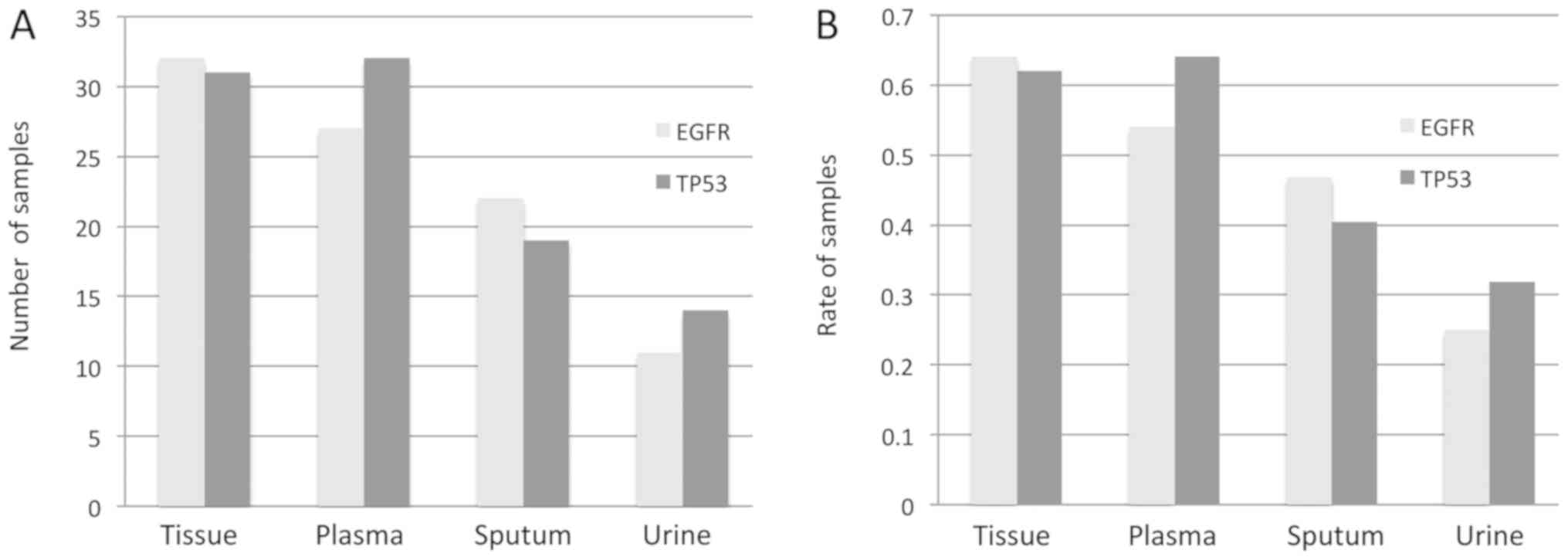
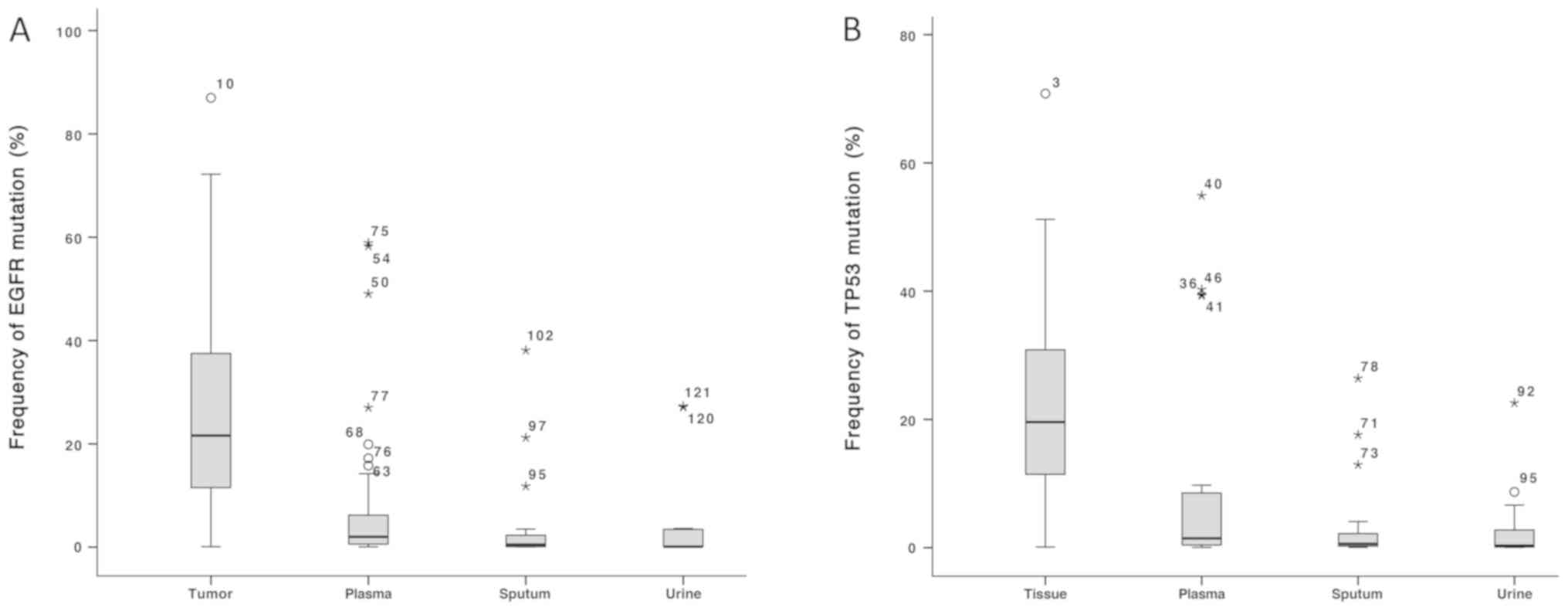
|
1
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ettinger DS, Aisner DL, Wood DE, Akerley
W, Bauman J, Chang JY, Chirieac LR, D'Amico TA, Dilling TJ,
Dobelbower M, et al: NCCN guidelines insights: Non-small cell lung
cancer, version 5.2018. J Natl Compr Canc Netw. 16:807–821. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwaederlé MC, Patel SP, Husain H, Ikeda
M, Lanman RB, Banks KC, Talasaz A, Bazhenova L and Kurzrock R:
Utility of genomic assessment of blood-derived circulating tumor
DNA (ctDNA) in patients with advanced lung adenocarcinoma. Clin
Cancer Res. 23:5101–5111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Canale M, Petracci E, Delmonte A, Chiadini
E, Dazzi C, Papi M, Capelli L, Casanova C, De Luigi N, Mariotti M,
et al: Impact of TP53 mutations on outcome in EGFR-mutated patients
treated with first-line tyrosine kinase inhibitors. Clin Cancer
Res. 23:2195–2202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi Y, Au JS, Thongprasert S, Srinivasan
S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G and Yang PC: A
prospective, molecular epidemiology study of EGFR mutations in
Asian patients with advanced non-small-cell lung cancer of
adenocarcinoma histology (PIONEER). J Thorac Oncol. 9:154–162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kohno T, Nakaoku T, Tsuta K, Tsuchihara K,
Matsumoto S, Yoh K and Goto K: Beyond ALK-RET, ROS1 and other
oncogene fusions in lung cancer. Transl Lung Cancer Res. 4:156–164.
2015.PubMed/NCBI
|
|
7
|
Labbé C, Cabanero M, Korpanty GJ, Tomasini
P, Doherty MK, Mascaux C, Jao K, Pitcher B, Wang R, Pintilie M, et
al: Prognostic and predictive effects of TP53 co-mutation in
patients with EGFR-mutated non-small cell lung cancer (NSCLC). Lung
Cancer. 111:23–29. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu Y, Tong X, Yan J, Wu X, Shao YW and Fan
Y: Short-term responders of non-small cell lung cancer patients to
EGFR tyrosine kinase inhibitors display high prevalence of TP53
mutations and primary resistance mechanisms. Transl Oncol.
11:1364–1369. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Douillard JY, Ostoros G, Cobo M, Ciuleanu
T, Cole R, McWalter G, Walker J, Dearden S, Webster A, Milenkova T
and McCormack R: Gefitinib treatment in EGFR mutated caucasian
NSCLC: Circulating-free tumor DNA as a surrogate for determination
of EGFR status. J Thorac Oncol. 9:1345–1353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Accordino MK, Wright JD, Buono D, Neugut
AI and Hershman DL: Trends in use and safety of image-guided
transthoracic needle biopsies in patients with cancer. J Oncol
Pract. 11:e351–e359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Piotrowska Z, Niederst MJ, Karlovich CA,
Wakelee HA, Neal JW, Mino-Kenudson M, Fulton L, Hata AN, Lockerman
EL, Kalsy A, et al: Heterogeneity underlies the emergence of
EGFRT790 wild-type clones following treatment of T790M-positive
cancers with a third-generation EGFR inhibitor. Cancer Discov.
5:713–722. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reck M, Hagiwara K, Han B, Tjulandin S,
Grohé C, Yokoi T, Morabito A, Novello S, Arriola E, Molinier O, et
al: ctDNA Determination of EGFR mutation status in European and
Japanese patients with advanced NSCLC: The ASSESS study. J Thorac
Oncol. 11:1682–1689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han B, Tjulandin S, Hagiwara K, Normanno
N, Wulandari L, Laktionov K, Hudoyo A, He Y, Zhang YP, Wang MZ, et
al: EGFR mutation prevalence in Asia-Pacific and Russian patients
with advanced NSCLC of adenocarcinoma and non-adenocarcinoma
histology: The IGNITE study. Lung Cancer. 113:37–44. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Janku F, Zhang S, Waters J, Liu L, Huang
HJ, Subbiah V, Hong DS, Karp DD, Fu S, Cai X, et al: Development
and validation of an ultradeep next-generation sequencing assay for
testing of plasma cell-Free DNA from patients with advanced cancer.
Clin Cancer Res. 23:5648–5656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Möhrmann L, Huang HJ, Hong DS, Tsimberidou
AM, Fu S, Piha-Paul SA, Subbiah V, Karp DD, Naing A, Krug A, et al:
Liquid biopsies using plasma exosomal nucleic acids and plasma
cell-free DNA compared with clinical outcomes of patients with
advanced cancers. Clin Cancer Res. 24:181–188. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thompson JC, Yee SS, Troxel AB, Savitch
SL, Fan R, Balli D, Lieberman DB, Morrissette JD, Evans TL, Bauml
J, et al: Detection of therapeutically targetable driver and
resistance mutations in lung cancer patients by next-generation
sequencing of cell-free circulating tumor DNA. Clin Cancer Res.
22:5772–5782. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JY, Qing X, Xiumin W, Yali B, Chi S,
Bak SH, Lee HY, Sun JM, Lee SH, Ahn JS, et al: Longitudinal
monitoring of EGFR mutations in plasma predicts outcomes of NSCLC
patients treated with EGFR TKIs: Korean lung cancer consortium
(KLCC-12-02). Oncotarget. 7:6984–6993. 2016.PubMed/NCBI
|
|
18
|
Su YH, Wang M, Brenner DE, Ng A, Melkonyan
H, Umansky S, Syngal S and Block TM: Human urine contains small,
150 to 250 nucleotide-sized, soluble DNA derived from the
circulation and may be useful in the detection of colorectal
cancer. J Mol Diagn. 6:101–107. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Togneri FS, Ward DG, Foster JM, Devall AJ,
Wojtowicz P, Alyas S, Vasques FR, Oumie A, James ND, Cheng KK, et
al: Genomic complexity of urothelial bladder cancer revealed in
urinary cfDNA. Eur J Hum Genet. 24:1167–1174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Husain H, Melnikova VO, Kosco K, Woodward
B, More S, Pingle SC, Weihe E, Park BH, Tewari M, Erlander MG, et
al: Monitoring daily dynamics of early tumor response to targeted
therapy by detecting circulating tumor DNA in urine. Clin Cancer
Res. 23:4716–4723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen S, Zhao J, Cui L and Liu Y: Urinary
circulating DNA detection for dynamic tracking of EGFR mutations
for NSCLC patients treated with EGFR-TKIs. Clin Transl Oncol.
19:332–340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li YS, Jiang BY, Yang JJ, Zhang XC, Zhang
Z, Ye JY, Zhong WZ, Tu HY, Chen HJ, Wang Z, et al: Unique genetic
profiles from cerebrospinal fluid cell-free DNA in leptomeningeal
metastases of EGFR-mutant non-small-cell lung cancer: A new medium
of liquid biopsy. Ann Oncol. 29:945–952. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vollbrecht C, Lehmann A, Lenze D and
Hummel M: Validation and comparison of two NGS assays for the
detection of EGFR T790M resistance mutation in liquid biopsies of
NSCLC patients. Oncotarget. 9:18529–18539. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reckamp KL, Melnikova VO, Karlovich C,
Sequist LV, Camidge DR, Wakelee H, Perol M, Oxnard GR, Kosco K,
Croucher P, et al: A highly sensitive and quantitative test
platform for detection of NSCLC EGFR mutations in urine and plasma.
J Thorac Oncol. 11:1690–1700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van der Drift MA, Prinsen CF, Hol BE,
Bolijn AS, Jeunink MA, Dekhuijzen PN and Thunnissen FB: Can free
DNA be detected in sputum of lung cancer patients? Lung Cancer.
61:385–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu Z, Yang Z, Li CS, Zhao W, Liang ZX, Dai
Y, Zhu Q, Miao KL, Cui DH and Chen LA: Differences in the genomic
profiles of cell-free DNA between plasma, sputum, urine, and tumor
tissue in advanced NSCLC. Cancer Med. 8:910–919. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Z, Yang N, Ou Q, Xiang Y, Jiang T, Wu
X, Bao H, Tong X, Wang X, Shao YW, et al: Investigating novel
resistance mechanisms to third-generation EGFR tyrosine kinase
inhibitor osimertinib in non-small cell lung cancer patients. Clin
Cancer Res. 24:3097–3107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu G, Ye X, Dong Z, Lu YC, Sun Y, Liu Y,
McCormack R, Gu Y and Liu X: Highly sensitive droplet digital PCR
method for detection of EGFR-activating mutations in plasma
cell-free DNA from patients with advanced non-small cell lung
cancer. J Mol Diagn. 17:265–272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pender A, Garcia-Murillas I, Rana S, Cutts
RJ, Kelly G, Fenwick K, Kozarewa I, Gonzalez de Castro D, Bhosle J,
O'Brien M, et al: Efficient genotyping of KRAS mutant non-small
cell lung cancer using a multiplexed droplet digital PCR approach.
PLoS One. 10:e01390742015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu S, Lou F, Wu Y, Sun DQ, Zhang JB, Chen
W, Ye H, Liu JH, Wei S, Zhao MY, et al: Circulating tumor DNA
identified by targeted sequencing in advanced-stage non-small cell
lung cancer patients. Cancer Lett. 370:324–331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mok T, Wu YL, Lee JS, Yu CJ, Sriuranpong
V, Sandoval-Tan J, Ladrera G, Thongprasert S, Srimuninnimit V, Liao
M, et al: Detection and dynamic changes of EGFR mutations from
circulating tumor DNA as a predictor of survival outcomes in NSCLC
patients treated with first-line intercalated erlotinib and
chemotherapy. Clin Cancer Res. 21:3196–3203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sacher AG, Paweletz C, Dahlberg SE, Alden
RS, O'Connell A, Feeney N, Mach SL, Jänne PA and Oxnard GR:
Prospective validation of rapid plasma genotyping for the detection
of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol.
2:1014–1022. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jovelet C, Ileana E, Le Deley MC, Motté N,
Rosellini S, Romero A, Lefebvre C, Pedrero M, Pata-Merci N, Droin
N, et al: Circulating cell-free tumor DNA analysis of 50 genes by
next-generation sequencing in the prospective MOSCATO trial. Clin
Cancer Res. 22:2960–2968. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Xu H, Su S, Ye J, Chen J, Jin X, Lin
Q, Zhang D, Ye C and Chen C: Clinical validation of a highly
sensitive assay to detect EGFR mutations in plasma cell-free DNA
from patients with advanced lung adenocarcinoma. PLoS One.
12:e01833312017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goto K, Ichinose Y, Ohe Y, Yamamoto N,
Negoro S, Nishio K, Itoh Y, Jiang H, Duffield E, McCormack R, et
al: Epidermal growth factor receptor mutation status in circulating
free DNA in serum: From IPASS, a phase III study of gefitinib or
carboplatin/paclitaxel in non-small cell lung cancer. J Thorac
Oncol. 7:115–121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oxnard GR, Thress KS, Alden RS, Lawrance
R, Paweletz CP, Cantarini M, Yang JC, Barrett JC and Jänne PA:
Association between plasma genotyping and outcomes of treatment
with osimertinib (AZD9291) in advanced non-small-cell lung cancer.
J Clin Oncol. 34:3375–3382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu T, Kang X, You X, Dai L, Tian D, Yan W,
Yang Y, Xiong H, Liang Z, Zhao GQ, et al: Cross-platform comparison
of four leading technologies for detecting EGFR mutations in
circulating tumor DNA from non-small cell lung carcinoma patient
plasma. Theranostics. 7:1437–1446. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu Y, Ulrich BC, Supplee J, Kuang Y,
Lizotte PH, Feeney NB, Guibert NM, Awad MM, Wong KK, Jänne PA, et
al: False positive plasma genotyping due to clonal hematopoiesis.
Clin Cancer Res. 24:4437–4443. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sundaresan TK, Sequist LV, Heymach JV,
Riely GJ, Jänne PA, Koch WH, Sullivan JP, Fox DB, Maher R,
Muzikansky A, et al: Detection of T790M, the acquired resistance
EGFR mutation, by tumor biopsy versus noninvasive blood-based
analyses. Clin Cancer Res. 22:1103–1110. 2016. View Article : Google Scholar : PubMed/NCBI
|