Introduction
Accounting for 15% of breast cancer cases worldwide,
triple-negative breast cancer (TNBC) is an aggressive subtype of
breast cancer, which is characterized by the lack of estrogen
receptor, progesterone receptor and human epidermal growth factor
receptor 2 (HER2) (1). Despite high
sensitivity towards chemotherapy, the overall survival rate of
patients with TNBC remains poor due to the frequently occurring
relapse (2).
Gemcitabine is a chemotherapy agent, which is
derived from deoxycytidine, and is commonly used for the treatment
of patients with breast cancer (3).
Gemcitabine requires intracellular transport and induces cell cycle
arrest via incorporating into DNA or inhibition of ribonucleotide
reductase (4). Although patients
respond to gemcitabine at the beginning of treatment, numerous
patients eventually develop secondary resistance, which may result
in patient mortality (5). Thus,
further investigation regarding the molecular mechanism of
gemcitabine resistance would facilitate the development of novel
therapeutic approaches and improve patient outcomes.
microRNAs (miRNAs) are small, non-coding, single
strand RNAs with a length of ~20 nucleotides (6). Through binding to 3′ untranslated
region (UTR) of target gene mRNAs, miRNAs directly inhibit gene
expression at the post-transcriptional level (7). Deregulation of numerous miRNAs has been
determined to be associated with the chemotherapy resistance of
breast cancer (8). For example,
miR-105 and miR-93-3p can target secreted frizzle related protein 1
to activate Wnt signaling, which leads to chemoresistance in TNBC
cells (9). miR-873 was reported to
be downregulated in numerous cancer types, including breast cancer
(10,11). Previous studies indicated that
miR-873 may function as a sensitizer of cancer cells towards
chemotherapeutic agents (12,13).
However, whether and how miR-873 influences chemosensitivity in
TNBC remains unclear.
Zinc finger E-box binding homeobox 1 (ZEB1) is a
transcription factor that is well-known for its role as an
epithelial-mesenchymal transition (EMT) inducer (14). Through the induction of EMT, ZEB1
promotes cancer cell dissociation, resulting in metastasis and poor
prognosis of patients with cancer (15,16).
Upregulation of ZEB1 was identified in TNBC, compared with other
breast cancer subtypes (17).
Notably, ZEB1 elevation was detected in circulating breast cancer
cells, which were characterized with cancer stem cell traits
including self-renewal and strong metastatic potential (18). Mechanistically, ZEB1 coordinated with
Hippo-pathway effector Yes associated protein (YAP) to maintain
cell stemness and promote metastasis (19). Via maintenance of cancer cell
stemness, ZEB1 has been identified to contribute to the development
of cancer cell chemoresistance (14).
In the present study, the role of miR-873 in the
regulation of chemosensitivity in TNBC was demonstrated, and it was
revealed that miR-873 downregulation led to gemcitabine resistance
of TNBC cells via regulation of ZEB1. The results depicted a novel
role of miR-873 in mediating gemcitabine sensitivity in TNBC cells,
indicating that the expression of miR-873 may serve as a predictor
for gemcitabine sensitivity of patients with TNBC.
Materials and methods
Cell culture and agents
The human kidney cell line 293 and human TNBC cell
lines (MDA-MB-231 and BT549) were purchased from American Type
Culture Collection (Manassas, MA, USA). All cell lines were
cultured in Dulbecco's minimum essential medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Hyclone; GE Healthcare Life Sciences,
Logan UT, USA) in an incubator containing 5% CO2 at 37°C.
The gemcitabine resistant MDA-MB-231 cell line
(MDA-MB-231GEMr) was generated by continuous exposure of MDA-MB-231
to increasing concentrations (0.1–15 nM) of gemcitabine
(Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) for 12 months at
37°C, according to the method of a precious report (20). The MDA-MB-231GEMr cells were cultured
in DMEM with 10% FBS and 15 nM gemcitabine, and incubated in an
incubator with 5% CO2 at 37°C prior to experiments. Prior to
additional experiments, 1×106 MDA-MB-231GEMr cell passage in DMEM
without gemcitabine was conducted twice.
Overexpression and downregulation of
miR-873
miR-873 mimics, miR-negative control (miR-NC)
mimics, miR-873 inhibitor and miR-NC inhibitor were obtained from
Chang Jing Bio-Tech, Ltd. (Changsha, China). For overexpression or
downregulation of miR-873, 100 nM miR-873 mimic or miR-873
inhibitor was transfected into MDA-MB-231 or BT549 cells with
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to manufacturer's protocol. Following
48 h, the cells were subjected to the further experiments. The
sequences are listed in Table I.
 | Table I.Sequences of miR mimics and miR
inhibitors. |
Table I.
Sequences of miR mimics and miR
inhibitors.
| Name | Sequence |
|---|
| miR-873 mimic |
5′-GCAGGAACUUGUGAGUCUCCUTT-3′ |
| miR-NC mimic |
5′-UCGCUUGGUGCAGGUCGGGAATT-3′ |
| miR-873
inhibitor |
5′-AGGAGACUCACAAGUUCCUGCTT-3′ |
| miR-NC inhibitor |
5′-UUCUCCGAACGUGUCACGUTT-3′ |
Silencing of ZEB1 in TNBC cells
Control small interfering RNA (siRNA), ZEB1 siRNA1
and ZEB1 siRNA2 were obtained from Chang Jing Bio-Tech, Ltd.. For
silencing of ZEB1 expression, 50 nM ZEB1 siRNA1 or ZEB1 siRNA2 were
transfected into MDA-MB-231GEMr cells using
Lipofectamine® RNAiMax (Invitrogen; Thermo Fisher
Scientific). Following 48 h, the cells were subjected to further
experiments. The sequences are listed in Table II.
 | Table II.Sequences of control siRNA and ZEB
siRNAs. |
Table II.
Sequences of control siRNA and ZEB
siRNAs.
| Name | Sequence |
|---|
| Control siRNA |
5′-UUCUCCGAACGUGUCACGUTT-3′ |
| ZEB1 siRNA1 |
5′-GUCGCUACAAACAGUUGUATT-3′ |
| ZEB1 siRNA2 |
5′-CCUAGUCAGCCACCUUUAATT-3′ |
Western blot analysis
Antibodies for E-cadherin (catalog no. 14472;
1:2,000), AXL receptor tyrosine kinase (AXL; catalog no. 8661;
1:2,000), connective tissue growth factor (CTGF; catalog no. 86641;
1:2,000), cysteine rich angiogenic inducer 61 (CYR61; catalog no.
14479; 1:2,000) and ZEB1 (catalog no. 3396; 1:2,000) were purchased
from Cell Signaling Technology (Cell Signaling Technology, Inc.,
Danvers, MA, USA). The GAPDH antibody (catalog no. SAB1403850;
1:10,000) was purchased from Sigma-Aldrich (Merck KGaA).
Horseradish peroxidase-conjugated secondary antibodies against
rabbit (catalog no. SA00001-2; 1:10,000) and mouse (catalog no.
SA00001-1; 1:10,000) were obtained from ProteinTech Group, Inc.
(Chicago, IL, USA). Protein lysates were prepared with
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). The concentration of lysates was
determined with a BCA Protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Protein extracts (20 µg) were then separated on
an 8% SDS-PAGE gel and transferred to polyvinylidene fluoride
membrane. The membrane was then blocked with 5% non-fat milk at
room temperature for 0.5 h, followed by incubation of indicated
primary antibodies overnight at 4°C. The following day, the
membrane was washed three times with TBS with 0.1% Tween 20 (TBST)
at room temperature for 10 mins and then incubated with secondary
antibodies for 1 h at room temperature. Subsequently, the membrane
was washed with TBST (0.1% Tween 20) at room temperature for 10
mins (three times), developed with enhanced chemiluminescence
detection agent (Thermo Fisher Scientific, Inc.) and visualized on
ImageQuant TL version 1.1.0.1 (GE Healthcare, Chicago, IL,
USA).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The extraction of total RNA from 293, MDA-MB-231 and
BT549 cells were achieved using a miRNeasy Mini kit (Qiagen GmbH,
Hilden, Germany), according to the manufacturer's protocol. The
synthesis of first strand cDNA was conducted using a M-MLV kit
(Thermo Fisher Scientific, Inc.). RT-qPCR was conducted to analyze
specific gene levels on CFX96 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) using SYBR® Premix Ex Taq (Takara
Bio, Inc., Otsu, Japan). The thermocycling conditions were as
follows: pre-denaturing at 95°C for 30 sec, denaturing at 95°C for
30 sec, and 35 cycles of annealing and elongation at 60°C for 30
sec. GAPDH and U6 served as internal controls for mRNA and miRNA,
respectively. The relative expression levels of indicated genes
were calculated using the 2−ΔΔCq method (21). The primer sequences are listed in
Table III.
 | Table III.Sequences of primers for reverse
transcription-quantitative polymerase chain reaction. |
Table III.
Sequences of primers for reverse
transcription-quantitative polymerase chain reaction.
| Name | Sequence |
|---|
| Stem loop
primer |
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAGGAGA-3′ |
|
miR-873-forward |
5′-TCGGCAGGGCAGGAACTTGTGA-3′ |
|
miR-873-reverse |
5′-CTCAACTGGTGTCGTGGA-3′ |
| U6-forward |
5′-CTCGCTTCGGCAGCACA-3′ |
| U6-reverse |
5′-AACGCTTCACGAATTTGCGT-3′ |
| AXL-forward |
5′-GTGGGCAACCCAGGGAATATC-3′ |
| AXL-reverse |
5′-GTACTGTCCCGTGTCGGAAAG-3′ |
| CTGF-forward |
5′-CAGCATGGACGTTCGTCTG-3′ |
| CTGF-reverse |
5′-AACCACGGTTTGGTCCTTGG-3′ |
| CYR61-forward |
5′-CTCGCCTTAGTCGTCACCC-3′ |
| CYR61-reverse |
5′-CGCCGAAGTTGCATTCCAG-3′ |
| ZEB1-forward |
5′-GATGATGAATGCGAGTCAGATGC-3′ |
| ZEB1-reverse |
5′-ACAGCAGTGTCTTGTTGTTGT-3′ |
| GAPDH-forward |
5′-CCTGCACCACCAACTGCTTA-3′ |
| GAPDH-reverse |
5′-GGCCATCCACAGTCTTCTGAG-3′ |
Cell viability assay
The cell viability was detected using Cell Counting
kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan), according to the manufacturer's protocol. Briefly, 1,000
MDA-MB-231 or BT549 cells/well were seeded in 96-well plates.
Following the transfection of miR-873 mimics or miR-NC mimics for
48 h, 10 µl CCK-8 solution was added into each well and incubated
for 1 h in an incubator with 5% CO2 at 37°C. Following incubation
the medium was transferred into another 96-well plate and the
absorbance at 450 nm of each well was detected using a microplate
reader (Bio-Rad Laboratories, Inc.).
Dual luciferase assay
Prediction of the putative binding site between ZEB1
3′UTR and miR-873 was achieved using TargetScan software version
7.2 (http://www.targetscan.org/vert_72/). The cDNA was
prepared by RNA extraction using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed into cDNA with RevertAid First Strand cDNA Synthesis
kit (Thermo Fisher Scientific, Inc.). The 3′UTR of ZEB1 mRNA was
amplified from cDNA of 293 cells and was inserted into pGL3 plasmid
(Promega Corporation, Madison, WI, USA) to construct pGL3-ZEB1
3′UTR-wild type (WT). pGL3-ZEB1 3′UTR-mutant (Mut) with mutation of
predicted miR-873 binding sites was constructed by site mutation of
pGL3-ZEB1 3′UTR-WT using a QuikChange Site-directed Mutagenesis kit
(Agilent Technologies, Inc., Santa Clara, CA, USA). For dual
luciferase assay, 293 cells were transfected with pGL3-ZEB1
3′UTR-WT or pGL3-ZEB1 3′UTR-Mut accompanied with miR-873 mimics or
miR-negative control (NC) mimics and an internal control
Renilla plasmid with Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.). The firefly
luciferase activity was firstly normalized to Renilla
luciferase activity, followed by normalization to the control
group. At 24 h post-transfection, the relative luciferase activity
of each well was detected using a Dual Luciferase Reporter assay
kit (Promega Corporation), according to the manufacturer's
protocol.
Statistical analysis
All data were calculated and analyzed using GraphPad
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) and
presented as mean ± standard deviation. The differences between two
groups were compared with an unpaired Student's t-test. The
differences among three groups were analyzed using two-way analysis
of variance followed by Newman-Keuls post-hoc analysis. P<0.05
was considered to indicate a statistically significant difference
significance. All experiments were repeated a minimum of three
times.
Results
miR-873 is negatively associated with
gemcitabine sensitivity in TNBC cells
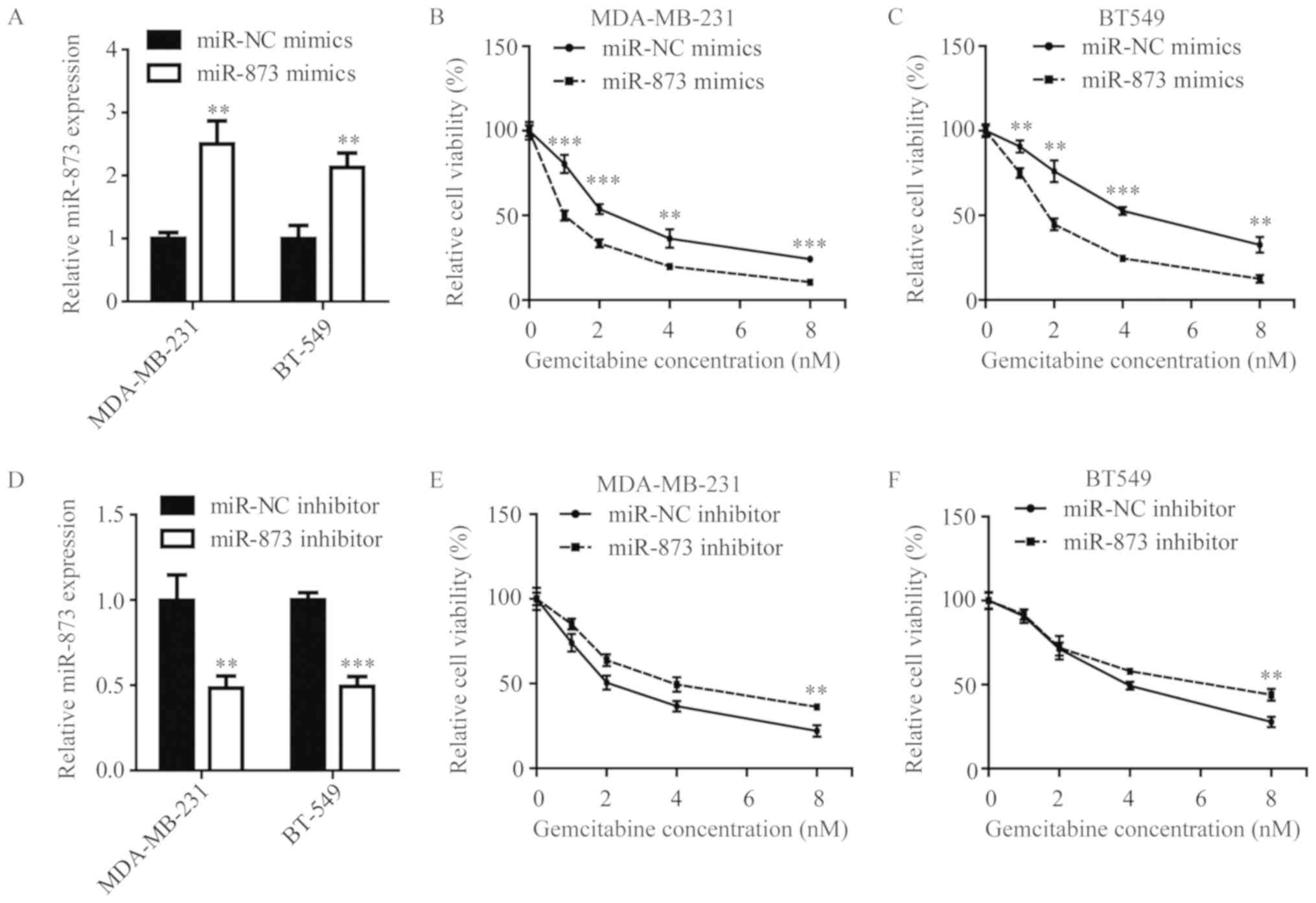
To investigate the role of miR-873 in mediating
gemcitabine sensitivity in TNBC cells, the present study examined
the gemcitabine sensitivity of MDA-MB-231 cells and BT549 cells
following miR-873 elevation and downregulation via transfection of
miR-873 mimics or inhibitors, respectively. Following
overexpression of miR-873 (Fig. 1A),
MDA-MB-231 cells and BT549 cells became increasingly sensitive
towards increasing concentrations of gemcitabine (0–8 nM), compared
with their corresponding control groups (Fig. 1B-C). Conversely, antagonist of
miR-873 (Fig. 1D) caused MDA-MB-231
cells and BT549 cells to be more insensitive upon gemcitabine
exposure, compared with cells transfected with miR-NC inhibitor
(Fig. 1E-F). These data indicate
that miR-873 could mediate gemcitabine sensitivity in TNBC
cells.
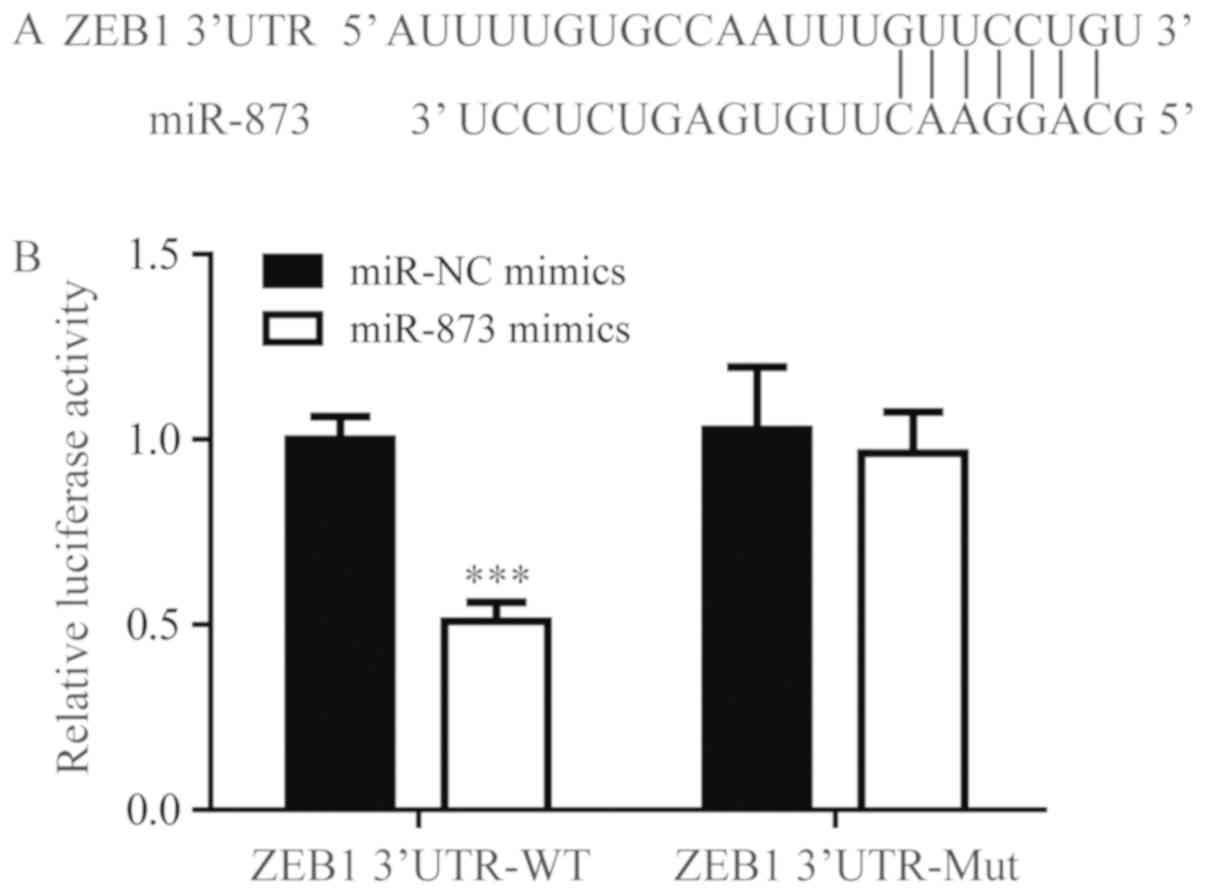
miR-873 directly represses ZEB1
expression via binding to its 3′UTR
Using TargetScan, miR-873 was predicted to bind to
3′UTR of ZEB1 mRNA (Fig. 2A). To
confirm the regulatory association between miR-873 and 3′UTR of
ZEB1, a dual luciferase assay was performed. miR-873 mimics were
demonstrated to significantly downregulate the relative luciferase
activity of ZEB1 3′UTR-WT but not ZEB1 3′UTR-Mut (Fig. 2B). This result validated ZEB1 as a
direct target of miR-873.
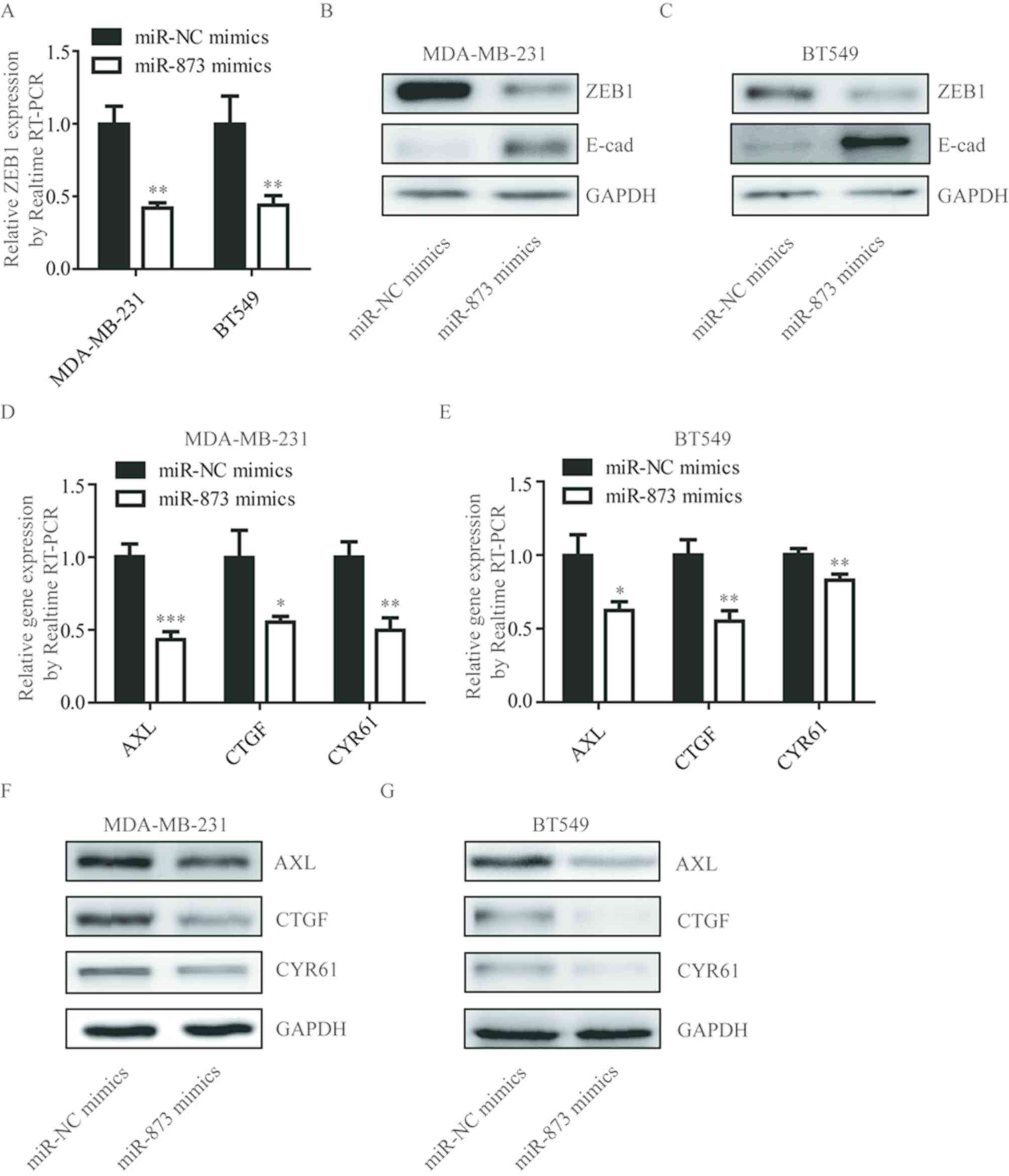
miR-873 regulates ZEB1 and its target
genes in TNBC cells
To investigate whether miR-873 regulated ZEB1 in
TNBC cells, the present study detected ZEB1 mRNA levels following
miR-873 overexpression in MDA-MB-231 and BT549 cells. As
illustrated in Fig. 3A,
overexpression of miR-873 significantly decreased the ZEB1 mRNA
level in MDA-MB-231 and BT549 cells. ZEB1 was determined to be a
classic transcription suppressor and functions as an EMT inducer
via repression of E-cadherin expression (22). Consistently, in MDA-MB-231 and BT549
cells, miR-873 mimics markedly decreased ZEB1 protein levels and
upregulated E-cadherin protein levels (Fig. 3B and C). A recent study demonstrated
that ZEB1 functions as a transcription activator through
interacting with YAP1 (19).
Transfection of miR-873 mimics predominantly decreased YAP target
genes (AXL, CTGF and CYR61) at mRNA and protein levels in
MDA-MB-231 and BT549 cells (Fig.
3D-G). These data support the notion that miR-873 regulates the
target genes of ZEB1 in TNBC cells.
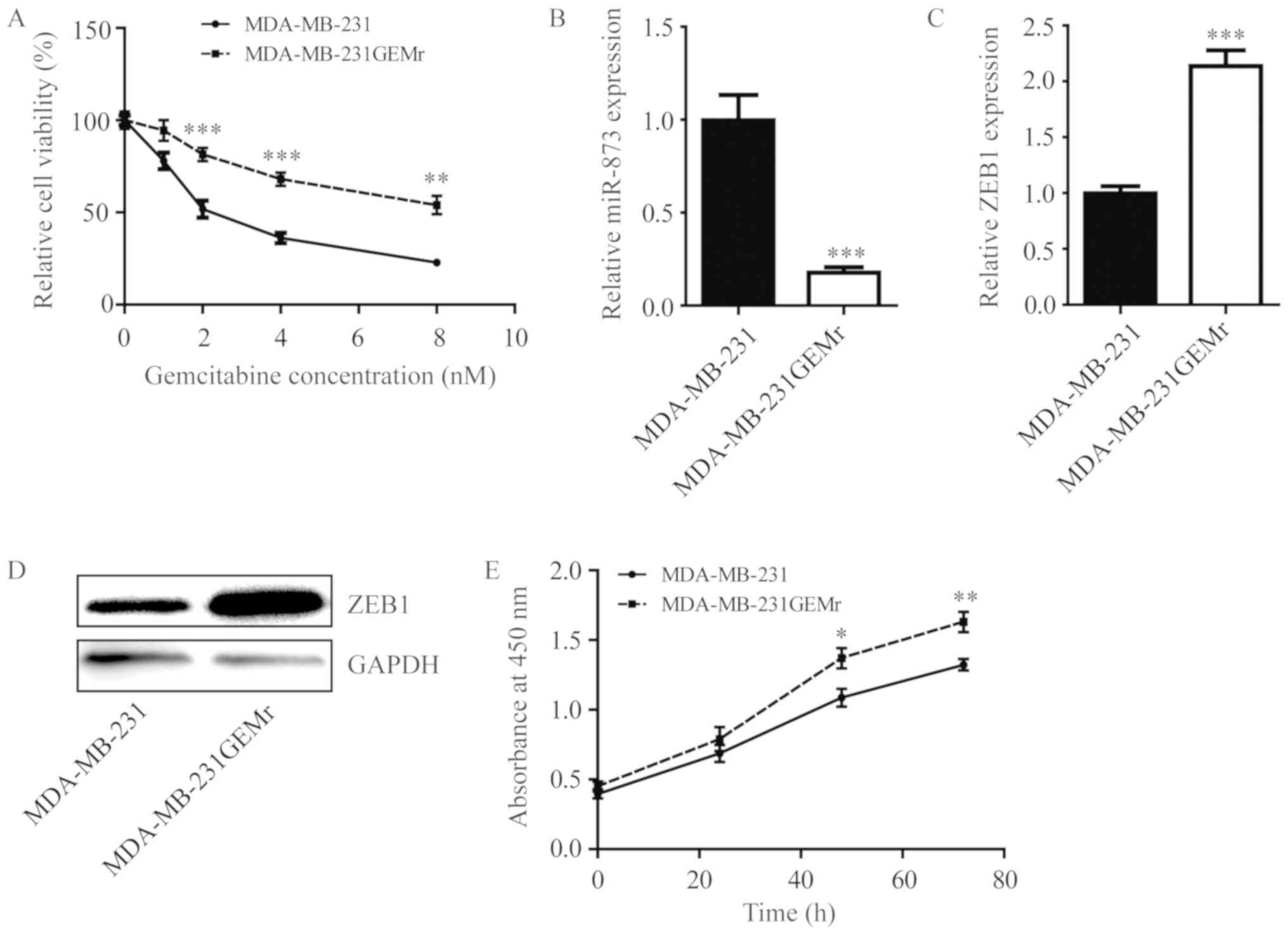
Aberrant expression of miR-873 and
ZEB1 in gemcitabine-resistant MDA-MB-231 cells
The present study then sought to investigate the
role of miR-873 during the development of gemcitabine resistance
using MDA-MB-231GEMr cells. Compared with parental MDA-MB-231
cells, MDA-MB-231GEMr cells were relatively insensitive towards
gemcitabine treatment (Fig. 4A).
Additionally, miR-873 level was significantly decreased (Fig. 4B) in MDA-MB-231GEMr cells accompanied
with elevation of ZEB1 at the mRNA and protein levels (Fig. 4C and D). Consistent with the
proliferation promotion role of ZEB1, the growth rate of
MDA-MB-231GEMr cells was significantly increased, compared with
that of MDA-MB-231 cells (Fig. 4E;
P<0.05 at 48 h; P<0.01 at 72 h).
Decrease of miR-873 contributes to
gemcitabine resistance in MDA-MB-231GEMr via regulation of
ZEB1
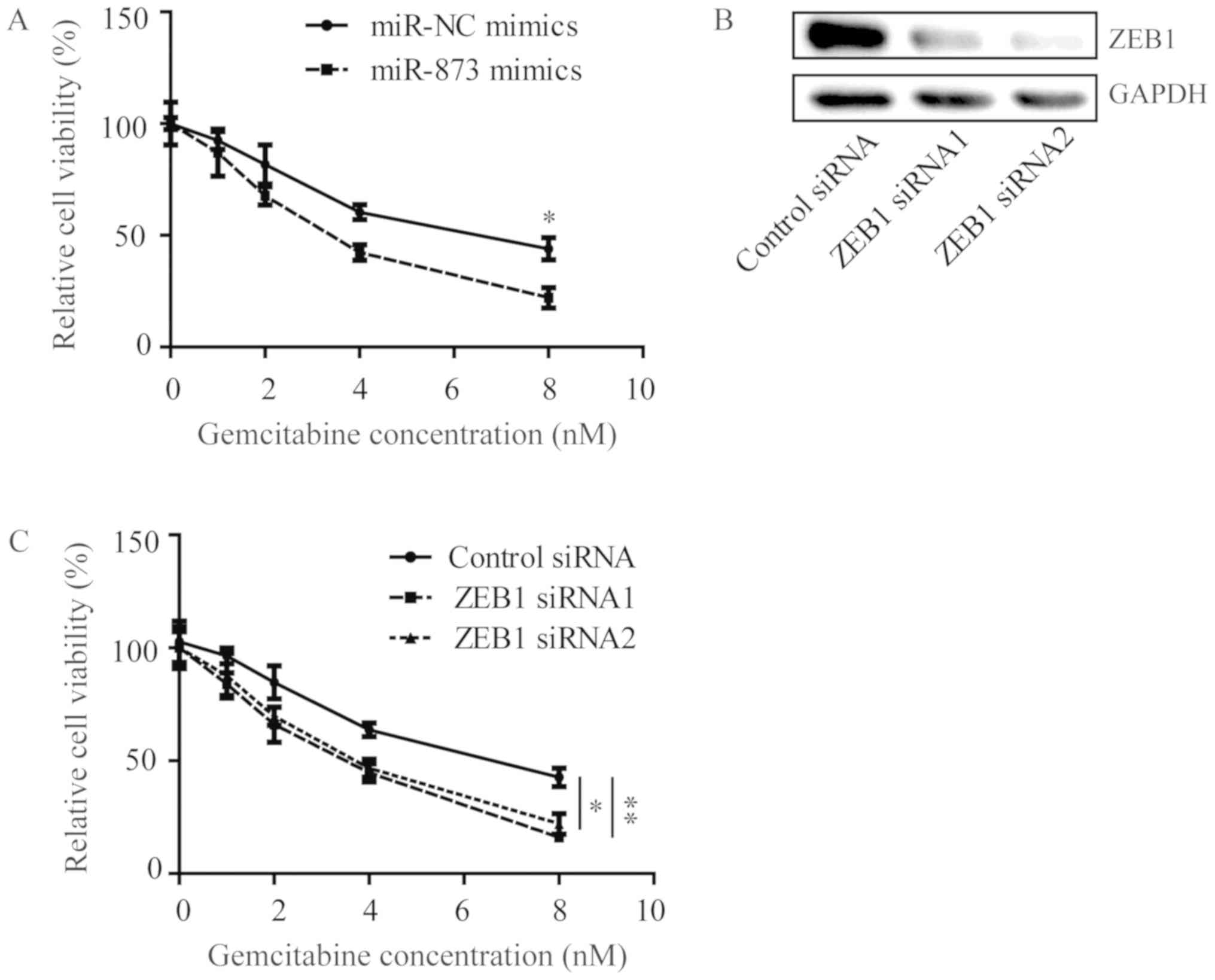
In MDA-MB-231GEMr cells, the overexpression of
miR-873 by transfection of miR-873 mimics sensitized cells towards
gemcitabine treatment (Fig. 5A).
Furthermore, silencing of ZEB1 using ZEB1 siRNAs also enhanced cell
viability inhibition, which was induced by gemcitabine treatment in
MDA-MB-231GEMr cells, indicating a significant reversion of
gemcitabine resistance in MDA-MB-231GEMr cells (Fig. 5B and C).
Discussion
Standard chemotherapy is a major effective treatment
approach for patients with TNBC that do not respond towards
endocrine therapy or HER2-target therapy (23). However, almost all patients with TNBC
develop chemoresistance, which eventually culminates in patient
mortality (24). Determining the
underlying mechanism of chemoresistance is important to improve
patient outcomes. In the present study, miR-873 was identified as a
pivotal molecule in the regulation of chemotherapy sensitivity in
TNBC.
Dysregulation of miRNAs was frequently observed in
TNBC and contributed to the initiation, progression and
chemoresistance (25). Multiple
miRNAs, including miR-638 and miR-101, were decreased in TNBC
tissues, compared with normal adjacent tissues, and these miRNAs
could sensitize TNBC cells towards chemotherapy, while reduction of
their expression contributed to chemoresistance (26,27).
miR-873 levels were downregulated in breast cancer tissues and
tamoxifen-resistant MCF7 cells, and forced overexpression of
miR-873 inhibited breast cancer cell growth and reversed tamoxifen
resistance of tamoxifen-resistant MCF7 cells (11). The present study demonstrated that
miR-873 regulated gemcitabine sensitivity in TNBC cells, and the
transfection of MDA-MB-231 and BT549 cells with miR-873 mimics
sensitized them towards gemcitabine treatment. Additionally,
antagonists of miR-873 by miR-873 inhibitors weakened
gemcitabine-induced cell viability inhibition in MDA-MB-231 and
BT549 cells. Notably, in MDA-MB-231GEMr cells, a decreased miR-873
level, compared with their parental cells, was observed.
Furthermore, silencing of miR-873 reversed gemcitabine resistance
of MDA-MB-231GEMr cells. These results indicated that loss of
miR-873 promotes the development of gemcitabine resistance in TNBC
cells.
ZEB1 is a transcription factor well-known for its
oncogenic role via inducing EMT (28), which functions in cells through
various mechanisms including Wnt, nuclear factor-κB and miRNAs
(29–31). Using TargetScan, miR-873 was
predicted as a direct regulator of 3′UTR of ZEB1 mRNA. In 293
cells, miR-873 mimics greatly repressed luciferase activity of ZEB1
3′UTR-WT in a dual luciferase assay. In MDA-MB-231 cells and BT549
cells, transfection of miR-873 mimics decreased ZEB1 expression.
ZEB1 could function as a transcription activator to activate YAP1
target genes, including AXL, CTGF and CYR61 expression, and as a
transcription suppressor to inhibit target gene (E-cadherin)
expression (19). In the present
study, miR-873 overexpression increased E-cadherin expression and
decreased AXL, CTGF and CYR61 expression, indicating that miR-873
could repress ZEB1 expression to regulate ZEB1 target genes levels.
With self-renewal ability, cancer stem cells have been demonstrated
to contribute to chemoresistance in numerous types of cancer,
including breast, colon and prostate cancer, through protecting
tumor cells from DNA damage and activating pathways involved in
maintaining cell survival (32,33).
Since ZEB1 serves a pivotal role in promoting the development of
cancer stem cell properties, overexpression of ZEB1 has been
implicated in chemoresistance in cancer (14). The present study detected an
elevation of ZEB1 in MDA-MB-231GEMr cells, indicating that ZEB1
expression was associated with chemoresistance in TNBC.
Additionally, silencing of ZEB1 reversed gemcitabine resistance of
MDA-MB-231GEMr cells. Collectively, the present study identified
miR-873 as a novel regulator of ZEB1 3′UTR and demonstrated that
miR-873 determined ZEB1 expression to alter gemcitabine sensitivity
in TNBC cells.
In conclusion, the present study demonstrated that
miR-873 could negatively regulate ZEB1 expression and enhance cell
growth inhibition induced by treatment with gemcitabine. These data
provide strong evidence that the loss of miR-873 contributes to the
development of gemcitabine resistance in TNBC by controlling ZEB1
expression, which implicates miR-873 as a potential predictor and
target for TNBC treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GW, YD, HL, NJ, JC, AL, XT and YR designed the study
and acquired the data. GW and YD established the cell line. GW, XT
and YR wrote and revised the manuscript. GW supervised the
study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Oakman C, Viale G and Di Leo A: Management
of triple negative breast cancer. Breast. 19:312–321. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carey LA, Dees EC, Sawyer L, Gatti L,
Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML and Perou
CM: The triple negative paradox: Primary tumor chemosensitivity of
breast cancer subtypes. Clin Cancer Res. 13:2329–2334. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Papa AL, Sidiqui A, Balasubramanian SU,
Sarangi S, Luchette M, Sengupta S and Harfouche R: PEGylated
liposomal Gemcitabine: Insights into a potential breast cancer
therapeutic. Cell Oncol (Dordr). 36:449–457. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ueno H, Kiyosawa K and Kaniwa N:
Pharmacogenomics of gemcitabine: Can genetic studies lead to
tailor-made therapy? Br J Cancer. 97:145–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Samanta D, Gilkes DM, Chaturvedi P, Xiang
L and Semenza GL: Hypoxia-inducible factors are required for
chemotherapy resistance of breast cancer stem cells. Proc Natl Acad
Sci USA. 111:E5429–E5438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu B, Li J and Cairns MJ: Identifying
miRNAs, targets and functions. Brief Bioinform. 15:1–19. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J, Yang M, Li Y and Han B: The role
of MicroRNAs in the chemoresistance of breast cancer. Drug Dev Res.
76:368–374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li HY, Liang JL, Kuo YL, Lee HH, Calkins
MJ, Chang HT, Lin FC, Chen YC, Hsu TI, Hsiao M, et al:
miR-105/93-3p promotes chemoresistance and circulating
miR-105/93-3p acts as a diagnostic biomarker for triple negative
breast cancer. Breast Cancer Res. 19:1332017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang RJ, Li JW, Bao BH, Wu HC, Du ZH, Su
JL, Zhang MH and Liang HQ: MicroRNA-873 (miRNA-873) inhibits
glioblastoma tumorigenesis and metastasis by suppressing the
expression of IGF2BP1. J Biol Chem. 290:8938–8948. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cui J, Yang Y, Li H, Leng Y, Qian K, Huang
Q, Zhang C, Lu Z, Chen J, Sun T, et al: MiR-873 regulates ERα
transcriptional activity and tamoxifen resistance via targeting
CDK3 in breast cancer cells. Oncogene. 34:40182015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen X, Zhang Y, Shi Y, Lian H, Tu H, Han
S, Peng B, Liu W and He X: MiR-873 acts as a novel sensitizer of
glioma cells to cisplatin by targeting Bcl-2. Int J Oncol.
47:1603–1611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu DD, Li XS, Meng XN, Yan J and Zong ZH:
MicroRNA-873 mediates multidrug resistance in ovarian cancer cells
by targeting ABCB1. Tumour Biol. 37:10499–10506. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang P, Sun Y and Ma L: ZEB1: At the
crossroads of epithelial-mesenchymal transition, metastasis and
therapy resistance. Cell Cycle. 14:481–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karihtala P, Auvinen P, Kauppila S,
Haapasaari KM, Jukkola-Vuorinen A and Soini Y: Vimentin, zeb1 and
Sip1 are up-regulated in triple-negative and basal-like breast
cancers: Association with an aggressive tumour phenotype. Breast
Cancer Res Treat. 138:81–90. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giordano A, Gao H, Anfossi S, Cohen E,
Mego M, Lee BN, Tin S, De Laurentiis M, Parker CA, Alvarez RH, et
al: Epithelial-mesenchymal transition and stem cell markers in
patients with HER2-positive metastatic breast cancer. Mol Cancer
Ther. 11:2526–2534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lehmann W, Mossmann D, Kleemann J, Mock K,
Meisinger C, Brummer T, Herr R, Brabletz S, Stemmler MP and
Brabletz T: ZEB1 turns into a transcriptional activator by
interacting with YAP1 in aggressive cancer types. Nat Commun.
7:104982016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Davidson JD, Ma L, Flagella M, Geeganage
S, Gelbert LM and Slapak CA: An increase in the expression of
ribonucleotide reductase large subunit 1 is associated with
gemcitabine resistance in non-small cell lung cancer cell lines.
Cancer Res. 64:3761–3766. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sánchez-Tilló E, Lázaro A, Torrent R,
Cuatrecasas M, Vaquero EC, Castells A, Engel P and Postigo A: ZEB1
represses E-cadherin and induces an EMT by recruiting the SWI/SNF
chromatin-remodeling protein BRG1. Oncogene. 29:3490–3500. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Podo F, Buydens LM, Degani H, Hilhorst R,
Klipp E, Gribbestad IS, Van Huffel S, van Laarhoven HW, Luts J,
Monleon D, et al: Triple-negative breast cancer: Present challenges
and new perspectives. Mol Oncol. 4:209–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
O'Reilly EA, Gubbins L, Sharma S, Tully R,
Guang MH, Weiner-Gorzel K, McCaffrey J, Harrison M, Furlong F, Kell
M and McCann A: The fate of chemoresistance in triple negative
breast cancer (TNBC). BBA Clin. 3:257–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang F, Zhang W, Shen Y and Guan X:
Identification of dysregulated microRNAs in triple-negative breast
cancer (review). Int J Oncol. 46:927–932. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu X, Tang H, Chen J, Song C, Yang L, Liu
P, Wang N and Xie X, Lin X and Xie X: MicroRNA-101 inhibits cell
progression and increases paclitaxel sensitivity by suppressing
MCL-1 expression in human triple-negative breast cancer.
Oncotarget. 6:20070–20083. 2015.PubMed/NCBI
|
|
27
|
Tan X, Peng J, Fu Y, An S, Rezaei K,
Tabbara S, Teal CB, Man YG, Brem RF and Fu SW: miR-638 mediated
regulation of BRCA1 affects DNA repair and sensitivity to UV and
cisplatin in triple-negative breast cancer. Breast Cancer Res.
16:4352014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aigner K, Dampier B, Descovich L, Mikula
M, Sultan A, Schreiber M, Mikulits W, Brabletz T, Strand D, Obrist
P, et al: The transcription factor ZEB1 (deltaEF1) promotes tumour
cell dedifferentiation by repressing master regulators of
epithelial polarity. Oncogene. 26:6979–6988. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kahlert UD, Maciaczyk D, Doostkam S, Orr
BA, Simons B, Bogiel T, Reithmeier T, Prinz M, Schubert J,
Niedermann G, et al: Activation of canonical WNT/β-catenin
signaling enhances in vitro motility of glioblastoma cells by
activation of ZEB1 and other activators of
epithelial-to-mesenchymal transition. Cancer Lett. 325:42–53. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chua HL, Bhat-Nakshatri P, Clare SE,
Morimiya A, Badve S and Nakshatri H: NF-kappaB represses E-cadherin
expression and enhances epithelial to mesenchymal transition of
mammary epithelial cells: Potential involvement of ZEB-1 and ZEB-2.
Oncogene. 26:711–724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Korpal M, Lee ES, Hu G and Kang Y: The
miR-200 family inhibits epithelial-mesenchymal transition and
cancer cell migration by direct targeting of E-cadherin
transcriptional repressors ZEB1 and ZEB2. J Biol Chem.
283:14910–14914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peitzsch C, Kurth I, Kunz-Schughart L,
Baumann M and Dubrovska A: Discovery of the cancer stem cell
related determinants of radioresistance. Radiother Oncol.
108:378–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Allegra A, Alonci A, Penna G, Innao V,
Gerace D, Rotondo F and Musolino C: The cancer stem cell
hypothesis: A guide to potential molecular targets. Cancer Invest.
32:470–495. 2014. View Article : Google Scholar : PubMed/NCBI
|