Introduction
Esophageal cancer is a common malignancy of the
digestive tract and the sixth for cancer death worldwide (1). The predominant histopathological types
are squamous cell carcinoma and adenocarcinoma, and in China,
squamous cell carcinoma is most frequently exhibited (2). Although there are numerous treatments
available for esophageal cancer, the 5-year survival rate remains
at ~20% (3). The discovery of
improved predictive markers, early detection and improved treatment
options are key to increasing the overall survival (OS) time of
patients with esophageal cancer.
There are ≥2 types of nuclear protein complex in the
polycomb group family, namely polycomb-repressive complex 1 (PRC1)
and PRC2 (4). Human PRC1 includes
polycomb (PC), polyhomeotic (PH), B-cell-specific Moloney murine
leukemia virus integration site-1 (BMI1), RING1a and RING1b
(5). PRC2 includes embryonic
ectoderm development (EED), enhancer of zeste homolog 2 (EZH2) and
suppressor of zeste 12 homolog (SUZ12) (6). In the PRC1 complex, BMI1 and RNF2
heterodimers form E3 ubiquitin ligase in the N-terminal area of the
RING (7–9). BMI1 expression is upregulated in
hepatocellular carcinoma (10) and
pancreatic cancer (11), and RNF2 is
overexpressed in many different types of tumors, such as
gastrointestinal tumors, lymphomas (12), breast cancer (13), ovarian tumor tissues (14). A previous biological study revealed
that silencing Ring finger protein 2 (RNF2) in esophageal cancer
cells may lead to defects in DNA damage pathways, and therefore
increase sensitivity to radiotherapy (15). Another previous study demonstrated
that a high expression level of BMI1 promoted the expression of
phosphor-protein kinase B (P-AKT) following radiotherapy, and that
the phosphoinositide 3-kinase (PI3K)/AKT pathway was involved in
resistance to radiotherapy (16).
However, the potential association between P-AKT and the RNF2
expression level, and the survival of patients with esophageal
squamous cell carcinoma (ESCC) treated with radiotherapy, requires
further investigation.
Therefore, in the present study, it was hypothesized
that an increased expression of RNF2 and P-AKT may result in
increased resistance to radiotherapy in patients with ESCC.
Immunohistochemistry was used to detect RNF2 and P-AKT protein
expression in patients prior to radiotherapy, and the association
between RNF2+P-AKT protein expression and clinicopathological
features and survival prognosis was retrospectively analyzed, in
order to investigate the role of RNF2+P-AKT protein expression in
patients with ESCC following radiotherapy.
Patients and methods
Patient sample selection
Between January 2010 and December 2013, 99 I–IVa
(American Joint Committee on Cancer, 2010) (17) stage ESCC patients, with a mean age of
66 years (range, 48–87) and a male:female ratio of 1.61 (61/38),
were selected from The Fourth Affiliated Hospital of Hebei Medical
University, and their tumor samples (formalin-fixed and
paraffin-embedded) were taken for the analysis of RNF2 and P-AKT.
Patients who had received any anti-cancer treatment prior to
diagnosis were excluded. The inclusion criteria were as follows: i)
Histological evidence of invasive squamous-cell carcinoma of the
esophagus; and ii) informed written consent to receive radiotherapy
(RT). Detailed clinical and follow-up data was obtained from all
patients, and written consent was obtained for the collection of
tissue specimens. The study protocols were approved by the Ethics
Committee for Clinical Research of the Fourth Affiliated Medical
University.
Immunohistochemistry (IHC)
IHC was performed using conventional methods.
Briefly, paraffin-embedded ESCC tissues were sliced into 4-µm-thick
sections, deparaffinized in xylene at 55°C, and rehydrated in a
descending alcohol series (100% alcohol first time, 100% alcohol
second time, 95% alcohol, 80% alcohol) for 10 min respectively.
Endogenous peroxidases were blocked using 3% hydrogen peroxide for
20 min at 37°C, and antigen retrieval was conducted using citrate
buffer (Thermo Fisher Scientific, Inc.) in a microwave oven for 15
min at 98°C. The sections were incubated with primary antibodies
against RNF2 (1:100; cat. no. ab101273; Abcam) and P-AKT (1:40;
cat. no. ab81283; Abcam) at 4°C overnight, followed by subsequent
incubation with horseradish peroxidase-conjugated goat anti-rabbit
polyclonal antibody (1:100; cat. no. SP-9000; Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd.) for 30 min at 37°C. The
sections were then processed with 3,3′-diaminobenzidine (DAB) for 5
min at 37°C and counterstained with hematoxylin for 16 min at 37°C
and assessed under a light microscope (Olympus Corporation) by two
pathologists who were blinded to the clinical parameters of the
patients.
Reactivity scoring and interpretation
of IHC
The expression of RNF2 and P-AKT was microscopically
observed using a light microscope, and this was identified to be
predominantly located in the nucleus. The IHC results were
independently evaluated by 2 pathologists with no prior knowledge
of the patients' clinicopathological data. If different scores were
assigned for the same sample, the sample was revaluated and, if
required, further discussed to determine a final score. For
positive staining in the nuclei, the most intensively stained
region was initially selected with a low-power magnification
(×100). The percentage of positively stained cells was then
calculated from the observation of 5 random sections at a higher
magnification (×200). A total of 100 tumor cells were counted in
each section, and the number and intensity classification of the
positively-stained cells was determined. The immunoreactive score
(IRS) system was used. The staining intensity classification
(18,19) was as follows: i) 0, unstained; ii) 1,
light yellow; iii) 2, brownish yellow; and iv) 3, tan. The
percentage score for positive cells was classified as follows: i)
0, positive cells ≤5%; ii) l, positive cells 6–25%; iii) 2,
positive cells 26–50%; iv) 3, positive cells 51–75%; and v) 4,
positive cells >75%. The total sum of the staining intensity and
percentage-positive scores was indicated as follows: i) 0–1,
negative (−); ii) 2–3, weakly positive (+);
iii) 4–5, moderately positive (++); and iv) 6–7,
strongly positive (+++). Where the degree of positive
staining is not being addressed, the word ‘positive’ has been used
to indicate positive staining in general, in place of the
percentage-positive scoring symbols, which is also suitable for the
negative staining.
Statistical analysis
SPSS 19.0 statistical software (IBM Corp.) was used
for all statistical analyses. The relationship between RNF2, P-AKT
and patient clinicopathological parameters was analyzed using the
χ2 test, extended Fisher's exact test, linear-by-linear
association and the Goodman-Kruskal γ test. The Kaplan-Meier method
was used to analyze survival prognosis, in addition to the log-rank
test. Univariate and multivariate analysis of survival prognosis
was performed using cox regression analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical data and characteristics
IHC analysis was performed on 99 patients with ESCC,
pathologically diagnosed at The Fourth Affiliated Hospital of Hebei
Medical University. Due to incomplete clinicopathological or IHC
data, or discontinued radiotherapy, a number of patients were
excluded from the study. As a result, a total of 83 patients were
assigned to the RNF2 group, 85 to the P-AKT group, and 80 to the
RNF2+P-AKT group. Detailed clinical data are outlined in Table I.
 | Table I.Demographic, baseline variables and
treatment characteristics of the study population. |
Table I.
Demographic, baseline variables and
treatment characteristics of the study population.
| Variables | RNF2 (n=83)
(%) | P-AKT (n=85)
(%) | RNF2/P-AKT (n=80)
(%) |
|---|
| Sex |
|
Male | 51 (61.4) | 53 (62.4) | 49 (61.2) |
|
Female | 32 (38.6) | 32 (37.6) | 31 (38.8) |
| Age (years) |
|
≤66 | 44 (53.0) | 45 (52.9) | 43 (53.8) |
|
>66 | 39 (47.0) | 40 (47.1) | 37 (46.3) |
| Tumor location |
|
Cervical | 6
(7.2) | 6
(7.1) | 6
(7.5) |
| Upper
thoracic | 24 (28.9) | 25 (29.4) | 24 (30.0) |
| Middle
thoracic | 37 (44.6) | 38 (44.7) | 34 (42.5) |
| Lower
thoracic | 16 (19.3) | 16 (18.8) | 16 (20.0) |
| Tumor length
(cm) |
| ≤3 | 4
(4.8) | 4
(4.7) | 4
(5.0) |
|
3–5 | 33 (39.8) | 33 (38.8) | 32 (40.0) |
|
5–7 | 24 (28.9) | 25 (29.4) | 23 (28.8) |
|
>7 | 22 (26.5) | 23 (27.1) | 21 (26.3) |
| Tumor volume
(cm3) |
|
≤25 | 25 (30.1) | 26 (30.6) | 24 (30.0) |
|
>25 | 58 (69.9) | 59 (69.4) | 56 (70.0) |
| T stage |
|
1+2 | 29 (34.9) | 29 (34.1) | 27 (33.8) |
|
3+4 | 54 (65.1) | 56 (65.9) | 53 (66.3) |
| N stage |
| 0 | 34 (41.0) | 35 (41.2) | 33 (41.3) |
| + | 49 (59.0) | 50 (58.8) | 47 (58.8) |
| Tumor- |
|
Node-Metastasis stage
(17) |
|
I+II | 36 (43.4) | 37 (43.5) | 34 (42.5) |
|
III+IV | 47 (56.6) | 48 (56.5) | 46 (57.5) |
| Radiation
field |
|
IFI | 45 (54.2) | 46 (54.1) | 43 (53.8) |
|
ENI | 38 (45.8) | 39 (45.9) | 37 (46.3) |
| Radiation dose
(Gy) |
|
≤60 | 42 (50.6) | 43 (50.6) | 40 (50.0) |
|
>60 | 41 (49.4) | 42 (49.4) | 40 (50.0) |
| Chemotherapy |
| No | 41 (49.4) | 43 (50.6) | 38 (47.5) |
|
Yes | 42 (50.6) | 42 (49.4) | 42 (52.5) |
Influence of RNF2 protein expression
level on ESCC
IHC analysis of patients' specimens revealed that
the expression of RNF2 protein was primarily localized to the
nuclei of tumor cells (Fig. 1).
Among the 83 cases of ESCC, 9 (10.8%) were RNF2-negative, while 74
(89.2%) exhibited positive expression levels of RNF2; of these
RNF2-positive patients, 20 (24.1%), 18 (21.7%) and 36 (43.4%)
demonstrated weak, moderate and strong-positive expression levels,
respectively. Furthermore, there was a significant association
between RNF2 expression and tumor volume (P<0.05), whereas no
significant association was revealed for any other
clinicopathological feature, including age, sex, tumor site, tumor
length and Tumor-Node-Metastasis (TNM) stage (17) (Table
II).
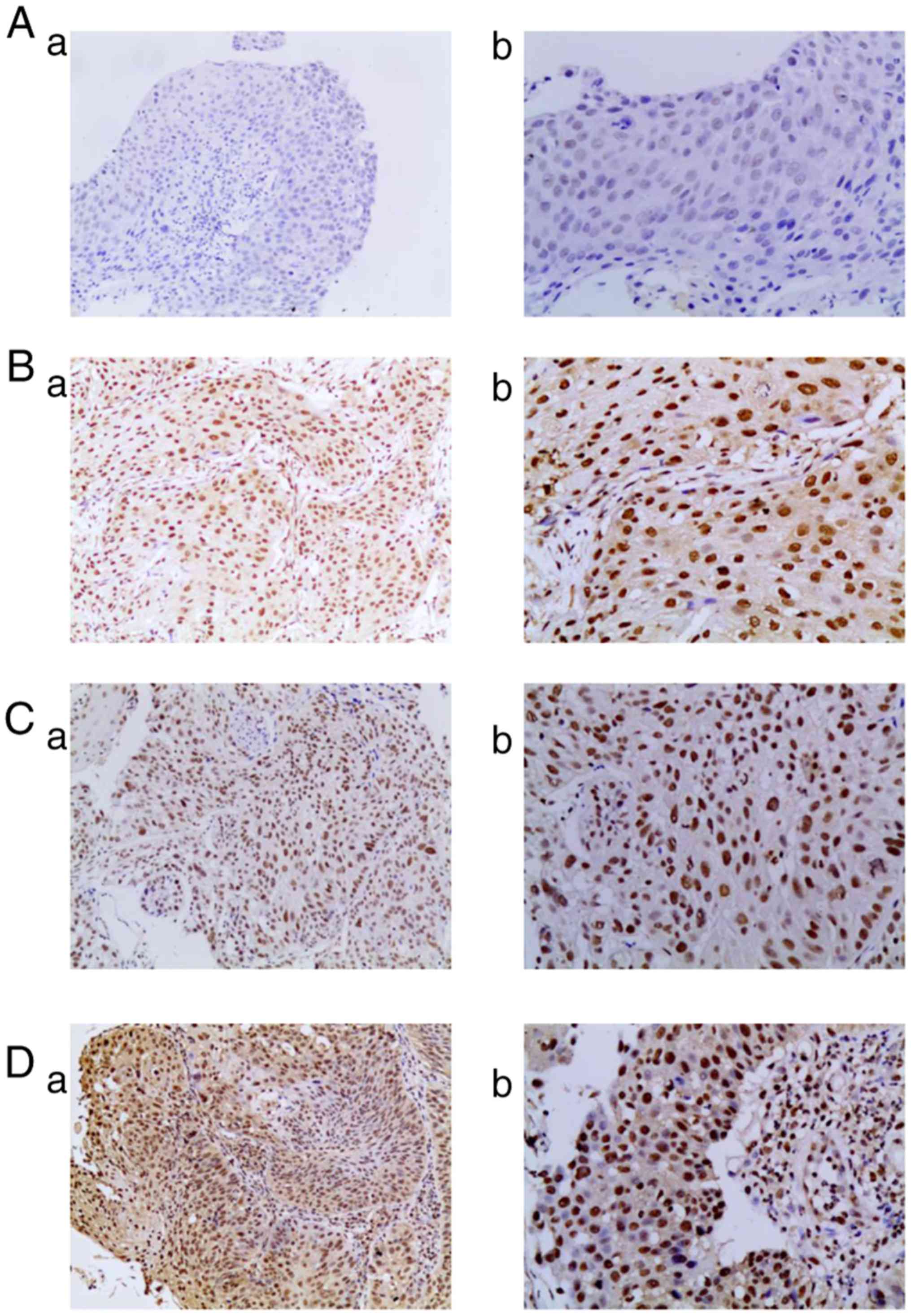 | Figure 1.Expression levels of RNF2 protein in
ESCC tissues. (A) No RNF2 expression in ESCC tissues; (a)
magnification, ×100 and (b) magnification, ×200. (B) Weak
expression of RNF2 in ESCC tissues; (a) magnification, ×100 and (b)
magnification, ×200. (C) Moderate expression of RNF2 in ESCC
tissues; (a) magnification, ×100 and (b) magnification, ×200. (D)
Strong expression of RNF2 in ESCC tissues; (a) magnification, ×100
and (b) magnification, ×200. RNF2, Ring finger protein 2; ESCC,
esophageal squamous cell carcinoma. |
 | Table II.Association between RNF2 expression
and the clinicopathological variables of patients with esophageal
squamous cell carcinoma. |
Table II.
Association between RNF2 expression
and the clinicopathological variables of patients with esophageal
squamous cell carcinoma.
|
| RNF2
expression |
|
|---|
|
|
|
|
|---|
| Variables | (−) (n=9) | % | (+) (n=20) | % | (++) (n=18) | % | (+++) (n=36) | % | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
|
|
Male | 4 | 44.4 | 12 | 60.0 | 14 | 77.8 | 21 | 58.3 | 0.635 |
|
Female | 5 | 55.6 | 8 | 40.0 | 4 | 22.2 | 15 | 41.7 |
|
| Age, years |
|
|
|
|
|
|
|
|
|
|
≤66 | 6 | 66.7 | 13 | 65.0 | 7 | 38.9 | 18 | 50.0 | 0.254 |
|
>66 | 3 | 33.3 | 7 | 35.0 | 11 | 61.1 | 18 | 50.0 |
|
| Tumor location |
|
|
|
|
|
|
|
|
|
|
Cervical | 1 | 11.1 | 2 | 10.0 | 2 | 11.1 | 1 | 2.8 | 0.579 |
| Upper
thoracic | 2 | 22.2 | 6 | 30.0 | 5 | 27.8 | 11 | 30.6 |
|
| Middle
thoracic | 4 | 44.4 | 8 | 40.0 | 9 | 50.0 | 16 | 44.4 |
|
| Lower
thoracic | 2 | 22.2 | 4 | 20.0 | 2 | 11.1 | 8 | 22.2 |
|
| Tumor length,
cm |
|
|
|
|
|
|
|
|
|
| ≤5 | 6 | 66.7 | 7 | 35.0 | 11 | 61.1 | 13 | 36.1 | 0.269 |
|
>5 | 3 | 33.3 | 13 | 65.0 | 7 | 38.9 | 23 | 63.9 |
|
| Tumor volume,
cm3 |
|
|
|
|
|
|
|
|
|
|
≤25 | 5 | 55.6 | 6 | 30.0 | 8 | 44.4 | 6 | 16.7 | 0.024 |
|
>25 | 4 | 44.4 | 14 | 70.0 | 10 | 55.6 | 30 | 83.3 |
|
| T stage |
|
|
|
|
|
|
|
|
|
|
1+2 | 4 | 44.4 | 7 | 35.0 | 7 | 38.9 | 11 | 30.6 | 0.467 |
|
3+4 | 5 | 55.6 | 13 | 65.0 | 11 | 61.1 | 25 | 69.4 |
|
| N stage |
|
|
|
|
|
|
|
|
|
| 0 | 4 | 44.4 | 8 | 40.0 | 10 | 55.6 | 12 | 33.3 | 0.503 |
| + | 5 | 55.6 | 12 | 60.0 | 8 | 44.4 | 24 | 66.7 |
|
|
Tumor-Node-Metastasis stage (17) |
|
|
|
|
|
|
|
|
|
|
I+II | 4 | 44.4 | 9 | 45.0 | 7 | 38.9 | 16 | 44.4 | 0.992 |
|
III+IV | 5 | 55.6 | 11 | 55.0 | 11 | 61.1 | 20 | 55.6 |
|
Influence of P-AKT protein expression
on ESCC
The assessment of the P-AKT expression level was
determined for 85 tumor samples, and this was identified to be
predominantly present in the nuclei of tumor cells (Fig. 2). A total of 29 (34.1%) patient
samples were classified as expression-negative, whereas 56 (65.9%)
exhibited positive P-AKT expression. In the P-AKT-positive group,
39 (45.9%), 16 (18.8%) and 1 (1.2%) samples exhibited weak,
moderate and strong-positive expression levels, respectively.
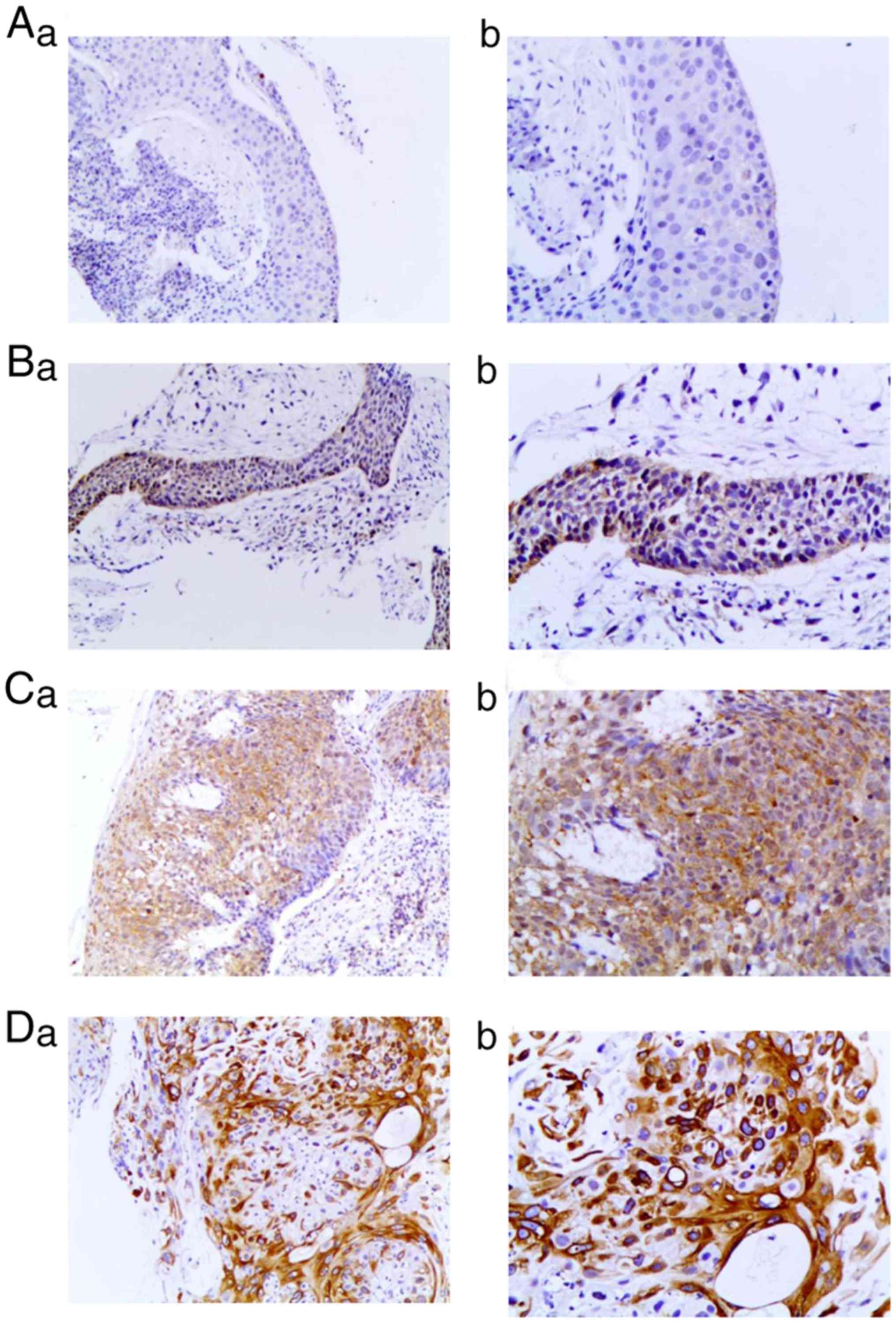 | Figure 2.Expression levels of P-AKT protein in
ESCC tissues. (A) No P-AKT expression in ESCC tissue; (a)
magnification, ×100 and (b) magnification, ×200. (B) Weak
expression of P-AKT in ESCC tissues: (a) magnification, ×100 and
(b) magnification, ×200. (C) Moderate expression of P-AKT in ESCC
tissues; (a) magnification, ×100 and (b) magnification, ×200. (D)
Strong expression of P-AKT in ESCC tissues; (a) magnification, ×100
and (b) magnification, ×200. P-AKT, phosphor-protein kinase B;
ESCC, esophageal squamous cell carcinoma. |
There was also a significant association between
P-AKT expression and the sex of the patient (P<0.05), whereas no
such association was observed for the other clinicopathological
features, including age, tumor site, tumor length and volume, T, N
and TNM stage (Table III).
 | Table III.Association between phosphor-protein
kinase B expression and the clinicopathological variables of
patients with esophageal squamous cell carcinoma. |
Table III.
Association between phosphor-protein
kinase B expression and the clinicopathological variables of
patients with esophageal squamous cell carcinoma.
|
| P-AKT
expression |
|
|---|
|
|
|
|
|---|
| Variables | (−) (n=29) | % | (+) (n=39) | % | (++) (n=16) | % | (+++) (n=1) | % | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
|
|
Male | 13 | 44.8 | 28 | 71.8 | 11 | 68.8 | 1 | 100.0 | 0.041 |
|
Female | 16 | 55.2 | 11 | 28.2 | 5 | 31.3 | 0 | 0.0 |
|
| Age, years |
|
|
|
|
|
|
|
|
|
|
≤66 | 14 | 48.3 | 21 | 53.8 | 9 | 56.3 | 1 | 100.0 | 0.456 |
|
>66 | 15 | 51.7 | 18 | 46.2 | 7 | 43.8 | 0 | 0.0 |
|
| Tumor location |
|
|
|
|
|
|
|
|
|
|
Cervical | 1 | 3.4 | 3 | 7.7 | 2 | 12.5 | 0 | 0.0 | 0.248 |
| Upper
thoracic | 6 | 20.7 | 13 | 33.3 | 6 | 37.5 | 0 | 0.0 |
|
| Middle
thoracic | 15 | 51.7 | 18 | 46.2 | 5 | 31.3 | 0 | 0.0 |
|
| Lower
thoracic | 7 | 24.1 | 5 | 12.8 | 3 | 18.8 | 1 | 100.0 |
|
| Tumor length,
cm |
|
|
|
|
|
|
|
|
|
| ≤5 | 10 | 34.5 | 19 | 48.7 | 7 | 43.8 | 1 | 100.0 | 0.281 |
|
>5 | 19 | 65.5 | 20 | 51.3 | 9 | 56.3 | 0 | 0.0 |
|
| Tumor volume,
cm3 |
|
|
|
|
|
|
|
|
|
|
≤25 | 8 | 27.6 | 12 | 30.8 | 6 | 37.5 | 0 | 0.0 | 0.611 |
|
>25 | 21 | 72.4 | 27 | 69.2 | 10 | 62.5 | 1 | 100.0 |
|
| T stage |
|
|
|
|
|
|
|
|
|
|
1+2 | 14 | 48.3 | 22 | 56.4 | 3 | 18.7 | 0 | 0.0 | 0.117 |
|
3+4 | 15 | 51.7 | 17 | 43.6 | 13 | 81.3 | 1 | 100.0 |
|
| N stage |
|
|
|
|
|
|
|
|
|
| 0 | 12 | 41.4 | 19 | 48.7 | 4 | 25.0 | 0 | 0.0 | 0.309 |
| 1 | 17 | 58.6 | 20 | 51.3 | 12 | 75.0 | 1 | 100.0 |
|
|
Tumor-Node-Metastasis stage (17) |
|
|
|
|
|
|
|
|
|
|
I+II | 11 | 37.9 | 25 | 64.1 | 6 | 37.5 | 0 | 0 | 0.688 |
|
III+IV | 18 | 62.1 | 14 | 35.9 | 10 | 62.5 | 1 | 100.0 |
|
Influence of RNF2+P-AKT protein
expression on ESCC
Among the 80 samples used to assess the level of
RNF2+P-AKT expression, 5 patients (6.25%) were
RNF2-negative/P-AKT-negative, 21 (26.25%) were
RNF2-positive/P-AKT-negative, 4 (5.00%) were
RNF2-negative/P-AKT-positive, and 50 patients (62.50%) were
RNF2-positive/P-AKT-positive. Furthermore, there was a significant
association between RNF2-positive/P-AKT-positive expression and sex
(χ2=9.132; P=0.003), and an association with T stage
(χ2=7.240; P=0.065), yet no significant association with
age, tumor length, N stage or TNM stage (Table IV).
 | Table IV.Association between RNF2+P-AKT
expression and the clinicopathological variables of patients with
esophageal squamous cell carcinoma. |
Table IV.
Association between RNF2+P-AKT
expression and the clinicopathological variables of patients with
esophageal squamous cell carcinoma.
|
| RNF2/P-AKT
expression |
|
|---|
|
|
|
|
|---|
|
| RNF2/P-AKT both
positive | Other |
|
|---|
|
|
|
|
|
|---|
| Variables | (n=50) | % | (n=30) | % | P-value |
|---|
| Sex |
|
Male | 37 | 74.00 | 12 | 40.00 | 0.003 |
|
Female | 13 | 26.00 | 18 | 60.00 |
|
| T stage |
| 1 | 0 | 0.00 | 2 | 6.67 | 0.065 |
| 2 | 17 | 34.00 | 8 | 26.67 |
|
| 3 | 6 | 12.00 | 8 | 26.67 |
|
| 4 | 27 | 54.00 | 12 | 40.00 |
|
Association between RNF2 and P-AKT
protein expression level and OS
The patient follow-up date was December 2016, with a
median follow-up time of 80.78 months (95%CI, 61.06–80.61 months)
and a loss rate of 4.0% (4/99) due to loss of contact. Kaplan-Meier
survival curves were generated to estimate the survival rates of
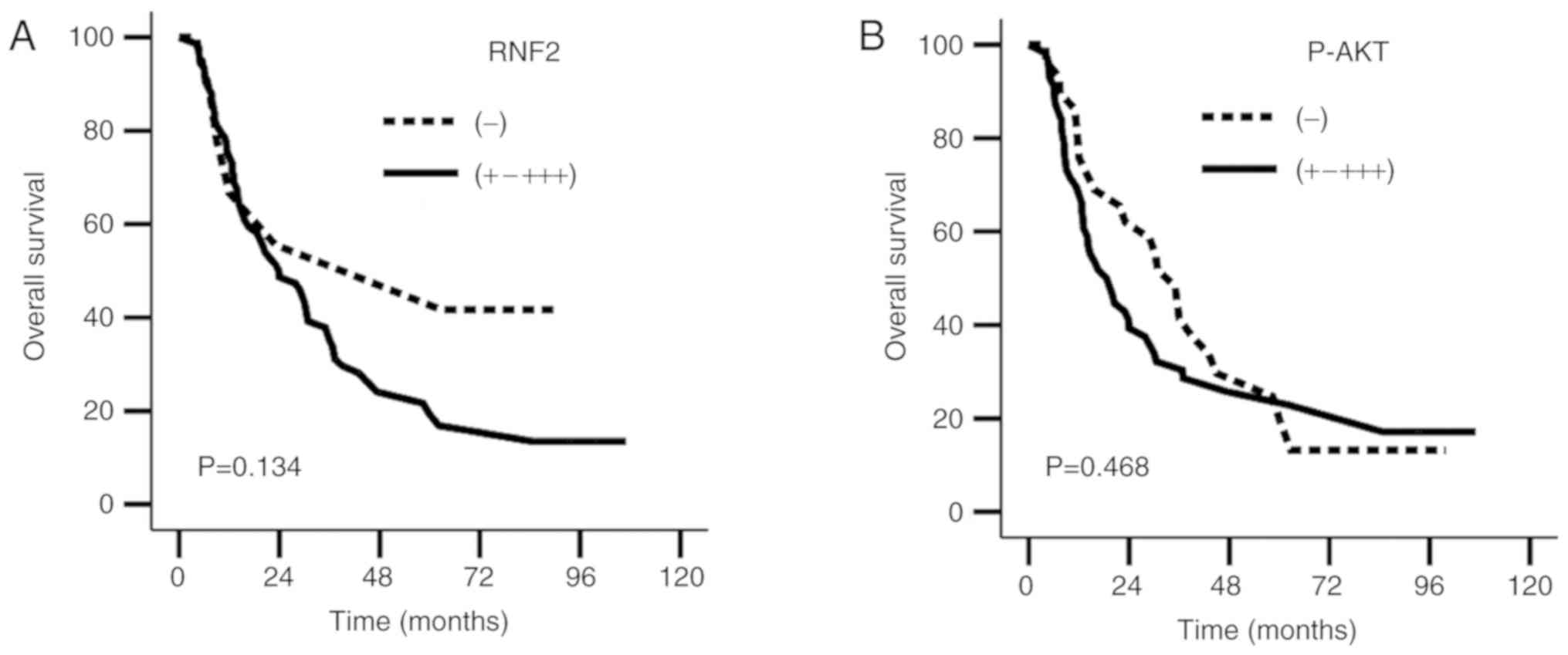
these patients. The results revealed no significant differences in
OS and progression-free survival (PFS) rates between the
RNF2− and RNF2+-+++ groups
(χ2=2.244, P=0.134; χ2=0.818, P=0.366), or
between the P-AKT− and P-AKT+-+++ groups
(χ2=0.526, P=0.468; χ2=0.306, P=0.580;
Fig. 3A and B; Table V).
 | Table V.Survival rates of patients with
esophageal squamous cell carcinoma with different RNF2+P-AKT
expression levels. |
Table V.
Survival rates of patients with
esophageal squamous cell carcinoma with different RNF2+P-AKT
expression levels.
|
| Overall survival
(%) |
|
| Progression-free
survival (%) |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Expression
levels | 1-year | 3-year | 5-year | χ2 | P-value | 1-year | 3-year | 5-year | χ2 | P-value |
|---|
| RNF2
expression |
|
Negative | 77.8 | 55.6 | 55.6 | 2.244 | 0.134 | 66.7 | 33.3 | 33.3 | 0.818 | 0.366 |
|
Positive | 75.7 | 37.8 | 21.6 |
|
| 62.2 | 25.7 | 15.5 |
|
|
| P-AKT
expression |
|
Negative | 82.8 | 48.3 | 24.7 | 0.526 | 0.468 | 69.0 | 27.6 | 11.0 | 0.306 | 0.580 |
|
Positive | 69.6 | 32.1 | 17.1 |
|
| 55.4 | 25.0 | 20.6 |
|
|
| RNF2/P-AKT
expression |
|
Positive | 68.0 | 28.0 | 20.0 | 4.205 | 0.040 | 52.0 | 20.0 | 14.4 | 3.407 | 0.065 |
|
Other | 86.7 | 53.3 | 31.1 |
|
| 76.7 | 33.3 | 18.3 |
|
|
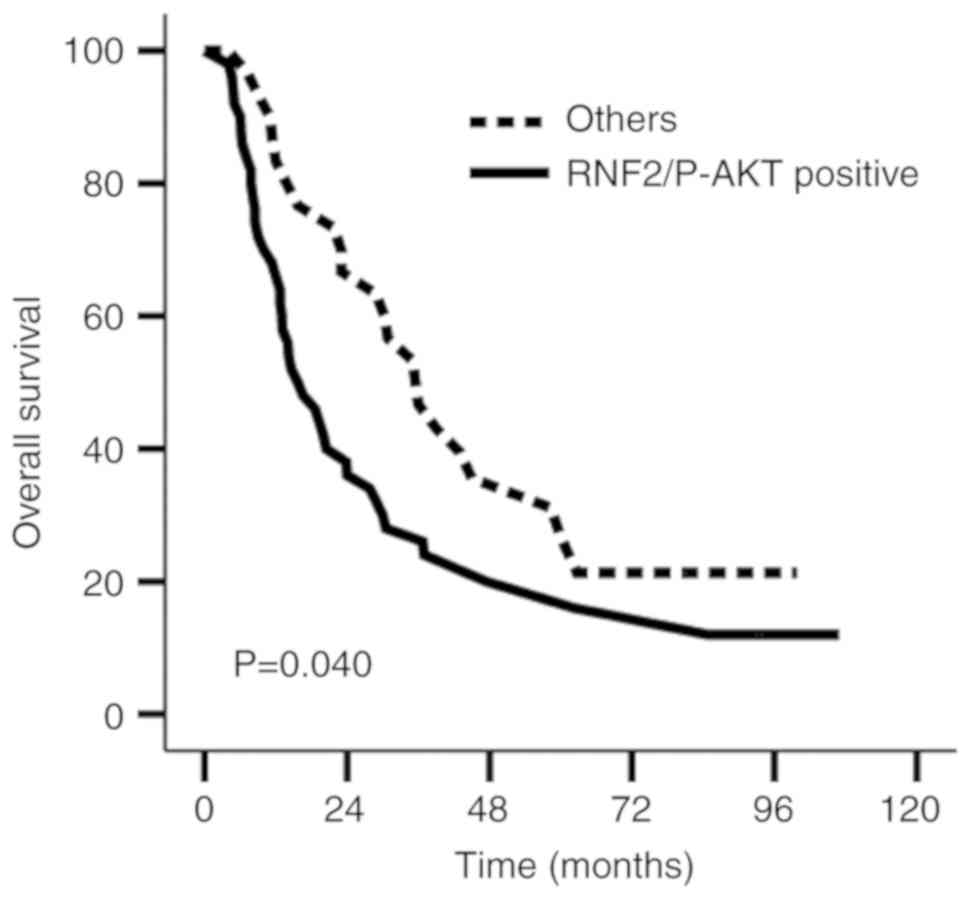
Furthermore, the results illustrated a median
survival time of 15.7 and 36.0 months; the 1, 3 and 5-year survival
rates were 68.0, 28.0 and 20.0%, and 86.7, 53.3 and 31.1%
(χ2=4.205; P=0.040) in the RNF2+P-AKT-positive
expression group and the Other group, respectively (Fig. 4). PFS was not significantly different
between the RNF2+P-AKT-positive expression and Other group
(χ2=3.407; P=0.065; Table
V).
Univariate analysis showed that age, T stage, tumor
volume, RNF2 protein expression, P-AKT protein expression and
RNF2+P-AKT expression were prognostic factors affecting OS
(P=0.036, 0.023, 0.039, 0.007, 0.003 and 0.040, respectively).
Furthermore, multivariate analysis demonstrated that age, T stage
and RNF2+P-AKT expression were independent prognostic factors for
ESCC (P=0.010, 0.008 and 0.010, respectively; Table VI).
 | Table VI.Univariate and multivariate analysis
for the 80 patients with esophageal squamous cell carcinoma with
RNF2+P-AKT positive expression. |
Table VI.
Univariate and multivariate analysis
for the 80 patients with esophageal squamous cell carcinoma with
RNF2+P-AKT positive expression.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
| Overall
survival | Progression-free
survival | Overall
survival | Progression-free
survival |
|---|
|
|
|
|
|
|
|---|
| Variables | HR | 95.0% CI | P-value | HR | 95.0% CI | P-value | HR | 95.0% CI | P-value | HR | 95.0% CI | P-value |
| Age (≤66/>66
years) | 1.704 | 1.030–2.819 | 0.036 | 1.477 | 0.908–2.402 | 0.116 | 1.957 | 1.175–3.259 | 0.010 | 1.620 | 0.990–2.650 | 0.055 |
| Tumor volume
(≤25/>25 cm3) | 1.586 | 1.298–2.802 | 0.039 | 1.863 | 1.058–3.279 | 0.031 | 0.856 | 0.423–1.735 | 0.667 | 1.204 | 0.587–2.469 | 0.613 |
| T stage
(1+2/3+4) | 1.871 | 1.081–3.239 | 0.023 | 1.932 | 1.133–3.296 | 0.016 | 2.125 | 1.222–3.698 | 0.008 | 2.047 | 1.196–3.504 | 0.009 |
| RNF2−
-RNF2+++ | 2.752 | 1.319–5.740 | 0.007 | 0.888 | 0.706–1.119 | 0.315 | 0.833 | 0.622–1.115 | 0.219 | 0.992 | 0.616–1.597 | 0.974 |
| P-AKT−
-P-AKT +++ | 0.045 | 0.005–0.420 | 0.003 | 1.025 | 0.755–1.392 | 0.872 | 0.739 | 0.513–1.065 | 0.105 | 1.533 | 0.622–3.781 | 0.353 |
| RNF2 and P-AKT | 1.713 | 1.017–2.886 | 0.040 | 1.596 | 0.966–2.636 | 0.068 | 2.010 | 1.183–3.415 | 0.010 | 3.341 | 1.758–6.351 | <0.001 |
| Expression
(other/both positive) |
Discussion
In the present study, IHC analysis was performed on
the pathological tissues of patients with ESCC, which revealed that
RNF2 and P-AKT protein expression levels were high in ESCC tissues.
Furthermore, patient survival rates in the RNF2+P-AKT-positive
expression group were lower compared with that in the other group,
suggesting that the expression of RNF2+P-AKT in patients with ESCC
who had previously received radiotherapy was associated with poor
prognosis.
The overexpression of RNF2 has been reported in
numerous types of solid tumor, such as gastrointestinal tumors,
lymphomas (12), breast cancer
(13) and ovarian tumor tissues
(14), and is associated with poor
prognosis in esophageal cancer (15). The present study identified that the
positive expression rate of RNF2 in ESCC was 89.2%, and that
localization of this protein to the nucleus was consistent with
previously published work (20). The
upregulation of RNF2 expression in other tumors has also been
associated with tumor size, pathological grade and poor patient
prognosis (21). In the present
study, a high RNF2 expression level was associated with tumor
volume, prompting the hypothesis that, in ESCC, RNF2 serves an
important role in early metastasis and tumor formation; this has
also been confirmed by other studies (22–24).
The PI3K signaling pathway is involved in numerous
cellular activities and sensitivity to radiotherapy, in addition to
tumor metastasis and apoptosis (25–27). The
phosphorylation of AKT to P-AKT is central to the PI3K pathway, and
its activation stimulates the proliferation of tumor cells
(28). Yoshioka et al
(29) reported that the prognosis of
a low P-AKT-expression group of patients with ESCC treated with
chemotherapy had improved prognoses compared with those exhibiting
high P-AKT expression levels. In addition, Schmitz et al
(30) revealed a negative
correlation between the P-AKT expression level and patient
survival. However, the results of the present study revealed no
significant differences between the survival rates of the
P-AKT-positive and -negative expression groups, which may result
from differences in clinical data and treatment methods between the
2 groups. The small sample size, particularly the
P-AKT(+++) number, was another factor that may have
affected the results; however, to a certain extent, the bias caused
by small sample size could be decreased by the analysis of
P-AKT(+-+++) expression. A larger sample size may
clarify this.
BMI1 is an important predictor of tumor progression
(13) that is able to promote
cellular activity, induce resistance to apoptosis, and increase the
likelihood of metastasis (14). It
may also be associated with treatment failure in a number of
malignancies, including breast and prostate cancer, and
hepatocellular carcinoma (31–33).
Previous studies have demonstrated that the overexpression of BMI1
and RNF2 was associated with tumor cell transformation in multiple
types of tumor tissue (12,34). BMI1 is also essential for the
ubiquitination of histone H2AX (35); when BMI1 is present, the capacity of
RNF2 to ubiquitinate H2AX is enhanced (36), while H2AX itself is associated with
the radiotherapeutic sensitivity of esophageal cancer cells
(37). RNF2 serves as an E3
ubiquitin ligase (35,38,39);
BMI1 is essential for the activity of this E3 ubiquitin ligase in
the PRC1 complex (40), and the
ubiquitination activity of RNF2 requires that BMI1 be present. In a
previous study, it was revealed that the knockdown of BMI1 resulted
in a DNA-damage response defect, and that the PI3K/AKT pathway
could be perturbed to increase sensitivity to radiotherapy
(16). Therefore, it was
hypothesized that RNF2 overexpression may increase radiotherapeutic
resistance by activating the PI3K/AKT pathway. IHC analysis was
conducted using tissue samples from patients with ESCC, which
revealed that the survival rate of those in the RNF2+P-AKT-positive
expression group was significantly lower compared with that of the
other group. In addition, multivariate analysis revealed that
RNF2+P-AKT-positive expression was an independent prognostic factor
affecting survival. To a degree, these results reflected the
synergistic effects of RNF2 and P-AKT in ESCC. Therefore, it was
hypothesized that the expression of RNF2 and P-AKT ESCC was an
important prognostic indicator, though the association between
these proteins requires further experimental confirmation.
There were several limitations associated with the
present study. Firstly, it was a single-center retrospective study
with a small number of cases, and the results require additional
confirmation from a larger prospective sample study. Secondly, the
tissue samples were all from biopsy specimens, and therefore the
tissues were small and not sufficient to fully evaluate the grading
of ESCC. Therefore, the association between the degree of RNF2 and
P-AKT expression, and the classification of tumor tissues, was not
analyzed. Finally, the association between RNF2 and P-AKT was not
assessed in molecular in vitro studies, which would
strengthen the conclusions drawn from the present study.
In conclusion, the present study revealed that the
RNF2 and P-AKT proteins may be important oncogenes in the prognosis
of patients with ESCC, and that their expression was associated
with tumor volume and OS rate, indicating their use as important
molecular markers of ESCC. Therefore, RNF2 and P-AKT are predicted
to be novel prognostic indicators for radical radiotherapy in
patients with ESCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
QL, SZ and XY analyzed and interpreted the patient
data regarding the research. QL, SZ and CS made substantial
contributions to conception and design. QL and CS drafted the
manuscript. QL, SL and CS were major contributors in writing the
manuscript. SL and XZ performed the histological examination of the
esophagus. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent to
participate prior to the collection of tissue specimens and
radiotherapy and/or chemotherapy. The present study was approved by
the Medical Ethics Committee of the Clinical Research of the Fourth
Affiliated Hebei Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, Maclntyre MF, Allen C, Hansen G, Woodbrook R, et
al: The global burden of cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics. 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Otte AP and Kwaks TH: Gene repression by
Polycomb group protein complexes: A distinct complex for every
occasion? Curr Opin Genet Dev. 13:448–454. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Levine SS, Weiss A, Erdjument-Bromage H,
Shao Z, Tempst P and Kingston RE: The core of the polycomb
repressive complex is compositionally and functionally conserved in
flies and humans. Mol Cell Biol. 22:6070–6078. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuzmichev A, Nishioka K, Erdjument-Bromage
H, Tempst P and Reinberg D: Histone methyltransferase activity
associated with a human multiprotein complex containing the
Enhancer of Zeste protein. Genes Dev. 16:2893–2905. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bentley ML, Corn JE, Dong KC, Phung Q,
Cheung TK and Cochran AG: Recognition of UbcH5c and the nucleosome
by the Bmi1/Ring1b ubiquitin ligase complex. EMBO J. 30:3285–3297.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buchwald G, van der Stoop P, Weichenrieder
O, Perrakis A, van Lohuizen M and Sixma TK: Structure and E3-ligase
activity of the Ring-Ring complex of polycomb proteins Bmi1 and
Ring1b. EMBO J. 25:2465–2474. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Z, Cao R, Wang M, Myers MP, Zhang Y and
Xu RM: Structure of a Bmi-1-Ring1B polycomb group ubiquitin ligase
complex. J Biol Chem. 281:20643–20649. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ruan ZP, Xu R, Lv Y, Tian T, Wang WJ, Guo
H and Nan KJ: Bmi1 knockdown inhibits hepatocarcinogenesis. Int J
Oncol. 42:261–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song W, Tao K, Li H, Jin C, Song Z, Li J,
Shi H, Li X, Dang Z and Dou K: Bmi-1 is related to proliferation,
survival and poor prognosis in pancreatic cancer. Cancer Sci.
101:1754–1760. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sánchez-Beato M, Sánchez E,
González-Carreró J, Morente M, Díez A, Sánchez-Verde L, Martín MC,
Cigudosa JC, Vidal M and Piris MA: Variability in the expression of
polycomb proteins in different normal and tumoral tissues. A pilot
study using tissue microarrays. Mod Pathol. 19:684–694. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bosch A, Panoutsopoulou K, Corominas JM,
Gimeno R, Moreno-Bueno G, Martín-Caballero J, Morales S, Lobato T,
Martínez-Romero C, Farias EF, et al: The Polycomb group protein
RING1B is overexpressed in ductal breast carcinoma and is required
to sustain FAK steady state levels in breast cancer epithelial
cells. Oncotarget. 5:2065–2076. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su WJ, Fang JS, Cheng F, Liu C, Zhou F and
Zhang J: RNF2/Ring1b negatively regulates p53 expression in
selective cancer cell types to promote tumor development. Proc Natl
Acad Sci USA. 110:1720–1725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang XX, Ma M, Sang MX, Wang XX, Song H,
Liu ZK and Zhu SC: Radiosensitization of esophageal carcinoma cells
by knockdown of RNF2 expression. Int J Oncol. 48:1985–1996. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang XX, Ma M, Sang MX, Zhang XY, Liu ZK,
Song H and Zhu SC: BMI-1 suppression increases the radiosensitivity
of oesophageal carcinoma via the PI3K/Akt signaling pathway. Oncol
Rep. 39:667–678. 2018.PubMed/NCBI
|
|
17
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Mamal. 7th.
Springer-Verlag; New York, NY: pp. 103–15. 2009
|
|
18
|
Masunaga R, Kohno H, Dhar DK, Ohno S,
Shibakita M, Kinugasa S, Yoshimura H, Tachibana M, Kubota H and
Nagasue N: Cyclooxygenase-2 expression correlates with tumor
neovascularization and prognosis in human colorectal carcinoma
patients. Clin Cancer Res. 6:4064–4068. 2000.PubMed/NCBI
|
|
19
|
Wu D, Ding Y, Wang S, Zhang Q and Liu L:
Increased expression of high mobility group box 1 (HMGB1) is
associated with progression and poor prognosis in human
nasopharyngeal carcinoma. J Pathol. 216:167–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Xu H, Zou X, Wang J, Zhu Y, Chen
H, Shen B, Deng X, Zhou A, Chin YE, et al: Snail recruits Ring1B to
mediate transcriptional repression and cell migration in pancreatic
cancer cells. Cancer Res. 74:4353–4363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen S, Chen J, Zhan Q, Zhu Y, Chen H,
Deng X, Hou Z, Shen B, Chen Y and Peng C: H2AK119Ub1 and H3K27Me3
in molecular staging for survival prediction of patients with
pancreatic ductal adenocarcinoma. Oncotarget. 5:10421–10433.
2014.PubMed/NCBI
|
|
22
|
Rai K, Akdemir KC, Kwong LN, Fiziev P, Wu
CJ, Keung EZ, Sharma S, Samant NS, Williams M, Axelrad JB, et al:
Dual Roles of RNF2 in melanoma progression. Cancer Discov.
5:1314–1327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van der Stoop P, Boutsma EA, Hulsman D,
Noback S, Heimerikx M, Kerkhoven RM, Voncken JW, Wessels LF and van
Lohuizen M: Ubiquitin E3 ligase Ring1b/Rnf2 of polycomb repressive
complex 1 contributes to stable maintenance of mouse embryonic stem
cells. PLoS One. 3:e22352008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van der Velden YU, Wang L, Querol Cano L
and Haramis AP: The polycomb group protein ring1b/rnf2 is
specifically required for craniofacial development. PLoS One.
8:e739972013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang XJ, Yu HY, Cai YJ and Ke M: Lycium
barbarum polysaccharides inhibit proliferation and migration of
bladder cancer cell lines BIU87 by suppressing Pi3K/AKT pathway.
Oncotarget. 8:5936–5942. 2017.PubMed/NCBI
|
|
26
|
Bussink J, van der Kogel AJ and Kaanders
JH: Activation of the PI3-K/AKT pathway and implications for
radioresistance mechanisms in head and neck cancer. Lancet Oncol.
9:288–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng R, Tang J, Ma JG, Chen SP, Xia LP,
Zhou WJ, Li DD, Feng GK, Zeng YX and Zhu XF: PKB/Akt promotes DSB
repair in cancer cells through upregulating Mre11 expression
following ionizing radiation. Oncogene. 30:944–955. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Zheng L, Ding Y, Li Q, Wang R,
Liu T, Sun Q, Yang H, Peng S, Wang W and Chen L: MiR-20a induces
cell radioresistance by activating the PTEN/PI3K/Akt signaling
pathway in hepatocellular carcinoma. Int J Radiat Oncol Biol Phys.
92:1132–1140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshioka A, Miyata H, Doki Y, Yasuda T,
Yamasaki M, Motoori M, Okada K, Matsuyama J, Makari Y, Sohma I, et
al: The activation of Akt during preoperative chemotherapy for
esophageal cancer correlates with poor prognosis. Oncol Rep.
19:1099–1107. 2008.PubMed/NCBI
|
|
30
|
Schmitz KJ, Otterbach F, Callies R, Levkau
B, Hölscher M, Hoffmann O, Grabellus F, Kimmig R, Schmid KW and
Baba HA: Prognostic relevance of activated Akt kinase in
node-negative breast cancer: A clinicopathological study of 99
cases. Mod Pathol. 17:15–21. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang L, Liu JL, Yu L, Liu XX, Wu HM, Lei
FY, Wu S and Wang X: Downregulated miR-495 [Corrected] inhibits the
G1-S Phase transition by targeting Bmi-1 in breast cancer. Medicine
(Baltimore). 94:e7182015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin M, Zhang T, Liu C, Badeaux MA, Liu B,
Liu R, Jeter C, Chen X, Vlassov AV and Tang DG: miRNA-128
suppresses prostate cancer by inhibiting BMI-1 to inhibit
tumor-initiating cells. Cancer Res. 74:4183–4195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang F, Lv LZ, Cai QC and Jiang Y:
Potential roles of EZH2, Bmi-1 and miR-203 in cell proliferation
and invasion in hepatocellular carcinoma cell line Hep3B. World J
Gastroenterol. 21:13268–13276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Richly H, Aloia L and Di Croce L: Roles of
the Polycomb group proteins in stem cells and cancer. Cell Death
Dis. 2:e2042011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang H, Wang L, Erdjument-Bromage H, Vidal
M, Tempst P, Jones RS and Zhang Y: Role of histone H2A
ubiquitination in Polycomb silencing. Nature. 431:873–878. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rao PS, Satelli A, Zhang S, Srivastava SK,
Srivenugopal KS and Rao US: RNF2 is the target for phosphorylation
by the p38 MAPK and ERK signaling pathways. Proteomics.
9:2776–2787. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi HY and Zhu SC: Radiosensitization of
esophageal cancer cells ECA109 by knockdown of H2AX. Thorac Cancer.
4:254–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ohtsubo M, Yasunaga S, Ohno Y, Tsumura M,
Okada S, Ishikawa N, Shirao K, Kikuchi A, Nishitani H, Kobayashi M
and Takihara Y: Polycomb-group complex 1 acts as an E3 ubiquitin
ligase for Geminin to sustain hematopoietic stem cell activity.
Proc Natl Acad Sci USA. 105:10396–10401. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sen N, Satija YK and Das S: PGC-1α, a key
modulator of p53, promotes cell survival upon metabolic stress. Mol
Cell. 44:621–634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ben-Saadon R, Zaaroor D, Ziv T and
Ciechanover A: The polycomb protein Ring1B generates self atypical
mixed ubiquitin chains required for its in vitro histone H2A ligase
activity. Mol Cell. 24:701–711. 2006. View Article : Google Scholar : PubMed/NCBI
|