Introduction
Osteosarcoma (OS) is the most common primary
malignant bone tumor which frequently develops in children and
adolescents. Although there have been improvements in diagnosis and
treatment, the 5-year survival rate of patients with OS remains
unchanged (1). This is due in part
to an 80% prevalence of micro-metastases to the lungs at diagnosis
(2). Lung metastasis is the leading
cause of mortality in patients with OS (2). As with other types of tumor, the
development of metastasis in OS is a complex, multi-step and
multi-gene process (3). Therefore,
clarification of the molecular mechanisms of invasion, and
identification of new molecular targets are necessary for the
development of effective treatments.
Valosin-containing protein (VCP), also known as p97
in mammals, is involved in a variety of cellular functions. Studies
have identified VCP expression in various tumor types, in addition
to an association between tumorigenesis and the development of
metastasis (4–7). In previous studies, the role of VCP in
metastatic OS was investigated. The results illustrated that VCP
was involved in the invasion and migration of OS cells, in part
through the activation of the PI3K/Akt signaling pathway, and the
upregulation of matrix metallopeptidase (MMP)-2 and MMP-9 (8). Furthermore, inhibition of the PI3K/Akt
pathway did not completely reverse the VCP-mediated invasion and
migration of OS, suggesting that VCP-mediated OS invasion and
migration may involve other mechanisms. Upon further investigation,
VCP was revealed to inhibit apoptosis by activating the NF-κβ
signaling. However, the details of this molecular mechanism are yet
to be elucidated.
The role of autophagy in tumorigenesis has become a
focus of biomedical research (9).
The role of autophagy in tumorigenesis and tumor drug resistance is
well delineated (10–13); however, the role of tumor cell
autophagy in tumor metastasis remains unclear. In the development
of metastasis, cells undergo local infiltration and penetration of
the vasculature, subsequently entering the circulation for
dissemination throughout the body (14,15).
Tumor cells must separate from the cell matrix during this process
(16). Apoptosis occurs following
separation from the matrix in a process called anoikis (17). A growing number of studies have
suggested that autophagy provides a mechanism for stromal-isolation
of cells to resist anoikis (18,19). In
a lung metastasis model of hepatocellular carcinoma, inhibition of
autophagy significantly reduced metastasis of hepatoma cells to the
lung. Inhibition of autophagy did not affect cell invasiveness,
migration or epithelial-mesenchymal transition, but decreased the
ability of liver cancer cells to resist anoikis and implant in the
lung (20). These studies strongly
suggested that autophagy promoted metastasis by mediating cellular
resistance to anoikis. However, the role of anoikis resistance in
the metastasis of OS remains to be investigated.
As a member of the adenosine triphosphate
superfamily, VCP is closely associated with energy metabolism. VCP
participates in the regulation of protein degradation by inducing
autophagy (21), and is also closely
associated with the development of numerous diseases. Ozsoy et
al (22) revealed that
VCP-induced autophagy is a prominent mechanism in the development
of pre-eclampsia. Whether VCP promotes OS metastasis via
autophagy-induced anoikis resistance is unclear, and requires
further investigation.
ERK, a member of the mitogen-activated protein
kinase (MAPK) family, transmits signals from cell surface receptors
to the nucleus, and serves a key role in signal transduction
(23,24). The MAPK family has a number of
members, including ERK1/2, p38 and c-Jun N-terminal kinase
(25). ERK1 (44 kDa) and ERK2 (42
kDa) are the most thoroughly studied (26–29).
They are widely expressed and integral to the regulation of cell
growth, development and differentiation (30). Phosphorylation of ERK1/2 and
activation of the nuclear transcription factor NF-κβ exerts a
biological effect, such as regulating other proteins. Copetti et
al (31,32) demonstrated that the
autophagy-promoting protein beclin-1 was able to bind to NF-κβ.
Activated NF-κβp65 is able to promote beclin-1 expression by
binding to the autophagy-promoting protein beclin-1. Numerous
studies (33–35) have also confirmed that VCP regulates
the ERK/NF-κβ signaling pathway, which is involved in a number of
biological processes, including cell proliferation, apoptosis,
protein degradation and DNA damage repair, in addition to
tumorigenesis. Therefore, it is hypothesized that VCP may activate
the ERK1/2/NF-κβ/beclin-1 pathway, inducing autophagy-mediated
anoikis resistance, and subsequently promoting OS metastasis.
The present study aimed to demonstrate that VCP
overexpression activates the ERK/NF-κβ/beclin-1 pathway, enhances
autophagy-mediated anoikis resistance and promotes the metastasis
of OS. Successful completion of this study may increase the
understanding of VCP in OS metastasis, and ultimately facilitate
the development of effective treatments for metastatic OS.
Materials and methods
Patient specimens
A total of 24 paired samples of OS and
paraneoplastic tissue were obtained from patients (10 males and 14
females; age range, 9–35 years; mean age, 17±5 years) with OS who
underwent surgery at The First Hospital Affiliated with Nanchang
University (Nanchang, China) between January 2010 and December
2017. None of the patients received chemotherapy or radiotherapy
prior to surgical resection. Written informed consent was obtained
from all patients, and the study was approved by the Ethics
Committee of The First Hospital Affiliated with Nanchang
University.
Cell lines
The human osteosarcoma cell line 143B was purchased
from the Cell Bank of the Type Culture Collection of the Chinese
Academy of Sciences. 143B cells were cultured in Dulbecco's
Modified Eagle Medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.), supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml of penicillin and 100 U/ml of
streptomycin. Cells were cultured at 37°C with 5%
CO2.
Lentivirus vector construction and
transfection
To construct siRNA vectors for the downregulation of
VCP, reverse complement sequences as well as nonfunctional negative
sequences (Invitrogen; Thermo Fisher Scientific, Inc.; Table I) were cloned into the lentivirus
vector GV159 (Invitrogen; Thermo Fisher Scientific, Inc.). Fresh
medium was administered to 143B cells, and they were cultured to
~80% confluence. Virus particles incorporating lentivirus vector
for the downregulation of VCP (LV-down-VCP; MOI=20) and the cell
transfection enhancer polybrene (6 µg/ml; Invitrogen; Thermo Fisher
Scientific, Inc.) were added to the appropriate cultures
[LV-down-VCP and negative lentivirus vector (Neg-LV)], and
incubated at 37°C, 5% CO2 for 6–8 h. The medium was
collected and replaced with fresh medium at 6 h after transfection.
Transfection efficiency was evaluated using a fluorescence
microscope 24 h post-transfection. 143B cells transfected with
Neg-LV served as the control. A total of 6 independent experiments
were performed, and subsequent experiments were started
324 h after transfection.
 | Table I.Small interfering RNA sequence
targeting valosin-containing protein and non-targeting negative
control sequence. |
Table I.
Small interfering RNA sequence
targeting valosin-containing protein and non-targeting negative
control sequence.
| Oligomer | Sequence
(5′→3′) |
|---|
| siRNA-F |
TGCTGTACAGGTCATCATCATTGTCTGTTTTGGCCACTGACTGACAGACAATGGATGACCTGTA |
| siRNA-R |
CCTGTACAGGTCATCCATTGTCTGTCAGTCAGTGGCCAAAACAGACAATGATGATGACCTGTAC |
| Negative-F |
TGCTGAAATGTACTGCGCGTGGAGACGTTTTGGCCACTGACTGACGTCTCCACGCAGTACATTT |
| Negative-R |
CCTGAAATGTACTGCGTGGAGACGTCAGTCAGTGGCCAAAACGTCTCCACGCGCAGTACATTTC |
Autophagy intervention experiment
Autophinib and spermidine trihydrochloride were
purchased form Sigma-Aldrich (Merck KGaA). Concentrations were
adjusted to 10 mM for storage according to the manufacturer's
instructions. For cell treatment, autophinib and spermidine
trihydrochloride were added to the cell culture medium at final
concentrations of 20 and 30 nM, respectively, and incubated at 37°C
with 5% CO2 for 6 h. For cells treated with RNA
interference and autophagy agonists, the drug treatment was
performed 24 h after transfection.
Western blotting
Total protein was extracted from the OS tissues and
cell lines using radioimmunoprecipitation assay lysis buffer
(Beijing Solarbio Science & Technology Co., Ltd.) containing 60
µg/ml phenylmethylsulfonyl fluoride. Protein concentrations were
determined using a Bradford assay. Proteins (15 µg/lane) were
separated by SDS-PAGE using a 5% concentrated gel and 15%
separating gel. Following electrophoresis, the protein was
transferred to a nitrocellulose membrane and the membrane was
blocked with 5% skimmed milk for 30 min at room temperature.
Western blot analysis was conducted using primary antibodies
against VCP (cat. no. ab36047), ERK (cat. no. ab79853), NF-κβ (cat.
no. ab16502), beclin-1 (cat. no. ab62557) and LC3 (cat. no.
ab62721; all Abcam; dilution, 1:2,000) and GAPDH (cat. no.
sc-48166, Santa Cruz Biotechnology, Inc.; dilution, 1:5,000) and
horseradish peroxidase-conjugated secondary antibodies (cat. nos.
sc-2004 and sc-2020, Santa Cruz Biotechnology, Inc.; dilution,
1:5,000). Membranes were incubated with primary antibodies at 4°C
for ~12 h (overnight), and subsequently with secondary antibodies
at room temperature for 2–3 h. Immune complexes were detected using
a pro-light HRP kit (Pierce; Thermo Fisher Scientific, Inc.). The
strip gray value was determined using ImageJ software (version
1.46; National Institutes of Health). A total of 6 independent
experiments were performed.
Migration assay
Cell migration was assessed using a wound healing
assay to determine the ability of cells to move into a cellular
space in two-dimensions, in vitro. In brief, cells were
cultured to confluence in six-well tissue culture dishes, to a
density of ~5×106 cells/well. The wound was created by
dragging a rubber policeman (Thermo Fisher Scientific, Inc.) across
the center of the plate. Cultures were rinsed with PBS and replaced
with fresh medium alone or containing 10 g/l BSA (Gibco; Thermo
Fisher Scientific, Inc.), and incubated at 37°C for 24 h. BSA was
only used in place of FBS in the wound healing experiments. Images
were captured using a light microscope at 0 and 24 h, and the
migration distance was determined using ImageJ Software (National
Institutes of Health).
Transwell invasion assay
The invasiveness of OS cells was assessed using the
BD BioCoat™ BD Matrigel™ Invasion Chamber (BD Bioscience) according
to the manufacturer's protocol. Cells (2×105) were
resuspended in serum-free DMEM and added to the upper chambers; the
medium in the lower chambers contained 5% FBS as a
chemo-attractant. At 24 h, cells that had migrated through the
Matrigel-coated membrane were stained with Diff-Quik (Sysmex
Corporation, Kobe) at room temperature in Diff-Quik A for 10–20
sec, Diff-Quik B for 5–10 sec and Diff-Quik C for 5–10 sec, and
images were captured under a light microscope (magnification,
×200).
MTT assay
Osteosarcoma 143B cells (2×106 cells)
were cultured in suspension for 7 days and subsequently cultured
for 6 h. MTT solution (5 mg/ml; 500 µl/well) was added to a 24-well
plate, incubated for 4 h at 37°C, 1 ml DMSO was added to each well,
and the plate was agitated for 10 min to completely dissolve the
crystals. The absorbance at 490 nm was measured using a microplate
spectrophotometer.
Statistical analysis
All statistical analyses were conducted using SPSS
software (version 13.0; SPSS Inc., Chicago, IL, USA). Data are
expressed as the mean ± standard deviation. Bivariate correlation
analysis (Spearman's Rho) was performed to evaluate the association
between VCP expression and autophagy in OS tissues. One-way
analysis of variance followed by a Fisher's Least Significant
Difference test was used to analyze multiple samples. P<0.05 was
considered to indicate a statistically significant difference.
Results
Correlation between VCP expression and
autophagy in OS tissues
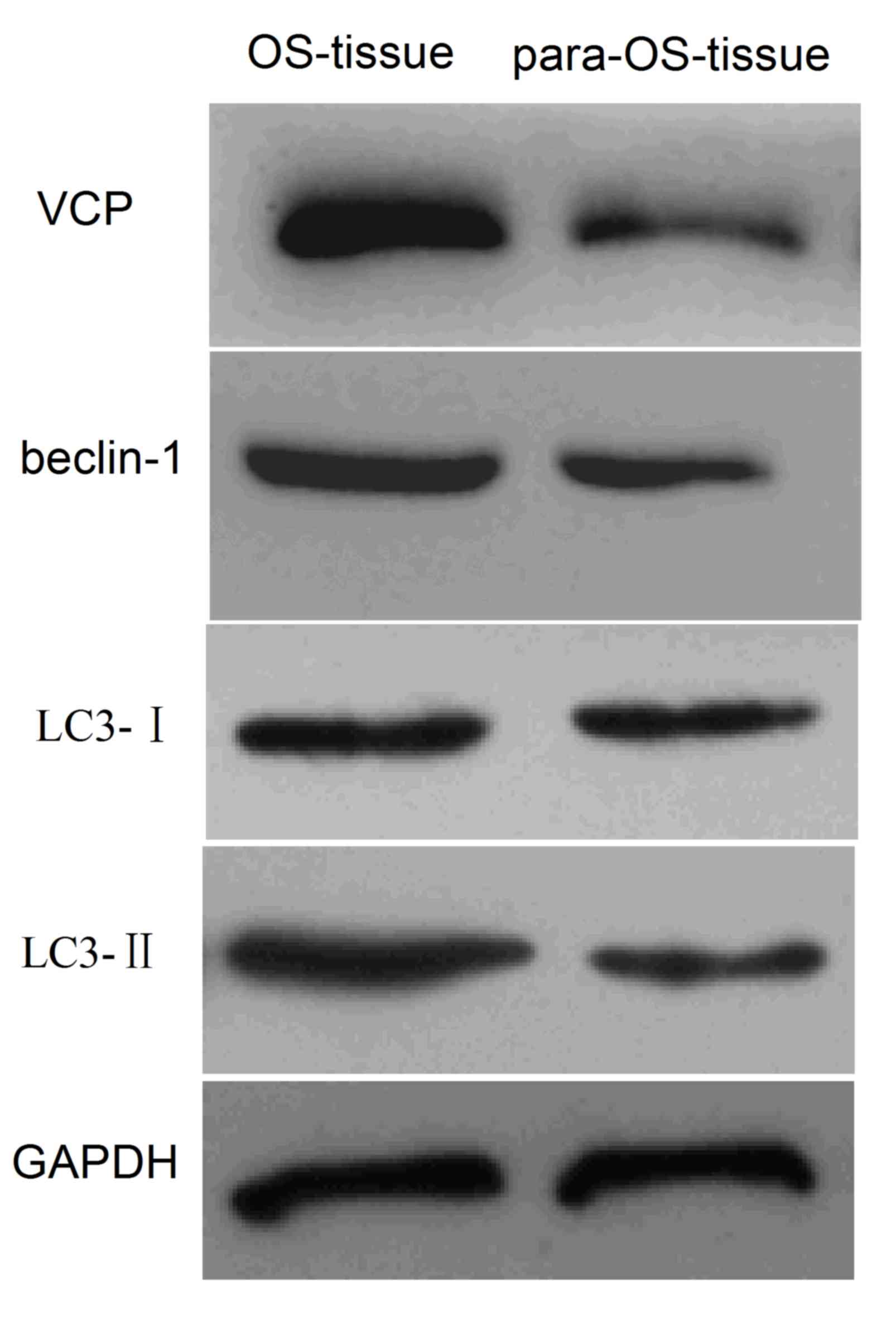
To investigate the association between VCP
expression and autophagy in OS, VCP and autophagy-associated
proteins beclin-1 and microtubule-associated protein 1A/1B-light
chain 3 (LC3)-I/II were analyzed in 24 tissue samples from patients
with OS, using western blot analysis. The association between VCP
and autophagy-associated proteins was evaluated by bivariate
correlation (Spearman's Rho). The results revealed that VCP,
beclin-1 and LC3-I/II expression was greater in OS tissues compared
with paracancerous tissues (Fig. 1).
There was a positive correlation between increased expression
levels of VCP and autophagy (Spearman's Rho=0.658; data not shown).
Therefore, increased expression levels of VCP may contribute to
cell autophagy in OS.
VCP induces autophagy to enhance
anoikis resistance
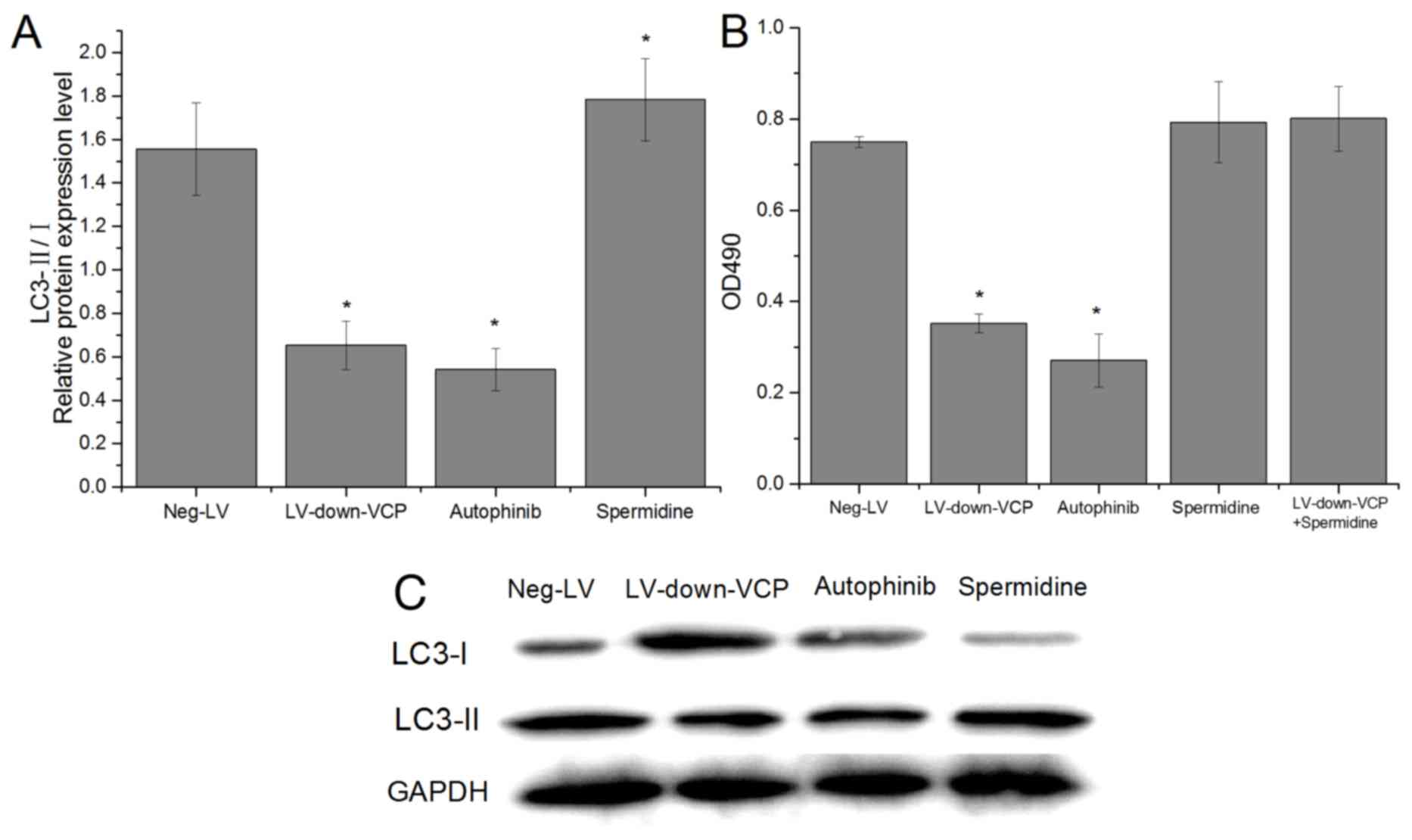
To determine if VCP was able to induce autophagy and
anoikis resistance, VCP expression was inhibited in 143B cells by
RNA interference. After 7 days in suspension culture, LC3-II/I
expression levels and cell survival were analyzed (Fig. 2). The results illustrated that VCP
inhibition lead to a significant decrease in LC3-II/I expression
(Fig. 2A) and cell survival
(Fig. 2B). Further studies performed
with an autophagy inhibitor autophinib and autophagy stimulator
spermidine trihydrochloride revealed that autophagy inhibitors and
stimulator could cause relative changes in the levels of
autophagy-related proteins in cells (Fig. 2A), and inhibiting autophagy decreased
cell survival; conversely, autophagy stimulation promoted cell
survival and counteracted the changes caused by VCP inhibition
(Fig. 2B). Collectively, these
results indicate that VCP induced autophagy to enhance cell
survival and possibly anoikis resistance.
VCP promotes migration and invasion in
OS cells via enhanced cell survival induced by autophagy
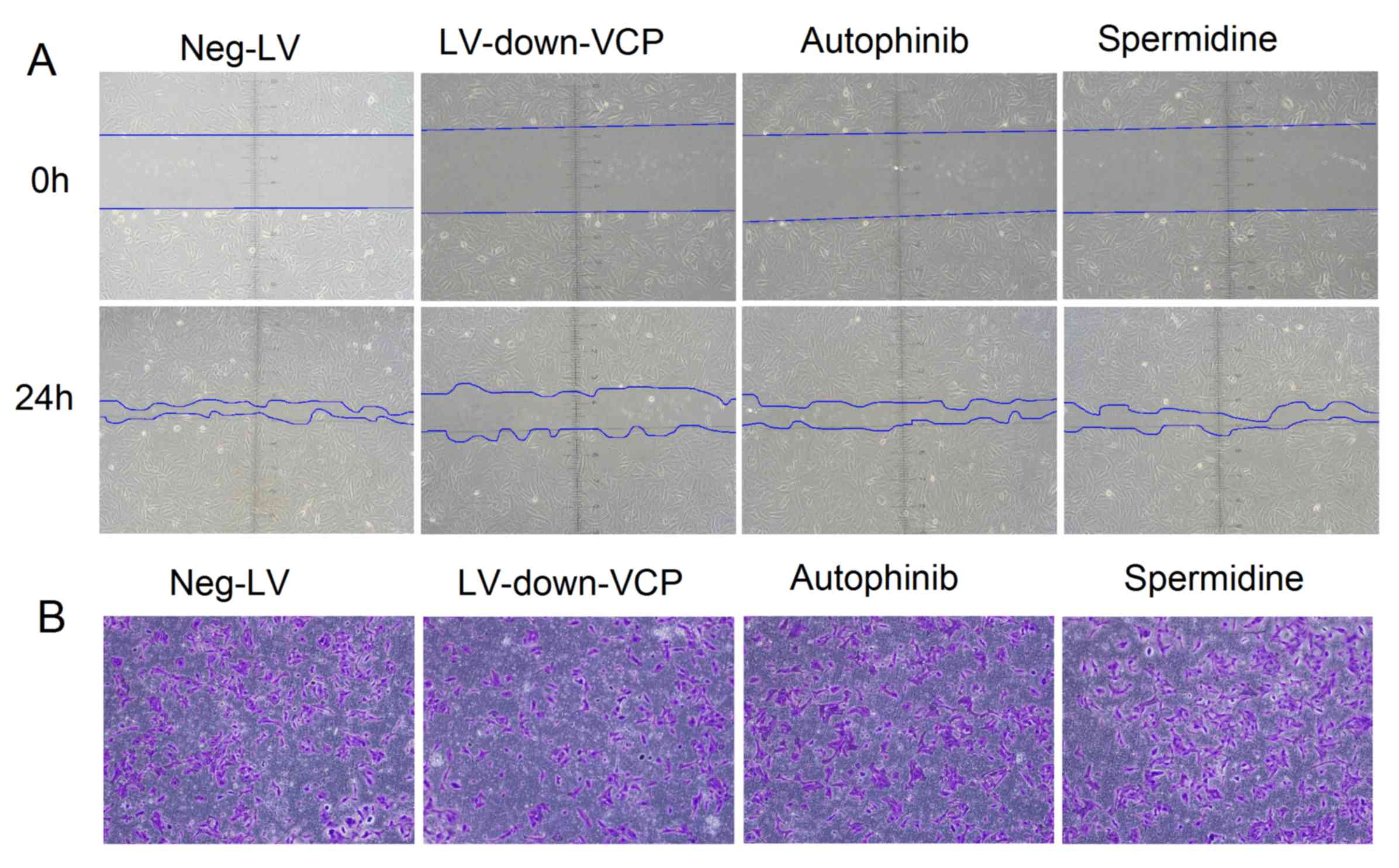
To identify whether VCP expression effects
autophagy, and may therefore alter the malignant phenotype of OS
cells, lentivirus vectors and autophagy-inhibitory and stimulating
agents were used to treat OS cells. Wound-healing and
Matrigel-invasion assays were used to evaluate the malignant
phenotype of OS cells. The results demonstrated that after 24 h,
the migration rate and number of invaded cells were reduced in
cells where VCP was downregulated, compared with those transfected
with Neg-LV. By contrast, there was no marked difference in the
migration rate and invasive ability of cells treated with autophagy
inhibitor or stimulator, compared with that of Neg-LV cells
(Fig. 3). Collectively, the results
revealed that VCP can induce cell autophagy and promote OS cell
invasiveness or migration, but altering autophagy levels in
vitro did not affect cell invasiveness or migration.
VCP induces autophagy via the
ERK/NF-κβ/beclin-1 signaling pathway
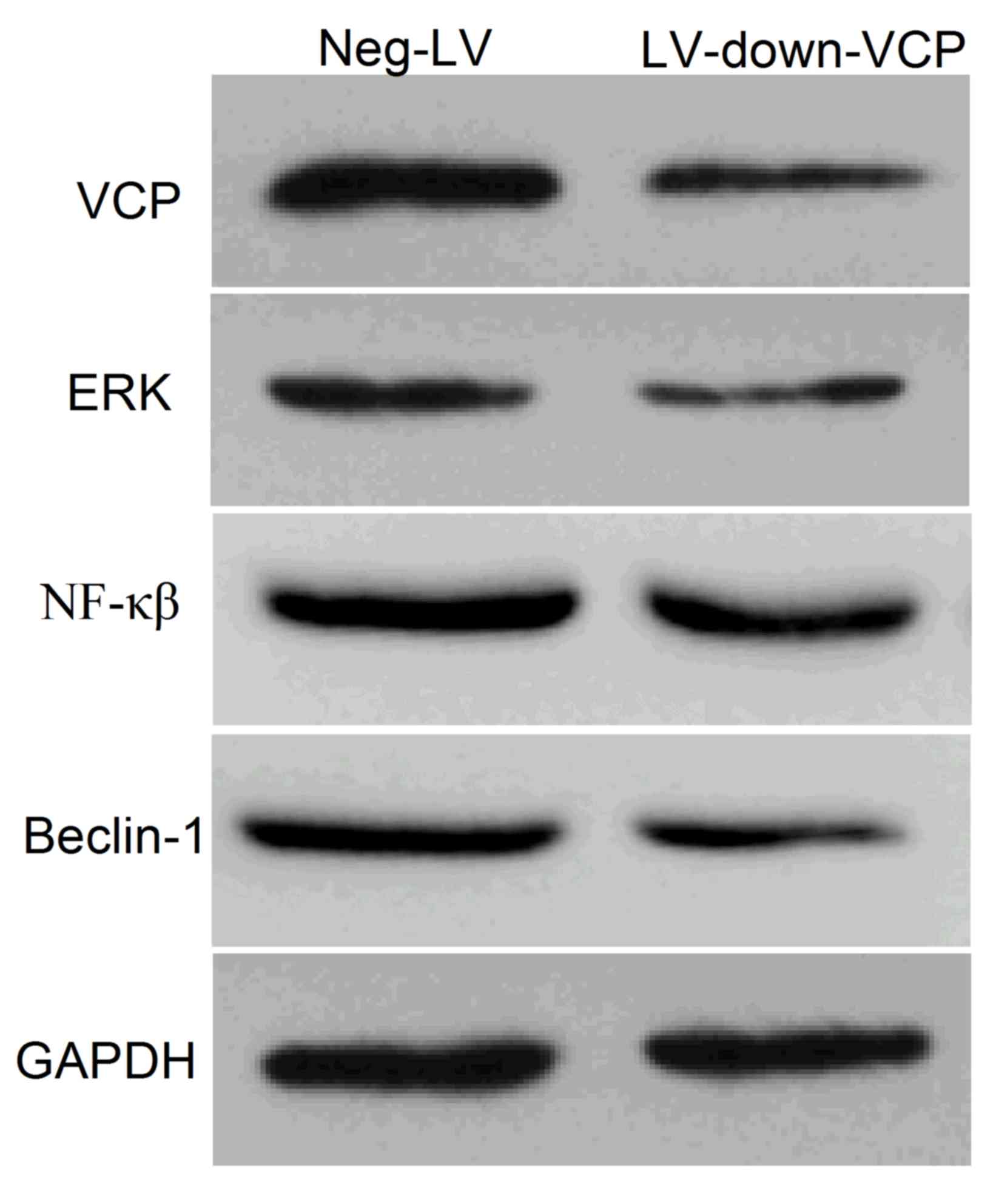
To investigate the mechanism by which VCP induced
autophagy, 143B cells were treated with LV-down-VCP vector, and the
expression levels of VCP, ERK, NF-κβ and autophagy-associated
protein beclin-1 were determined using western blotting. The
results illustrated that VCP inhibition led to a marked decrease in
the expression levels of ERK, NF-κβ and beclin-1 (Fig. 4).
Discussion
OS lung metastases are frequently present at
diagnosis and confer high rates of mortality in patients with OS.
Despite the emergence of a number of novel chemotherapeutical
regimens, the clinical outcome for patients with metastatic OS
remains poor (36). Therefore,
clarification of the molecular mechanism of invasion, and
identification of new molecular targets are imperative to the
development of effective treatments for OS.
VCP is involved in the development of various types
of tumor (37–40), and thus, may serve as a potential
tumor marker. Previous studies have revealed that VCP expression
levels in OS samples with pulmonary metastasis were higher compared
with those without pulmonary metastatic disease. Inhibition of VCP
expression may suppress OS metastasis by modulating the Akt/NF-κβ
signaling pathway (8). However, our
previous study revealed that inhibition of the PI3K/NF-κβ pathway
does not completely reverse VCP-mediated invasion and migration of
OS (Long et al, unpublished data). Other mechanisms of
VCP-mediated OS invasion and migration may be involved.
Further investigation into autophagy and anoikis
resistance may highlight novel mechanisms involved in OS
metastasis. In the late stages of metastasis, autophagy may
compensate for the loss of external signals that promote and
maintain metabolism, and delay apoptosis, therefore allowing cells
to reconnect with the extracellular matrix, and ultimately increase
viability. Autophagy supports the adjustment of metastatic cells to
the altered matrix environment, and allows cells to enter a dormant
state (41). In the present study,
the expression levels of VCP and autophagy-associated proteins
beclin-1 and LC3-II/I were detected in 24-paired samples of OS and
paracancerous tissues. The results revealed a positive correlation
between the expression of VCP and autophagy-associated proteins.
Therefore, an increase in VCP expression levels may promote
autophagy in OS. Silencing VCP expression in 143B cells using an
RNA interference technique resulted in significantly decreased
expression levels of LC3-II/I, suggesting that VCP is able to
induce autophagy. Furthermore, VCP inhibition resulted in reduced
cell survival following 7 days in suspension culture, suggesting
that VCP may also enhance anoikis resistance. Autophagy inhibition
decreased cell survival after seven days in suspension culture, and
conversely, autophagy stimulation promoted cell survival and
counteracted the decrease caused by VCP inhibition. Autophagy did
not affect cell invasiveness or migration. Collectively, the
results indicate that VCP induced autophagy and enhanced cell
survival. to promote OS metastasis, but altering autophagy levels
in vitro did not affect cell invasiveness or migration.
The ERK/NF-κβ signaling pathway induces anoikis and
promotes autophagy (42,43). Specifically, the inhibition of
autophagy by beclin-1 silencing reduces liver cancer metastasis, by
reducing the resistance of malignant cells to apoptosis (44). In the present study, VCP inhibition
resulted in decreased levels of ERK, NF-κβ and beclin-1 protein
expression, in addition to decreased migration and invasiveness
compared with the Neg-LV group. VCP may increase the metastatic
potential of OS through the promotion of autophagy via the
ERK/NF-κβ/Beclin-1 signaling pathway.
In conclusion, in the present study, VCP promoted
migration and invasion in OS by inducing autophagy and possibly
inhibiting anoikis via the ERK/NF-κβ/beclin-1 signaling pathway.
These results may enhance the development of novel treatment
strategies for patients with OS.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Education
Department Foundation of Jiangxi Province (grant no.
GTJ160237).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request
Authors' contributions
XHL organized and analysed the data, and wrote the
manuscript. YFZ and ML completed the cell experiments. SHH and ZLL
performed surgery, specimen collection and tissue experiments. YS
contributed to the project design and experiment management.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Hospital Affiliated with Nanchang
University. Written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Niswander LM and Kim SY: Stratifying
osteosarcoma: Minimizing and maximizing therapy. Curr Oncol Rep.
12:266–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Munajat I, Zulmi W, Norazman MZ and Wan
Faisham WI: Tumour volume and lung metastasis in patients with
osteosarcoma. J Orthop Surg (Hong Kong). 16:182–185. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ewens CA, Kloppsteck P, Förster A, Zhang X
and Freemont PS: Structural and functional implications of
phosphorylation and acetylation in the regulation of the AAA+
protein p97. Biochem Cell Biol. 88:41–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Q, Song C and Li CC: Molecular
perspectives on p97-VCP: Progress in understanding its structure
and diverse biological functions. J Struct Biol. 146:44–57. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lass A, Kujawa M, McConnell E, Paton AW,
Paton JC and Wójcik C: Decreased ER-associated degradation of
alpha-TCR induced by Grp78 depletion with the SubAB cytotoxin. Int
J Biochem Cell Biol. 40:2865–2879. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Livingstone M, Ruan H, Weiner J, Clauser
KR, Strack P, Jin S, Williams A, Greulich H, Gardner J, Venere M,
et al: Valosin-containing protein phosphorylation at Ser784 in
response to DNA damage. Cancer Res. 65:7533–7540. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Long XH, Zhang ZH, Liu ZL, Huang SH and
Luo QF: Inhibiting valosin-containing protein suppresses
osteosarcoma cell metastasis via AKT/nuclear factor of kappa B
signaling pathway in vitro. Indian J Pathol Microbiol. 56:190–195.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin L and Baehrecke EH: Autophagy, cell
death, and cancer. Mol Cell Oncol. 2:e9859132015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Z, Zhao J, Xue J, Zhao X and Liu P:
Autophagy inhibition promotes epithelial-mesenchymal transition
through ROS/HO-1 pathway in ovarian cancer cells. Am J Cancer Res.
6:2162–2177. 2016.PubMed/NCBI
|
|
11
|
Wu HM, Shao LJ, Jiang ZF and Liu RY:
Gemcitabine-induced autophagy protects human lung cancer cells from
apoptotic death. Lung. 194:959–966. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng Y, Miao H, Wu S, Yang W, Zhang Y, Xie
G, Xie X, Li J, Shi C, Ye L, et al: ABHD5 interacts with BECN1 to
regulate autophagy and tumorigenesis of colon cancer independent of
PNPLA2. Autophagy. 12:2167–2182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yin L, Liu S, Li C, Ding S, Bi D, Niu Z,
Han L, Li W, Gao D, Liu Z and Lu J: CYLD downregulates Livin and
synergistically improves gemcitabine chemosensitivity and decreases
migratory/invasive potential in bladder cancer: The effect is
autophagy-associated. Tumour Biol. 37:12731–12742. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Canc. 9:274–284. 2009. View
Article : Google Scholar
|
|
15
|
Vanharanta S and Massagué J: Origins of
metastatic traits. Cancer Cell. 24:410–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liotta LA and Kohn EC: The
microenvironment of the tumour-host interface. Nature. 411:375–379.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gilmore AP: Anoikis. Cell Death Differ. 12
(Suppl 2):S1473–S1477. 2005. View Article : Google Scholar
|
|
18
|
Lock R and Debnath J: Extracellular matrix
regulation of autophagy. Curr Opin Cell Biol. 20:583–588. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Debnath J: Detachment-induced autophagy
during anoikis and lumen formation in epithelial acini. Autophagy.
4:351–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng YF, Shi YH, Ding ZB, Ke AW, Gu CY,
Hui B, Zhou J, Qiu SJ, Dai Z and Fan J: Autophagy inhibition
suppresses pulmonary metastasis of HCC in mice via impairing
anoikis resistance and colonization of HCC cells. Autophagy.
9:2056–2068. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wiederstein JL, Nolte H, Günther S, Piller
T, Baraldo M, Kostin S, Bloch W, Schindler N, Sandri M, Blaauw B,
et al: Skeletal muscle-specific methyltransferase METTL21C
trimethylates p97 and regulates autophagy-associated protein
breakdown. Cell Rep. 23:1342–1356. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ozsoy AZ, Cayli S, Sahin C, Ocakli S,
Sanci TO and Ilhan DB: Altered expression of p97/Valosin containing
protein and impaired autophagy in preeclamptic human placenta.
Placenta. 67:45–53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Zhao GD, Shi Z, Qi LL, Zhou LY and
Fu ZX: The Ras/Raf/MEK/ERK signaling pathway and its role in the
occurrence and development of HCC. Oncol Lett. 12:3045–3050. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang S, Shi R, Chen S, Wei X, Zhou Q and
Wang Y: All-trans retinoic acid inhibits the proliferation of
SGC7901 cells by regulating caveolin-1 localization via the
ERK/MAPK signaling pathway. Oncol Lett. 15:1523–1528.
2018.PubMed/NCBI
|
|
25
|
Yan KH, Yao CJ, Hsiao CH, Lin KH, Lin YW,
Wen YC, Liu CC, Yan MD, Chuang SE, Lai GM and Lee LM: Mefloquine
exerts anticancer activity in prostate cancer cells via
ROS-mediated modulation of Akt, ERK, JNK and AMPK signaling. Oncol
Lett. 5:1541–1545. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi M and Elion EA: MAP kinase pathways. J
Cell Sci. 118:3569–3572. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang B, Liang W, Liao Y, Li Z, Wang Y and
Yan C: PEA15 promotes liver metastasis of colorectal cancer by
upregulating the ERK/MAPK signaling pathway. Oncol Rep. 41:43–56.
2019.PubMed/NCBI
|
|
28
|
Huang Y, Zou Y, Lin L, Ma X and Zheng R:
miR-101 regulates the cell proliferation and apoptosis in diffuse
large B-cell lymphoma by targeting MEK1 via regulation of the
ERK/MAPK signaling pathway. Oncol Rep. 41:377–386. 2019.PubMed/NCBI
|
|
29
|
Wang D, Xu Y, Feng L, Yin P, Song SS, Wu
F, Yan P and Liang Z: RGS5 decreases the proliferation of human
ovarian carcinoma-derived primary endothelial cells through the
MAPK/ERK signaling pathway in hypoxia. Oncol Rep. 41:165–177.
2019.PubMed/NCBI
|
|
30
|
Chen Z, Gibson TB, Robinson F, Silvestro
L, Pearson G, Xu B, Wright A, Vanderbilt C and Cobb MH: MAP kinase.
Chem Rev. 101:2449–2476. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Copetti T, Bertoli C, Dalla E, Demarchi F
and Schneider C: p65/RelA modulates BECN1 transcription and
autophagy. Mol Cell Biol. 29:2594–2608. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Copetti T, Demarchi F and Schneider C:
p65/RelA binds and activates the beclin 1 promoter. Autophagy.
5:858–859. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schweitzer K, Pralow A and Naumann M:
p97/VCP promotes Cullin-RING-ubiquitin-ligase/proteasome-dependent
degradation of IκBα and the preceding liberation of RelA from
ubiquitinated IκBα. J Cell Mol Med. 20:58–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McNeill H, Knebel A, Arthur JS, Cuenda A
and Cohen P: A novel UBA and UBX domain protein that binds
polyubiquitin and VCP and is a substrate for SAPKs. Biochem J.
384:391–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Z, Wang Y, Li C, Shi Z, Hao Q, Wang
W, Song X, Zhao Y, Jiao S and Zhou Z: The transitional endoplasmic
reticulum ATPase p97 regulates the alternative nuclear factor NF-κB
signaling via partial degradation of the NF-κB subunit p100. J Biol
Chem. 290:19558–19568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ando K, Heymann MF, Stresing V, Mori K,
Rédini F and Heymann D: Current therapeutic strategies and novel
approaches in osteosarcoma. Cancers (Basel). 5:591–616. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yamamoto S, Tomita Y, Hoshida Y, Takiguchi
S, Fujiwara Y, Yasuda T, Yano M, Nakamori S, Sakon M, Monden M and
Aozasa K: Expression level of valosin-containing protein is
strongly associated with progression and prognosis of gastric
carcinoma. J Clin Oncol. 21:2537–2544. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamamoto S, Tomita Y, Hoshida Y, Sakon M,
Kameyama M, Imaoka S, Sekimoto M, Nakamori S, Monden M and Aozasa
K: Expression of valosin-containing protein in colorectal
carcinomas as a predictor for disease recurrence and prognosis.
Clin Cancer Res. 10:651–657. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamamoto S, Tomita Y, Nakamori S, Hoshida
Y, Nagano H, Dono K, Umeshita K, Sakon M, Monden M and Aozasa K:
Elevated expression of valosin-containing protein (p97) in
hepatocellular carcinoma is correlated with increased incidence of
tumor recurrence. J Clin Oncol. 21:447–452. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yamamoto S, Tomita Y, Nakamori S, Hoshida
Y, Iizuka N, Okami J, Nagano H, Dono K, Umeshita K, Sakon M, et al:
Valosin-containing protein (p97) and Ki-67 expression is a useful
marker in detecting malignant behavior of pancreatic endocrine
neoplasms. Oncology. 66:468–475. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guadamillas MC, Cerezo A and del Pozo MA:
Overcoming anoikis-pathways to anchorage-independent growth in
cancer. J Cell Sci. 124:3189–3197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu L, Zhang Q, Dai W, Li S, Feng J, Li J,
Liu T, Xu S, Wang W, Lu X, et al: Quercetin pretreatment attenuates
hepatic ischemia reperfusion-induced apoptosis and autophagy by
inhibiting ERK/NF-κB pathway. Gastroenterol Res Pract 2017.
97242172017.
|
|
43
|
Paoli P, Giannoni E and Chiarugi P:
Anoikis molecular pathways and its role in cancer progression.
Biochim Biophys Acta 1833. 3481–3498. 2013.
|
|
44
|
Peng YF, Shi YH, Ding ZB, Ke AW, Gu CY,
Hui B, Zhou J, Qiu SJ, Dai Z and Fan J: Autophagy inhibition
suppresses pulmonary metastasis of HCC in mice via impairing
anoikis resistance and colonization of HCC cells. Autophagy.
9:2056–2068. 2013. View Article : Google Scholar : PubMed/NCBI
|