre-operative evaluation of
the extent and prognosis of breast cancer is critical to clinical
practice, its optimization may consequently improve the overall
survival rate and outcomes of patients with breast cancer. The aims
of the present study were as follows: i) Investigate the diagnostic
performance of DWI-derived ADC values in differentiating malignant
tumors from benign ones; and ii) examine the correlation between
ADC values and various prognostic factors in females with breast
cancer.
Materials and methods
Study cohort
The present cross-sectional study was performed at
the Department of Radiology of the Women's Hospital, School of
Medicine, Zhejiang University (Hangzhou, China) from November 2015
to September 2018. DWI was included in the clinical breast MRI
protocol for females with suspicious breast lesions detected by
mammogram and/or sonography. The study protocol was approved by the
Institutional Review Board and Ethics Committee of Women's
Hospital, School of Medicine, Zhejiang University. Written informed
consent was obtained from all the subjects prior to the study.
The present study retrospectively assessed 762
female patients with breast masses who had undergone DWI prior to
histopathological diagnosis from specimens obtained via core needle
biopsy or excision surgery. Patients with inflammatory cancer or
those receiving ongoing chemotherapy, and those without
histopathological confirmation of the lesion, were excluded.
Patients with tumorous lesions >10 mm in diameter were excluded
to avoid unreliable delineation of the tumor on MRI. Based on these
criteria, 223 patients were excluded and 539 patients were
eventually included in the present study.
Histological analysis
Histological analysis was performed on tissues
obtained by core needle biopsy and excision surgery. Histological
grading of breast lesions was performed considering three
morphological features: Tubule formation, nuclear pleomorphism and
the number of mitotic figures, according to the criteria of Elston
and Ellis (14). The total possible
score ranged from 3–9, with a total score of 3–5 representing grade
1, a total score of 6 or 7 resembling grade 2 and a total score of
8 or 9 resembling grade 3. The nuclear grade (1, differentiated; 2,
moderately differentiated; and 3, poorly differentiated) was
determined from 10% formalin-fixed paraffin-embedded tumor tissue
sections stained with hematoxylin and eosin based on Robinson's
grading system (15).
Immunohistochemistry (IHC) was performed on tissues fixed with 10%
neutral buffered formalin at room temperature for 18 h.
Paraffin-embedded materials were cut to 5-µm sections (16,17),
deparaffinized in xylene, treated with 100% ethanol at room
temperature and then heated in a microwave in 0.01 M citrate buffer
(pH 6.0) for 15 min for antigen retrieval. ER (ER: Era EP1), PR
(PR: PgR, 636) and HER-2 (c-erbB-2) status, and proliferation
index, determined using proliferation marker protein Ki-67 (DAKO;
Agilent Technologies, Inc.; Ki-67, MM-1) (1:100) antibody, were
examined on all specimens using a DAKO Autostainer (Dako; Agilent
Technologies, Inc.) and commercially available monoclonal
antibodies. The proportion of ER-positive and PR-positive tumor
cells was expressed as a percentage. ER and PR expression were
scored as positive or negative with a nuclear immunostaining
cut-off of 10%. For ER/PR status to be considered positive,
guidelines from the American Society of Clinical Oncology and
College of American Pathologists (18) recommended that ≥1% of tumor cells
must demonstrate positive nuclear staining on IHC. However, in
routine practice, a wide range of arbitrary cutoffs in proportion
of stained cells are used. For example, 1 (19), 5–10, and 20% (20). A number of clinicians (21–23),
including our hospital, consider 10% as the cut-off for eligibility
for endocrine therapy. In addition, Iwamoto et al (24) demonstrated that tumors with <10%
ER-positive staining on IHC have molecular characteristics more
similar to the ER-negative, basal-like phenotype. Considering the
fact that patients with ER-positive (1–9%) tumors do not appear to
benefit from endocrine therapy (25), a cut-off at 10% was adopted in the
present study. HER-2 expression was evaluated as positive when
membrane immunostaining scores were 3+, or when HER-2 gene
amplification was demonstrated by fluorescent in situ
hybridization in case of a sample with 2+ score, based on the
scoring guidelines of HercepTest (DAKO; Agilent Technologies, Inc.)
(26). Ki-67 staining results were
expressed as the percentage of Ki-67-positive malignant cells among
1,000 malignant cells assessed under high-power magnification (×40
objective). Samples with ≥14% Ki-67 staining were considered
positive, while those with <14% were considered negative
according to the St. Gallen consensus (27). Information on the microvascular lymph
node status was obtained by sentinel lymph node resection followed
by immediate lymph node dissection. A positive result was defined
as presence of metastasis. The prognostic markers considered in the
present study were histological and nuclear grade, lymph node
status and molecular markers, including ER, PR, HER-2 and
Ki-67.
MRI acquisition and analysis
Diagnostic MRI was performed on a GE Signa HDX 1.5 T
MRI machine (GE Healthcare) using a double breast coil with the
patient in a prone position. For all cases, a standardized MRI
protocol was applied.
Prior to contrast medium administration, axial
fat-suppressed T2-weighted short-tau inversion recovery images
[response time (TR), 5,220 msec; echo time (TE), 49.2 msec;
inversion time (TI), 145 msec; field of view (FOV), 36×36 cm; slice
thickness, 5.5 mm; total acquisition time, 2 min 32 sec] were
obtained. Axial DWI with spin-echo planar imaging was performed
with the following parameters: b=1,000 sec/mm2; TR,
4,250 msec; TE, 76.5 msec; FOV, 39×27.3 cm; slice thickness, 5.5
mm; acquisition time, 60 sec.
In all patients, a bolus of intravenous contrast
medium (gadopentetate dimeglumine) was administered at a dose of
0.2 mmol/kg body weight (0.5 mmol/ml Magnevist®),
followed by 10 ml saline solution (1%). Dynamic MRI
(VIBRANT®) with fat suppression was performed prior to
and 6 times after injection of the contrast medium. The parameters
for dynamic MRI were as follows: TR, 5.7 msec; TE, 2.8 msec; TI, 18
msec; FOV, 37×33.3 cm; slice thickness, 1.6 mm; acquisition time, 6
min 14 sec.
The ADC map was automatically generated by the
console of the manufacturer (AW VolumeShare 5; GE Healthcare).
Linear regression was used to calculate ADC maps. Breast lesions
were reviewed and manually delineated by 2 independent radiologists
with 10 and 5 years' experience in breast MRI, respectively, and
blinded to other imaging or clinicopathological findings other than
the presence of breast masses. The lesions were manually delineated
with a circular region of interest placed within the primary
lesions to include an area as large as possible within the confines
of the actual lesions. The mean and maximum ADC values of lesions
were denoted as ADCmean and ADCmax,
respectively. ADC measurements were performed at least 3 times by 2
independent observers and the average ADC was recorded as the final
result. The volume of the lesions was calculated by counting the
number of voxels delineated on lesion maps and then multiplied by
the size of the voxel.
Statistical analysis
For continuous variables, the Kolmogorov-Smirnov
test was performed. For comparisons between breast cancer and
different types of benign lesion, a Student's t-test was used. In
addition, a receiver operating characteristics (ROC) curve was
fitted and the area under the ROC curve (AUC) with 95% confidence
interval (CI) was determined to identify the best cut-off ADC value
for differentiating between benign and malignant breast masses.
Sensitivity, specificity, positive predictive values and negative
predictive values were calculated, respectively.
The associations between ADC values and prognostic
factors were calculated using Student's t-test with Bonferroni
correction. The ADC values of histological grade (1 vs. 2/3),
nuclear grade (1 vs. 2/3), lymph node status (positive vs.
negative), as well as status of ER, PR, HER-2 (positive vs.
negative) and Ki-67 (<14 vs. ≥14%) were compared. In the
univariate analysis, variables for which P<0.1, including
histological and nuclear grade, lymph node status, ER, PR, HER-2
and Ki-67, were subjected to multiple logistic regression analysis
to determine those that were independently associated with
ADCmax and ADCmean values. P<0.05 was
considered to indicate a statistically significant difference. SPSS
22.0 (IBM Corp.) was used for all statistical analyses.
Results
A total of 539 females (mean age, 43.9±8.3 years)
with breast lesions were included in the present study. Based on
the pathological results, malignant tumors were detected in 307
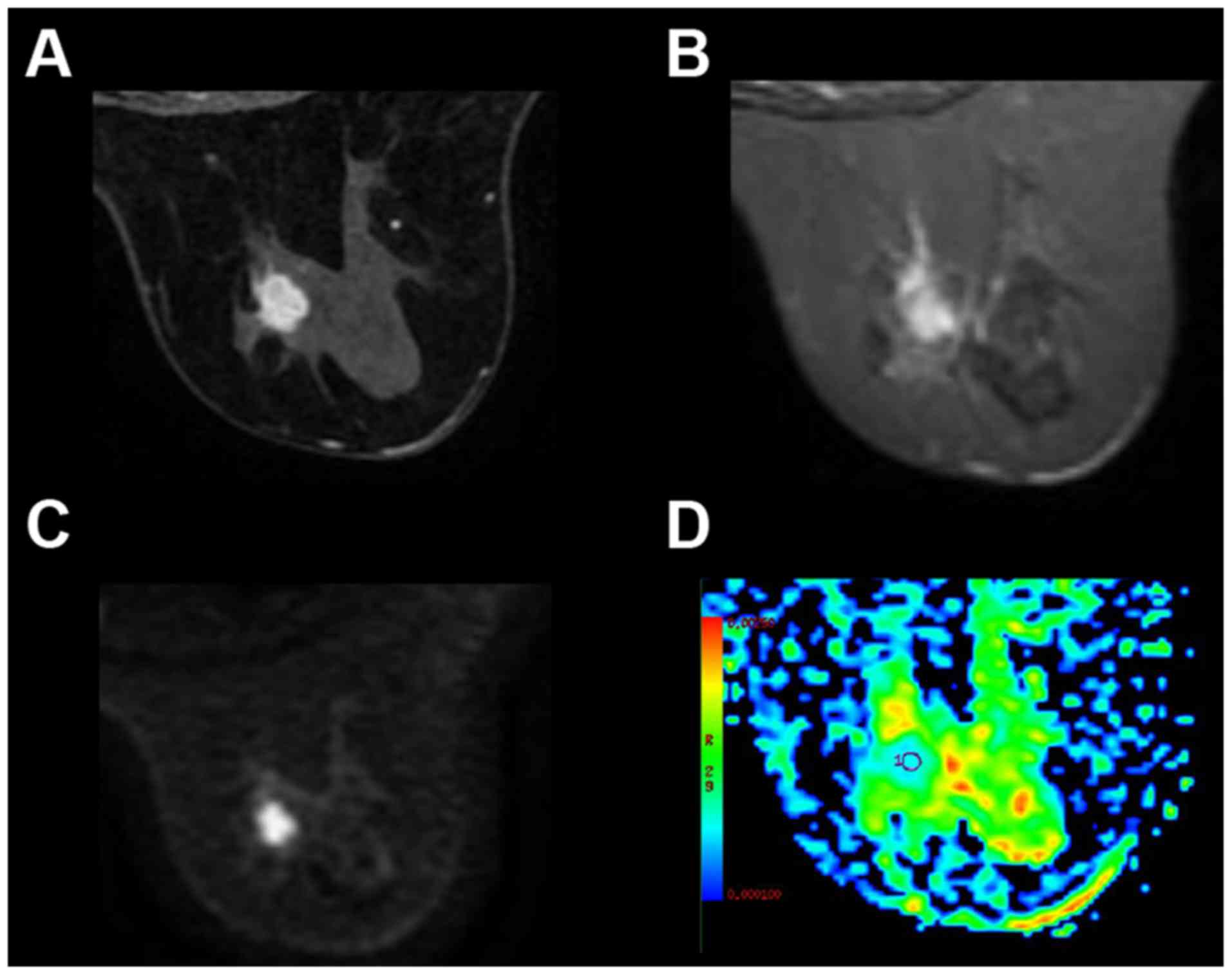
subjects (Fig. 1) and benign tumors
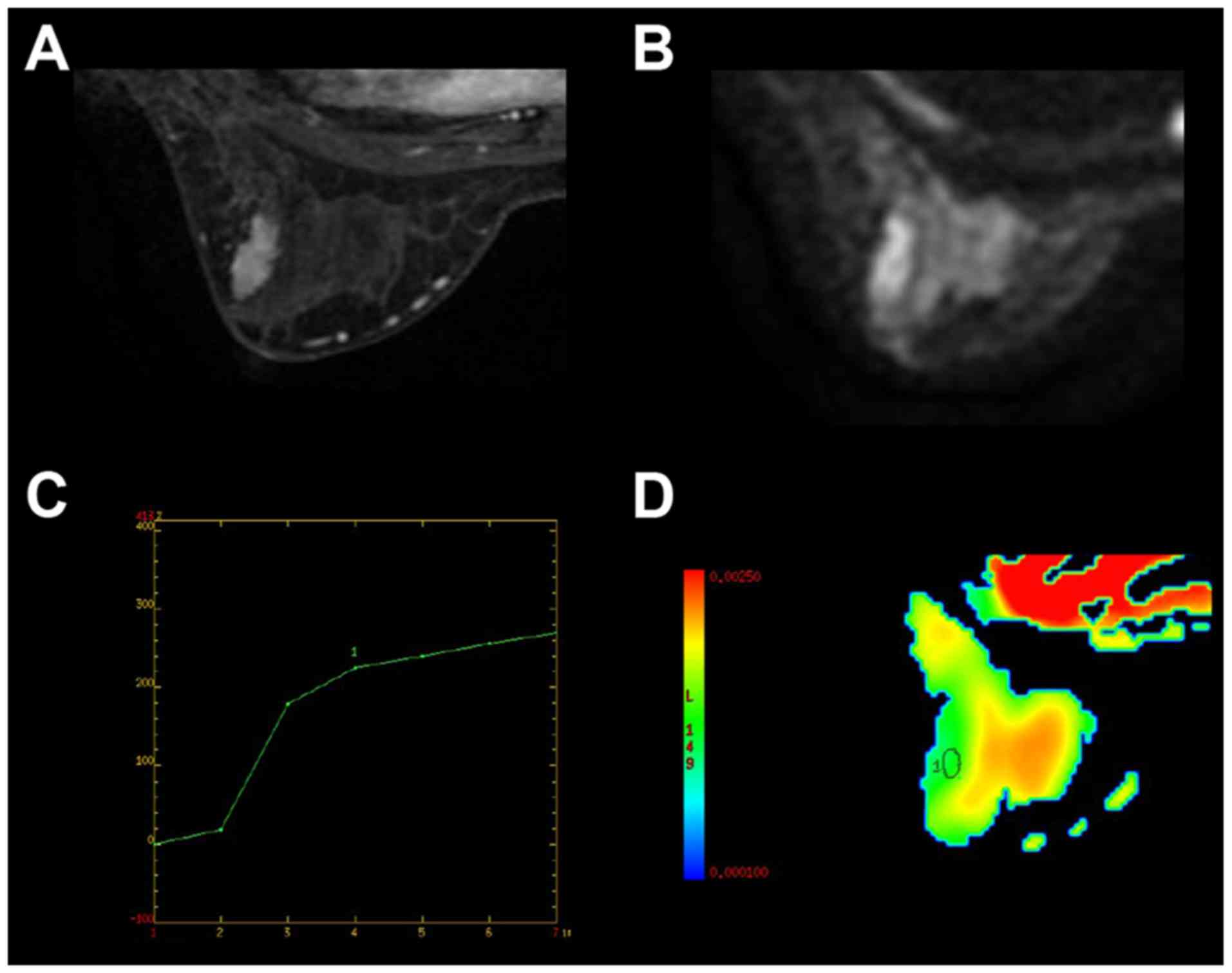
in 232 subjects (Fig. 2). Detailed
demographics of the patients and pathological types of their
lesions are summarized in Table I.
Patients with malignant breast lesions were significantly older
compared with those with benign lesions (P<0.001) and had a
lower body weight (P=0.007).
 | Table I.Patient demographics,
histopathological diagnosis and imaging biomarkers for patients
with benign and malignant breast lesions (n=539). |
Table I.
Patient demographics,
histopathological diagnosis and imaging biomarkers for patients
with benign and malignant breast lesions (n=539).
|
Characteristics | Benign (n=232) | Malignant
(n=307) | P value |
|---|
| Age, years | 41.3±7.5 | 45.7±7.7 | <0.001 |
| Weight, kg | 62.2±10.4 | 59.5±9.8 | 0.007 |
| Height, cm | 158.2±5.3 | 159.1±5.7 | 0.352 |
| Final diagnosis, n
(%) |
|
|
|
|
Fibrocystic changes | 12 (5.2) | – | – |
| Plasma
cell mastitis | 49 (21.0) | – | – |
|
Intraductal papilloma | 13 (5.5) | – |
|
|
Fibroadenoma | 77 (33.3) | – |
|
|
Mastopathy | 58 (25.1) | – |
|
|
Sclerosing mastopathy | 23 (10.0) | – |
|
|
Infiltrating ductal
carcinoma | – | 176 (57.3) |
|
|
Malignant phyllodes tumor | – | 10 (3.2) |
|
|
Mucinous carcinoma | – | 6 (2.0) |
|
|
Invasive lobular
carcinoma | – | 115 (37.5) |
|
| Volume, ml | 7.7±4.1 | 5.8±3.9 | <0.001 |
| ADCmax,
×10−3 mm2/s | 2.1±0.2 | 1.7±0.2 | <0.001 |
| ADCmean,
×10−3 mm2/s | 1.5±0.2 | 1.0±0.2 | <0.001 |
Analysis of the ADCmean and
ADCmax values of malignant and benign breast lesions
indicated that malignant lesions usually had a smaller size
(P<0.001) and lower ADCmax
(P<0.001)/ADCmean (P<0.001) compared with benign
lesions (Table I). The
ADCmean demonstrated a relatively improved performance
in distinguishing malignant from benign lesions compared with
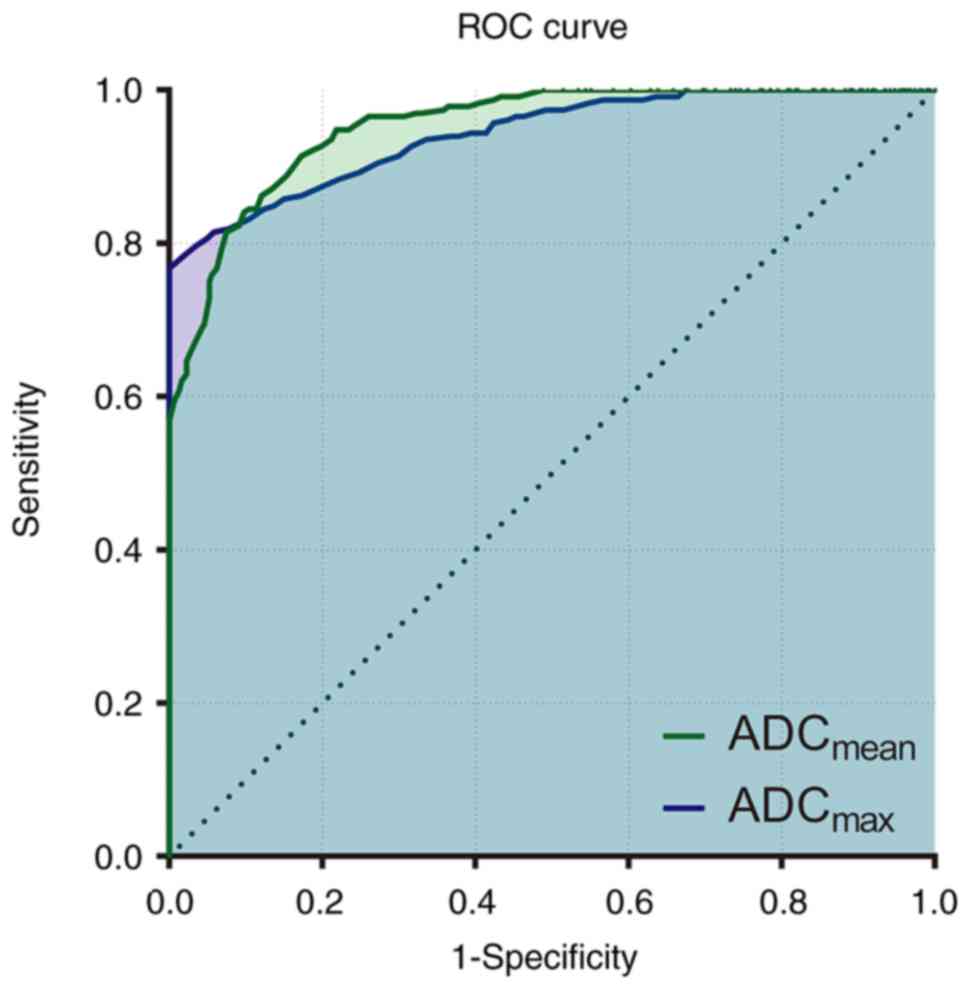
ADCmax, as indicated by the ROC curves in Fig. 3. The AUC for ADCmean and
ADCmax was 0.953 and 0.942, respectively. With the
optimum cut-off for the ADCmean value at
1.30×10−3 mm2/sec, DWI achieved a sensitivity
of 84.1% and a specificity of 90.2%, a positive predictive value of
86.7% and a negative predictive value of 88.2% for malignant vs.
benign lesions. Furthermore, with the optimum cut-off value at 1.98
mm2/sec, ADCmax yielded a sensitivity of
78.4%, a specificity of 98.0%, positive predictive value of 85.8%
and negative predictive value of 96.8% regarding the identification
of malignant vs. benign lesions.
Next, the association between ADC and independent
tumor prognostic factors was examined. Tumor prognostic factors
included histological grade, nuclear grade, lymph node status and
molecular markers, including ER, PR, HER-2 and Ki-67. The analysis
revealed significant associations between ADC and all prognostic
factors. For those patients with malignant breast lesions,
univariate analysis demonstrated significantly lower
ADCmean and ADCmax values for high
histological grade (grade 2 and 3), high nuclear grade (grade 2 and
3), and lymph node-positive, ER-negative, PR-negative,
HER-2-negative status, and Ki-67 ≥14%. Detailed ADCmean
and ADCmax values for patients with different prognostic
factors are presented in Table
II.
 | Table II.Associations between prognostic
factors and ADC measurements for patients with malignant breast
lesions (n=307). |
Table II.
Associations between prognostic
factors and ADC measurements for patients with malignant breast
lesions (n=307).
|
|
| ADCmax,
×10−3 mm2/s | ADCmean,
×10−3 mm2/s |
|---|
|
|
|
|
|
|---|
| Prognostic
factors | No. of cases | Mean ± SD | P value | Mean ± SD | P value |
|---|
| Histological
Grade |
|
| <0.001 |
| <0.001 |
| 1 | 153 | 1.79±0.12 |
| 1.20±0.12 |
|
|
2+3 | 154 | 1.48±0.11 |
| 0.83±0.11 |
|
| Nuclear grade |
|
| <0.001 |
| <0.001 |
| 1 | 160 | 1.85±0.09 |
| 1.18±0.12 |
|
|
2+3 | 147 | 1.56±0.11 |
| 0.87±0.08 |
|
| Lymph node
status |
|
| <0.001 |
| <0.001 |
|
Positive | 148 | 1.56±0.11 |
| 0.88±0.09 |
|
|
Negative | 159 | 1.85±0.09 |
| 1.17±0.12 |
|
| ER |
|
| <0.001 |
| <0.001 |
|
Positive | 91 | 1.80±0.15 |
| 1.14±0.17 |
|
|
Negative | 216 | 1.67±0.17 |
| 0.99±0.17 |
|
| PR |
|
| 0.006 |
| <0.001 |
|
Positive | 67 | 1.76±0.18 |
| 1.13±0.21 |
|
|
Negative | 240 | 1.69±0.17 |
| 1.00±0.17 |
|
| HER-2 |
|
| <0.001 |
| <0.001 |
|
Positive | 178 | 1.82±0.12 |
| 1.14±0.15 |
|
|
Negative | 129 | 1.56±0.12 |
| 0.88±0.09 |
|
| Ki-67 |
|
| <0.001 |
| <0.001 |
|
<14% | 103 | 1.89±0.08 |
| 1.24±0.10 |
|
|
≥14% | 204 | 1.62±0.13 |
| 0.92±0.11 |
|
The present study also performed multiple regression
analyses regarding the relative association between prognostic
factors for patients with malignant breast lesions and MRI
biomarkers (ADCmax and ADCmean). Histological
grade (P<0.001), nuclear grade (P<0.001), Ki-67 (P<0.001)
and PR (P=0.005) were the variables demonstrated to independently
affect the ADCmax. Furthermore, Ki-67 (P<0.001),
lymph node status (P<0.001), HER-2 (P=0.01) and PR (P=0.01) were
all indicated to independently affect the ADCmean.
Discussion
To the best of our knowledge, the present study is
the first large-scale study performed in a Chinese population to
validate the use of the ADC for distinguishing malignant from
benign breast lesions, and to determine its association with common
biomarkers of breast malignancy and prognosis. The results
demonstrated that ADC is a promising tool in differentiating
malignant tumors from benign masses with high sensitivity and
specificity, with optional cutoff values of 1.30×10−3
and 1.98 mm2/sec for ADCmean and
ADCmax, respectively. Furthermore, ADC biomarkers have
potential in predicting the clinical outcomes of malignant breast
cancer. The present results suggested that ADCmax and
ADC mean were significantly decreased in patients with high nuclear
grade, and lymph node-positive, ER-negative, PR-negative and
HER-2-negative status, and Ki-67 ≥14%. Subsequent analysis of the
effects of independent prognostic factors on ADC biomarkers
suggested that ADCmax was affected by nuclear grade,
Ki-67 and PR, whereas ADCmean was affected by lymph node
status, HER-2, PR and Ki-67.
Breast cancer is one of the most common types of
cancer in females worldwide and DCE-MRI is an established technique
for detection, diagnosis and staging of breast cancer. Despite an
inherently high sensitivity, DCE only has moderate specificity for
the characterization of breast lesions (14). In clinical practice, a standardized
imaging protocol allows for the investigation of morphological and
kinetic patterns of benign and malignant breast lesions screened by
mammography and ultrasound. However, this standardized protocol is
also prone to a high false-positive rate and may therefore result
in unnecessary biopsies. Conversely, being able to measure the
biophysical characteristics of tissues, DWI and its derived ADC
maps were mostly applied in breast imaging to decrease
false-positives on conventional DCE-MRI. Numerous studies have
demonstrated that malignant breast lesions usually exhibit a high
signal on DWI and a low signal on ADC maps compared with normal
fibroglandular tissues (15),
indicating decreased water diffusion in malignant tissues.
Significantly increased cell density was the major contributor to
this decreased water diffusion, consequently resulting in an
increased hindrance of water motion in the tortuous extracellular
space and increased volume of restricted intracellular fluid
(16,17). For example, simple cysts demonstrate
higher water diffusion rates compared with breast fibroglandular
tissue due to the relatively unrestricted microenvironment. The
potential application of DWI and ADC may avoid unnecessary biopsies
and provides a fast approach to assess the malignancy of suspicious
breast lesions (18). A
meta-analysis of 14 studies revealed that ADCs demonstrated an
excellent performance in classifying suspicious breast lesions, and
their inclusion may therefore increase the accuracy of conventional
clinical breast assessments (19).
ADCmax and ADCmean values demonstrated a good
performance in the differentiation between malignant and benign
breast masses. The ADCmean for malignant and benign
tumors in the present study were 1.0±0.2 and 1.5±0.2, respectively.
This was, to a certain degree, consistent with the results of
previous studies on breast lesions (9,17,20,21).
Compared with previous studies, the present study included a large
population and yielded more comprehensive results. The
ADCmean alone had the best performance in
differentiating between benign and malignant diseases, and with the
best cut-off value at 1.98 mm2/sec, it had a sensitivity
of 84.1%, specificity of 90.2%, positive predictive value of 86.7%
and negative predictive value of 88.2%. While the present study did
not compare the accuracy of DCE with DWI for breast cancer
detection, a previous meta-analysis obtained a pooled sensitivity
and specificity of DCE-MRI of 93.2 and 71.1% (19). This suggested that DWI alone had an
improved specificity compared with DCE-MRI; therefore, this
technique may be implemented to complement conventional parameters
and improve the accuracy of breast cancer detection. As the
measurement of the extracellular water content provides information
on additional features, this may increase the specificity of the
classification of pathological breast lesions. More importantly,
although a generalizable ADC threshold should always be avoided due
to dependence of lesion selection and combination of b-values
(22), the sensitivity and
specificity of DWI were robust and not significantly affected by
choice of b-values as long as a suggested maximum b-value of 1,000
sec/mm2 was adopted (23). Despite the fact that DWI is not to be
used as a stand-alone diagnostic criterion, it is useful if
integrated into the conventional clinical protocol.
Considering that personalized and targeted
therapeutic approaches largely depend on the accuracy of tumor
characterization in terms of histological type and biological
aggressiveness, a non-invasive method capable of measuring all
prognostic features is more favorable compared with other
techniques. From the results of the present study, which
demonstrated that malignant breast lesions had lower ADCs, it may
be expected that lower ADCs, likely due to higher levels of
proliferation and cellularity, would generally correlate with
higher aggressiveness. Cellularity is an important indicator of
tumor grade. As increased cellular density of high-grade tumors is
associated with smaller extracellular volume fractions, tumor
cellularity is inversely correlated with tumor ADC. Indeed, Jiang
et al (28) demonstrated a
marked negative correlation between cellularity and ADC. However,
the exact association between restricted diffusion and well-known
prognostic factors has remained largely unknown. For example, a
number of undifferentiated breast tumors have very few neoplastic
cells in an abundant fibrous stroma, and this may result in
increased ADC values. In certain cases, the complex
microarchitecture and strong desmoplasia in tumors makes the
reliable detection of disease and early diagnosis difficult. In
addition, although fibrous stroma with higher stroma grades
(3–5)
demonstrate low ADCs just like neoplastic cells (29), the microstructural models for
diffusion MRI are always too simplistic to describe the underlying
tissue microstructure in clinical settings. This issue was also
raised by a previous study (30),
and efforts to build more complex models to better describe the
data are being made. Therefore, at present, histological
assessments remain irreplaceable, and clinical decisions should be
made based on the results of multiple tests, to compensate for the
limitations of any single treatment modality. In the present study,
the correlation between the ADC values and various prognostic
factors was assessed. The results suggested that patients with
detected lymph node metastasis had a significantly decreased ADC
value in the primary breast tumor. This is in concordance with
several previous studies (6,25), and it is commonly accepted that a
lower ADC is an indicator of higher aggressiveness and metastatic
potential. Furthermore, although the prognosis of malignant tumors
does not exclusively depend on the histological type of cancer
cells, the histopathological characteristics of the tumor,
particularly tumor grade, are markedly correlated with tumor
progression. The results of the present study demonstrated a
statistically significant association between higher nuclear grade
and lower ADCs. This is in accordance with other studies (6,26), which
all observed increased cellular density in high-grade tumors by
measuring ADCs. Furthermore, previous studies have investigated the
association between histological grade and ADC (31,32). The
results of the present study were in concordance with those
identified previously (29,30), and demonstrated an association
between lower ADC values and higher histological grades. This
subsequently supports the hypothesis that the increased cellular
density observed in high-grade malignancies is associated with low
ADC values (33). Overexpression of
HER-2 accelerates cell growth, thereby contributing to
carcinogenesis. Consequently, HER-2-positive cells exhibit a more
malignant phenotype compared HER-2-negative cells, comprising
increased cell proliferation, invasion and metastatic potential.
However, the present study determined increased ADC values in
HER-2-positive samples compared with HER-2-negative breast cancer
samples. Notably, HER-2 also induces angiogenesis, which leads to
increased perfusion in tumors. Based on this, the increased ADC
values observed in HER-2-positive lesions in the present study may
be explained by an increased proportion of total extracellular
fluid due to high vascularity. Although to the best of our
knowledge, there is no study investigating the association between
vascularity of breast lesions and HER-2 expression, different
imaging studies indirectly supported our hypothesis. Zhang et
al (34) and Rashmi et al
(35) demonstrated that tumors with
high Adler degrees of vascularity were associated with positive
HER-2 expression in power doppler studies. Due to the lack of
pathological evidence available at present, above statement is just
speculative, and histological examination is always required to
verify this high vascularity hypothesis. Martincich et al
(36) also observed high ADCs in
tumors with high HER-2 expression compared with tumors without
HER-2 expression. Of note, although ER-positive breast cancer
generally has an improved prognosis compared with ER-negative
cancer, previous studies have described conflicting results, as
several studies indicated that ER-positive breast lesions exhibited
lower ADCs (12,37,38). The
results of the present study indicated increased ADCs in
ER-positive lesions, which is only consistent with the study by
Kitajima et al (39).
Similarly, while most studies did not observe any significant
association between PR status and ADC, the data from the present
study indicated that PR-positive lesions exhibited increased ADC
values. Therefore, the correlation of ADC with other prognostic
factors, including ER, PR, HER-2 and Ki-67, is less consistent
among studies and may vary between different populations. Future
large-population studies may be required to determine the
association between DWI biomarkers and these prognostic
factors.
The present study had several limitations. Firstly,
the present study had a retrospective design, was performed at a
single institution, and it was not possible to evaluate the
long-term follow-up results of the study population. The
association between MRI imaging biomarkers and treatment response
may be an important topic for future study. Secondly, the present
study did not compare the diagnostic performance of the ADC with
conventional DCE-MRI. Although the combination of 2 methods
provides an improved diagnostic value compared with either alone,
it may be worthwhile to assess this specifically in future studies.
Finally, all of the patients included in the present study were
screened by mammography and/or sonography and had a tumor size of
>10 mm, which may have potentially yielded selection bias. One
possible consequence of this selection bias may be the increased
prevalence of ER-negative, PR-negative and HER-2-postive breast
lesions in the present study compared to others. Therefore, the
data should be interpreted the with caution and direct comparisons
between different studies should be avoided.
In conclusion, the present study revealed that
malignant tissues exhibited lower ADCs compared with benign tumors.
This suggested that ADCs may be promising imaging parameters that
may help identify tumors with higher malignancies. In addition, the
present study provided additional confirmatory evidence for the
utility of the ADC values in the characterization of breast
lesions, and the ADCmean and ADCmax were
effective parameters to distinguish malignant from benign breast
lesions. Therefore, DWI-derived ADCs are useful biomarkers that may
contribute to improved concordance between radiological and
pathological data.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data are available upon reasonable request.
Authors' contributions
CR conducted experiments, analyzed data and drafted
the manuscript. YZ assisted with the collection of pathological
results and patient recruitment. XZ and KL facilitated lesion
delineation and radiological examination. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Review Board and Ethics Committee of Women's Hospital, School of
Medicine, Zhejiang University. Written informed consent was
obtained from all the subjects prior to the study.
Patient consent for publication
Written informed consent was obtained from all the
subjects prior to the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van Bodegraven EA, van Raaij JC, Van
Goethem M and Tjalma WAA: Guidelines and recommendations for MRI in
breast cancer follow-up: A review. Eur J Obstet Gynecol Reprod
Biol. 218:5–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quinn EM, Coveney AP and Redmond HP: Use
of magnetic resonance imaging in detection of breast cancer
recurrence: A systematic review. Ann Surg Oncol. 19:3035–3041.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mehnati P and Tirtash MJ: Comparative
efficacy of four imaging instruments for breast cancer screening.
Asian Pac J Cancer Prev. 16:6177–6186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koh DM and Collins DJ: Diffusion-weighted
MRI in the body: Applications and challenges in oncology. Am J
Roentgenol. 188:1622–1635. 2007. View Article : Google Scholar
|
|
5
|
Chen X, Li WL, Zhang YL, Wu Q, Guo YM and
Bai ZL: Meta-analysis of quantitative diffusion-weighted MR imaging
in the differential diagnosis of breast lesions. BMC Cancer.
10:6932010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Belli P, Costantini M, Bufi E, Giardina
GG, Rinaldi P, Franceschini G and Bonomo L: Diffusion magnetic
resonance imaging in breast cancer characterisation: Correlations
between the apparent diffusion coefficient and major prognostic
factors. Radiol Medica. 120:268–276. 2015. View Article : Google Scholar
|
|
7
|
Min Q, Shao K, Zhai L, Liu W, Zhu C, Yuan
L and Yang J: Differential diagnosis of benign and malignant breast
masses using diffusion-weighted magnetic resonance imaging. World J
Surg Oncol. 13:322015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Partridge SC, DeMartini WB, Kurland BF,
Eby PR, White SW and Lehman CD: Quantitative diffusion-weighted
imaging as an adjunct to conventional breast MRI for improved
positive predictive value. Am J Roentgenol. 193:1716–1722. 2009.
View Article : Google Scholar
|
|
9
|
Peters NH, Vincken KL, van den Bosch MA,
Luijten PR, Mali WP and Bartels LW: Quantitative diffusion weighted
imaging for differentiation of benign and malignant breast lesions:
The influence of the choice of b-values. J Magn Reson Imaging.
31:1100–1105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Savas P, Salgado R, Denkert C, Sotiriou C,
Darcy PK, Smyth MJ and Loi S: Clinical relevance of host immunity
in breast cancer: From TILs to the clinic. Nat Rev Clin Oncol.
13:228–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheang MCU, Chia SK, Voduc D, Gao D, Leung
S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al: Ki67
Index, HER2 Status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi SY, Chang YW, Park HJ, Kim HJ, Hong
SS and Seo DY: Correlation of the apparent diffusion coefficiency
values on diffusion-weighted imaging with prognostic factors for
breast cancer. Br J Radiol. 85:e474–e479. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim SH, Cha ES, Kim HS, Kang BJ, Choi JJ,
Jung JH, Park YG and Suh YJ: Diffusion-weighted imaging of breast
cancer: Correlation of the apparent diffusion coefficient value
with prognostic factors. J Magn Reson Imaging. 30:615–620. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Robinson IA, McKee G, Nicholson A, D'Arcy
J, Jackson PA, Cook MG and Kissin MW: Prognostic value of
cytological grading of fine-needle aspirates from breast
carcinomas. Lancet. 343:947–949. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jacobs GH, Rippey JJ and Altini M:
Prediction of aggressive behavior in basal cell carcinoma. Cancer.
49:533–537. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei M, Qin J, Yan R, Bi K, Liu C, Yao Z
and Lu Q: Association of resting-state network dysfunction with
their dynamics of inter-network interactions in depression. J
Affect Disord. 174:527–534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hammond ME, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American society of clinical oncology/college of
american pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. J Clin Oncol. 28:2784–2795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dowsett M, Cuzick J, Wale C, Howell T,
Houghton J and Baum M: Retrospective analysis of time to recurrence
in the ATAC trial according to hormone receptor status: An
hypothesis-generating study. J Clin Oncol. 23:7512–7517. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Layfield LJ, Gupta D and Mooney EE:
Assessment of tissue estrogen and progesterone receptor levels: A
survey of current practice, techniques, and quantitation methods.
Breast J. 6:189–196. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Regan MM, Viale G, Mastropasqua MG,
Maiorano E, Golouh R, Carbone A, Brown B, Suurküla M, Langman G,
Mazzucchelli L, et al: Re-evaluating adjuvant breast cancer trials:
Assessing hormone receptor status by immunohistochemical versus
extraction assays. J Natl Cancer Inst. 98:1571–1581. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dowsett M, Allred C, Knox J, Quinn E,
Salter J, Wale C, Cuzick J, Houghton J, Williams N, Mallon E, et
al: Relationship between quantitative estrogen and progesterone
receptor expression and human epidermal growth factor receptor 2
(HER-2) status with recurrence in the arimidex, tamoxifen, alone or
in combination trial. J Clin Oncol. 26:1059–1065. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Viale G, Regan MM, Maiorano E,
Mastropasqua MG, Dell'Orto P, Rasmussen BB, Raffoul J, Neven P,
Orosz Z, Braye S, et al: Prognostic and predictive value of
centrally reviewed expression of estrogen and progesterone
receptors in a randomized trial comparing letrozole and tamoxifen
adjuvant therapy for postmenopausal early breast cancer: BIG 1–98.
J Clin Oncol. 25:3846–3852. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iwamoto T, Booser D, Valero V, Murray JL,
Koenig K, Esteva FJ, Ueno NT, Zhang J, Shi W, Qi Y, et al: Estrogen
receptor (ER) mRNA and ER-related gene expression in breast cancers
that are 1% to 10% ER-positive by immunohistochemistry. J Clin
Oncol. 30:729–734. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yi M, Huo L, Koenig KB, Mittendorf EA,
Meric-Bernstam F, Kuerer HM, Bedrosian I, Buzdar AU, Symmans WF,
Crow JR, et al: Which threshold for ER positivity? a retrospective
study based on 9639 patients. Ann Oncol. 25:1004–1011. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yaziji H, Goldstein LC, Barry TS, Werling
R, Hwang H, Ellis GK, Gralow JR, Livingston RB and Gown AM: HER-2
testing in breast cancer using parallel tissue-based methods. JAMA.
291:1972–1977. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ; Panel members, : Strategies for
subtypes-dealing with the diversity of breast cancer: Highlights of
the St. Gallen international expert consensus on the primary
therapy of early breast cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang R, Ma Z, Dong H, Sun S, Zeng X and
Li X: Diffusion tensor imaging of breast lesions: Evaluation of
apparent diffusion coefficient and fractional anisotropy and tissue
cellularity. Br J Radiol. 89:201600762016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Winfield JM, Miah AB, Strauss D, Thway K,
Collins DJ, deSouza NM, Leach MO, Morgan VA, Giles SL, Moskovic E,
et al: Utility of multi-parametric quantitative magnetic resonance
imaging for characterization and radiotherapy response assessment
in Soft-tissue sarcomas and correlation with histopathology. Front
Oncol. 9:2802019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Strosberg JR, Cheema A, Weber J, Han G,
Coppola D and Kvols LK: Prognostic validity of a novel American
Joint Committee on Cancer Staging Classification for pancreatic
neuroendocrine tumors. J Clin Oncol. 29:3044–3049. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Razek AA, Gaballa G, Denewer A and Nada N:
Invasive ductal carcinoma: Correlation of apparent diffusion
coefficient value with pathological prognostic factors. NMR Biomed.
23:619–623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Byun BH, Noh WC, Lim I, Lee SS, Cho AR,
Park JA, Kim KM, Kim HA, Kim EK, Kim BI, et al: A new method for
apparent diffusion coefficient measurement using sequential 18F-FDG
PET and MRI: Correlation with histological grade of invasive ductal
carcinoma of the breast. Ann Nucl Med. 27:720–728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo Y, Cai YQ, Cai ZL, Gao YG, An NY, Ma
L, Mahankali S and Gao JH: Differentiation of clinically benign and
malignant breast lesions using diffusion-weighted imaging. J Magn
Reson Imaging. 16:172–178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang L, Li J, Xiao Y, Cui H, Du G, Wang
Y, Li Z, Wu T, Li X and Tian J: Identifying ultrasound and clinical
features of breast cancer molecular subtypes by ensemble decision.
Sci Rep. 5:110852015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rashmi S, Kamala S, Murthy SS, Kotha S,
Rao YS and Chaudhary KV: Predicting the molecular subtype of breast
cancer based on mammography and ultrasound findings. Indian J
Radiol Imaging. 28:354–361. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Martincich L, Deantoni V, Bertotto I,
Redana S, Kubatzki F, Sarotto I, Rossi V, Liotti M, Ponzone R,
Aglietta M, et al: Correlations between diffusion-weighted imaging
and breast cancer biomarkers. Eur Radiol. 22:1519–1528. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Orel SG: MR imaging of the breast. Radiol
Clin North Am. 38:899–913. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Partridge SC, Nissan N, Rahbar H, Kitsch
AE and Sigmund EE: Diffusion-weighted breast MRI: Clinical
applications and emerging techniques. J Magn Reson Imaging.
45:337–355. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kitajima K, Yamano T, Fukushima K, Miyoshi
Y and Hirota S, Kawanaka Y, Miya M, Doi H, Yamakado K and Hirota S:
Correlation of the SUVmaxof FDG-PET and ADC values of
diffusion-weighted MR imaging with pathologic prognostic factors in
breast carcinoma. Eur J Radiol. 85:943–949. 2016. View Article : Google Scholar : PubMed/NCBI
|