Introduction
Precise molecular targeted therapy based on specific
driver gene mutations has improved the prognosis of patients with
advanced non-small cell lung cancer (NSCLC) (1). Mutations of driver genes, including the
epidermal growth factor receptor (EGFR) and anaplastic lymphoma
kinase, substitution in the serine/threonine-protein kinase B-raf
V600E and alterations of the repressor of silencing 1, are used to
guide targeted therapy (2).
Furthermore, alterations in driver genes, including cellular tumor
antigen p53 (TP53), are associated with treatment efficacy in NSCLC
(3,4). Mutations of EGFR are present in 40–55
and 5–15% of lung adenocarcinoma cases in patients from Asia and
Europe/USA, respectively (5,6). Examining the mutational status of EGFR,
including sensitive and drug-resistant mutations such as T790M
and/or TP53, may improve tumor treatment by providing targeted
therapy through the use of EGFR-tyrosine kinase inhibitors (TKIs)
(7,8).
Although tumor biopsy is considered as the gold
standard for detecting mutations in driver genes, poor patient
health status, the invasive nature of biopsies and inadequate tumor
tissue samples make the completion of gene testing from tumor
biopsies difficult (9,10). In particular, in patients receiving
targeted therapy, it is not possible to use tissue biopsies to
monitor drug resistance because of the invasive nature of the
biopsy procedure. Furthermore, the underlying inter- and
intratumoral heterogeneity may lead to false-negative results in
gene detection (11). Liquid
biopsies therefore represent an effective and non-invasive
alternative to detect gene mutations, and a relatively
comprehensive method to monitor changes during treatment (12,13).
It has been hypothesized that circulating tumor DNA
(ctDNA) may originate from tumor cells in the peripheral
circulation and may be representative of the cancer genomic
profile. The concordance rate of matched mutations in ctDNA with
those in the tumor tissue DNA is >90% in certain cases (14,15).
Plasma ctDNA may overcome the shortcomings of tumor heterogeneity
by accurately identifying the extent of present mutations (16), and may also be used to detect drug
resistance-associated mutations in driver genes prior to imaging
results of computed tomography (CT) scans through dynamic
monitoring during the treatment process (17). ctDNA in urine is derived from
systemic circulation and may reflect the genetic information of a
tumor (18–20). A previous study reported that
detection of ctDNA in urine represents a non-invasive and highly
accurate method of genetic profiling in patients with advanced
NSCLC (21). However, some
limitations exist, including the low amount of ctDNA in the
circulating free DNA (~0.01% of circulating free DNA under certain
circumstances, particularly for primary cancer) (22). It is therefore difficult to determine
a unique genomic profile of NSCLC based on a single fluid sample.
Highly sensitive assays are required to improve the genetic
profiling accuracy and address this challenge (23). As ctDNA in urine and plasma have
different genomic profiles and contain unique gene alterations
(24), they may help to overcome the
tumor heterogeneity present in a biopsy. In addition, early
monitoring of dynamic changes in driver gene mutations could
predict the outcome of targeted therapy and provide superior
predictive value compared with imaging evaluation of CT scan
(3).
Cell-free DNA (cfDNA) and tumor-associated DNA are
present in the sputum of patients with NSCLC (25). Free DNA may originate from malignant
cells and inflammatory cells in upper airway cancer, and may be
used to detect genetic alterations (25). A previous study demonstrated that
cfDNA in sputum from patients with NSCLC can be used to detect gene
variations efficiently, and that the cfDNA profiles obtained from
plasma, urine and sputum were different, which increased the
overall rate of concurrence of mutations between cfDNA and tumor
DNA significantly when the three samples were combined (26). However, to the best of our knowledge,
detailed information on the consistency of individual gene
detection in different sample types has not yet been described.
In the present study, the combination of plasma,
sputum and urine from patients with NSCLC was used to evaluate the
genomic profiles of liquid samples and tumor tissue using
next-generation sequencing (NGS). Furthermore, this study aimed to
evaluate the clinical performance of NGS to identify EGFR and TP53
mutations in matched pretreatment sputum, urine and plasma samples
compared with tumor tissue.
Materials and methods
Patients
A total of 50 patients diagnosed with advanced NSCLC
admitted at The Chinese People's Liberation Army General Hospital
(Beijing, China) were recruited for the present study between
October 2015 and December 2017. The inclusion and exclusion
criteria for patient enrollment are stated in our previous study
(26). Advanced NSCLC patients
having enough tumor tissue and fluid samples including plasma,
urine and sputum to analyze genetic mutations were recruited, while
patients who not want to receive regular follow-up were excluded.
Patients were divided into two groups as follows: i) A total of 32
patients newly diagnosed with advanced NSCLC who had not received
any treatment; and ii) 18 patients with drug resistance acquired
following first-line EGFR-TKI treatment and who did not receive
further treatment. All patients with an EGFR sensitizing mutation
detected by NGS received first-line EGFR-TKI, whereas other
patients were given standard chemotherapy that consisted of
cisplatin with pemetrexed for adenocarcinoma or gemcitabine with
cisplatin for squamous cell carcinoma. Patients with a EGFR T790M
mutation were treated with osimertinib according to guidelines
(2). Signed informed consent was
obtained from all patients or their families, and the present study
was approved by The Ethics Committee of The Chinese People's
Liberation Army General Hospital.
Sample collection and processing
Matching sputum, plasma and urine samples and tumor
tissues were collected prior to first-line therapy from newly
diagnosed patients or prior to changing treatment regimen from
patients who had acquired drug resistance. The four types of
samples were collected or extracted and stored as described
previously (26). All extraction and
analyses procedures were performed in a CAP/CLIA-certified
diagnostic laboratory.
Library preparation and NGS
A KAPA Hyper Prep kit (Kapa Biosystems; Roche
Diagnostics) was used to prepare the sequencing libraries according
to the manufacturer's protocol for liquid samples (26). Targeted sequencing was conducted
using GeneseeqOne™ 416-gene panel (Nanjing Geneseeq Technology
Inc.). The protocol for cfDNA isolation were similar among the
different liquid samples and details of the procedures were
described in our previous studies (26,27).
Statistical analysis
Gene detection from tumor tissue is considered as
the gold standard. The definitions of sensitivity, specificity and
positive predictive value of gene alterations in each liquid sample
are assessed in our previous study (26). Sensitivity is the ratio of mutations
detected from liquid samples to the mutations detected from tissue
sample in the same patients. The specificity is the ratio of the
wild-type of driver gene detected from liquid samples to that
detected from tissue samples in the same patients (28). The positive predictive value is the
ratio of actual mutations to all of the mutations detected from
liquid samples obtained from the same patient. The sensitivity and
specificity of EGFR and TP53 mutations were compared between each
liquid sample and the combination of all liquid samples was
analyzed using χ2 test. A Fisher's exact test and a
one-way ANOVA with Bonferroni's post hoc test were used to compare
the objective response rate (ORR) and progression free survival
(PFS) for EGFR and TP53 mutation in different kinds of samples,
respectively, between subgroups with or without EGFR mutations in
the different types of liquid samples from patients receiving a
first-line EGFR-TKI. The effect of a TP53 mutation on ORR in
patients receiving a first line EGFR-TKI was analyzed using a
Fisher's exact test, whereas a one-way ANOVA was used to determine
the effect of TP53 mutations on PFS in patients treated with a
first-line EGFR-TKI. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS version 22 (IBM Corp.).
Results
Patient characteristics
Similarly to our previous study, 60% of participants
were women, 86% had stage IV lung cancer and 96% were diagnosed
with adenocarcinoma (26). Detailed
information on each group of patients is presented in Table I. As previously demonstrated, the
most frequently mutated genes were TP53 and EGFR, which were
present in 52.2 and 48.3% of all kinds of successfully tested
samples (blood, urine, sputum and tumor tissue), respectively
(26). A total of 32 patients
carried an EGFR sensitizing mutation and 9 patients had the T790M
mutation, and 31 patients were diagnosed with TP53 mutations and 18
patients had both EGFR and TP53 mutations. The distribution of EGFR
and TP53 mutations in the different types of samples are presented
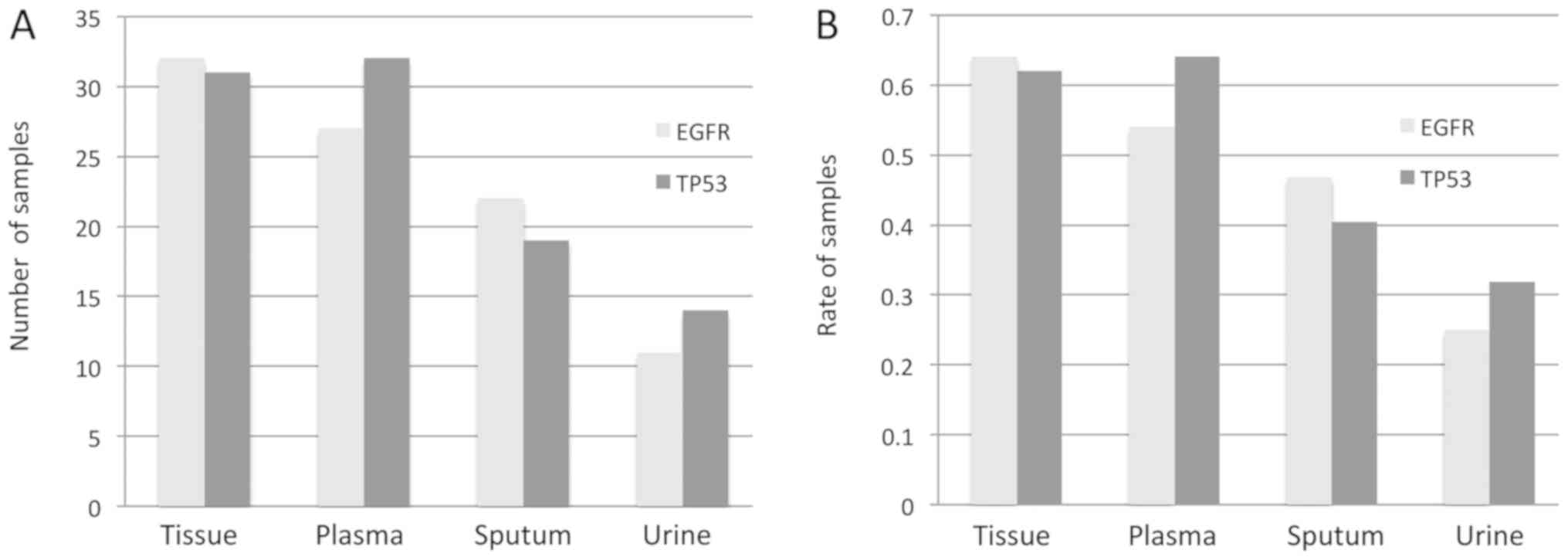
in Fig. 1. The appropriate
therapeutic regimen was based on the genetic profiling results and
each patient enrolled was followed up regularly every three months
until death.
 | Table I.Clinical and demographic
characteristics of patients with non-small cell lung cancer. |
Table I.
Clinical and demographic
characteristics of patients with non-small cell lung cancer.
|
Characteristics | All patients,
(n=50) | Newly diagnosed
(n=32) | Acquired resistance
(n=18) |
|---|
| Age, years
(range) | 61 (36–81) | 61 (36–81) | 60 (43–67) |
| Sex, n (%) |
|
|
|
|
Male | 20 (40) | 16 (50) | 4 (22) |
|
Female | 30 (60) | 16 (50) | 14 (78) |
| Smoking, n (%) |
|
|
|
|
Yes | 15 (30) | 12 (38) | 3 (17) |
| No | 35 (70) | 20 (62) | 15 (83) |
| Histology, n
(%) |
|
|
|
|
Adenocarcinoma | 48 (96) | 30 (94) | 18 (100) |
|
Squamous | 1 (2) | 1 (3) | 0 (0) |
|
Non-specific NSCLC | 1 (2) | 1(3) | 0 (0) |
| Disease stage, n
(%) |
|
|
|
|
IIIb | 7 (14) | 6 (19) | 1 (6) |
| IV | 43 (86) | 26 (81) | 17 (94) |
| Number of
metastases, n (%) |
|
|
|
| 0 | 14 (16) | 8 (25) | 2 (11) |
| 1 | 10 (18) | 4 (12) | 10 (56) |
|
>1 | 26 (66) | 20 (63) | 6 (33) |
| Biopsy site for
genotyping, n (%) |
|
|
|
|
Lung | 47 (94) | 29 (91) | 10 (100) |
|
Liver | 1 (2) | 1 (3) | 0 (0) |
|
Bone | 1 (2) | 1 (3) | 0 (0) |
| Lymph
node | 1 (2) | 1 (3) | 0 (0) |
Assay characteristics of EGFR and TP53
mutations in tumor tissue and liquid biopsies
The presence of EGFR and TP53 mutations in the three
liquid biopsies and in the tissue sample of patients newly
diagnosed with NSCLC and of patients with acquired drug-resistant
NSCLC are presented in Tables SI
and SII, respectively.
The frequency of EGFR or TP53 mutations in the
different types of samples is presented in Fig. 2. The mean frequency of EGFR mutations
was 27.0, 8.2, 3.3 and 4.6% in tumor tissues, plasma samples,
sputum samples and urine samples, respectively. Furthermore, a
significant difference was observed between tumor tissue and each
liquid biopsy (all P<0.001). In addition, a significant
difference in EGFR mutations frequency was observed between tumor
tissues and each type of liquid sample independently (all
P<0.001). The mean frequency of TP53 mutations was 24.0, 7.9,
3.7 and 3.2% in tumor tissue, plasma, sputum and urine,
respectively, and a significant difference in TP53 mutations
frequency was observed between tumor tissues and each liquid sample
independently (all P<0.001). The distribution of EGFR and TP53
mutations in tissue and matched liquid samples are presented in
Table II. Overall, the sensitivity
of detecting an EGFR sensitizing mutation was 84% for plasma, 63%
for sputum and 28% for urine samples, with a combined sensitivity
of 91% (P=0.001). In addition, the sensitivity of detecting EGFR
sensitizing mutations in exons 18–20 was improved when the three
types of liquid samples were combined. This sensitivity was 81% for
plasma, 63% for sputum, 31% for urine and 94% when samples were
combined (P=0.003). The sensitivity of detecting a mutation in exon
21 of EGFR gene in the different body fluids was as follows:
Plasma, 86%; sputum, 67%; urine, 29%; and 90% when the three
samples types were combined (P=0.001). Combining the three samples
did not affect the sensitivity of detecting T790M mutation [89%
(plasma) vs. 89% (combination); Table
III]. Furthermore, an EGFR exon 19 deletion with a frequency of
11.8% was detected in the sputum and tumor tissue; however, it was
not detected in the plasma or urine samples of newly diagnosed
patient (Table SI). A patient
accepted EGFR-TKIs as a first-line therapy and had a PFS of 10
months from clinical observation. In addition, T790M mutation
(Table SII) was detected in plasma
and sputum with frequencies of 4.84 and 0.10%, respectively;
however, it was not detected in the tumor tissue of patients with
drug-resistance. The patient was subsequently treated with
osimertinib as second line therapy and had a PFS of 13 months.
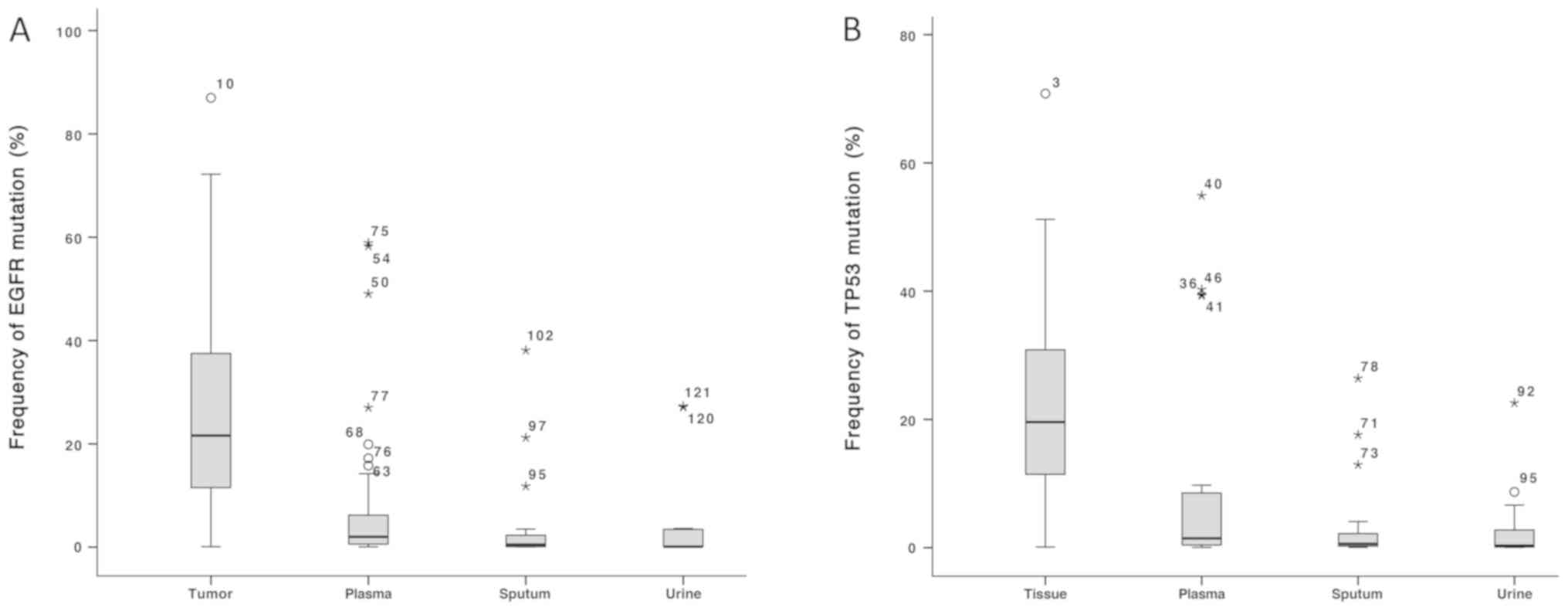 | Figure 2.Frequency of EGFR and TP53 mutations
in tissue, plasma, sputum and urine samples. (A) Frequency of EGFR
mutations, and (B) frequency of TP53 mutations in tissue, plasma,
sputum and urine samples. EGFR and TP53 mutation frequency was
significantly higher in tumor samples compared with plasma, urine
and sputum samples. EGFR, epidermal growth factor; TP53, cellular
tumor antigen p53; *,Oextreme outliers and mild
outliners, respectively. Numbers on the outlier symbols represent
the number of each outlier. |
 | Table II.Number of EGFR and TP53 mutations in
tissue and matched liquid samples. |
Table II.
Number of EGFR and TP53 mutations in
tissue and matched liquid samples.
|
| Plasma | Sputum | Urine | Combination |
|---|
|
|
|
|
|
|
|---|
| Mutation | + | − | Total | + | − | Total | + | − | Total | + | − | Total |
|---|
| EGFR E18-20
sensitizing mutation, n |
| + | 13 | 3 | 16 | 10 | 6 | 16 | 5 | 11 | 16 | 15 | 1 | 16 |
| − | 0 | 34 | 34 | 0 | 31 | 31 | 0 | 28 | 28 | 0 | 34 | 34 |
|
Total | 13 | 37 | 50 | 10 | 37 | 47 | 5 | 39 | 44 | 15 | 35 | 50 |
| EGFR E21
sensitizing mutation, n |
| + | 18 | 3 | 21 | 14 | 7 | 21 | 6 | 15 | 21 | 19 | 2 | 21 |
| − | 1 | 28 | 29 | 1 | 25 | 26 | 0 | 23 | 23 | 1 | 28 | 29 |
|
Total | 19 | 31 | 50 | 15 | 32 | 47 | 6 | 38 | 44 | 20 | 30 | 50 |
| EGFR T790M, n |
| + | 8 | 1 | 9 | 6 | 3 | 9 | 3 | 6 | 9 | 8 | 1 | 9 |
| − | 1 | 8 | 9 | 1 | 8 | 9 | 0 | 9 | 9 | 1 | 8 | 9 |
|
Total | 9 | 9 | 18 | 7 | 11 | 18 | 3 | 15 | 18 | 9 | 9 | 18 |
| TP53, n |
| + | 27 | 4 | 31 | 14 | 17 | 31 | 7 | 20 | 27 | 29 | 2 | 31 |
| − | 5 | 14 | 19 | 4 | 12 | 16 | 4 | 13 | 17 | 9 | 10 | 19 |
|
Total | 32 | 18 | 50 | 18 | 29 | 47 | 11 | 33 | 44 | 38 | 12 | 50 |
 | Table III.Sensitivity, specificity and positive
predictive value of EGFR and TP53 mutations in liquid samples
compared with tissue samples. |
Table III.
Sensitivity, specificity and positive
predictive value of EGFR and TP53 mutations in liquid samples
compared with tissue samples.
| A, sensitive
mutation in exons 18/19/20 of EGFR |
|---|
|
|---|
|
| Sensitivity, % (95%
CI) | Specificity, % (95%
CI) | Positive predictive
value, % (95% CI) |
|---|
|
|
|
|
|
|---|
| Treatment
stage | Plasma | Sputum | Urine | All | P-value | Plasma | Sputum | Urine | All | P-value | Plasma | Sputum | Urine | All | P-value |
|---|
| Newly
diagnosed | 60 (17–93) | 40 (7–83) | 0 (0–54) | 80 (30–99) | 0.070 | 100 (85–100) | 100 (85–100) | 100 (85–100) | 100 (85–100) | / | 100 (31–100) | 100 (20–100) | / | 100 (40–100) | / |
| Drug-resistant | 91 (57–100) | 73 (39–93) | 45 (18–75) | 100 (68–100) | 0.018 | 100 (60–100) | 100 (60–100) | 100 (60–100) | 100 (60–100) | / | 100 (66–100) | 100 (60–100) | 100 (46–100) | 100 (68–100) | / |
| All | 81 (54–95) | 63 (36–84) | 31 (12–59) | 94 (68–100) | 0.003 | 100 (88–100) | 100 (88–100) | 100 (88–100) | 100 (88–100) | / | 100 (72–100) | 100 (66–100) | 100 (46–100) | 100 (73–100) | / |
|
| B, sensitive
mutation in 21 exon of EGFR |
|
|
| Sensitivity, %
(95% CI) | Specificity, %
(95% CI) | Positive
predictive value, % (95% CI) |
|
|
|
|
|
| Treatment
stage | Plasma | Sputum | Urine | All | P-value | Plasma | Sputum | Urine | All | P-value | Plasma | Sputum | Urine | All | P-value |
|
| Newly
diagnosed | 93 (64–100) | 57 (30–81) | 36 (14–64) | 93 (64–100) | 0.001 | 100 (81–100) | 100 (81–100) | 100 (81–100) | 100 (80–100) | / | 100 (72–100) | 100 (60–100) | 100 (46–100) | 100 (72–100) | / |
| Drug-resistant | 71 (30–95) | 86 (42–96) | 14 (1–60) | 86 (42–96) | 0.012 | 91 (57–100) | 91 (57–100) | 100 (68–100) | 91 (57–100) | 0.615 | 83 (36–99) | 86 (42–96) | 100 (5–100) | 86 (42–96) | 0.008 |
| All | 86 (63–96) | 67 (43–85) | 29 (12–52) | 90 (68–98) | 0.001 | 97 (82–100) | 97 (82–100) | 100 (87–100) | 97 (82–100) | 0.626 | 95 (72–100) | 93 (66–100) | 100 (52–100) | 95 (73–100) | 0.874 |
|
| C, T790M of
EGFR |
|
|
| Sensitivity, %
(95% CI) | Specificity, %
(95% CI) | Positive
predictive value, % (95% CI) |
|
|
|
|
|
| Treatment
stage | Plasma | Sputum | Urine | All | P-value | Plasma | Sputum | Urine | All | P-value | Plasma | Sputum | Urine | All | P-value |
|
| Drug-resistant | 89 (51–99) | 56 (23–85) | 33 (9–69) | 89 (51–99) | 0.024 | 89 (51–99) | 89 (51–99) | 100 (63–100) | 89 (51–99) | 0.612 | 89 (51–99) | 83 (36–99) | 100 (31–100) | 89 (51–99) | 0.832 |
|
| D, TP53
mutation |
|
|
| Sensitivity, %
(95% CI) | Specificity, %
(95% CI) | Positive
predictive value, % (95% CI) |
|
|
|
|
|
| Treatment
stage | Plasma | Sputum | Urine | All | P-value | Plasma | Sputum | Urine | All | P-value | Plasma | Sputum | Urine | All | P-value |
|
| Newly
diagnosed | 90 (68–98) | 48 (26–70) | 33 (15–57) | 95 (74–100) | 0.001 | 73 (39–93) | 64 (32–88) | 64 (32–88) | 36 (12–68) | 0.336 | 86 (64–96) | 72 (42–90) | 64 (32–88) | 74 (53–88) | 0.474 |
| Drug-resistant | 80 (44–96) | 40 (14–73) | 20 (4–56) | 90 (54–99) | 0.002 | 75 (36–96) | 83 (36–99) | 88 (47–99) | 75 (36–96) | 0.841 | 80 (44–96) | 86 (42–99) | 75 (22–99) | 82 (48–97) | 0.959 |
| All | 87 (69–96) | 45 (28–64) | 26 (12–47) | 94 (77–100) | 0.001 | 74 (49–90) | 75 (47–92) | 76 (50–92) | 53 (29–75) | 0.404 | 84 (66–94) | 74 (49–90) | 64 (36–86) | 76 (59–88) | 0.504 |
In all patients enrolled in the present study, the
sensitivity of TP53 mutation detection was 87% in plasma samples,
45% in sputum samples and 26% in urine samples. By combining the
three body fluid samples, the sensitivity of TP53 mutation
detection increased to 94% (P=0.001). In the newly diagnosed
patients, the sensitivity of TP53 mutations in plasma, urine and
sputum sample was 90, 48 and 33%, respectively, and the combined
sensitivity increased to 95% (P=0.001). In the drug-resistant
group, the sensitivity also significantly increased from 80% in the
plasma sample to 90% when all samples were combined (P=0.002;
Table III).
Predictors of EGFR and TP53 mutations
detection sensitivity
To further examine the factors affecting the
detection sensitivity of driver gene mutations in the different
body fluid samples, a subgroup analysis based on the clinical
characteristics of patients was performed. The results demonstrated
an association between the number of metastases and the detection
sensitivity of EGFR and TP53 mutations (Table IV). The sensitivity of detecting
EGFR sensitizing mutations in the group with >1 metastases was
greater compared with that in the group with ≤1 metastasis and was
as follows: Sputum, 77% compared with 36% (P=0.025); urine, 42%
compared with 0% (P=0.009); and combined, 100% compared with 73%
(P=0.021). Detection sensitivity for TP53 mutations in urine was
also higher in patients with >1 metastases compared with that in
patients with ≤1 metastasis (39% compared with 0%; P=0.041).
However, there was no significant difference in sensitivities based
on sex, age and smoking history.
 | Table IV.Association between sensitivity of
detection for EGFR and TP53 mutations and clinical
characteristics. |
Table IV.
Association between sensitivity of
detection for EGFR and TP53 mutations and clinical
characteristics.
| A, Sensitizing
mutation in EGFR |
|---|
|
|---|
| Characteristic | Plasma | Sputum | Urine | Total |
|---|
| Sex |
|
|
|
|
| Male, n
(%) | 8
(89) | 6
(67) | 5
(56) | 9
(100) |
| Female,
n (%) | 23 (82) | 18 (64) | 6
(21) | 25 (89) |
|
P-value |
0.523 |
0.614 |
0.066 |
0.422 |
| Number of
metastases, n |
|
|
|
|
| >1,
n (%) | 23 (88) | 20 (77) | 11 (42) | 26 (100) |
| ≤1, n
(%) | 8
(73) | 4
(36) | 0
(0) | 8
(73) |
|
P-value |
0.236 |
0.025 |
0.009 |
0.021 |
| Smoking |
|
|
|
|
| Yes, n
(%) | 7
(88) | 6
(75) | 4
(50) | 8
(100) |
| No, n
(%) | 24 (83) | 18 (62) | 7
(24) | 26
(90) |
|
P-value |
0.560 |
0.409 |
0.163 |
0.470 |
| Age, years |
|
|
|
|
| >60,
n (%) | 6
(86) | 6
(86) | 1
(14) | 6
(86) |
| ≤60, n
(%) | 25 (83) | 18 (60) | 10 (33) | 28
(93) |
|
P-value |
0.685 |
0.204 |
0.310 |
0.477 |
|
| B, TP53
mutation |
|
|
Characteristic | Plasma | Sputum | Urine | Total |
|
| Sex |
|
|
|
|
| Male, n
(%) | 15 (94) | 7
(44) | 5 (31) | 16 (100) |
| Female,
n (%) | 12 (80) | 7
(47) | 4 (27) | 13 (87) |
|
P-value |
0.275 |
0.578 | 0.546 |
0.226 |
| Number of
metastases, n |
|
|
|
|
| >1,
n (%) | 21 (91) | 12 (52) | 9 (39) | 22 (96) |
| ≤1, n
(%) | 6
(75) | 2
(25) | 0 (0) | 7
(88) |
|
P-value |
0.268 |
0.180 | 0.041 |
0.456 |
| Smoking |
|
|
|
|
| Yes, n
(%) | 14 (93) | 7
(47) | 5 (33) | 15 (100) |
| No, n
(%) | 13 (81) | 7
(44) | 4 (25) | 14 (88) |
|
P-value |
0.325 |
0.578 | 0.454 |
0.258 |
| Age, years |
|
|
|
|
| >60,
n (%) | 8
(80) | 4
(40) | 4 (40) | 9
(94) |
| ≤60, n
(%) | 19 (90) | 10 (48) | 5 (24) | 20 (95) |
|
P-value |
0.387 |
0.497 | 0.302 |
0.548 |
Prognostic predictions of first-line
EGFR-TKIs efficiency based on cfDNA
In the newly diagnosed group, 15 patients were
identified as having tumors with EGFR sensitizing mutations and
therefore received a first-line EGFR-TKI treatment. Among these
patients, the ORRs were 67, 67, 63 and 66% in the tissue, plasma,
sputum and urine, respectively, and 75% when the three types of
samples were combined (P=0.981). The PFS times were similar in
patients with EGFR sensitizing mutations in tissue (9.0 months),
plasma (7.5 months), sputum (7.9 months) and urine (7.3 months;
P=0.721; Table V). The ORR values
were 56% in the tissue samples from patients with TP53 mutations
and 83% in the tissue samples from patients without TP53 mutations
(P=0.580). The ORR values were 60% in the plasma samples from
patients with TP53 mutations and 80% in the plasma samples from
patients without TP53 mutations (P=0.6). The ORR values were 64% in
the combined liquid samples from patients with TP53 mutations and
75% in the combined liquid samples from patients without TP53
mutations (P=0.566). By excluding the group of patients with TP53
mutations in the urine samples, as patients with or without TP53
mutation in urine had the same PFS, the PFS times of patients with
TP53 mutations were decreased compared with the patients without
TP53 mutations, and were as follows: Tissue, 8.2 months compared
with 10.2 months (P=0.412); plasma, 8.4 months compared with 10.2
months (P=0.466); sputum, 8.3 months compared with 9.1 months
(P=0.904); and when combined, 8.8 months compared with 10.3 months
(P=0.599; Table VI). However, there
was no significant difference in these comparisons. The DCR of
patients receiving first-line EGFR-TKI among different groups also
demonstrated no significant difference (Tables V and VI).
 | Table V.Prediction of first-line
EGFR-tyrosine kinase inhibitor therapy effect based on EGFR
mutation status in patients newly diagnosed with non-small cell
lung cancer. |
Table V.
Prediction of first-line
EGFR-tyrosine kinase inhibitor therapy effect based on EGFR
mutation status in patients newly diagnosed with non-small cell
lung cancer.
| Variable | Tissue | Plasma | Sputum | Urine | Combination | P-value |
|---|
| EGFR mutation, n
(%) | 15 (47) | 12 (38) | 8 (30) | 3 (13) | 13 (41) | 0.044 |
| ORR, n (%) | 10 (67) | 8 (67) | 5 (63) | 2 (66) | 9 (75) | 0.981 |
| DCR, n (%) | 13 (87) | 11 (91) | 7 (88) | 3 (100) | 12 (92) | 0.903 |
| PFS, months | 9.0 | 7.5 | 7.9 | 7.3 | 9.3 | 0.721 |
 | Table VI.Prediction of first-line
EGFR-tyrosine kinase inhibitor therapy effect based on TP53
mutation status in patients newly diagnosed with non-small cell
lung cancer. |
Table VI.
Prediction of first-line
EGFR-tyrosine kinase inhibitor therapy effect based on TP53
mutation status in patients newly diagnosed with non-small cell
lung cancer.
| Sample type | ORR, n (%) | DCR, n (%) | PFS, months |
|---|
| Tissue |
|
|
|
|
Wild-type | 5 (83) | 6 (100) | 10.2 |
|
Mutant | 5 (56) | 7 (78) | 8.2 |
|
P-value | 0.580 | 0.586 | 0.412 |
| Plasma |
|
|
|
|
Wild-type | 4 (80) | 5 (100) | 10.2 |
|
Mutant | 6 (60) | 8 (80) | 8.4 |
|
P-value | 0.600 | 0.524 | 0.466 |
| Sputum |
|
|
|
|
Wild-type | 6 (60) | 8 (80) | 9.1 |
|
Mutant | 4 (80) | 5 (100) | 8.8 |
|
P-value | 0.600 | 0.524 | 0.904 |
| Urine |
|
|
|
|
Wild-type | 6 (60) | 8 (80) | 8.3 |
|
Mutant | 4 (80) | 5 (100) | 10.4 |
|
P-value | 0.600 | 0.524 | 0.393 |
|
Combinationa |
|
|
|
| Wild-type | 3 (75) | 4 (100) | 10.3 |
| Mutant | 7 (64) | 9 (82) | 8.8 |
| P-value | 1.000 | 1.000 | 0.599 |
Discussion
In the present prospective study, the combination of
plasma, sputum and urine biopsies with matching tumor tissues from
patients with NSCLC was used for the first time to detect mutations
in EGFR and TP53 using a NGS platform. The present study determined
the association of EGFR and TP53 mutations in plasma, sputum, urine
and tumor tissue and demonstrated the value of combining samples
for gene detection, with the prediction efficacy of first line
EGFR-TKI therapy predicted by EGFR and TP53 gene variations
detected through the different types of samples. The results
demonstrated that the differences in the mean frequency of EGFR or
TP53 mutation in the plasma, sputum and urine were not
statistically significant compared with the tumor tissue, which
suggested that liquid samples may potentially be used to detect
gene variations. Furthermore, the present study reported that the
combination of these three body fluid samples increased the
sensitivity of detecting EGFR sensitizing mutations and TP53
mutations. The sensitivity of combining liquid biopsy was improved
compared with previous studies that used single liquid sample
(29–31). The present study provided an
effective method to detect mutations in common driver genes,
including EGFR and TP53, with a relatively high sensitivity and
specificity in patients with advanced NSCLC.
In the present study, the sensitivity of detecting
EGFR sensitizing mutations in sputum and urine cfDNA was associated
with the number of metastatic sites. Previous studies demonstrated
that an increased number of metastatic sites is associated with
increased cfDNA present in the circulation (32,33).
Increased cfDNA in circulation from tumor cells may therefore be
associated with the upper limit of the assay sensitivity. The
present study demonstrated that cfDNA was also present in urine and
sputum and that the detection sensitivity of EGFR or TP53 mutations
in sputum or urine was higher in patients with >1 metastatic
site.
The ORR following first-line EGFR-TKIs was similar
in patients with EGFR sensitizing mutations detected in plasma,
sputum, urine and tissue samples. There was no significant
difference in the PFS in patients receiving first-line EGFR-TKIs
among patients with mutations of EGFR, EGFR mutations in the plasma
samples, EGFR mutations in the sputum and EGFR mutations in the
urine. These results suggested that the value of EGFR mutations
detected in liquid samples was similar to the value detected in
tissue samples and that they may both be used to predict the
efficacy of first line EGFR-TKIs. Previous studies demonstrated
that the ORR was 65.7–75.0% for first-line EGFR-TKIs in patients
with an EGFR mutation detected in plasma or serum cfDNA (34,35). In
the present study, results from urine and sputum samples indicated
that additional liquid samples may be useful in clinical practice
for genetic profiling, and may help to determine which therapy
would be best, in particular in patients for whom it may not be
possible to obtain a tissue biopsy. The ORR for first line EGFR-TKI
in patients with TP53 mutations was decreased compared with
patients without TP53 mutations in the tumor biopsy, plasma samples
and when the liquid samples were combined. When evaluating the PFS
of first line EGFR-TKIs, excluding urine samples, TP53 mutations in
tumor and liquid biopsies predicted a decreased PFS. TP53 mutations
were associated with lower disease control rate and ORR in patients
receiving first-line EGFR-TKIs based on the tissue or plasma
samples. The results from the present study were similar to
previous studies that demonstrated that TP53 mutations detected in
tumor tissues or plasma samples were associated with poor ORR and
shorter PFS, and that TP53 mutations detection in sputum was
possible and could be used to predict the efficacy of first-line
EGFR-TKIs (3,4,8).
However, the aforementioned differences were not statistically
significant in the present study, which may be due to the small
sample size.
To the best of our knowledge, cfDNA from sputum was
used for the first time to detect gene variations in the present
study. The detection sensitivity of EFGR sensitizing mutations
using sputum was lower compared with that obtained when using
plasma samples; however, it was higher than when using urine
samples. The ORR of treatment with EGFR-TKI in patients with EGFR
mutations detected in the sputum was similar to that of patients
with EGFR mutations detected in the tissue samples. Furthermore,
certain EGFR sensitizing mutations, including an EGFR exon 19
deletion, were not detected in the plasma or urine samples;
however, they were detected in the cfDNA from sputum in a newly
diagnosed patient. These results demonstrated that combining cfDNA
from different body fluid samples may improve the sensitivity of
liquid biopsy, enabling the detection of more sensitive mutations
using fluid samples and allowing more patients to receive
individualized treatment.
Similar to previous studies, driver gene mutations,
including EGFR and TP53, were not detected in tumor tissues, but
were detected in body fluid samples (36,37).
Previous studies reported inconsistent results between tissue
biopsies and liquid biopsies, where certain driver gene mutations
were identified in liquid samples but not in tumor tissue; however,
this may be due to tumor heterogeneity and clonal hematopoiesis
(CH) (38,39). In the present study, as the genomic
DNA from peripheral blood leukocytes was also included in the
sequencing analysis as a background reference, but excluded when
the result was analyzed, the probability of inconsistent results
caused by CH was reduced. As most patients enrolled in the present
study had >1 metastasis site, a single biopsy of the tumor
tissue did not reflect the overall tumor genomic profile.
Therefore, cfDNA from body fluids came from multiple disease sites
throughout the body, which may allow the detection of a more
diverse and representative genomic profile than from single tumor
biopsy (36). In the present study,
tumor heterogeneity may have been the primary cause of the
inconsistent results observed in gene detection from body fluid
samples and tumor tissues. For example, one case of T790M mutation
was detected in the plasma and sputum, but was not detected in the
tumor tissue; the patient received osimertinib treatment and had a
PFS of 13 months, which confirmed the clinical efficacy of the
liquid biopsy. Subsequently, the results from the liquid biopsy
that were inconsistent with the tissue biopsy may not always be a
false positive. Instead, the tissue biopsy may be a false negative
due to tumor heterogeneity.
In conclusion, the present study confirmed that the
use of target sequencing with HiSeq4000 and the GeneSeqOne™ gene
panel may be used to detect with a high sensitivity EGFR and TP53
mutations in cfDNA from plasma, sputum and urine samples of
patients with advanced NSCLC. Although the sensitivity based on
liquid samples individually may not meet the needs of clinical
practice, it was possible to detect that the genomic profiles were
distinct from each other, and that the combination of results from
liquid samples increased the overall sensitivity of EGFR and TP53
mutations. In addition, liquid samples provided a method to predict
the efficacy of a personalized therapeutic regimen. Further
investigation will require a bigger sample size in order to
validate the differences between genomic profiles in unique liquid
samples and the utility of combining liquid biopsy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZW and ZY contributed equally to the conception and
design of the present study. ZW and ZY analyzed and interpreted
data and wrote the manuscript. YD, QZ, DHC and KLM selected
patients, collected liquid and tissue samples and completed the
follow-up of the recruited patients. CSL, WZ and ZXL performed the
experimental procedures. JZ contributed to statistical analysis.
LAC conceived and designed the study and revised the manuscript
critically for intellectual content. All authors approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of the Chinese People's Liberation Army General Hospital
(Beijing, China; approval no. S2015-099-001). All patients provided
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ettinger DS, Aisner DL, Wood DE, Akerley
W, Bauman J, Chang JY, Chirieac LR, D'Amico TA, Dilling TJ,
Dobelbower M, et al: NCCN guidelines insights: Non-small cell lung
cancer, version 5.2018. J Natl Compr Canc Netw. 16:807–821. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwaederlé MC, Patel SP, Husain H, Ikeda
M, Lanman RB, Banks KC, Talasaz A, Bazhenova L and Kurzrock R:
Utility of genomic assessment of blood-derived circulating tumor
DNA (ctDNA) in patients with advanced lung adenocarcinoma. Clin
Cancer Res. 23:5101–5111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Canale M, Petracci E, Delmonte A, Chiadini
E, Dazzi C, Papi M, Capelli L, Casanova C, De Luigi N, Mariotti M,
et al: Impact of TP53 mutations on outcome in EGFR-mutated patients
treated with first-line tyrosine kinase inhibitors. Clin Cancer
Res. 23:2195–2202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi Y, Au JS, Thongprasert S, Srinivasan
S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G and Yang PC: A
prospective, molecular epidemiology study of EGFR mutations in
Asian patients with advanced non-small-cell lung cancer of
adenocarcinoma histology (PIONEER). J Thorac Oncol. 9:154–162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kohno T, Nakaoku T, Tsuta K, Tsuchihara K,
Matsumoto S, Yoh K and Goto K: Beyond ALK-RET, ROS1 and other
oncogene fusions in lung cancer. Transl Lung Cancer Res. 4:156–164.
2015.PubMed/NCBI
|
|
7
|
Labbé C, Cabanero M, Korpanty GJ, Tomasini
P, Doherty MK, Mascaux C, Jao K, Pitcher B, Wang R, Pintilie M, et
al: Prognostic and predictive effects of TP53 co-mutation in
patients with EGFR-mutated non-small cell lung cancer (NSCLC). Lung
Cancer. 111:23–29. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu Y, Tong X, Yan J, Wu X, Shao YW and Fan
Y: Short-term responders of non-small cell lung cancer patients to
EGFR tyrosine kinase inhibitors display high prevalence of TP53
mutations and primary resistance mechanisms. Transl Oncol.
11:1364–1369. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Douillard JY, Ostoros G, Cobo M, Ciuleanu
T, Cole R, McWalter G, Walker J, Dearden S, Webster A, Milenkova T
and McCormack R: Gefitinib treatment in EGFR mutated caucasian
NSCLC: Circulating-free tumor DNA as a surrogate for determination
of EGFR status. J Thorac Oncol. 9:1345–1353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Accordino MK, Wright JD, Buono D, Neugut
AI and Hershman DL: Trends in use and safety of image-guided
transthoracic needle biopsies in patients with cancer. J Oncol
Pract. 11:e351–e359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Piotrowska Z, Niederst MJ, Karlovich CA,
Wakelee HA, Neal JW, Mino-Kenudson M, Fulton L, Hata AN, Lockerman
EL, Kalsy A, et al: Heterogeneity underlies the emergence of
EGFRT790 wild-type clones following treatment of T790M-positive
cancers with a third-generation EGFR inhibitor. Cancer Discov.
5:713–722. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reck M, Hagiwara K, Han B, Tjulandin S,
Grohé C, Yokoi T, Morabito A, Novello S, Arriola E, Molinier O, et
al: ctDNA Determination of EGFR mutation status in European and
Japanese patients with advanced NSCLC: The ASSESS study. J Thorac
Oncol. 11:1682–1689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han B, Tjulandin S, Hagiwara K, Normanno
N, Wulandari L, Laktionov K, Hudoyo A, He Y, Zhang YP, Wang MZ, et
al: EGFR mutation prevalence in Asia-Pacific and Russian patients
with advanced NSCLC of adenocarcinoma and non-adenocarcinoma
histology: The IGNITE study. Lung Cancer. 113:37–44. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Janku F, Zhang S, Waters J, Liu L, Huang
HJ, Subbiah V, Hong DS, Karp DD, Fu S, Cai X, et al: Development
and validation of an ultradeep next-generation sequencing assay for
testing of plasma cell-Free DNA from patients with advanced cancer.
Clin Cancer Res. 23:5648–5656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Möhrmann L, Huang HJ, Hong DS, Tsimberidou
AM, Fu S, Piha-Paul SA, Subbiah V, Karp DD, Naing A, Krug A, et al:
Liquid biopsies using plasma exosomal nucleic acids and plasma
cell-free DNA compared with clinical outcomes of patients with
advanced cancers. Clin Cancer Res. 24:181–188. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thompson JC, Yee SS, Troxel AB, Savitch
SL, Fan R, Balli D, Lieberman DB, Morrissette JD, Evans TL, Bauml
J, et al: Detection of therapeutically targetable driver and
resistance mutations in lung cancer patients by next-generation
sequencing of cell-free circulating tumor DNA. Clin Cancer Res.
22:5772–5782. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JY, Qing X, Xiumin W, Yali B, Chi S,
Bak SH, Lee HY, Sun JM, Lee SH, Ahn JS, et al: Longitudinal
monitoring of EGFR mutations in plasma predicts outcomes of NSCLC
patients treated with EGFR TKIs: Korean lung cancer consortium
(KLCC-12-02). Oncotarget. 7:6984–6993. 2016.PubMed/NCBI
|
|
18
|
Su YH, Wang M, Brenner DE, Ng A, Melkonyan
H, Umansky S, Syngal S and Block TM: Human urine contains small,
150 to 250 nucleotide-sized, soluble DNA derived from the
circulation and may be useful in the detection of colorectal
cancer. J Mol Diagn. 6:101–107. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Togneri FS, Ward DG, Foster JM, Devall AJ,
Wojtowicz P, Alyas S, Vasques FR, Oumie A, James ND, Cheng KK, et
al: Genomic complexity of urothelial bladder cancer revealed in
urinary cfDNA. Eur J Hum Genet. 24:1167–1174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Husain H, Melnikova VO, Kosco K, Woodward
B, More S, Pingle SC, Weihe E, Park BH, Tewari M, Erlander MG, et
al: Monitoring daily dynamics of early tumor response to targeted
therapy by detecting circulating tumor DNA in urine. Clin Cancer
Res. 23:4716–4723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen S, Zhao J, Cui L and Liu Y: Urinary
circulating DNA detection for dynamic tracking of EGFR mutations
for NSCLC patients treated with EGFR-TKIs. Clin Transl Oncol.
19:332–340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li YS, Jiang BY, Yang JJ, Zhang XC, Zhang
Z, Ye JY, Zhong WZ, Tu HY, Chen HJ, Wang Z, et al: Unique genetic
profiles from cerebrospinal fluid cell-free DNA in leptomeningeal
metastases of EGFR-mutant non-small-cell lung cancer: A new medium
of liquid biopsy. Ann Oncol. 29:945–952. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vollbrecht C, Lehmann A, Lenze D and
Hummel M: Validation and comparison of two NGS assays for the
detection of EGFR T790M resistance mutation in liquid biopsies of
NSCLC patients. Oncotarget. 9:18529–18539. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reckamp KL, Melnikova VO, Karlovich C,
Sequist LV, Camidge DR, Wakelee H, Perol M, Oxnard GR, Kosco K,
Croucher P, et al: A highly sensitive and quantitative test
platform for detection of NSCLC EGFR mutations in urine and plasma.
J Thorac Oncol. 11:1690–1700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van der Drift MA, Prinsen CF, Hol BE,
Bolijn AS, Jeunink MA, Dekhuijzen PN and Thunnissen FB: Can free
DNA be detected in sputum of lung cancer patients? Lung Cancer.
61:385–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu Z, Yang Z, Li CS, Zhao W, Liang ZX, Dai
Y, Zhu Q, Miao KL, Cui DH and Chen LA: Differences in the genomic
profiles of cell-free DNA between plasma, sputum, urine, and tumor
tissue in advanced NSCLC. Cancer Med. 8:910–919. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Z, Yang N, Ou Q, Xiang Y, Jiang T, Wu
X, Bao H, Tong X, Wang X, Shao YW, et al: Investigating novel
resistance mechanisms to third-generation EGFR tyrosine kinase
inhibitor osimertinib in non-small cell lung cancer patients. Clin
Cancer Res. 24:3097–3107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu G, Ye X, Dong Z, Lu YC, Sun Y, Liu Y,
McCormack R, Gu Y and Liu X: Highly sensitive droplet digital PCR
method for detection of EGFR-activating mutations in plasma
cell-free DNA from patients with advanced non-small cell lung
cancer. J Mol Diagn. 17:265–272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pender A, Garcia-Murillas I, Rana S, Cutts
RJ, Kelly G, Fenwick K, Kozarewa I, Gonzalez de Castro D, Bhosle J,
O'Brien M, et al: Efficient genotyping of KRAS mutant non-small
cell lung cancer using a multiplexed droplet digital PCR approach.
PLoS One. 10:e01390742015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu S, Lou F, Wu Y, Sun DQ, Zhang JB, Chen
W, Ye H, Liu JH, Wei S, Zhao MY, et al: Circulating tumor DNA
identified by targeted sequencing in advanced-stage non-small cell
lung cancer patients. Cancer Lett. 370:324–331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mok T, Wu YL, Lee JS, Yu CJ, Sriuranpong
V, Sandoval-Tan J, Ladrera G, Thongprasert S, Srimuninnimit V, Liao
M, et al: Detection and dynamic changes of EGFR mutations from
circulating tumor DNA as a predictor of survival outcomes in NSCLC
patients treated with first-line intercalated erlotinib and
chemotherapy. Clin Cancer Res. 21:3196–3203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sacher AG, Paweletz C, Dahlberg SE, Alden
RS, O'Connell A, Feeney N, Mach SL, Jänne PA and Oxnard GR:
Prospective validation of rapid plasma genotyping for the detection
of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol.
2:1014–1022. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jovelet C, Ileana E, Le Deley MC, Motté N,
Rosellini S, Romero A, Lefebvre C, Pedrero M, Pata-Merci N, Droin
N, et al: Circulating cell-free tumor DNA analysis of 50 genes by
next-generation sequencing in the prospective MOSCATO trial. Clin
Cancer Res. 22:2960–2968. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Xu H, Su S, Ye J, Chen J, Jin X, Lin
Q, Zhang D, Ye C and Chen C: Clinical validation of a highly
sensitive assay to detect EGFR mutations in plasma cell-free DNA
from patients with advanced lung adenocarcinoma. PLoS One.
12:e01833312017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goto K, Ichinose Y, Ohe Y, Yamamoto N,
Negoro S, Nishio K, Itoh Y, Jiang H, Duffield E, McCormack R, et
al: Epidermal growth factor receptor mutation status in circulating
free DNA in serum: From IPASS, a phase III study of gefitinib or
carboplatin/paclitaxel in non-small cell lung cancer. J Thorac
Oncol. 7:115–121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oxnard GR, Thress KS, Alden RS, Lawrance
R, Paweletz CP, Cantarini M, Yang JC, Barrett JC and Jänne PA:
Association between plasma genotyping and outcomes of treatment
with osimertinib (AZD9291) in advanced non-small-cell lung cancer.
J Clin Oncol. 34:3375–3382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu T, Kang X, You X, Dai L, Tian D, Yan W,
Yang Y, Xiong H, Liang Z, Zhao GQ, et al: Cross-platform comparison
of four leading technologies for detecting EGFR mutations in
circulating tumor DNA from non-small cell lung carcinoma patient
plasma. Theranostics. 7:1437–1446. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu Y, Ulrich BC, Supplee J, Kuang Y,
Lizotte PH, Feeney NB, Guibert NM, Awad MM, Wong KK, Jänne PA, et
al: False positive plasma genotyping due to clonal hematopoiesis.
Clin Cancer Res. 24:4437–4443. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sundaresan TK, Sequist LV, Heymach JV,
Riely GJ, Jänne PA, Koch WH, Sullivan JP, Fox DB, Maher R,
Muzikansky A, et al: Detection of T790M, the acquired resistance
EGFR mutation, by tumor biopsy versus noninvasive blood-based
analyses. Clin Cancer Res. 22:1103–1110. 2016. View Article : Google Scholar : PubMed/NCBI
|