Introduction
Prostate cancer (PCa) was the most commonly
diagnosed and third most fatal cancer among males in 2008 in the
developing world (1).
Prostate-specific antigen (PSA) is widely used as a tumor marker
and aids the diagnosis of PCa at an early stage (2). The most common curative treatment for
localized PCa is radical prostatectomy (RP) (3), with robot-assisted radical
prostatectomy (RARP) becoming a widely adopted procedure. According
to a number of previous studies, RARP improves perioperative and
functional outcomes, and at least comparable oncologic outcomes
compared with open RP in the localized PCa (4–8). Pound
et al (9) contributed in the
establishment of the natural history of high-risk PCa in
surgically-treated cases. After a median of 8 years, Pound et
al (9) identified biochemical
recurrence (BCR) in 15% of cases and reported the development of
metastatic disease in 34% of the cohort. In survival analysis, time
to biochemical progression, the Gleason score (GS) (10) and PSA doubling time are predictive
factors of the probability and time to develop metastatic disease
(9). Boorjian et al (11) reported that the risks of BCR and
cancer-specific mortality are 3.3 and 11.5 times greater,
respectively, in cases with high-risk PCa compared with cases of
low-risk PCa. Therefore, high-risk localized PCa cases have been
formerly characterized as having an increased risk of metastasis
and requiring complex treatments, such as surgery (12). However, a number of previous studies
support surgery as monotherapy for high-risk localized PCa cases
and have revealed optimal outcomes (13,14).
In the RARP procedure, the excision of the
neurovascular bundle (NVB) is often performed in patients with
intermediate- or high-risk PCa to reduce the probability of a
positive surgical margin (PSM) (15). When performing nerve sparing (NS)
RARP, there should be a number of cases who obtain successful
oncological and functional outcomes, since the ‘high-risk’ group is
notably heterogenous (16).
The present study selected high-risk PCa cases based
on original criteria. The patients underwent RARP with NS, to
evaluate the feasibility, oncologic safety as compared with non-NS
in the intermediate-term and functional outcomes.
Materials and methods
Study design
A total of 767 male cases received RARP at Fujita
Health University Hospital (Toyoake, Japan) between August 2009 and
December 2016. Median age was 66 years old and the range was from
45 to 88 years. Among the 767 cases screened for the present study,
230 high-risk PCa cases who were observed for >6 months
comprised the study cohort for retrospective analysis. The mean age
for the high-risk cohort is provided in Table I. All cases had non-metastatic and
clinically high-risk PCa, as defined according to the D'Amico risk
stratification system (17), and
exhibited at least one of the following: i) A serum PSA level of
>20 ng/ml (measured using a American Cancer Society-PSA kit
(Ciba Corning Diagnostics Corp.) with chemiluminescent immunoassay,
according to the manufacturer's instructions; ii) GS ≥8; or iii)
clinical stage ≥T2c. TNM classification was defined using the
American Joint Committee on Cancer staging manual (18). The following clinical variables were
evaluated: Age, serum PSA level (ng/ml), clinical T stage, GS and
neoadjuvant treatment. The criteria for NS RARP were: Bilateral NS,
at least two factors (PSA <10 ng/ml, cT1c, <GS 7, <30% of
positive-core ratio on the NS side); unilateral NS, <cT2b or
<30% positive-core ratio on the NS side; non-NS, other than the
aforementioned criteria. All patients received pelvic lymph node
dissection. Surgery time, estimated blood loss (EBL), console time,
pathological stage (pT stage), positive lymph node metastases [pN
(+)], and surgical margin positivity were recorded to assess
perioperative parameters. The schedule after RARP surgery consisted
of a PSA assay every 3 months for the first 2 years, every 6 months
for the following 3 years and annually thereafter. The number of
pads used daily, at 3 months and 6 months after RARP, was checked
to assess urinary continence recovery. The onset of BCR was defined
as the date when the serum PSA level was >0.2 ng/ml. The time to
events was calculated from the day of RARP.
 | Table I.Clinical characteristics of NS and
non-NS robot-assisted radical prostatectomy cases. |
Table I.
Clinical characteristics of NS and
non-NS robot-assisted radical prostatectomy cases.
| Baseline patient
characteristics | NS cohort (n=133), n
(range or %) | non-NS cohort (n=97),
n (range or %) | P-value |
|---|
| Mean age, years | 64.6 (45–76) | 67.1 (49–77) | <0.01a |
| Mean serum PSA level,
ng/ml | 10.2 (1.6–57.1) | 14.5 (3.9–158.3) | 0.01a |
| T stage |
| cT1c | 12 (9.0) | 1 (1.0) |
<0.01a |
|
cT2a | 31 (23.3) | 7 (7.2) |
|
|
cT2b | 37 (27.8) | 9 (9.3) |
|
|
cT2c | 46 (34.6) | 65 (67.0) |
|
|
cT3a | 7 (5.3) | 14 (14.4) |
|
|
cT3b | 0 (0.0) | 1 (1.0) |
|
| Gleason score |
| 6 | 12 (9.0) | 15 (15.5) |
<0.01a |
| 7 | 43 (32.3) | 51 (52.6) |
|
| 8 | 55 (41.4) | 17 (17.5) |
|
| 9 | 23 (17.3) | 12 (12.4) |
|
| 10 | 0 (0) | 2 (2.1) |
|
| Neoadjuvant
treatment |
|
Anti-androgen monotherapy | 46 (34.6) | 29 (29.9) | 0.10 |
| LHRH
agonist alone | 5 (3.8) | 4 (4.1) |
|
|
Combined androgen
blockade | 11 (8.3) | 16 (16.5) |
|
|
Others | 3 (2.3) | 7 (7.2) |
|
|
None | 68 (51.1) | 41 (42.3) |
|
The protocol of the present study was approved by
the Ethics Committee of Fujita Health University Hospital (approval
no. HM 18-115), and the present study was performed in accordance
with the ethical standards laid down in the most recent version of
the Declaration of Helsinki.
Statistical analysis
All values are presented as the mean ± standard
deviation, and statistical comparison of the results was performed
using a Student's t-test, a Mann-Whitney test, the χ2
test or Fisher's exact test. BCR-free survival was estimated using
the Kaplan-Meier method, and a log-rank test was used to compare
the survival curves. To assess prognostic factors, univariate
analysis was performed using the following variables: Age, initial
PSA, cT stage, GS, NS, neoadjuvant hormonal therapy (NHT) and
resection margin. Significant preoperative variables in the
univariate analysis were included in the multivariate analysis
using a Cox proportional hazards regression model. P<0.05 was
considered to indicate a statistically significant difference. All
data were analyzed using IBM SPSS Statistics version 23 (IBM
Corp.).
Results
Clinical characteristics of cases
Out of the 230 high-risk cases, 133 underwent RARP
with NS, while 97 underwent RARP without NS (Table I). The mean age in the NS and non-NS
cohorts was 64.6 and 67.1 years, respectively. The serum PSA level
in the NS and non-NS cohorts was 10.2 and 14.5 ng/ml, respectively.
The clinical stage with the highest number of cases, in each
cohort, was T2c. GSs of 8 in the NS cohort and 7 in the non-NS
cohort were observed most frequently. For neoadjuvant treatment, 68
cases (51.1%) in the NS cohort and 41 cases (42.3%) in the non-NS
cohort did not receive NHT. Among the factors of age, PSA level, T
stage, GS and NHT, only NHT exhibited no significant difference
between the two cohorts (P=0.102).
Perioperative parameters
The mean operation times in the NS and non-NS
cohorts were 171 and 179 min, respectively. The mean console times
in the NS and non-NS cohorts were 131 and 137 min, respectively.
The mean EBL was 177 ml in the NS cohort and 171 ml in the non-NS
cohort. There were no significant differences identified for these
three factors between cohorts (operation time, P=0.189; console
tine, P=0.259; EBL, P=0.697; Fig.
1).
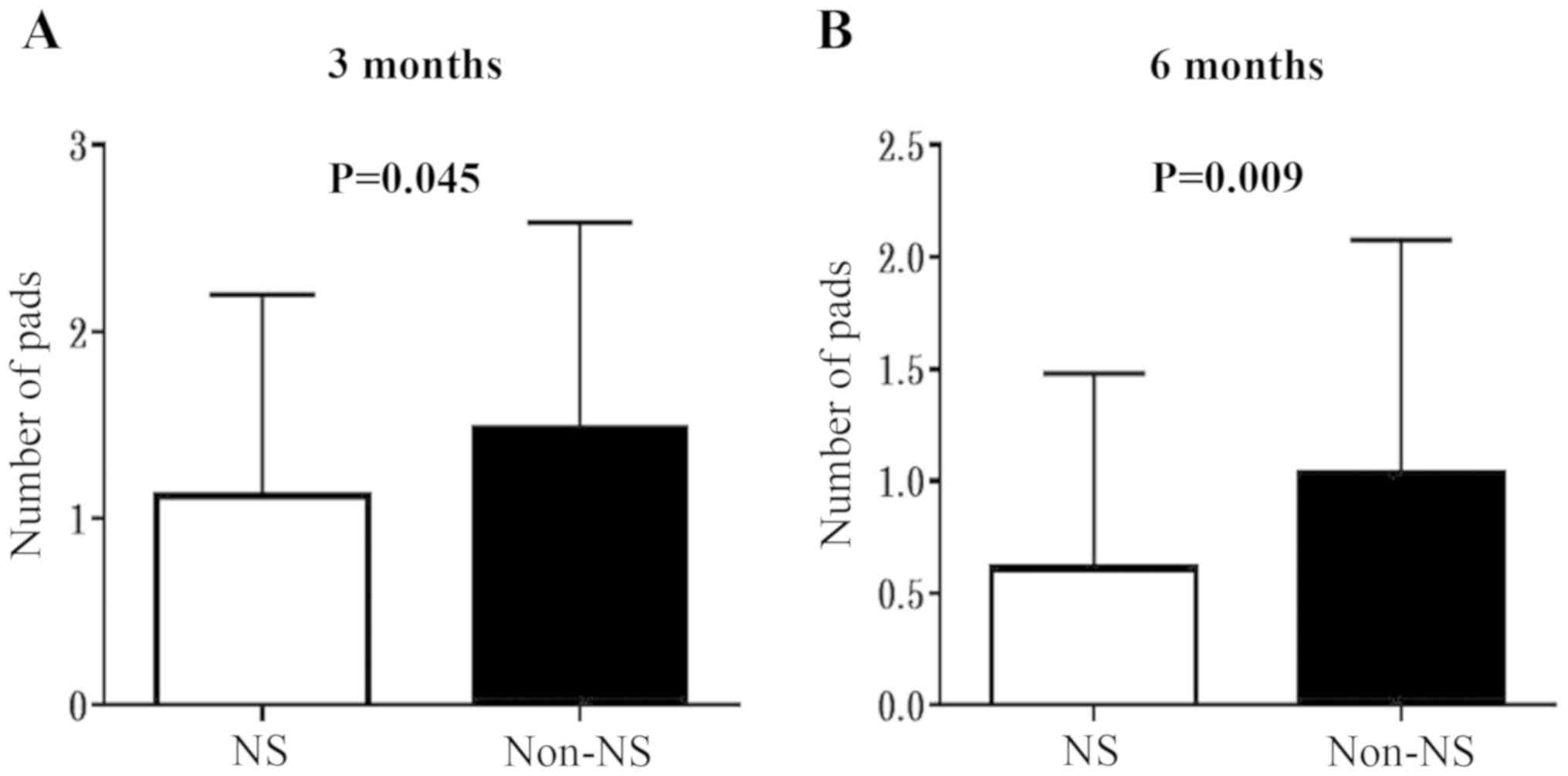
Urinary continence recovery
In the present study, urinary continence recovery
was assessed by evaluating the quantity of pads used daily at 3 and
6 months after RARP. The mean ± standard deviation quantity of pads
used daily at 3 months in the NS/non-NS cohorts was
1.12±1.08/1.48±1.11, and that at 6 months was 0.61±0.87/1.03±1.05
(Fig. 2). As expected, the NS
procedure resulted in significantly improved outcomes regarding
urinary continence.
Oncological findings
Oncological findings, including pT stage, PSM, pN
(+) and BCR, are shown in Table II.
pT stage T2c was most frequent in the NS and non-NS cohorts. The
overall PSM rate was 22.6% (Fig.
1D), with rates of 18.0% in the NS group and 28.9% in the
non-NS group. No significant difference in the PSM rates between
cohorts was observed (P=0.053). The PSM rates in the NHT/non-NHT
cohorts were 24.8 and 20.2%, respectively, with no significant
differences identified between them (P=0.406). For pN (+), only one
case was observed in each cohort. The BCR rates in the NS and
non-NS cohorts were 23.3 and 19.6%, respectively, with no
significant difference identified between them (P=0.501).
 | Table II.Oncological findings of NS and non-NS
robot-assisted radical prostatectomy cases. |
Table II.
Oncological findings of NS and non-NS
robot-assisted radical prostatectomy cases.
| Oncological
findings | NS cohort (n=133),
n (%) | Non-NS cohort
(n=97), n (%) | P-value |
|---|
| Pathological
stage |
|
pT0 | 2 (1.5) | 2 (2.1) | 0.358 |
|
pT2a | 23 (17.3) | 9 (9.3) |
|
|
pT2b | 12 (9.0) | 5 (5.2) |
|
|
pT2c | 69 (51.9) | 61 (62.9) |
|
|
pT3a | 15 (11.3) | 11 (11.3) |
|
|
pT3b | 12 (9.0) | 8 (8.2) |
|
|
pT4 | 0 (0.0) | 1 (1.0) |
|
| Positive surgical
margin | 24 (18.0) | 28 (28.9) | 0.053 |
| pN(+) | 1 (0.8) | 1 (1.0) | 0.823 |
| Biochemical
recurrence | 31 (23.3) | 19 (19.6) | 0.501 |
BCR-free survival rates
BCR-free survival rates in the NS and NHT categories
are indicated in Figs. S1 and
S2. A total of 16 cases (7.0%) were
observed with PSA ≥0.2 ng/ml at the first postoperative
measurement. The BCR-free survival rates at 3 years after RARP in
the NS and non-NS cohorts were 72.7 and 75.0%, respectively
(Fig. S1). When BCR-free survival
rates within the NS and NHT categories were compared, no
significant differences were observed (NS, P=0.6572; NHT, P=0.0812;
Figs. S1 and S2). These results suggest that the NS and
NHT treatments did not affect cancer control in D'Amico high-risk
PCa cases.
Cox regression analysis for time to
BCR
When risk parameters in high-risk PCa cases were
compared, the factors of age, initial PSA, GS 7, and resection
margin exhibited significant differences. In multivariate analysis,
the factors of age, initial PSA, GS 8–10, and resection margin were
associated with time to BCR, whereas the factors of cT stage, NS
and NHT were not associated with time to BCR (Table III). These results suggest that NS
and NHT did not affect BCR-free survival following RARP in
high-risk PCa cases.
 | Table III.Cox regression analysis for time to
biochemical recurrence. |
Table III.
Cox regression analysis for time to
biochemical recurrence.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 0.95
(0.91–0.99) | 0.019a | 0.95
(0.91–0.99) | 0.024a |
| Initial PSA | 1.02
(1.01–1.03) | 0.002a | 1.01
(1.00–1.03) | 0.042a |
| cT stage |
| T1c,
T2a, T2b |
|
T2c | 0.71
(0.29–1.75) | 0.462 |
|
|
| T3a,
T3b | 0.48
(0.19–1.21) | 0.120 |
|
|
| Gleason score |
| 6 |
| 7 | 0.23
(0.05–0.94) | 0.041a | 0.25
(0.06–1.03) | 0.055 |
|
8–10 | 0.58
(0.32–1.06) | 0.077 | 0.50
(0.27–0.92) | 0.025a |
| Nerve sparing |
| No |
|
Yes | 0.93
(0.52–1.64) | 0.789 |
|
|
| Neoadjuvant
hormonal therapy |
| No |
|
Yes | 0.59
(0.33–1.04) | 0.068 |
|
|
| Resection
margin |
|
None |
|
Positive | 0.43
(0.25–0.77) | 0.004a | 0.43
(0.24–0.78) | 0.006a |
Discussion
Walsh (19) was the
first to demonstrate that NVBs run posterolateral to the prostate
between two layers of lateral pelvic fascia, the prostatic fascia
medially and levator fascia laterally, in an intraoperative study.
The effect of the preservation of the NVBs during RP on erectile
function is evident (20); however,
its influence on urinary continence remains unclear. According to a
recent cohort study, the NS technique is not associated with worse
cancer outcomes but is associated with improved urinary and
erectile function (21).
Additionally, Michl et al (22) indicated that the meticulous apical
dissection associated with the NS technique rather than the
preservation of the NVBs, can impart a positive impact on long-term
urinary continence rates.
RP with NS is challenging for D'Amico-classified
high-risk PCa cases, as such cases are more likely to have
‘non-organ-confined disease’, which possibly leads to BCR (23). However, Shikanov et al
(24) reported that even PCa cases
whose preoperative biopsy GS is 8 had organ-confined (pT2N0)
disease in 47% of this population. These findings indicate that the
‘high-risk’ group is heterogeneous, and it is important to select
cases in the high-risk group when performing RP with NS. The
criteria of RP with NS for high-risk PCa cases are unclear. In the
present study, the criteria for NS RARP were: Bilateral NS, at
least two factors (PSA <10 ng/ml, cT1c, <GS 7, <30% of
positive-core ratio on the NS side); unilateral NS, <cT2b or
<30% positive-core ratio on the NS side and non-NS, other than
the aforementioned criteria. However, a recent study has reported
their criteria as follows: Complete, non-palpable disease with
<3 cores involved on the prostate biopsy; partial, non-palpable
disease with <4 cores involved on the prostate biopsy; and none,
clinically palpable disease with ≥4 cores involved on the prostate
biopsy and intraoperative visual cues of locally advanced disease
(25).
In the present study, there were no significant
differences between the perioperative parameters of operation time,
EBL, console time and PSM rates in the NS and non-NS cohorts of
high-risk cases. Yossepowitch et al (15) reported that the long-term impact on
survival of patients with PCa after radical prostatectomy is
variable and largely affected by risk modifiers other than surgical
margin positivity; however, it is still considered as an adverse
oncological outcome. The overall PSM rate in the present study was
22.6%, whereas PSM rates have been reported as 35% (12–53%)
following RARP in high-risk PCa cases in previous studies (24,26,27). The
present study demonstrated that the factor of PSM was important for
time to BCR regardless of the NS technique in RARP in high-risk
cases. A number of studies have reported significant positive
associations between NS and surgical margin positivity; however,
other studies have not identified them (6,7,15). As a result, the association between
NS and surgical margin positivity remains controversial.
In the context of NHT treatment, a number of
previous studies have reported favorable BCR-free survival in
high-risk PCa cases treated with a neoadjuvant
gonadotropin-releasing hormone agonist or antagonist, and
estramustine phosphate followed by RP surgery (28,29).
However, in the present study, NHT treatment did not affect
BCR-free survival rates in D'Amico high-risk PCa cases.
The role of NS during RARP in high-risk PCa cases
has been reported in only a few previous studies (30,31).
Kumar et al (25) reported
that the overall BCR rate, at a mean follow-up of 24.3 months, was
19.2% and the mean time to BCR was 7.9 months in high-risk PCa
cases, which is comparable to other previous studies (24,26,27,32,33).
Kumar et al (25) performed
RARP with NS in 89.4% of cases of high-risk PCa without
compromising the PSM/BCR rate, while also providing improved
postoperative continence and potency outcomes, using preoperative
clinical variables along with intraoperative visual cues as a guide
for NS. Consistent with these results, the present study reported
that NS RARP in high-risk PCa resulted in equivalent oncological
outcomes compared with non-NS RARP according to the estimation of
BCR-free survival rates. Additionally, we demonstrated that NS and
NHT did not affect BCR-free survival following RARP of high-risk
PCa cases.
Limitations of the present study included the
retrospective collection, its small sample size of a single center,
a lack of well-designed analyses and short follow-up duration.
Therefore, further studies are required to demonstrate the clinical
utility of RARP with NS in high-risk PCa cases in the future.
In conclusion, NS RARP could provide
intermediate-term oncological safety and successful functional
outcomes in selected high-risk PCa cases based on the original
criteria used in the present study. However, rigorous selection is
required when performing NS RARP in high-risk PCa cases.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KT, MS, HS, MK and RS conceived and designed the
study. KT, KF, TJ, MN, MH, KZ, TN, MI and NF acquired the data. KT,
MS, KZ and TN drafted the manuscript. KT, MS, KZ and MK performed
the statistical analysis. RS supervised the study.
Ethics approval and consent to
participate
The protocol of the present study was approved by
the Ethics Committee of Fujita Health University Hospital (approval
no. HM 18-115), and the present study was performed in accordance
with the ethical standards laid down in the most recent version of
the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reed AB and Parekh DJ: Biomarkers for
prostate cancer detection. Expert Rev Anticancer Ther. 10:103–114.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bill-Axelson A, Holmberg L, Garmo H, Rider
JR, Taari K, Busch C, Nordling S, Häggman M, Andersson SO,
Spångberg A, et al: Radical prostatectomy or watchful waiting in
early prostate cancer. N Engl J Med. 370:932–942. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coelho RF, Rocco B, Patel MB, Orvieto MA,
Chauhan S, Ficarra V, Melegari S, Palmer KJ and Patel VR:
Retropubic, laparoscopic, and robot-assisted radical prostatectomy:
A critical review of outcomes reported by high-volume centers. J
Endourol. 24:2003–2015. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ficarra V, Borghesi M, Suardi N, De Naeyer
G, Novara G, Schatteman P, De Groote R, Carpentier P and Mottrie A:
Long-term evaluation of survival, continence and potency (SCP)
outcomes after robot-assisted radical prostatectomy (RARP). BJU
Int. 112:338–345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ficarra V, Cavalleri S, Novara G, Aragona
M and Artibani W: Evidence from robot-assisted laparoscopic radical
prostatectomy: A systematic review. Eur Urol. 51:45–56. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ficarra V, Novara G, Artibani W, Cestari
A, Galfano A, Graefen M, Guazzoni G, Guillonneau B, Menon M,
Montorsi F, et al: Retropubic, laparoscopic and robot-assisted
radical prostatectomy: A systematic review and cumulative analysis
of comparative studies. Eur Urol. 55:1037–1063. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Srivastava A, Chopra S, Pham A,
Sooriakumaran P, Durand M, Chughtai B, Gruschow S, Peyser A,
Harneja N, Leung R, et al: Effect of a risk-stratified grade of
nerve-sparing technique on early return of continence after
robot-assisted laparoscopic radical prostatectomy. Eur Urol.
63:438–444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pound CR, Partin AW, Eisenberger MA, Chan
DW, Pearson JD and Walsh PC: Natural history of progression after
PSA elevation following radical prostatectomy. JAMA. 281:1591–1597.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gleason DF and Mellinger GT: Prediction of
prognosis for prostatic adenocarcinoma by combined histological
grading and clinical staging. J Urol. 111:58–64. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boorjian SA, Karnes RJ, Rangel LJ,
Bergstralh EJ and Blute ML: Mayo Clinic validation of the D'amico
risk group classification for predicting survival following radical
prostatectomy. J Urol. 179:1354–1361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bastian PJ, Boorjian SA, Bossi A, Briganti
A, Heidenreich A, Freedland SJ, Montorsi F, Roach M III, Schröder
F, van Poppel H, et al: High-risk prostate cancer: From definition
to contemporary management. Eur Urol. 61:1096–1106. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cooperberg MR, Vickers AJ, Broering JM and
Carroll PR: Comparative risk-adjusted mortality outcomes after
primary surgery, radiotherapy, or androgen-deprivation therapy for
localized prostate cancer. Cancer. 116:5226–5234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Petrelli F, Vavassori I, Coinu A,
Borgonovo K, Sarti E and Barni S: Radical prostatectomy or
radiotherapy in high-risk prostate cancer: A systematic review and
metaanalysis. Clin Genitourin Cancer. 12:215–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yossepowitch O, Briganti A, Eastham JA,
Epstein J, Graefen M, Montironi R and Touijer K: Positive surgical
margins after radical prostatectomy: A systematic review and
contemporary update. Eur Urol. 65:303–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mossanen M, Nepple KG, Grubb RL III,
Androile GL, Kallogjeri D, Klein EA, Stephenson AJ and Kibel AS:
Heterogeneity in definitions of high-risk prostate cancer and
varying impact on mortality rates after radical prostatectomy. Eur
Urol Oncol. 1:143–148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
D'Amico AV, Whittington R, Malkowicz SB,
Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA,
Kaplan I, Beard CJ and Wein A: Biochemical outcome after radical
prostatectomy, external beam radiation therapy, or interstitial
radiation therapy for clinically localized prostate cancer. JAMA.
280:969–974. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC Cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Walsh PC: Anatomic radical prostatectomy:
Evolution of the surgical technique. J Urol. 160:2418–2424. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Castiglione F, Ralph DJ and Muneer A:
Surgical techniques for managing post-prostatectomy erectile
dysfunction. Curr Urol Rep. 18:902017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nguyen LN, Head L, Witiuk K, Punjani N,
Mallick R, Cnossen S, Fergusson DA, Cagiannos I, Lavallée LT,
Morash C and Breau RH: The risks and benefits of cavernous
neurovascular bundle sparing during radical prostatectomy: A
systematic review and meta-analysis. J Urol. 198:760–769. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Michl U, Tennstedt P, Feldmeier L, Mandel
P, Oh SJ, Ahyai S, Budäus L, Chun FKH, Haese A, Heinzer H, et al:
Nerve-sparing surgery technique, not the preservation of the
neurovascular bundles, leads to improved long-term continence rates
after radical prostatectomy. Eur Urol. 69:584–589. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
D'Amico AV, Whittington R, Malkowicz SB,
Fondurulia J, Chen MH, Kaplan I, Beard CJ, Tomaszewski JE, Renshaw
AA, Wein A and Coleman CN: Pretreatment nomogram for
prostate-specific antigen recurrence after radical prostatectomy or
external-beam radiation therapy for clinically localized prostate
cancer. J Clin Oncol. 17:168–172. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shikanov SA, Thong A, Gofrit ON, Zagaja
GP, Steinberg GD, Shalhav AL and Zorn KC: Robotic laparoscopic
radical prostatectomy for biopsy Gleason 8 to 10: Prediction of
favorable pathologic outcome with preoperative parameters. J
Endourol. 22:1477–1481. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kumar A, Samavedi S, Bates AS, Mouraviev
V, Coelho RF, Rocco B and Patel VR: Safety of selective nerve
sparing in high risk prostate cancer during robot-assisted radical
prostatectomy. J Robot Surg. 11:129–138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yuh B, Artibani W, Heidenreich A, Kimm S,
Menon M, Novara G, Tewari A, Touijer K, Wilson T, Zorn KC and
Eggener SE: The role of robot-assisted radical prostatectomy and
pelvic lymph node dissection in the management of high-risk
prostate cancer: A systematic review. Eur Urol. 65:918–927. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuh BE, Ruel NH, Mejia R, Wilson CM and
Wilson TG: Robotic extended pelvic lymphadenectomy for
intermediate- and high-risk prostate cancer. Eur Urol.
61:1004–1010. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fujita N, Koie T, Ohyama C, Tanaka Y, Soma
O, Matsumoto T, Yamamoto H, Imai A, Tobisawa Y, Yoneyama T, et al:
Overall survival of high-risk prostate cancer patients who received
neoadjuvant chemohormonal therapy followed by radical prostatectomy
at a single institution. Int J Clin Oncol. 22:1087–1093. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koie T, Mitsuzuka K, Yoneyama T, Narita S,
Kawamura S, Kaiho Y, Tsuchiya N, Tochigi T, Habuchi T, Arai Y, et
al: Neoadjuvant luteinizing-hormone-releasing hormone agonist plus
low-dose estramustine phosphate improves prostate-specific
antigen-free survival in high-risk prostate cancer patients: A
propensity score-matched analysis. Int J Clin Oncol. 20:1018–1025.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Casey JT, Meeks JJ, Greco KA, Wu SD and
Nadler RB: Outcomes of locally advanced (T3 or greater) prostate
cancer in men undergoing robot-assisted laparoscopic prostatectomy.
J Endourol. 23:1519–1522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lavery HJ, Nabizada-Pace F, Carlucci JR,
Brajtbord JS and Samadi DB: Nerve-sparing robotic prostatectomy in
preoperatively high-risk patients is safe and efficacious. Urol
Oncol. 30:26–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Connolly SS, Cathcart PJ, Gilmore P,
Kerger M, Crowe H, Peters JS, Murphy DG and Costello AJ: Robotic
radical prostatectomy as the initial step in multimodal therapy for
men with high-risk localised prostate cancer: Initial experience of
160 men. BJU Int. 109:752–759. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jayram G, Decastro GJ, Large MC, Razmaria
A, Zagaja GP, Shalhav AL and Brendler CB: Robotic radical
prostatectomy in patients with high-risk disease: A review of
short-term outcomes from a high-volume center. J Endourol.
25:455–457. 2011. View Article : Google Scholar : PubMed/NCBI
|