Introduction
Astrocytoma is the most common type of adult primary
brain tumor (1,2), and accurate grading of this tumor is
critical to prepare appropriate treatment and to evaluate the
prognosis of the patient. Classical histological classification and
malignancy grading are based on the criteria of the 2007 World
Health Organization (WHO) classification of tumors of the central
nervous system (3), and astrocytoma
are classified into I–IV grades; grade I–II, benign: Grade III–IV,
malignant. However, for patients treated without surgery, it is not
possible to obtain a pathological grade. Additionally, grades of
pathological diagnosis made from surgical resection samples may be
underestimated due to tumoral heterogeneity.
Functional magnetic resonance imaging (MRI) scanning
technologies, such as diffusion-weighted imaging (DWI), may provide
data on tumor cell density and proliferation and serve as an
important supplement to conventional MRI scans such as T1-weighted
image (T1WI), T2-weighted image (T2WI), T2-fluid attenuated
inversion recovery (FLAIR) scans (4–8). DWI may
quantify the diffusion rate of extracellular water molecules, and
the limited diffusion of water molecules in high-grade astrocytoma
results in a low apparent diffusion coefficient (ADC) value. In
addition, the astrocytoma grade is associated with the vasculature
of the tumor, and may be reflected by relative cerebral blood
volume (rCBV), thus guiding astrocytoma grading (8,9).
However, functional MRI scanning technology with DWI
or DSC cannot incorporate all features of astrocytomas
comprehensively. A previous study demonstrated that the accuracy of
DWI or dynamic susceptibility contrast-enhanced (DSC) measurements
in differentiating between grades of astrocytoma exhibited
unsatisfactory consequences (4). For
example, DWI has been revealed to increase diagnostic accuracy of
astrocytoma grade, but a singular DWI-MRI scan cannot provide
sufficient quantitative data concerning tumor structure (10). Additionally, previous studies have
not investigated the associations between DWI- and DSC-MRI scans
and immunohistochemical (IHC) indices.
In the present study, the efficacy of the
combination of DWI- and DSC-MRI scans in astrocytoma grading, and
the associations between these MRI scans and the histologic indices
of glial fibrillary acidic protein (GFAP), topoisomerase IIα (Topo
IIα) and O 6-methylguanine-DNA methyltransferase (MGMT) were
investigated. Histologic measurement of GFAP, Topo IIα and MGMT is
widely used in evaluating the levels of tumor infiltration and
proliferation, and has been demonstrated to correlate with tumor
grade and prognosis (11–13). The main aim of the present study was
to identify the accuracy of combined diagnostic techniques in
differentiating between grades II–IV astrocytoma, and to predict
astrocytoma malignancy through comparing histologic measurement of
levels of GFAP, Topo IIα and MGMT.
Materials and methods
Ethical considerations
The present study was approved by Shanxi Medical
University review board. All manuscripts comply with the guidelines
of the February 2006 consensus statement of the International
Committee of Medical Journal Editors, and all patients provided
written informed consent.
Patient selection
A total of 123 patients, 62 male and 61 female, with
an age range between 25 and 77 years, and a mean age of 51.3±11.2
years, with histologically confirmed astrocytoma subsequent to
surgical resection in The First Hospital of Shanxi Medical
University between April 2010 and December 2015 were included. All
patients underwent DWI, DSC and conventional MRI scans within 2
weeks prior to surgical resection. Pathological specimens were
classified according to the WHO 2007 central nervous system tumor
classification guidelines (3).
Astrocytoma were grouped into low-grade astrocytoma, WHO grade II,
23 patients; anaplastic astrocytomas, WHO grade III, 44 patients;
and glioblastoma multiforme, WHO grade IV, 56 patients.
MRI acquisition
MRI scans were performed using the General Electric
(GE) SIGNA HDx 3.0 Tesla MR scanner with an 8-channel phased-array
coil with a head and neck combination. All patients underwent
conventional T1WI, T2WI, T2-FLAIR and DWI axial scanning, followed
by DSC-MR perfusion scanning. DSC-MR perfusion images were captured
by elbow vein bolus injection of gadopentetate dimeglumine
(Magnevist®, Bayer Schering Pharma AG, Berlin, Germany)
at a flow rate of 4.5 ml/s and a dose of 0.1 mmol/kg. Finally,
conventional T1WI enhancement scanning was performed.
The scan protocol of each conventional MRI scan
included: T1WI, with a repetition time (TR)/echo time (TE) of
1677/24 ms; T2WI, with a TR/TE of 6800/105 ms; T2-FLAIR, with a
TR/TE of 8002/132 ms, a thickness of 6 mm, spacing of 1.2 mm
between two adjacent images, a field of view (FOV) of 240×240 mm,
matrix 320×256 mm, and number of excitations (NEX)=1. DWI scans
used a spin echo/echo planar imaging sequence, a TR/TE of 5,000/74
ms, a thickness of 6 mm, spacing of 1.2 mm, FOV 240×240 mm, matrix
160×160 mm, and NEX=2; the diffusion coefficient of sensitivity was
selected as 0.1000 s/mm2. The parameters of the DSC MRI
perfusion scans were as follows: TR/TE of 1500/14.5 ms, FOV of
240×255 mm, a matrix of 128×128 mm, a flip angle of 90° and a
NEX=1. The elbow intravenous bolus injections of gadopentetate
dimeglumine were administered with a flow rate of 4.5 ml/s,
followed by injection of normal saline at the same flow rate.
DWI and DSC images post-processing and
analysis
The original DWI and DSC maps were transmitted to an
advanced workstation 4.4 to generate the ADC and rCBV maps,
respectively. A total of three regions of interest (ROIs) in the
tumor parenchyma region on the ADC map were selected, and ADC
values were measured and averaged. ROI selection avoided the areas
of hemorrhage, necrosis, cystic degeneration and larger vessel
areas. A total of three ROIs at the tumor parenchyma on the rCBV
maps were drawn to measure the rCBV values and averaged. The ROIs
of the contralateral normal-appearing white matter (NAWM) were also
drawn. The rCBV included in the present study was defined as the
normalized rCBV value; rCBV value=the averaged rCBV value in
parenchyma/the rCBV value in contralateral NAWM. The ADC and rCBV
values were confirmed focus of the present study. A total of two
blinded, independent radiologists performed the image analyses.
Histopathology
The specimens were paraffin embedded subsequent to
4% formalin fixation and buffered in PBS, and 1-µm sections were
prepared for hematoxylin-eosin (HE) staining. Astrocytoma were
histopathologically classified according to the 2007 WHO central
nervous system classification criteria (3). The IHC indexes for GFAP, Topo IIα and
MGMT were assessed. The tumor parenchyma underwent corresponding
paraffin cuts and conventional dewaxing into water, and
avidin-biotin complex (ABC) IHC staining was performed. The main
reagents and instruments including GFAP, Topo IIα and MGMT
monoclonal antibodies were supplied by Dako; Agilent Technologies,
Inc. (Santa Clara, CA, USA), the ABC kit from Sigma-Aldrich Merck
KGaA (Darmstadt, Germany), the DAB chromogenic agent from
Sigma-Aldrich; Merck KGaA and the Digital Scan Scope case scanning
system (Merck KGaA). The Aperio Digital Pathology image analysis
system (Leica Microsystems GmbH, Wetzlar, Germany) and the software
Cytoplasmic V2 (Leica Microsystems GmbH) were used to select richly
stained tumor tissue sections. A total of three standard fields of
vision were randomly selected and the average optical density was
measured to compute an average for GFAP, Topo IIα and MGMT
expression levels of cells.
Statistical analysis
The ADC and rCBV values of different grades of
astrocytoma were compared using a two sample unpaired t-test
analysis. ROC curves were used to assess the astrocytoma grading
efficiency of ADC, rCBV and combined values of ADC and rCBV.
Associations between the MRI parameters and the IHC indices of
GFAP, Topo IIα and MGMT were analyzed using the Pearson correlation
method. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparisons of ADC and rCBV values
among grade II–IV astrocytoma
The DWI parameter value, ADC, and DSC parameter
value, rCBV, of the tumoral parenchyma are illustrated in Table I. The ADC values in the grade II
astrocytoma were significantly higher compared with the grade III
astrocytoma (P=0.003). The rCBV values in the grade II astrocytoma
were significantly lower compared with the grade III astrocytoma
(P=0.012). The ADC values in the grade III astrocytoma were
significantly higher compared with the grade III astrocytoma
(P=0.041). The rCBV values in the grade III astrocytoma were
significantly lower compared with the grade IV astrocytoma
(P=0.035).
 | Table I.Comparison of DWI and DSC parameters
among grade II–IV astrocytoma. |
Table I.
Comparison of DWI and DSC parameters
among grade II–IV astrocytoma.
|
| Grade II vs. III |
| Grade III vs. IV |
|
|---|
|
|
|
|
|
|
|---|
| Parameters | Grade II (n=23) | Grade III (n=44) | P-value | Grade III (n=44) | Grade IV (n=56) | P-value |
|---|
| ADC | 1.299±0.294 | 0.929±0.170 | 0.003a | 0.929±0.170 | 0.790±0.176 | 0.041a |
| rCBV | 2.552±0.705 | 5.195±1.883 | 0.012a | 5.195±1.883 | 7.070±1.721 | 0.035a |
The maps of the aforementioned conventional MRI
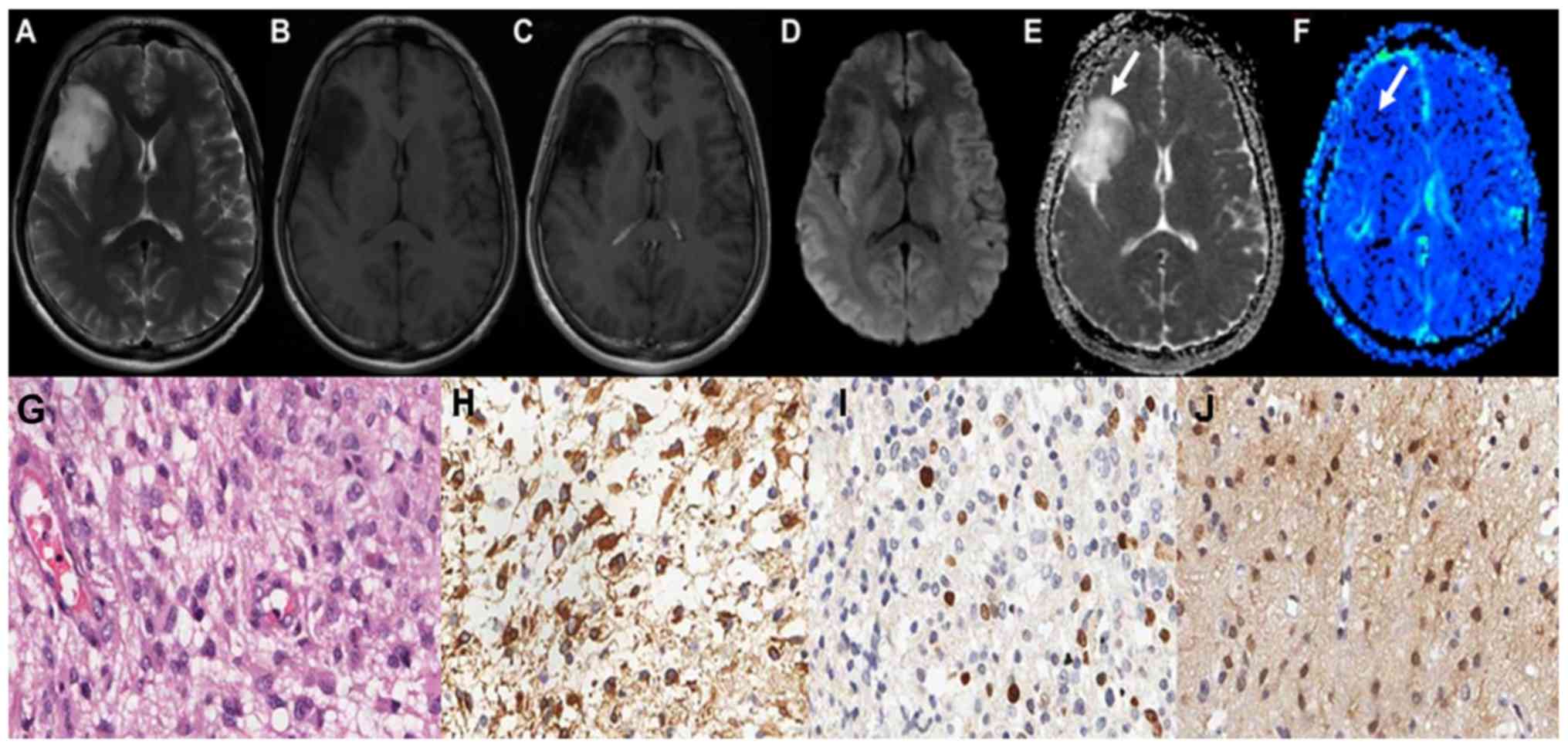
scans of grade II astrocytoma are demonstrated in Fig. 1A-C. Grade II astrocytoma exhibited
low signal on DWI sequence, and high ADC values, as illustrated in
Fig. 1D and E. The parenchyma of
grades II astrocytoma on the rCBV maps exhibited low signals, as
illustrated in Fig. 1F-J demonstrate
the HE staining map, IHC. GFAP map, IHC. Topo IIα map and IHC. MGMT
map of grades II astrocytoma respectively. The conventional MRI
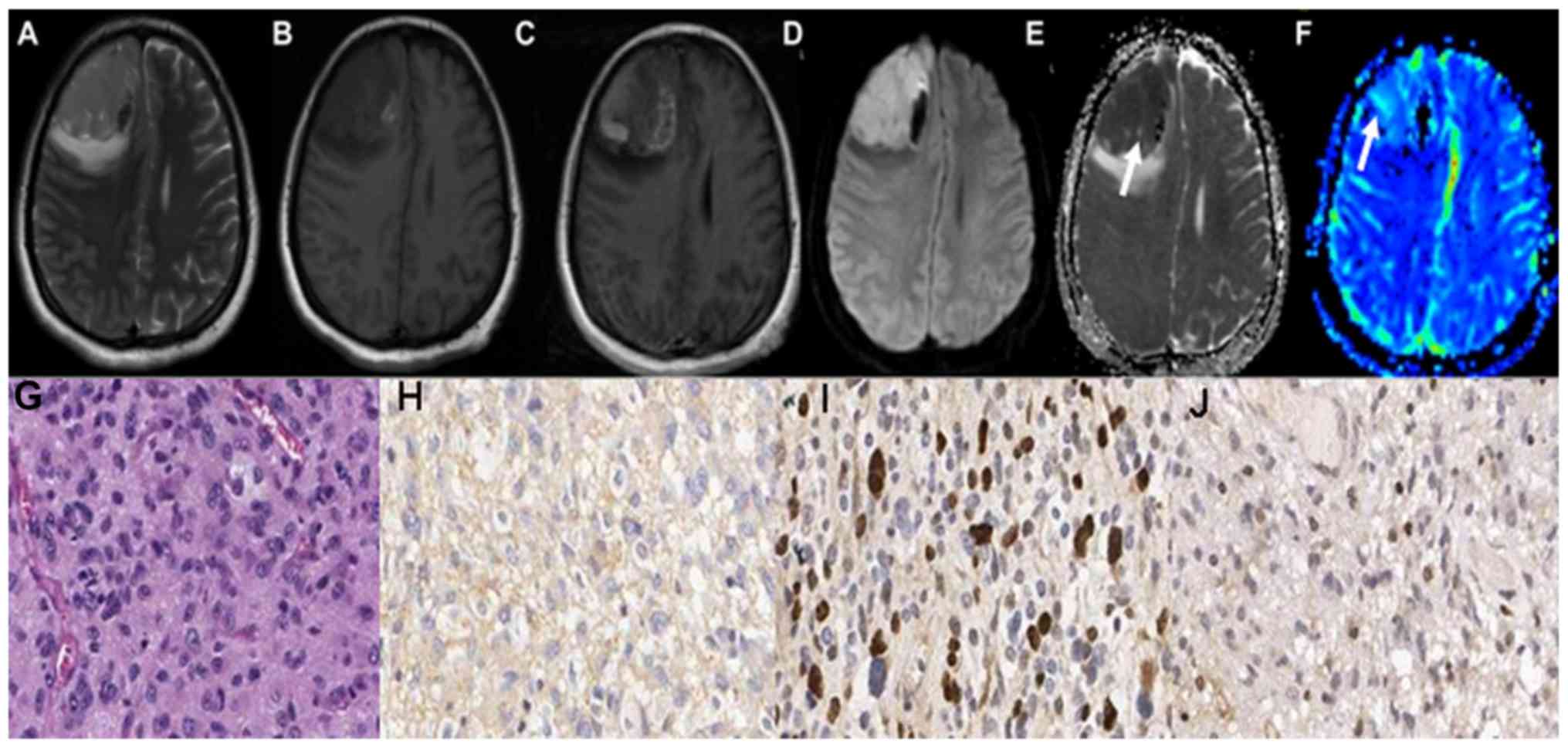
scans of grade III astrocytoma are demonstrated in Fig. 2A-C. Grade III astrocytoma
demonstrated high signal on DWI sequence, and the ADC values of the
tumor parenchyma were lower, as illustrated in Fig. 2D and E. The parenchyma of grades III
astrocytoma on the rCBV maps exhibited high signals, as illustrated
in Fig. 2F-J demonstrate the HE
staining map, IHC. GFAP map, IHC. Topo IIα map and IHC. MGMT map of
grades III astrocytoma respectively. The conventional MRI scans of
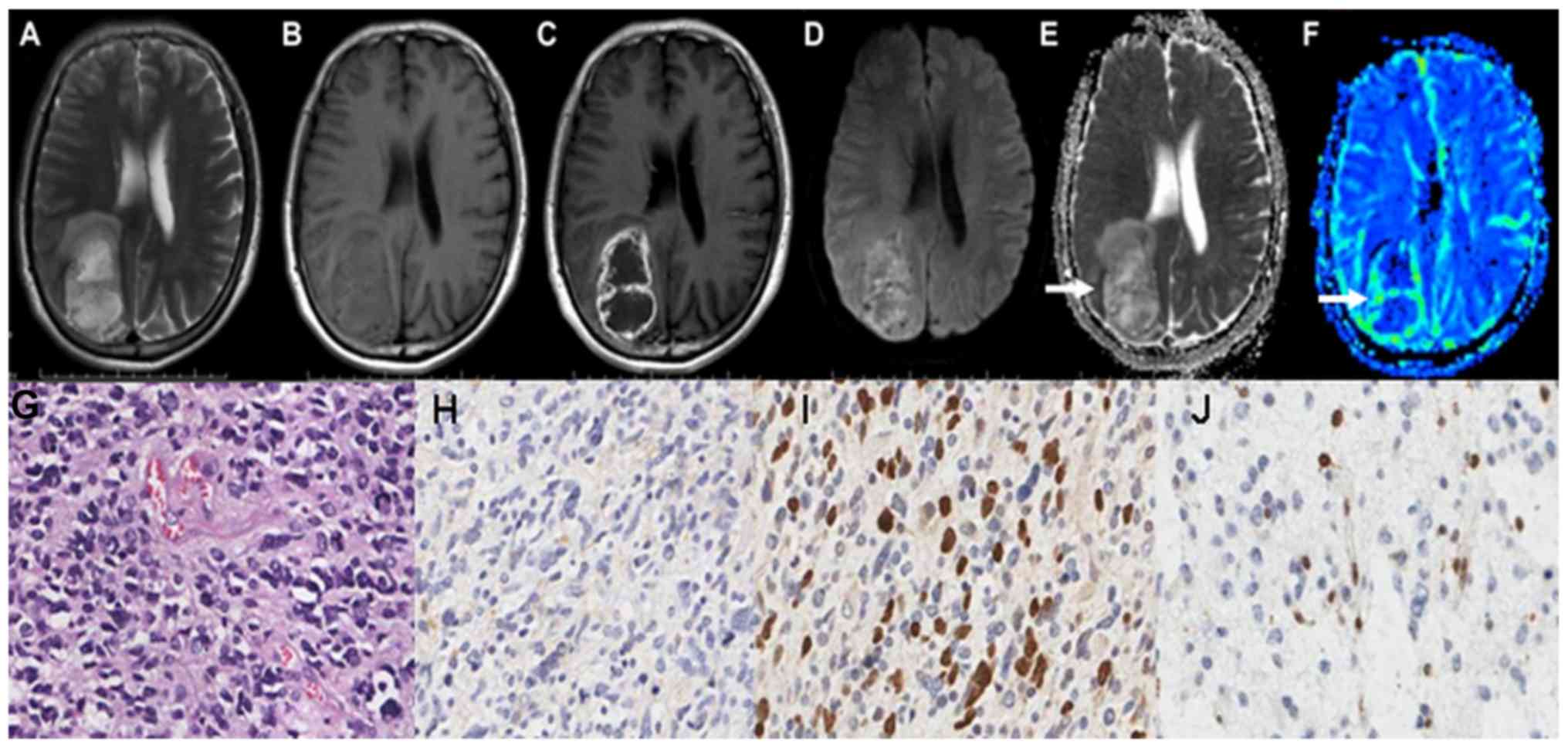
grade IV astrocytoma are demonstrated in Fig. 3A-C. Grade IV astrocytoma demonstrated
the highest signal on the DWI maps, and the ADC values were the
lowest between all of the astrocytoma grades, as illustrated in
Fig. 3D and E. The grade IV
astrocytoma on the rCBV map demonstrated the highest signal, as
illustrated in Fig. 3F-J demonstrate
the HE staining map, IHC. GFAP map, IHC. Topo IIα map and IHC. MGMT
map of grades IV astrocytoma respectively.
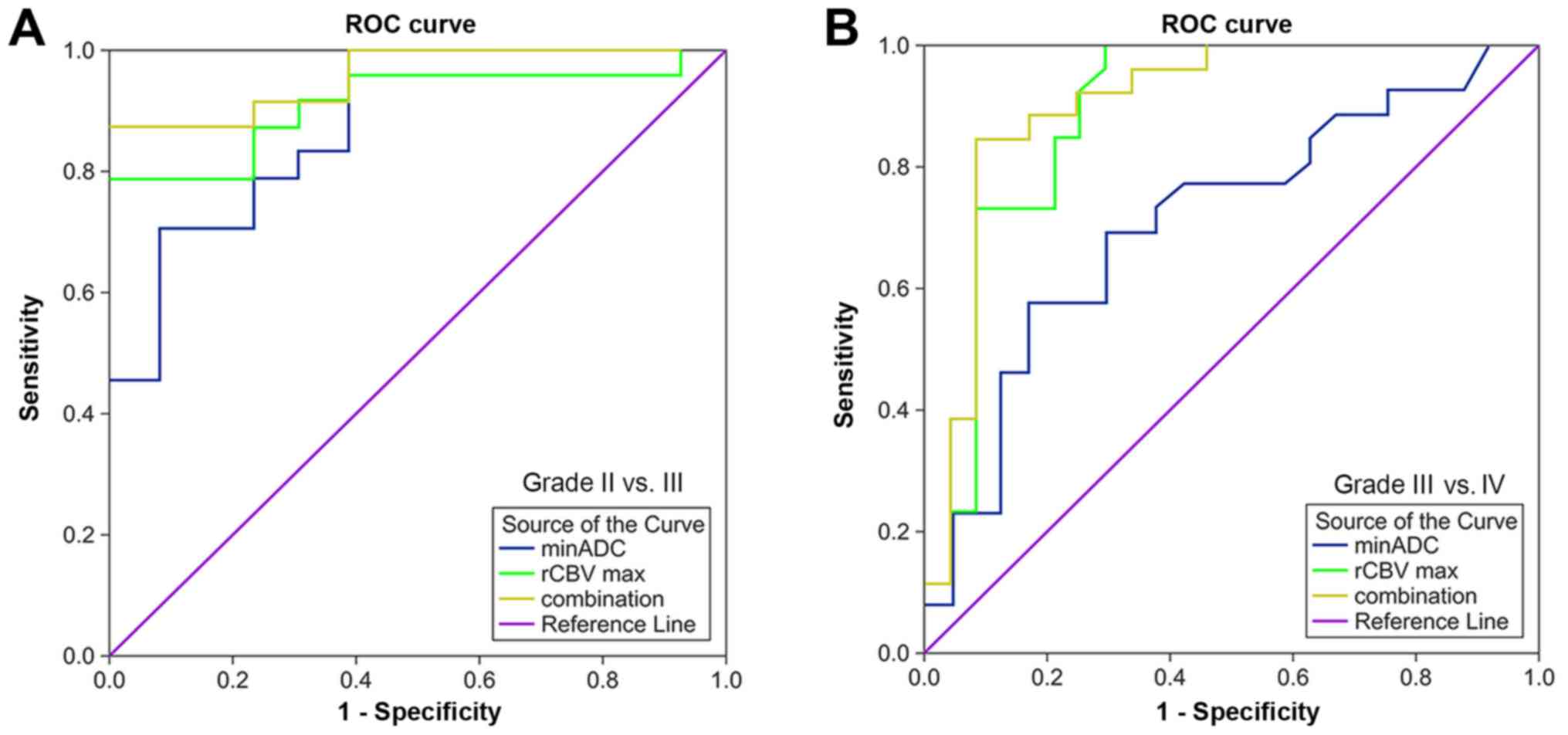
ROC analysis of DWI, DSC and combined
parameters in identifying grade II–III and III–IV astrocytoma
ROC analysis of value of ADC, value of rCBV, and the
combined value of ADC and rCBV in differentiating between grades II
and III astrocytoma was illustrated in Fig. 4A and Table II. The combined diagnostics method
had the highest area under the curve (AUC), 0.958, in
distinguishing between grades II and III astrocytoma, followed by
rCBV, 0.913, and ADC, 0.885. ROC analysis of ADC, rCBV and the
combined diagnostics method in differentiating between grades III
and IV astrocytoma is demonstrated in Fig. 4B and Table II. The combined parameter had the
highest AUC, 0.904, in distinguishing between grades III and IV
astrocytoma, followed by rCBV, 0.889, and ADC, 0.712.
 | Table II.Receiver operating characteristic
analysis of diffusion-weighted imaging, dynamic susceptibility
contrast-enhanced imaging and combined values in differentiating
grade II–IV astrocytoma. |
Table II.
Receiver operating characteristic
analysis of diffusion-weighted imaging, dynamic susceptibility
contrast-enhanced imaging and combined values in differentiating
grade II–IV astrocytoma.
|
| Grade II vs. III | Grade III vs. IV |
|---|
|
|
|
|
|---|
| Parameters | AUC | P-value | Cut-off value | Sensitivity (%) | Specificity (%) | AUC | P-value | Cut-off value | Sensitivity (%) | Specificity (%) |
|---|
| ADC | 0.885 | 0.034 | 1.021 | 70.8 | 92.9 | 0.712 | 0.035 | 0.783 | 57.7 | 83.3 |
| rCBV | 0.913 | 0.024 | 3.760 | 79.2 | 100 | 0.889 | 0.043 | 5.870 | 100 | 70.8 |
| Combined value | 0.958 | 0.001 |
| 87.5 | 100 | 0.904 | 0.002 |
| 84.6 | 91.7 |
Correlations between MRI parameters
and IHC indices
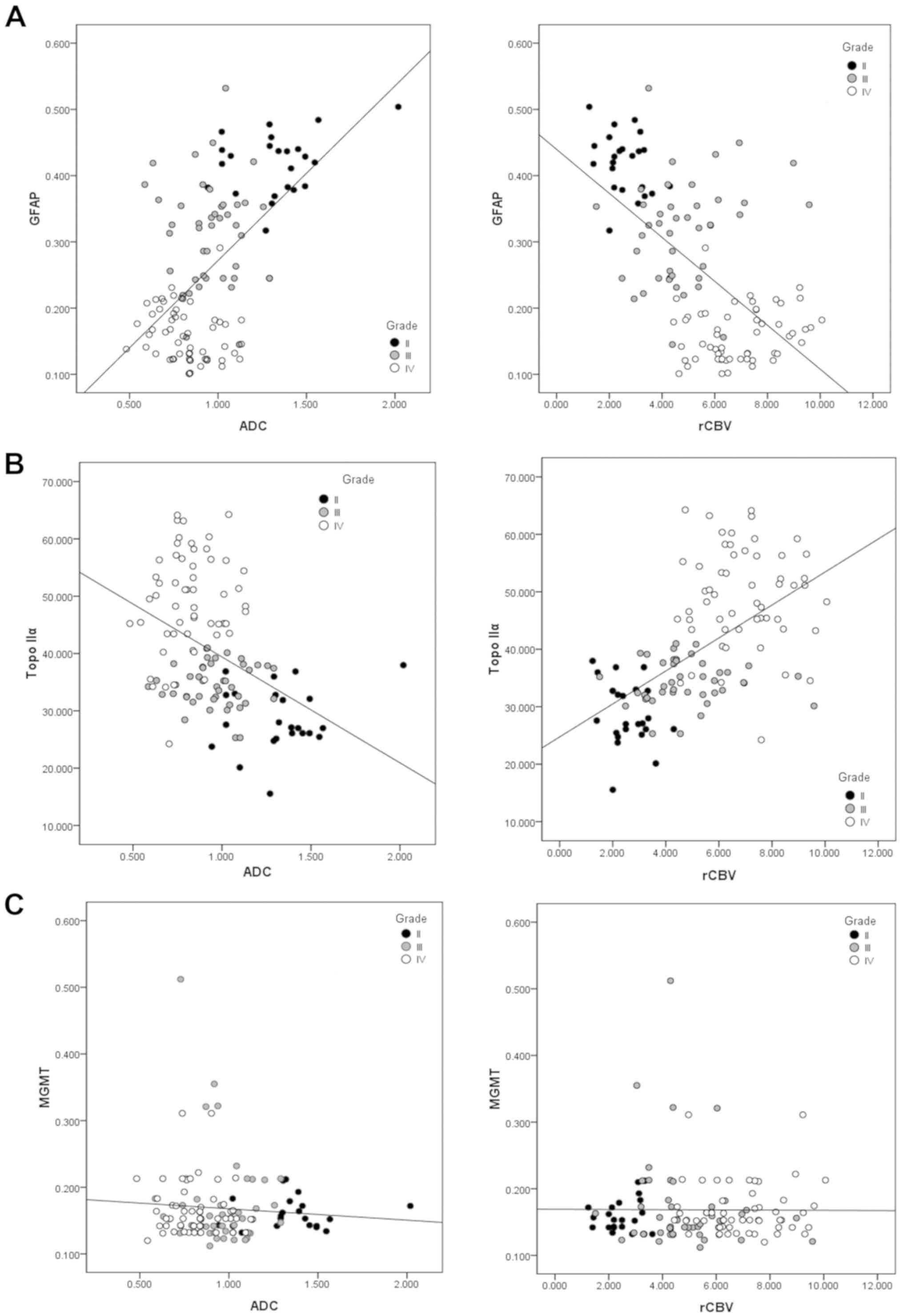
A positive correlation was exhibited between levels
of GFAP and ADC (r=0.574, P<0.001), whilst a negative
correlation was demonstrated between levels of GFAP and rCBV
(r=−0.610, P<0.001; Fig. 5A). A
negative correlation was demonstrated between levels of Topo IIα
and ADC (r=−0.435, P<0.001; Fig.
5B), and a positive correlation was exhibited between levels of
Topo IIα and rCBV (r=0.571, P<0.001; Fig. 5C). No correlation was observed
between levels of MGMT and ADC (r=−0.082, P=0.364 or rCBV of the
astrocytoma (r=0.024, P=0.790). These correlations between the MRI
parameters and the IHC indices are summarized in Table III.
 | Table III.Correlation between the values of
DWI/DSC and IHC parameters. |
Table III.
Correlation between the values of
DWI/DSC and IHC parameters.
| MRI and IHC
parameters | r-value | P-value |
|---|
| GFAP |
|
|
| ADC-GFAP | 0.574 | <0.001 |
| rCBV-GFAP | −0.610 | <0.001 |
| Topo IIα |
|
|
| ADC-Topo IIα | −0.435 | <0.001 |
| rCBV-Topo IIα | 0.571 | <0.001 |
| MGMT |
|
|
| ADC-MGMT | −0.082 | 0.364 |
| rCBV-MGMT | 0.024 | 0.790 |
Discussion
In the present study, the DWI and DSC parameters of
different grade astrocytoma were compared and the potential of
combing DWI and DSC data in astrocytoma grading was evaluated. In
addition, the correlation between DWI and DSC parameters and IHC
indices were examined. The results demonstrate that the ADC and
rCBV data exhibited significant differences between the grades
II–IV astrocytoma. Concurrently, the combined diagnostic method
exhibited the highest accuracy in differentiating between grades
II–IV astrocytoma. ADC and rCBV measurements demonstrated
correlations with levels of GFAP and Topo IIα. No association
between MRI parameters and levels of MGMT was observed.
Cells and subcellular structures limit the diffusion
of water molecules (14), and ADC
values can quantify this limited degree of diffusion. Thus, DWI can
non-invasively assess tumor cell density (15–17). In
the present study, there was a significant difference in ADC values
of tumor parenchyma between grades II and III (P<0.05); this
result is consistent with the findings of Lee et al
(15), Kono et al (16) and Calli et al (18). These previous studies revealed that
the ADC values of high-grade astrocytoma decreased significantly,
and the DWI signal increased. Previous studies on DWI of gliomas
examined the differences in ADC value between high-grade and
low-grade gliomas, yet the present study explored the values of DWI
in differentiating between grades II, III, and IV astrocytoma. In
the present study, the ADC value for grade IV astrocytoma was
0.790±0.176×10−3 mm2/s, which was lower
compared with the value revealed by Stadnik et al,
1.14×10−3 mm2/s (19). This difference may be associated with
the selection of ROI in the tumor parenchyma.
Invasiveness and tumor growth are closely associated
with neovascularization (20).
Therefore, utilizing a rCBV map of MR perfusion images for the
description of the characteristics of astrocytoma exhibits
potential that an rCBV map may be able to evaluate the degree of
angiogenesis. One study suggested that DSC-MR perfusion-weighted
imaging serves a major role in the identification of high- and
low-grade gliomas, and the rCBV values are significantly different
(6). The aforementioned study is
consistent with the results reported in the present study, in which
grades II and IV were compared with grade III astrocytoma, and a
statistically significant difference in rCBV values was revealed.
The results of the present study are different from those reported
in the study by Hakyemez et al (2), in which no significant difference as
observed between grades III and IV; this may be due to the
correction of the rCBV value with the contralateral NAWM in the
present study.
The present study focused on examining whether the
combined methods of ADC and rCBV measurements may improve
efficiency in astrocytoma grading. This is the first study in which
the combination of DWI and DSC MRI scanning may be used as a
classifying instrument in the grading of astrocytoma. The combined
diagnostic method increased the diagnostic power and had the
highest AUC, 0.943, and highest sensitivity, 87.5%, compared with
ADC and rCBV measurements alone, and a higher specificity, 100%,
compared with ADC measurements in astrocytoma grading. Through the
joint application of an arterial spin labeling technique and ADC
values to glioma grading, Kim et al (21) also concluded that the use of multiple
techniques improves the diagnostic accuracy of gliomas, serving as
an effective supplement to conventional MRI techniques. Hilario
et al (22) demonstrated that
the combination of minimum ADC and maximum rCBV measurements
improves the diagnostic accuracy of glioma grading. However, these
previous studies focus on gliomas as the type of cancer studied,
which covers several types of brain tumor. There have been fewer
reports investigating astrocytoma grading which apply DWI and DSC
imaging. In the present study, the range was narrowed to
astrocytoma from gliomas, which may provide increased precision for
preoperative astrocytoma grading systems, and generate data
pertinent for decisions concerning treatments.
GFAP is an intermediate filament cytoskeleton
protein which is expressed specifically by the gliocyte (11). Ilhan-Mutlu et al (23) reported that a decreased GFAP
expression was associated with an increasing malignancy grade in
gliomas. In the present study, a significant negative correlation
between GFAP and rCBV (r=−0.610, P<0.001), and a positive
correlation between GFAP and ADC (r=0.574, P<0.001) was
revealed. The decreased GFAP expression level correlated with the
aggressiveness and malignancy of astrocytoma, whilst rCBV and ADC
levels reflected the vasculation and cell density, which were
associated with the malignancy of tumors, which many be potential
explanations for the above correlation.
It was suggested that Topo IIα was associated with
cellular proliferation (24). In the
present study, it was demonstrated that Topo IIα was negatively
correlated with ADC (r=−0.435, P<0.001), and positively
correlated with rCBV (r=0.571, P<0.001). ADC reflects the cell
density of tumor, which may explain the correlation between Topo
IIα and ADC. Therefore, ADC may reveal the Topo IIα expression
levels, to predict the malignancy of astrocytoma. In addition, it
was revealed that rCBV also reflected the levels of Topo IIα
expression (25). Further studies
are required to examine the internal association between rCBV and
Topo IIα expression.
MGMT promoter methylation reflects the sensitivity
of chemotherapeutic drugs (temozolomide) to astrocytoma patients.
The existence of an association between expression levels of MGMT
protein and MGMT promoter methylation remains unknown (26). In the present study, no correlation
was observed between values of ADC and rCBV and MGMT protein
expression. As aforementioned, this association requires additional
study.
The present study included certain limitations.
Firstly, cystic-solid tumors may possess thin cystic wall tissues,
and the selected ROIs of ADC or rCBV may contain a portion of
normal brain parenchyma outside the cystic wall of the tumor
(27). This may lead to inaccurate
assessment of ADC and rCBV values to tumor cell proliferation and
vascular proliferation. Secondly, pathological misdiagnosis may
occur due to sampling errors caused by tumor heterogeneity,
particularly for malignant astrocytoma: For example, the tumor
samples may contain grades II and III astrocytoma cells. In
addition, manually drawing ROIs is a tedious procedure and may
incur personal error. Finally, the investigation did not involve
analysis of prognoses, which should be explored in future
studies.
In conclusion, the present study demonstrated that
the combination of DWI and DSC measurements may improve the
accuracy of astrocytoma grading. The DWI and DSC measurements which
exhibit correlations with IHC indices of GFAP and Topo IIα may be
useful biomarkers in predicting the levels of malignancy in
astrocytoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81471652,
81771824 and 81701681), the Precision Medicine Key Innovation Team
Project (grant no. YT1601), the Social Development Projects of Key
R&D Programs in the Shanxi Province (grant no. 201703D321016)
and the Natural Science Foundation of Shanxi Province (grant no.
201601D021162).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ proposed the notion of the present study and
interpreted the patient data. JQ performed the majority of the
experiments and was a major contributor in writing the manuscript.
XWa analyzed the data and designed the figures. YT and XWu
collected and managed the samples. All authors read and approved
the final manuscript for publication.
Ethics approval and consent to
participate
The present study was approved by Shanxi Medical
University review board. All manuscripts comply with the guidelines
of the February 2006 consensus statement of the International
Committee of Medical Journal Editors, and all patients provided
written informed consent prior to their inclusion within the
study.
Consent for publication
All patients provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Warmuth C, Gunther M and Zimmer C:
Quantification of blood flow in brain tumors: Comparison of
arterial spin labeling and dynamic susceptibility-weighted
contrast-enhanced MR imaging. Radiology. 228:523–532. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hakyemez B, Erdogan C, Ercan I, Ergin N,
Uysal S and Atahan S: High-grade and low-grade gliomas:
Differentiation by using perfusion MR imaging. Clin Radiol.
60:493–502. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirai T, Murakami R, Nakamura H, Kitajima
M, Fukuoka H, Sasao A, Akter M, Hayashida Y, Toya R, Oya N, et al:
Prognostic value of perfusion MR imaging of high-grade
astrocytomas: Long-term follow-up study. AJNR Am J Neuroradiol.
29:1505–1510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bulakbasi N, Kocaoglu M, Farzaliyev A,
Tayfun C, Ucoz T and Somuncu I: Assessment of diagnostic accuracy
of perfusion MR imaging in primary and metastatic solitary
malignant brain tumors. AJNR Am J Neuroradiol. 26:2187–2199.
2005.PubMed/NCBI
|
|
6
|
Sadeghi N, D'Haene N, Decaestecker C,
Levivier M, Metens T, Maris C, Wikler D, Baleriaux D, Salmon I and
Goldman S: Apparent diffusion coefficient and cerebral blood volume
in brain gliomas: Relation to tumor cell density and tumor
microvessel density based on stereotactic biopsies. AJNR Am J
Neuroradiol. 29:476–482. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prager AJ, Martinez N, Beal K, Omuro A,
Zhang Z and Young RJ: Diffusion and perfusion MRI to differentiate
treatment-related changes including pseudoprogression from
recurrent tumors in high-grade gliomas with histopathologic
evidence. AJNR Am J Neuroradiol. 36:877–885. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao HF, Chen ZY, Lou X, Wang YL, Gui QP,
Wang Y, Shi KN, Zhou ZY, Zheng DD, Wang DJ and Ma L: Astrocytic
tumour grading: A comparative study of three-dimensional
pseudocontinuous arterial spin labelling, dynamic susceptibility
contrast-enhanced perfusion-weighted imaging, and
diffusion-weighted imaging. Eur Radiol. 25:3423–3430. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leu K, Enzmann DR, Woodworth DC, Harris
RJ, Tran AN, Lai A, Nghiemphu PL, Pope WB, Cloughesy TF and
Ellingson BM: Hypervascular tumor volume estimated by comparison to
a large-scale cerebral blood volume radiographic atlas predicts
survival in recurrent glioblastoma treated with bevacizumab. Cancer
Imaging. 14:312014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Le Bihan D: Apparent diffusion coefficient
and beyond: What diffusion MR imaging can tell us about tissue
structure. Radiology. 268:318–322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elobeid A, Bongcam-Rudloff E, Westermark B
and Nister M: Effects of inducible glial fibrillary acidic protein
on glioma cell motility and proliferation. J Neurosci Res.
60:245–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Faria MH, Goncalves BP, do Patrocinio RM,
de Moraes-Filho MO and Rabenhorst SH: Expression of Ki-67,
topoisomerase IIalpha and c-MYC in astrocytic tumors: Correlation
with the histopathological grade and proliferative status.
26:519–527. 2006.PubMed/NCBI
|
|
13
|
Christmann M, Nagel G, Horn S, Krahn U,
Wiewrodt D, Sommer C and Kaina B: MGMT activity, promoter
methylation and immunohistochemistry of pretreatment and recurrent
malignant gliomas: A comparative study on astrocytoma and
glioblastoma. Int J Cancer. 127:2106–2118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kwee TC, Galbán CJ, Tsien C, Junck L,
Sundgren PC, Ivancevic MK, Johnson TD, Meyer CR, Rehemtulla A, Ross
BD and Chenevert TL: Comparison of apparent diffusion coefficients
and distributed diffusion coefficients in high-grade gliomas. J
Magn Reson Imaging. 31:531–537. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee EJ, Lee SK, Agid R, Bae JM, Keller A
and Terbrugge K: Preoperative grading of presumptive low-grade
astrocytomas on MR imaging: Diagnostic value of minimum apparent
diffusion coefficient. AJNR Am J Neuroradiol. 29:1872–1877. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kono K, Inoue Y, Nakayama K, Shakudo M,
Morino M, Ohata K, Wakasa K and Yamada R: The role of
diffusion-weighted imaging in patients with brain tumors. AJNR Am J
Neuroradiol. 22:1081–1088. 2001.PubMed/NCBI
|
|
17
|
Yamasaki F, Kurisu K, Satoh K, Arita K,
Sugiyama K, Ohtaki M, Takaba J, Tominaga A, Hanaya R, Yoshioka H,
et al: Apparent diffusion coefficient of human brain tumors at MR
imaging. Radiology. 235:985–991. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Calli C, Kitis O, Yunten N, Yurtseven T,
Islekel S and Akalin T: Perfusion and diffusion MR imaging in
enhancing malignant cerebral tumors. Eur J Radiol. 58:394–403.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stadnik TW, Chaskis C, Michotte A, Shabana
WM, van Rompaey K, Luypaert R, Budinsky L, Jellus V and Osteaux M:
Diffusion-weighted MR imaging of intracerebral masses: Comparison
with conventional MR imaging and histologic findings. AJNR Am J
Neuroradiol. 22:969–976. 2001.PubMed/NCBI
|
|
20
|
Ueda M, Terai Y, Kumagai K, Ueki K,
Yamaguchi H, Akise D and Ueki M: Vascular endothelial growth factor
C gene expression is closely related to invasion phenotype in
gynecological tumor cells. Gynencol Oncol. 82:162–166. 2001.
View Article : Google Scholar
|
|
21
|
Kim HS and Kim SY: A prospective study on
the added value of pulsed arterial spin-labeling and apparent
diffusion coefficients in the grading of gliomas. AJNR Am J
Neuroradiol. 28:1693–1699. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hilario A, Ramos A, Perez-Nuñez A,
Salvador E, Millan JM, Lagares A, Sepulveda JM, Gonzalez-Leon P,
Hernandez-Lain A and Ricoy JR: The added value of apparent
diffusion coefficient to cerebral blood volume in the preoperative
grading of diffuse gliomas. AJNR Am J Neuroradiol. 33:701–707.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ilhan-Mutlu A, Wagner L, Widhalm G, Wöhrer
A, Bartsch S, Czech T, Heinzl H, Leutmezer F, Prayer D, Marosi C,
et al: Exploratory investigation of eight circulating plasma
markers in brain tumor patients. Neurosurg Rev. 36:45–56. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kimura F, Okayasu I, Kakinuma H, Satoh Y,
Kuwao S, Saegusa M and Watanabe J: Differential diagnosis of
reactive mesothelial cells and malignant mesothelioma cells using
the cell proliferation markers minichromosome maintenance protein
7, geminin, topoisomerase II alpha and Ki-67. Acta Cytol.
57:384–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maia AC Jr, Malheiros SM, da Rocha AJ, da
Silva CJ, Gabbai AA, Ferraz FA and Stávale JN: MR cerebral blood
volume maps correlated with vascular endothelial growth factor
expression and tumor grade in nonenhancing gliomas. AJNR Am J
Neuroradiol. 26:777–783. 2005.PubMed/NCBI
|
|
26
|
Felsberg J, Thon N, Eigenbrod S, Hentschel
B, Sabel MC, Westphal M, Schackert G, Kreth FW, Pietsch T, Löffler
M, et al: Promoter methylation and expression of MGMT and
the DNA mismatch repair genes MLH1, MSH2, MSH6 and
PMS2 in paired primary and recurrent glioblastomas. Int J
Cancer. 129:659–670. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Svolos P, Kousi E, Kapsalaki E, Theodorou
K, Fezoulidis I, Kappas C and Tsougos I: The role of diffusion and
perfusion weighted imaging in the differential diagnosis of
cerebral tumors: A review and future perspectives. Cancer Imaging.
14:202014.PubMed/NCBI
|