Introduction
Esophageal cancer (EC) is the eigth most common
malignancy and the seventh leading cause of cancer-associated
mortality worldwide (1). There are
two main histological types of EC, including esophageal squamous
cell carcinoma (ESCC) and esophageal adenocarcinoma (2). Nearly 50% of EC cases worldwide occur
in China, and EC had the third highest incidence rate and fourth
higher mortality rate in China according to the Cancer Statistics
in China for 2015 (3). Additionally,
the majority of EC cases in China are ESCC, which accounts for
>90% of new EC cases (4). Despite
advancements in diagnostic and therapeutic techniques over the past
three decades, the prognosis of ESCC remains poor, with a 5-year
survival rate of <10% (5). Thus,
the search for an effective therapy for controlling this
devastating disease is ongoing. Since ESCC cells avoid apoptosis,
novel therapeutic strategies should be investigated for the
induction of apoptosis in ESCC cells.
The antineoplastic drug paclitaxel is a
microtubule-targeting agent widely utilized in the clinic for the
treatment of patients with ESCC and several other types of solid
tumor (6). Paclitaxel blocks cells
in the G2/M phase of the cell cycle by stabilizing microtubules
(7). As a result, chromosomes fail
to achieve the metaphase spindle configuration, which further
interferes with chromosome separation, leading to apoptosis
(8–10). Paclitaxel has offered substantial
improvements in patient survival; however, numerous patients
acquire resistance to paclitaxel, which reduces the anti-cancer
effect of paclitaxel (11).
Therefore, it is important to identify non-cytotoxic drugs to
increase paclitaxel sensitivity.
Shikonin, a natural product isolated from the plant
Lithospermum erythrohizon, which has long been used in
Traditional Chinese Medicine, is understood to act on a variety of
molecular targets associated with carcinogenesis, including
pyruvate kinase M2 (12). Previous
studies have demonstrated that shikonin exhibits significant
antitumor potential by inducing apoptosis and necroptosis in cancer
cell lines of various types, including breast cancer,
hepatocellular carcinoma, leukemia, glioma and osteosarcoma
(13–17). These findings indicate that shikonin
exhibits therapeutic promise for the treatment of cancer. In
addition, shikonin can enhance the sensitivity of cells to the
anticancer agents in various types of cancer. For instance,
shikonin has been identified to enhance adriamycin antitumor
effects in lung cancer (18).
Additionally, there is a potential for combined synergistic
effects of shikonin and arsenic trioxide against human
hepatocellular carcinoma (19).
The present study identified that the combination of
paclitaxel with shikonin has significantly improved effects on
esophageal cancer in vitro. The present study provides
molecular insights into the apoptosis process involved in combined
paclitaxel and shikonin treatment by detecting alterations of p53
and Bcl-2, and the data indicates that the combination of
paclitaxel and shikonin may be a promising alternative
chemotherapeutic strategy for esophageal cancer.
Materials and methods
Cell culture and chemicals
The ESCC cell lines KYSE270 and KYSE150, kindly
provided by Dr Hui Zhang (Sun Yat-Sen University Cancer Center,
Guangzhou, China), were cultured in RPMI-1640 medium (Thermo fisher
Scientific, Inc., Waltham, MA, USA), supplemented with 10% (v/v)
fetal bovine serum (Thermo fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Thermo Fisher Scientific Inc.). Cells were
incubated at 37°C in a 5% CO2 humidified atmosphere.
Paclitaxel, purchased from the National Institute for the Control
of Pharmaceutical and Biological Products (Beijing, China), and
shikonin, purchased from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany), were dissolved in DMSO.
MTT assay
MTT cytotoxicity assay was performed according to
manufacturer's protocol (Sigma-Aldrich; Merck KGaA). Briefly,
KYSE270 and KYSE150 cells were plated in 96-well plates, at a
density of 2,000 cells per well in 100 µl medium. Following
overnight culture, cells were treated with different treatments for
four days, including DMSO, paclitaxel (10, 100 and 200 nM),
shikonin (1 µM) and paclitaxel (100 nM) combined with shikonin (1
µM). On days 1, 2, 3 and 4, 10 µl MTT solution was added to each
well and incubated at room temperature for 3 h. Subsequently, 100
µl DMSO was added to each well and the plates were incubated
overnight. The absorbance was then measured using SpectraMax 190
(Molecular Devices, LLC, Sunnyvale, CA, USA) at a wavelength of 490
nm. The experiment was performed three times.
Cell cycle analysis and Annexin-V
apoptosis assay by flow cytometry
KYSE 270 and KYSE150 cells were plated into 12-well
plates at 1×105/well, and treated with DMSO, shikonin (1
µM), paclitaxel (100 nM) or a combination of shikonin (1 µM) and
paclitaxel (100 nM) at 37°C in a 5% CO2 humidified
atmosphere. Following 24 h, the cells were collected and fixed in
70% ethanol at −20°C overnight. Subsequently, the cells were washed
twice with ice-cold PBS. For cell cycle analysis, the cells were
incubated with RNase A (100 µg/ml; Sigma-Aldrich; Merck KGaA) and
propidium iodide (PI; 50 µg/ml; Sigma-Aldrich; Merck KGaA) at room
temperature for 30 min. The cells were then analyzed by flow
cytometry with a BD FACSCalibur (BD Biosciences, San Jose, CA,
USA). For the Annexin-V apoptosis assay, the cells were double
stained with PI and Annexin-V-FITC according to the manufacturer's
protocol (Nanjing KeyGen Biotech, Co., Ltd., Nanjing, China). The
cells were then immediately analyzed on BD FACSCalibur flow
cytometer. All FACS data were analyzed with FlowJo v7.6 software
(FlowJo, LLC, Ashland, OR, USA). All experiments were repeated
three times.
Reverse transcription-quantitative PCR
analysis
KYSE270 cells were collected for RNA isolation at 48
h following treatment with DMSO, shikonin (1 µM), paclitaxel (100
nM) or a combination of shikonin (1 µM) and paclitaxel (100 nM) at
37°C in a 5% CO2 humidified atmosphere. Total RNA was
extracted from KYSE270 cells using TRIzol® (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. Total RNA (1 µg) was reverse transcribed
into cDNA using MMLV reverse transcriptase according to the
manufacturer's protocol. (Invitrogen; Thermo Fisher Scientific,
Inc.). Bcl-2 and p53 gene expression was analyzed by quantitative
PCR in the presence of SYBR Green Master mix (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), with the following primers: Bcl-2
forward, 5′-CGACGACTTCTCCCGCCGCTACCGC-3′ and reverse,
5′-CCGCATGCTGGGGCCGTACAGTTCC-3′; p53 forward,
5′-TGTGGGATGGGGTGAGATTTC-3′ and reverse, 5′-CTGTTGGTCGGTGGGTTG-3′.
GAPDH was amplified as an endogenous control using the following
primers: forward, 5′-ACGGATTTGGTCGTATTGGG-3′ and reverse,
5′-TGATTTTGGAGGGATGTCGC-3′. The relative expression levels of Bcl-2
and p53 compared were GAPDH in each sample were calculated
according to 2−ΔΔCq method (20). The thermocycling conditions were: i)
An initial 95°C for 10 min; ii) 40 cycles of denaturation at 95°C
for 30 sec, annealing with primer at 57°C for 30 sec and extension
at 72°C for 30 sec; and ii) final extension at 72°C for 10 min. The
experiments were performed three times.
Western blot analysis
KYSE270 and KYSE150 cells were collected for protein
extraction at 48 h following the indicated treatments. The cell
lysates were prepared in RIPA buffer (Thermo Fisher Scientific,
Inc.). Following protein quantification with a BCA kit (Bio-Rad
Laboratories, Inc.), 20 µg protein was loaded per lane, separated
by 8% SDS-PAGE, and transferred to a PVDF membrane. Following
blocking with 5% non-fat milk for 1 h at room temperature, the
membranes were incubated with anti-Bcl-2 (cat. no. 4223T; 1:1,000),
anti-cleaved caspase 3 (cat. no. 9664T; 1:1,000), anti-cleaved poly
(ADP-ribose) polymerase (PARP; cat. no. 5625T; 1:1,000), anti-p53
(cat. no. 2527T; 1:1,000) and anti-GAPDH (cat. no. 2118S; 1:1,000)
antibodies overnight at 4°C. The membranes were then washed with
TBS-T (TBS with 0.05% Tween-20) three times for 5 min each time.
Subsequently, the membranes were incubated with a horseradish
peroxidase-conjugated anti-rabbit secondary antibody (cat. no.
7074S; 1:2,000) at room temperature for 1 h. All antibodies were
purchased from Cell signaling Technology, Inc., (Danvers, MA, USA).
After washing three times, the signals were detected using an ECL
kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All data are presented as the mean ± standard error
of the mean. The data from two groups or multiple groups were
compared using a Student's t-test or one-way ANOVA with Dunnett's
multiple comparisons test. All Statistical analysis was implemented
using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). All
experiments were repeated at least three times. P<0.05 was
considered to indicate a statistically significant difference.
Results
Shikonin significantly enhances the
cytotoxic effects of paclitaxel in KYSE270 cells
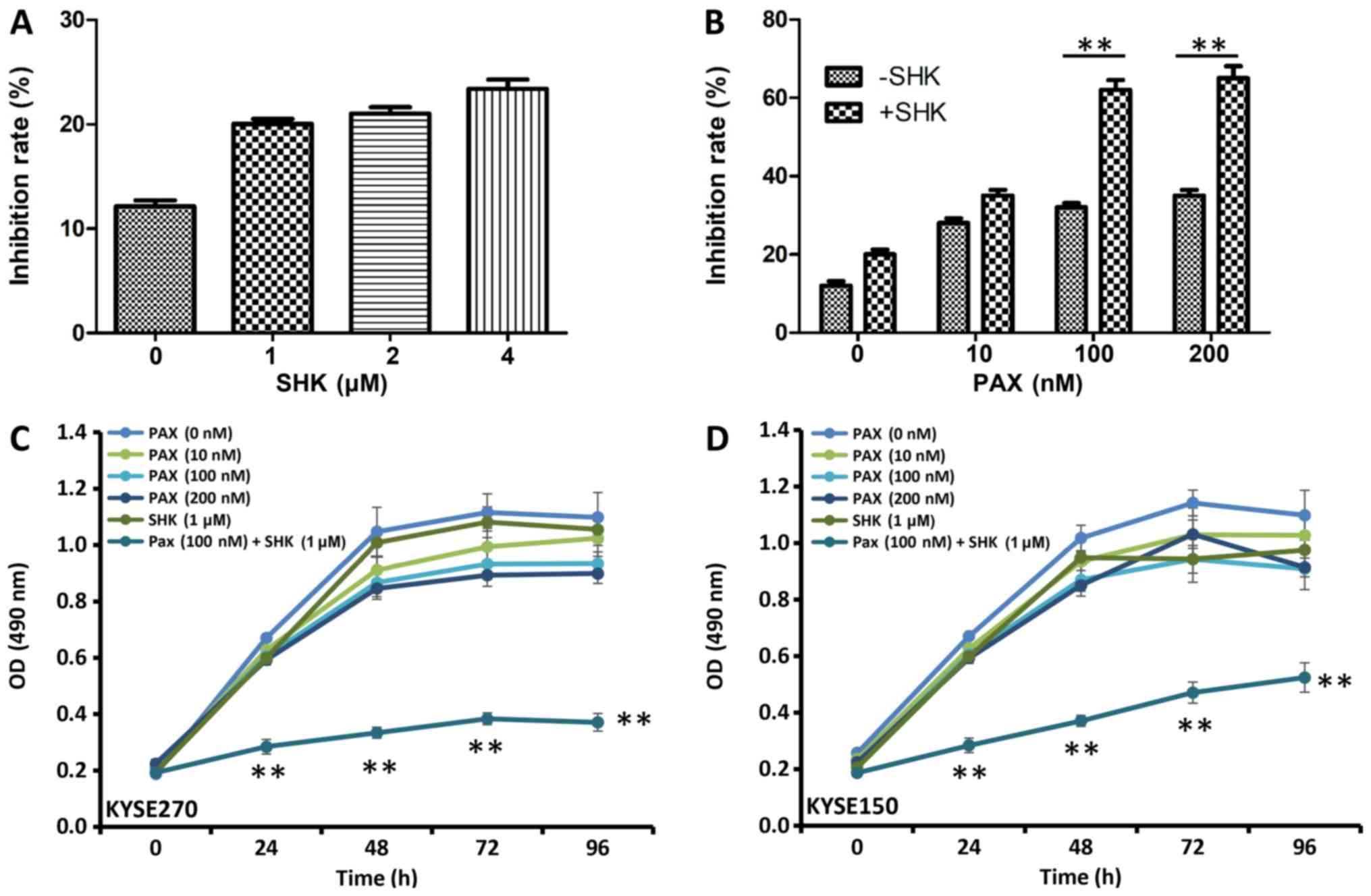
To determine the cytotoxic effect of shikonin in
ESCC carcinoma cells, the viability of cells treated with shikonin
was measured using MTT assay. As presented in Fig. 1A, B, shikonin and paclitaxel
inhibited KYSE270 and KYSE150 cell proliferation at 48 h following
treatment. Although treatments with the single drugs modestly
inhibited cell growth, increasing doses of either shikonin
(Fig. 1A) or paclitaxel (Fig. 1B) alone did not significantly inhibit
KYSE270 and KYSE150 cell growth. The effects of shikonin and
paclitaxel co-treatment on KYSE270 and KYSE150 cell proliferation
were assessed using various concentrations of paclitaxel, and a low
concentration of shikonin at 1 µM. Significant growth inhibition
was observed with 100 or 200 µM paclitaxel and 1 µM shikonin when
compared with cells treated with 100 or 200 µM paclitaxel alone
(Fig. 1B; P<0.001).
Analysis of the effects of shikonin and paclitaxel
alone or in combination on KYSE270 and KYSE150 cell proliferation
over time were also assessed (Fig.
1C-D). Between 24 and 96 h, cell survival rates were
significantly reduced in the combined treatment group (100 nM
paclitaxel and 1 µM shikonin) when compared with cell survival in
all single agent treatment groups (P<0.001). These data indicate
that shikonin increases paclitaxel-induced cell inhibitory effects
in ESCC cells.
Shikonin facilitates mitotic arrest
induction caused by paclitaxel
Paclitaxel exerts its cytotoxic effect by
interacting with β-tubulin, which stabilizes the structure of
microtubules and prevents the depolymerization of microtubules.
This microtubule stabilization leads to cell cycle arrest in the
G2/M phase and eventually causes cell death by apoptosis (21). Therefore, the present study
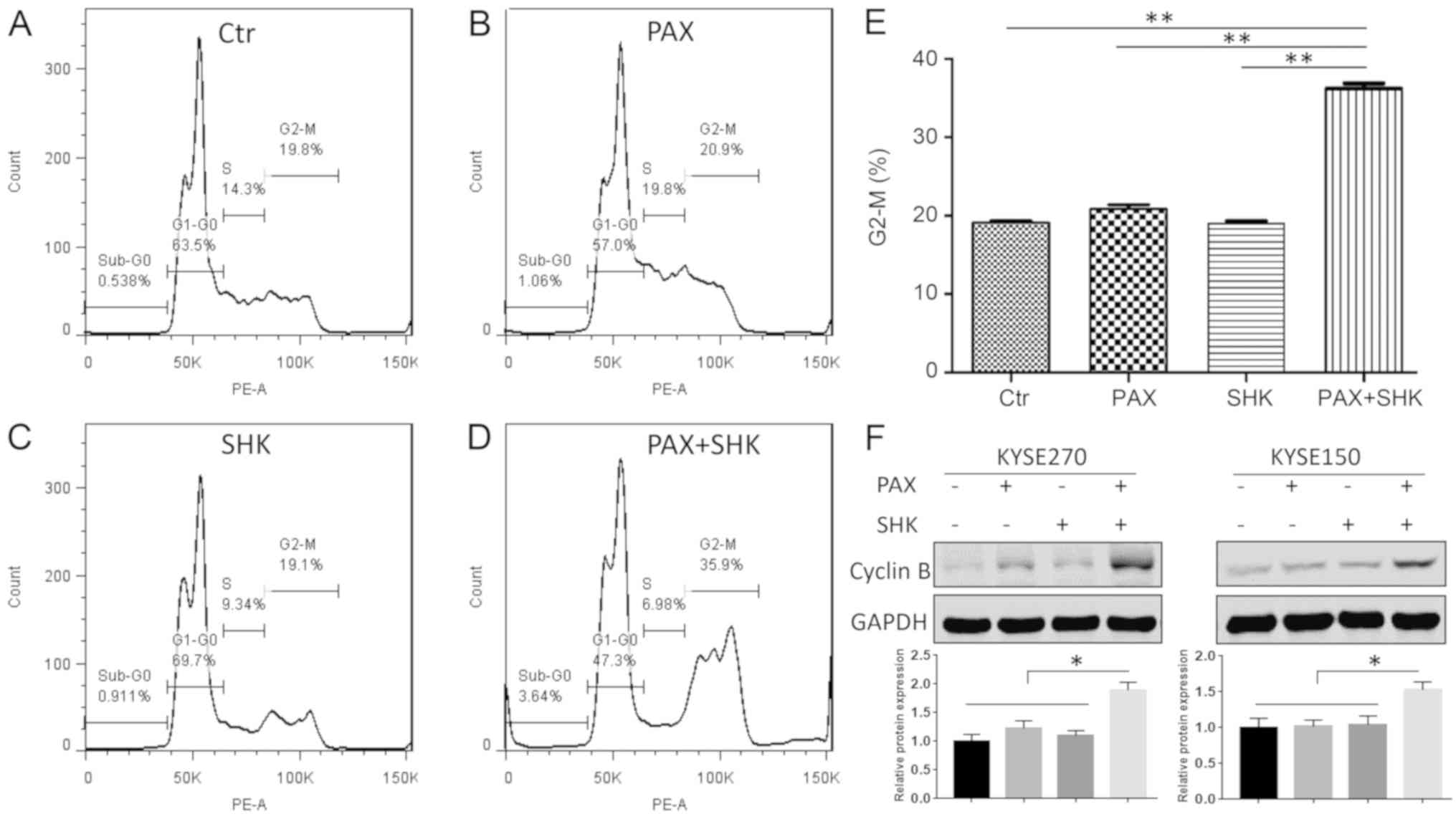
determined whether shikonin affects mitotic arrest caused by
paclitaxel through flow cytometric analysis. As presented in
Fig. 2A-E, following 24 h of
treatment, neither shikonin (1 µM) or paclitaxel (100 nM) alone
significantly altered the cell cycle compared with the control
group. However, compared with treatment with paclitaxel alone, the
combined treatment with shikonin and paclitaxel significantly
enhanced the cell percentage at the G2/M stage. In addition, to
assess the mitotic status of the ESCC cells, the mitotic protein
cyclin B was detected. A significant increased protein level of
cyclin B was detected following 24 h of shikonin and paclitaxel
combined treatment (P<0.05), indicating that combined treatment
induced mitotic arrest (Fig.
2F).
Shikonin enhances the ability of
paclitaxel to induce apoptosis of KYSE270 cells
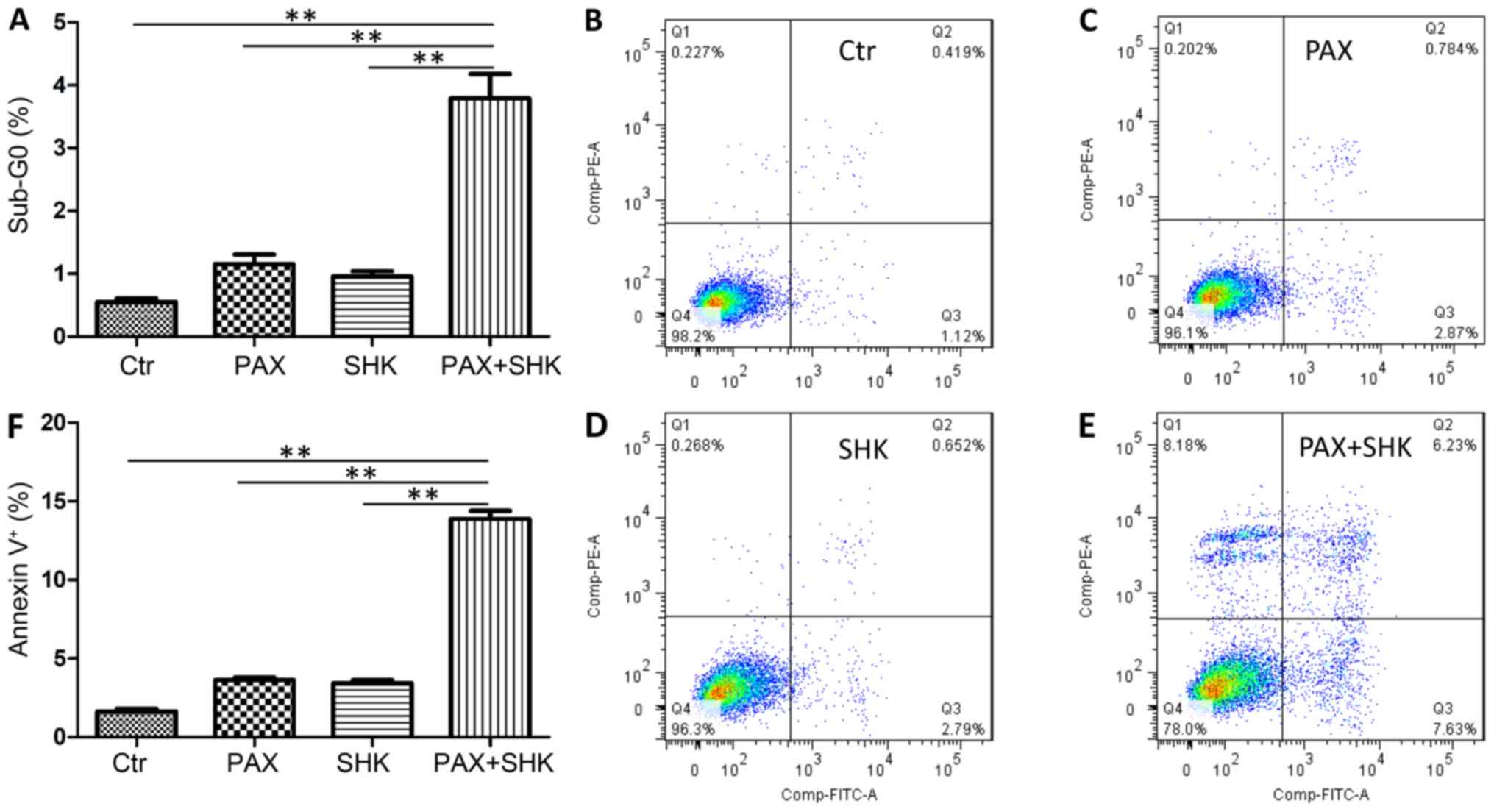
The present study further assessed the level of cell
apoptosis following treatment with either single or combined agents
using two methods. First, the percentage of sub-G0 cells was
calculated following 72 h of exposure to the mock treatment,
shikonin, paclitaxel, or a combination of shikonin and paclitaxel.
As presented in Fig. 3A, the
combined treatment significantly increased the cell apoptosis level
in KYSE270 cells when compared with both shikonin or
paclitaxel-treatment alone (P<0.001). The present study
subsequently detected cell apoptosis through Annexin-V and PI
staining. Consistent with the sub-G0 analysis, the shikonin and
paclitaxel combined treatment significantly increased the
percentage of apoptotic cells compared with both shikonin or
paclitaxel-treatment alone (P<0.001, Fig. 3B-F).
Shikonin activates caspase-3,
increases p53 expression and suppresses Bcl-2 expression
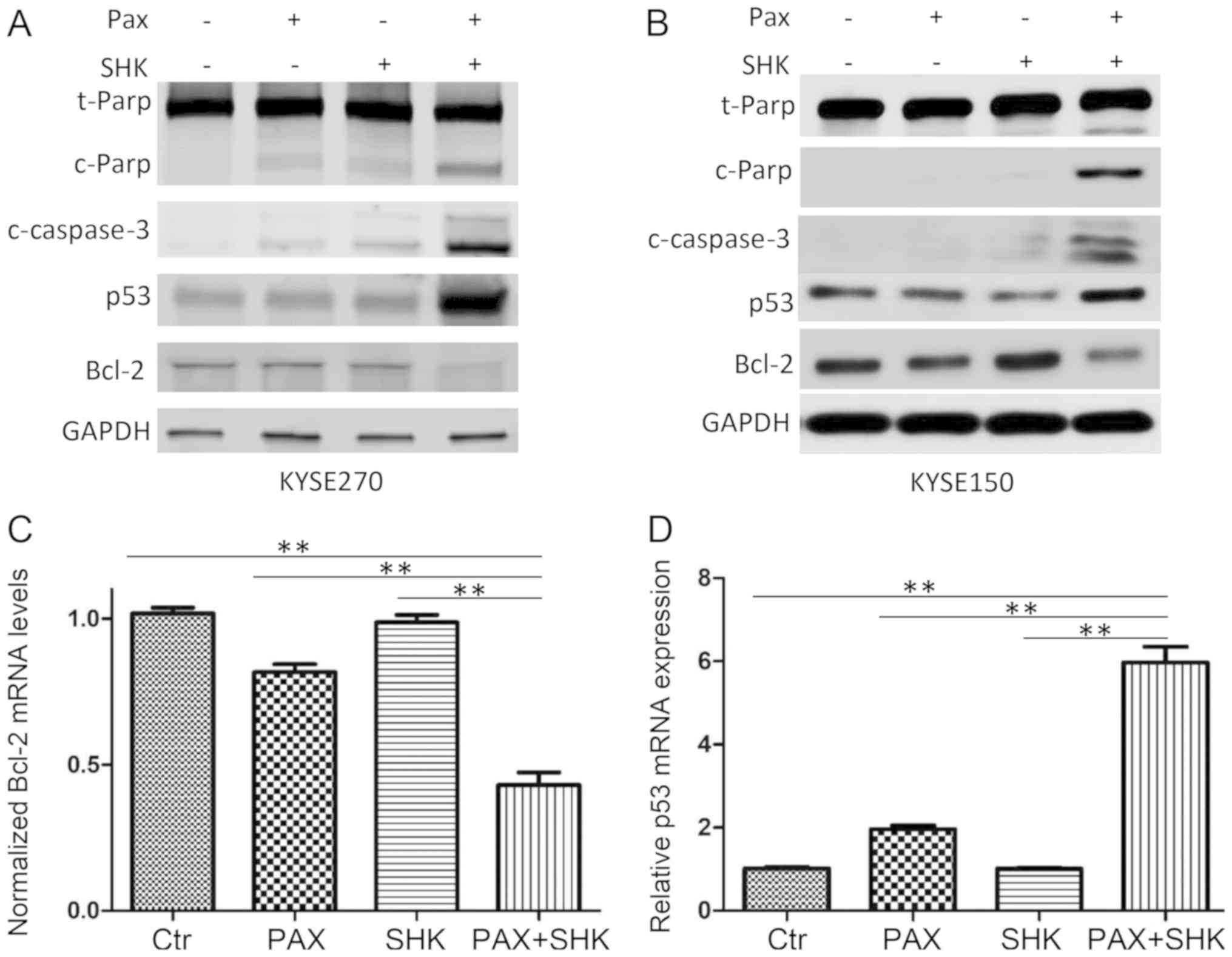
Caspases, particularly caspase-3, are
well-established markers of cell apoptosis (22). To further confirm cell apoptosis was
induced by various treatments, western blotting was performed to
detect cleaved caspase-3 and cleaved PARP. As presented in Fig. 4A and B, while single treatment with
paclitaxel only led to low cleaved caspase-3 and cleaved PARP
expression, combined shikonin and paclitaxel treatment markedly
enhanced expression levels of cleaved caspase-3 and cleaved
PARP.
To further evaluate the mechanism of shikonin
promotion of paclitaxel-induced apoptosis in ESCC cells, the
present study examined the changes in apoptosis-associated
molecules, including p53 and Bcl-2, at the mRNA and protein levels
following the indicated drug treatments. The results demonstrated
that compared with either shikonin or paclitaxel single treatment,
combined treatment significantly reduced Bcl-2 expression and
significantly increased p53 expression (P<0.001, Fig. 4A-D).
Discussion
The results of the present study demonstrated that
shikonin plus paclitaxel combination therapy enhanced the treatment
efficacy in ESCC cells. The addition of shikonin to paclitaxel
therapy enhanced paclitaxel drug cytotoxicity, which was
demonstrated by suppressed cell proliferation. In addition,
combined treatment increased cell mitotic arrest, and altered the
expression of p53 and Bcl-2, which further affected cell
apoptosis.
Paclitaxel is one of the most active agents
currently used for the clinical treatment of breast, ovarian, lung,
bladder, and head and neck cancer, and has been used for advanced
ESCC (23). However, the resistance
of cancer cells to paclitaxel is an important issue and can lead to
subsequent recurrence and metastasis of malignant tumors (24,25). The
synergy of chemotherapy drugs and shikonin can significantly
enhance the sensitivity of tumor cells (26). Previous studies have demonstrated
that the combination of Shikonin and docetaxel or taxotere can
enhance drug cytotoxicity in human breast cancer and ovarian
carcinoma (27,28). Consistently, the results of the
present study indicated that the combination of shikonin and
paclitaxel significantly improved effects on ESCC cells. In the
present study, MTT results demonstrated that the proliferation
inhibition of KYSE270 and KYSE150 cells following treatment with
shikonin and paclitaxel was significantly improved compared with
either drug alone. Sub-G1 and Annexin-V staining assays
demonstrated that paclitaxel could induce apoptosis of KYSE270 and
KYSE150 cells; however, the apoptosis rate increased significantly
when paclitaxel and shikonin were used in combination. These
results were similar to those presented by Yang et al
(29) regarding human osteosarcoma.
The current study demonstrates that paclitaxel combined with
shikonin can dramatically inhibit ESCC cell proliferation and
induce cell apoptosis, which supports the potential use of shikonin
in ESCC chemotherapy.
The Bcl-2 family consists of more than six
anti-apoptotic genes, including Bcl-2, and numerous pro-apoptotic
members that regulate cell death and survival (30,31). The
overexpression of Bcl-2 protects cancer cells from apoptosis
induced by a variety of anticancer agents (32–35).
Other studies have also reported that inhibiting or reducing Bcl-2
expression can sensitize cancer cells to chemotherapeutic agents.
For example, Bcl-2 inhibitors sensitize leukemia cells to vesicular
stomatitis virus oncolysis (36).
p53 is a nuclear protein that acts as a tumor suppressor (37). It has been implicated in multiple
cellular processes, including inhibition of proliferation (38), mediation of cell cycle arrest
(39) and apoptosis (40). Previous studies have demonstrated
that p53 can suppress Bcl-2 gene expression transcriptionally by
directly binding to the Bcl-2 promoter (41,42). In
the present study, while the treatment of shikonin or paclitaxel
had little influence on p53 and Bcl-2 expression levels, the
combined treatment significantly increased p53 expression, and
downregulated Bcl-2 expression, which revealed the role of p53 and
Bcl-2 in the mediation of shikonin and paclitaxel-induced
apoptosis.
In conclusion, the current study demonstrated that a
combination of shikonin and paclitaxel significantly improved
cancer cell growth inhibition, altered the expression of
apoptosis-associated molecules and enhanced cell apoptosis. The
present study indicates that this combination therapy may be
investigated as a potential treatment strategy for patients with
ESCC. In the future, it may be beneficial to establish primary
esophageal cancer cells from patient surgical tissue and perform
functional assays on patient derived cells. A patient derived tumor
xenograft experiment may also be performed to examine the efficacy
and toxicity of combined shikonin and paclitaxel. Finally, future
studies should investigate the effect of shikonin on radiation
sensitivity and chemo-radiosensitivity.
Acknowledgements
The authors would like to thank Dr Hui Zhang (Sun
Yat-Sen University Cancer Center, Guangzhou, China) for providing
the KYSE270 and KYSE150 cell lines used in the present study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL, WD and XH designed the research. WD, XH, ZY, YW
and XZ performed the experiments. JL wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y: Epidemiology of esophageal
cancer. World J Gastroenterol. 19:5598–5606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Law S and Wong J: Changing disease burden
and management issues for esophageal cancer in the Asia-Pacific
region. J Gastroenterol Hepatol. 17:374–381. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holmes RS and Vaughan TL: Epidemiology and
pathogenesis of esophageal cancer. Semin Radiat Oncol. 17:2–9.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stanton RA, Gernert KM, Nettles JH and
Aneja R: Drugs that target dynamic microtubules: A new molecular
perspective. Med Res Rev. 31:443–481. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yvon AM, Wadsworth P and Jordan MA: Taxol
suppresses dynamics of individual microtubules in living human
tumor cells. Mol Biol Cell. 10:947–959. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blagosklonny MV: Prolonged mitosis versus
tetraploid checkpoint: How p53 measures the duration of mitosis.
Cell Cycle. 5:971–975. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dumontet C and Jordan MA:
Microtubule-binding agents: A dynamic field of cancer therapeutics.
Nat Rev Drug Discov. 9:790–803. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ikui AE, Yang CP, Matsumoto T and Horwitz
SB: Low concentrations of taxol cause mitotic delay followed by
premature dissociation of p55CDC from Mad2 and BubR1 and abrogation
of the spindle checkpoint, leading to aneuploidy. Cell Cycle.
4:1385–1388. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Orr GA, Verdier-Pinard P, McDaid H and
Horwitz SB: Mechanisms of Taxol resistance related to microtubules.
Oncogene. 22:7280–7295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hou Y, Guo T, Wu C, He X and Zhao M:
Effect of shikonin on human breast cancer cells proliferation and
apoptosis in vitro. Yakugaku Zasshi. 126:1383–1386. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han W, Li L, Qiu S, Lu Q, Pan Q, Gu Y, Luo
J and Hu X: Shikonin circumvents cancer drug resistance by
induction of a necroptotic death. Mol Cancer Ther. 6:1641–1649.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang H, Zhou P, Huang H, Chen D, Ma N, Cui
QC, Shen S, Dong W, Zhang X, Lian W, et al: Shikonin exerts
antitumor activity via proteasome inhibition and cell death
induction in vitro and in vivo. Int J Cancer. 124:2450–2459. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang IC, Huang YJ, Chiang TI, Yeh CW and
Hsu LS: Shikonin induces apoptosis through reactive oxygen
species/extracellular signal-regulated kinase pathway in
osteosarcoma cells. Biol Pharm Bull. 33:816–824. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Long S, GuangZhi Y, BaoJie G, Wei X,
YanYong H, YingLi W, Yang Z and LiHua L: Shikonin derivatives
protect immune organs from damage and promote immune responses in
vivo in tumour-bearing mice. Phytother Res. 26:26–33. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao Q, Assimopoulou AN, Klauck SM,
Damianakos H, Chinou I, Kretschmer N, Rios JL, Papageorgiou VP,
Bauer R and Efferth T: Inhibition of c-MYC with involvement of
ERK/JNK/MAPK and AKT pathways as a novel mechanism for shikonin and
its derivatives in killing leukemia cells. Oncotarget.
6:38934–38951. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X and Sun G: Shikonin enhances
Adriamycin antitumor effects by inhibiting efflux pumps in A549
cells. Oncol Lett. 14:4270–4276. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song J, Zhao Z, Fan X, Chen M, Cheng X,
Zhang D, Wu F, Ying X and Ji J: Shikonin potentiates the effect of
arsenic trioxide against human hepatocellular carcinoma in vitro
and in vivo. Oncotarget. 7:70504–70515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mukhtar E, Adhami VM and Mukhtar H:
Targeting microtubules by natural agents for cancer therapy. Mol
Cancer Ther. 13:275–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Henley D, Isbill M, Fernando R, Foster JS
and Wimalasena J: Paclitaxel induced apoptosis in breast cancer
cells requires cell cycle transit but not Cdc2 activity. Cancer
Chemother Pharmacol. 59:235–249. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Januchowski R, Zawierucha P, Andrzejewska
M, Ruciński M and Zabel M: Microarray-based detection and
expression analysis of ABC and SLC transporters in drug-resistant
ovarian cancer cell lines. Biomed Pharmacother. 67:240–245. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun QL, Sha HF, Yang XH, Bao GL, Lu J and
Xie YY: Comparative proteomic analysis of paclitaxel sensitive A549
lung adenocarcinoma cell line and its resistant counterpart
A549-Taxol. J Cancer Res Clin Oncol. 137:521–532. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao Q, Kretschmer N, Bauer R and Efferth
T: Shikonin and its derivatives inhibit the epidermal growth factor
receptor signaling and synergistically kill glioblastoma cells in
combination with erlotinib. Int J Cancer. 137:1446–1456. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li W, Liu J, Jackson K, Shi R and Zhao Y:
Sensitizing the therapeutic efficacy of taxol with shikonin in
human breast cancer cells. PLoS One. 9:e940792014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Z, Yin J, Li M, Shen J, Xiao Z, Zhao
Y, Huang C, Zhang H, Zhang Z, Cho CH and Wu X: Combination of
shikonin with paclitaxel overcomes multidrug resistance in human
ovarian carcinoma cells in a P-gp-independent manner through
enhanced ROS generation. Chin Med. 14:72019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Q, Li S, Fu Z, Lin B, Zhou Z, Wang Z,
Hua Y and Cai Z: Shikonin promotes adriamycininduced apoptosis by
upregulating caspase-3 and caspase-8 in osteosarcoma. Mol Med Rep.
16:1347–1352. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aravind L, Dixit VM and Koonin EV:
Apoptotic molecular machinery: Vastly increased complexity in
vertebrates revealed by genome comparisons. Science. 291:1279–1284.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ogura T, Tanaka Y, Tamaki H and Harada M:
Docetaxel induces Bcl-2- and pro-apoptotic caspase-independent
death of human prostate cancer DU145 cells. Int J Oncol.
48:2330–2338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rong YP, Barr P, Yee VC and Distelhorst
CW: Targeting Bcl-2 based on the interaction of its BH4 domain with
the inositol 1,4,5-trisphosphate receptor. Biochim Biophys Acta.
1793:971–978. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Srivastava RK, Sasaki CY, Hardwick JM and
Longo DL: Bcl-2-mediated drug resistance: Inhibition of apoptosis
by blocking nuclear factor of activated T lymphocytes
(NFAT)-induced Fas ligand transcription. J Exp Med. 190:253–265.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang T, Xu F, Sheng Y, Zhang W and Chen Y:
A targeted proteomics approach to the quantitative analysis of
ERK/Bcl-2-mediated anti-apoptosis and multi-drug resistance in
breast cancer. Anal Bioanal Chem. 408:7491–7503. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Samuel S, Beljanski V, Van Grevenynghe J,
Richards S, Ben Yebdri F, He Z, Nichols C, Belgnaoui SM, Steel C,
Goulet ML, et al: BCL-2 inhibitors sensitize therapy-resistant
chronic lymphocytic leukemia cells to VSV oncolysis. Mol Ther.
21:1413–1423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Smith ND, Rubenstein JN, Eggener SE and
Kozlowski JM: The p53 tumor suppressor gene and nuclear protein:
Basic science review and relevance in the management of bladder
cancer. J Urol. 169:1219–1228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xiong Y, Hannon GJ, Zhang H, Casso D,
Kobayashi R and Beach D: p21 is a universal inhibitor of cyclin
kinases. Nature. 366:701–704. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yew PR and Berk AJ: Inhibition of p53
transactivation required for transformation by adenovirus early 1B
protein. Nature. 357:82–85. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yonish-Rouach E, Deguin V, Zaitchouk T,
Breugnot C, Mishal Z, Jenkins JR and May E: Transcriptional
activation plays a role in the induction of apoptosis by
transiently transfected wild-type p53. Oncogene. 11:2197–2205.
1995.PubMed/NCBI
|
|
41
|
Wu Y, Mehew JW, Heckman CA, Arcinas M and
Boxer LM: Negative regulation of bcl-2 expression by p53 in
hematopoietic cells. Oncogene. 20:240–251. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
May P and May E: Twenty years of p53
research: structural and functional aspects of the p53 protein.
Oncogene. 18:7621–7636. 1999. View Article : Google Scholar : PubMed/NCBI
|