Introduction
Preoperative neoadjuvant chemotherapy (NAC) is
established as a therapeutic avenue for selected high-risk BCs,
with tumors ≥2 cm and for locally advanced (including initially
ineligible for resection) disease (1). Breast cancer is divided into three
groups based on the expression or not of different markers such as
estrogen receptor (ER), progesterone receptor (PgR) and human
epidermal growth factor receptor 2 (HER2): Hormone receptor
positive/HER2 negative, HER2 positive, and triple-negative that
does not have any of the three molecular markers. The therapeutic
intervention consists of endocrine therapy for all Hormone Receptor
(HR) positive tumors with some patients requiring chemotherapy
(adriamycin, cyclophosphamide, paclitaxel or docetaxel);
chemotherapy plus HER2-targeted therapy for HER2 positive and
chemotherapy alone for triple-negative breast cancer (2). The use of NAC in BC treated with
surgery is currently increasing because of the chance of measuring
early in-vivo response to systemic treatment and to achieve higher
rates of breast conserving surgery (3). Neoadjuvant treatment modalities require
a close collaboration between oncologists, surgeons, radiologists
and pathologists to attain the best results.
The most important parameter for successful
treatment and improvement in overall survival (OS) is the
achievement of a pCR although the identification of clinical and
pathological parameters predictive of treatment response and best
survival outcomes remains not still clearly defined. Preliminary
identification of patient subgroups with high pCR rates could
preferably refer them to neoadjuvant treatment strategies. Despite
extensive clinical investigations, it has not yet been clarified
whether NAC would result in improved survival in comparison with
the standard adjuvant setting in any subgroups of patients with BC
(4).
However, comparable data between adjuvant and
neoadjuvant settings (5,6) have changed the BC treatment, with an
increased use of NAC. These data have stimulated the formation of
an international expert panel consensus to provide recommendations
on the use of neoadjuvant therapy. In short, the panel suggests
that NAC should be considered in any individual patient for whom
adjuvant chemotherapy is indicated (7).
BC is increasingly recognized as a heterogeneous
disease showing a different response to treatment according to
defined clinical and pathological parameters. Additionally, changes
in some pathological parameters during each BC history require new
tumor biopsy and even complicate the BC treatment. The prognostic
value of these changes is also not well defined (8,9).
Chemotherapy is especially effective in the treatment of endocrine
insensitive tumors, and such therapeutic benefit can be assumed for
patients with triple-negative, or HR negative and HER2 positive BC
(10,11). In case of HER2 positive tumors, an
anti-HER2 agent can be administered as part of the preoperative
treatment, and according to preliminary clinical data, dual HER2
blockade can offer an additional therapeutic value (12). However, dose escalation, kind of
chemotherapy regimen and number of cycles are still debating
matters.
In this study, we reported retrospective data
concerning 117 BC patients treated with NAC in a single
institution. All available clinic-pathological parameters were
analyzed to evaluate the correlation, if any, with response and
survival outcomes.
Patients and methods
Patient selection
Data from 117 patients with BC diagnosed by needle
aspiration cytology and histopathology biopsy and treated from
March 2010 to December 2015 with various regimens of NAC at a
single center were reviewed. All patients provided informed consent
and Local Ethical Committee of San Giovanni di Dio Hospital of
Frattamaggiore gave formal approval to this retrospective study
(approval no. 1250 on 20th February 2018). Eligible patients were
women aged ≥18 years with localized primary BC suitable for primary
medical treatment with or without regional lymph node metastases,
with adequate bone marrow, renal, hepatic, and cardiac functions,
no other uncontrolled medical or psychiatric disorders and with an
ECOG performance status of 0–1. The main exclusion criteria were
distant metastases, other malignancy in the past two years (except
for radically treated basal or squamous cell carcinoma of the skin
or carcinoma in situ of the cervix), and pregnancy or
lactation.
Clinical assessment
At baseline all patients underwent clinical
assessment, hematology and chemistry and core needle biopsies that
were performed either free-hand or under ultra-sound guidance. For
each patient we recorded baseline tumor size by ultrasound and
mammography, nodal status involvement by ultrasound-guided
fine-needle aspiration or, when negative, by sentinel node biopsy,
type of NAC and type of surgery (S). pCR defined as the absence of
invasive cells in the breast and the lymph nodes regardless of
Ductal Carcinoma In Situ (DCIS). Immunohistochemistry (IHC)
subtypes were defined according to Estrogen (ER) and progesterone
receptor (PgR), Ki-67 level (nuclear antigen expressed in cycling
cells), and HER2 status.
Clinical and radiological response evaluations were
performed after two, four and six courses of treatment. All
patients underwent Positron Emission Tomography (PET) at baseline
and before surgery.
Tumors were staged according to Tumor Node
Metastasis (TNM) staging system (7th edition). ER and PgR status
were determined by IHC and was considered positive if tumor cells
were ≥1%. Tumors with a score of 3+ (strong homogeneous staining)
were considered HER2-positive. In case of 2+ scores (moderate
homogeneous staining) chromogenic in situ hybridization
(CISH) was used to determine amplification. The tumor margins were
defined as a clear pathologic margin if the distance was >2 mm
by microscopy evaluation. The clinic-pathological data at baseline
are summarized in Table I. The
clinical pathological protocols used for the collection of the data
were recorded at the Unit of Oncology of San Giovanni di Dio
Hospital in Frattamaggiore.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Result |
|---|
| Age |
|
| Age
median (years) | 52.8 |
| Age range
(years) | 35–85 |
| Menstrual status |
|
|
Premenopausal | 53 |
|
Postmenopausal | 64 |
| Histology |
|
|
Ductal | 105 |
|
Lobular | 7 |
|
Mixed | 5 |
| T-stage |
|
| T1 | 12 |
| T2 | 49 |
| T3 | 15 |
| T4 | 42 |
| N-stage |
|
| N0 | 18 |
| N1 | 26 |
|
N2/N3 | 66 |
| NX | 7 |
| Receptor-based
subtype |
|
|
ER-positive (ER+; HER2-) | 67 |
|
Triple-negative (ER-; PR-;
HER2-) | 15 |
|
HER2+ | 35 |
| Ki67 status |
|
| Low
(<14%) | 24 |
|
Intermediate (14–30%) | 52 |
| High
(>30%) | 41 |
Therapeutic management of
patients
Neoadjuvant regimens of chemotherapy were summarized
in Table II. Number of chemotherapy
cycles administered was guided by tumor evaluation and ranged
between 4 and at most 6 cycles. Recommendation for surgery was made
after evaluation of a team of BC specialists including
radiologists, medical oncologists and surgeons. Treatment decisions
were mainly based on patient's desires and surgical considerations
involving the breast-tumor index, age, multifocality, localization
and response were obtained after NAC. All patients with proven
axillary lymph node metastases prior to NAC underwent to an
axillary lymph node dissection (ALND) at levels I and II with level
III sampling after NAC. Level III sampling was done to stage for
adjuvant radiotherapy indications. Patients undergoing to
breast-conserving surgery received radiation to the breast with a
boost to the tumor bed. The indication for loco-regional radiation
therapy (chest wall and regional nodal basins) was based on the
original staging. Hormone receptor-positive patients received
adjuvant endocrine treatment for at least 5 years and HER2-positive
patients were treated with trastuzumab for 1 year.
 | Table II.pCR correlation with neoadjuvant
treatment. |
Table II.
pCR correlation with neoadjuvant
treatment.
| Chemotherapy
regimen | Patient number | pCR number | pCR rate (%) |
|---|
| EC → TXT | 5 | 0 | 0 |
| EC → TXT + H | 2 | 0 | 0 |
| TMC | 16 | 1 | 6 |
| TEC + H | 11 | 2 | 18 |
| TEC | 60 | 17 | 28 |
| TMC + H | 6 | 2 | 33 |
| PERT/H/TXT | 17 | 13 | 76 |
Statistical anlaysis
RFS was estimated by the Kaplan-Meier method and
compared using the log rank analysis. P-values were considered
statistically significant when <0.05. The statistical analyses
were performed using the chi-square test and conducted by SPSS
software (version 17.0).
Results
Patient characteristics
The median age of the patients was 52.8 years (range
35–85 years). Fifty-three (45%) were pre- and 64 (55%) were
post-menopausal women. Most patients had ductal invasive carcinoma
(approximately 90%). T2 and T4 tumors were the most represented in
our series: 42 and 36%, respectively. Lymph node involvement
classified as N2/N3 was present in 56% of the patients. Hormone
positive, HER2 negative tumors were 57% of the patients. HER2
positive tumors were about 30% of the patients. Intermediate-high
proliferating tumors, expressed by Ki-67, were about 79% (Table I).
Effects of NAC regimen
Chemotherapy used for NAC was predominantly an
anthracycline-based regimen (67% of the patients) as shown in
Table II. A HER2 targeted therapy
was used in 16% of the patients. In 19% of the patients a liposomal
doxorubicin was used. In 14% of the patients, belonging to the last
enrolled patients, the novel treatment with double antibodies
(pertuzumab and trastuzumab) plus docetaxel was used (Table II). The number of patients treated
with trastuzumab was low because the combination of trastuzumab
with anthracyclines could induce sub-clinical or clinical cardiac
failure.
All patients had a positive PET scan at the tumor
site. About 73% of the patients showed significant uptake at the
level of the axilla. Overall Response Rate (ORR) was 76 and 72%
when assessed by radiological and pathological evaluation,
respectively. In 40 out of 117 patients (34%) a conservative
treatment was allowed. In details, we recorded 46 CRs and 43
partial responses (PRs) by radiology (Table III). Thirty-five pCRs (pCR rate
about 30%) and 49 pPRs were documented by pathology (Table III). Among the 15 patients with
triple-negative tumors, a conservative surgical treatment was
performed in 6 patients and in 6 instances a pathological response
was obtained. Three triple negative patients relapsed during
follow-up: One patient at lymph nodes, one at lymph nodes and bone
and one at bone and visceral sites.
 | Table III.Response rates by method of
assessment. |
Table III.
Response rates by method of
assessment.
| Clinical
assessment | Total number | ORR (%) | CR number | PR number | SD number | PD number |
|---|
| Radiological | 117 | 76 | 46 | 43 | 23 | 5 |
| Pathological | 117 | 72 | 35 | 49 | 28 | 5 |
Statistical correlation between
pathological responses, and clinical and pathological features
Comparing all together complete and partial
pathologically responders to the patients with SD and PD, the RFS
difference was highly statistically significant (P=0.0210) as shown
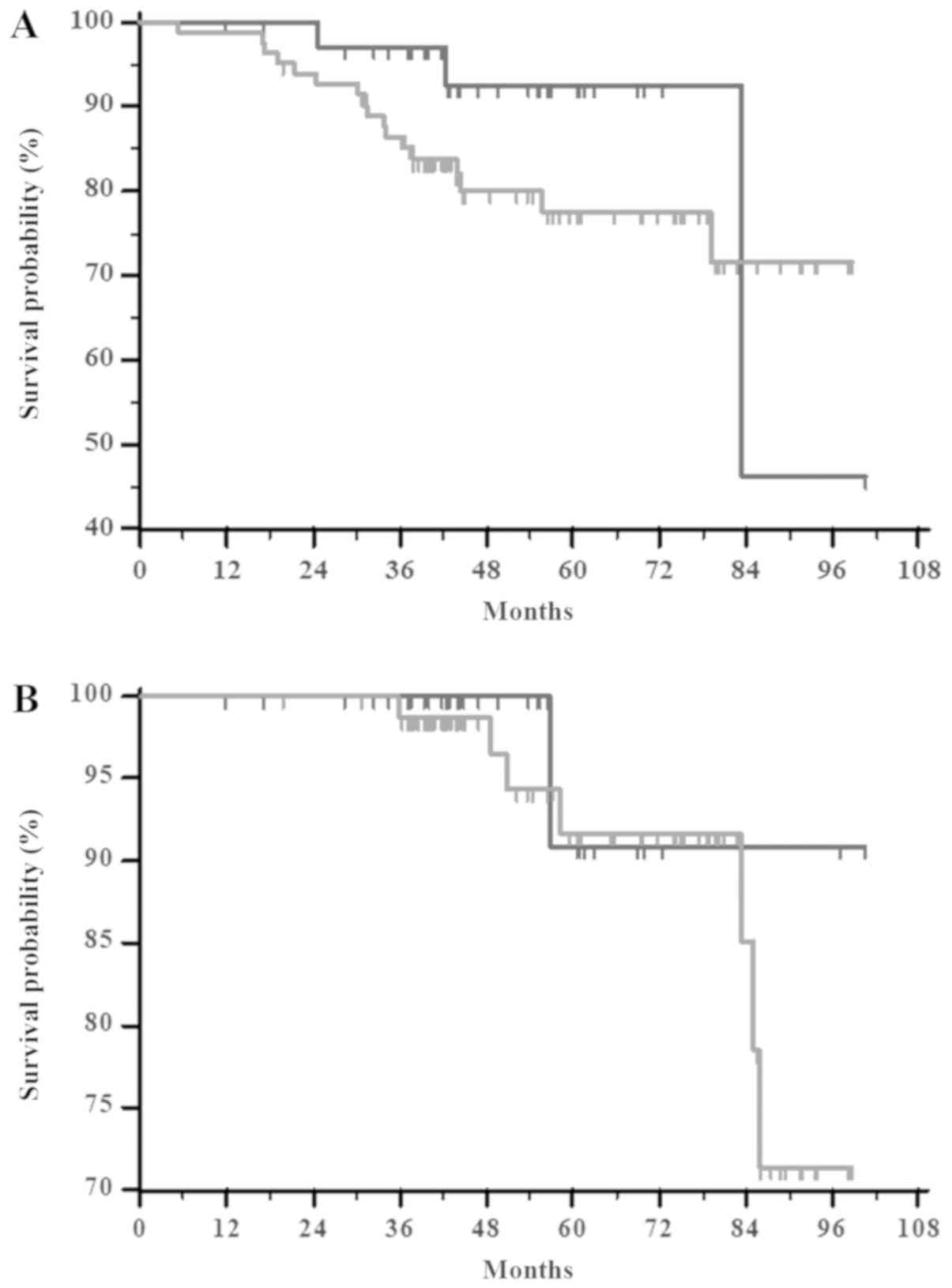
in Fig. 1. However, pCR does not
significantly correlate with either RFS or overall survival (OS)
(P=0.1852 and P=0.5599, respectively) (Fig. 2A and B, respectively), when compared
to patients with pPRs + pSD + PD. There was no significant
difference between clinical response and Ki-67 labeling index
(P=0.9705). Likewise, the statistical analysis showed no
differences between pathological responses and Ki-67 labeling index
(P=0.2867) (Table IV).
 | Table IV.Clinical and pathological response
after neoadjuvant chemotherapy by Ki67 labeling index. |
Table IV.
Clinical and pathological response
after neoadjuvant chemotherapy by Ki67 labeling index.
|
| Ki67 labeling
index |
|
|---|
|
|
|
|
|---|
| Therapy
response | Low | Intermediate | High | P-value |
|---|
| Clinical
response |
|
|
|
|
| PR +
CR | 17 | 37 | 30 | 0.9705 |
| SD +
PD | 7 | 15 | 11 |
|
| Pathological
response |
|
|
|
|
|
pCR | 6 | 13 | 16 | 0.2867 |
|
Not-pCR | 18 | 39 | 25 |
|
By chi-square test the combination of an
anthracycline-based regimen plus an anti-HER2 therapy was
significantly associated with pCR (P=0.00025712).
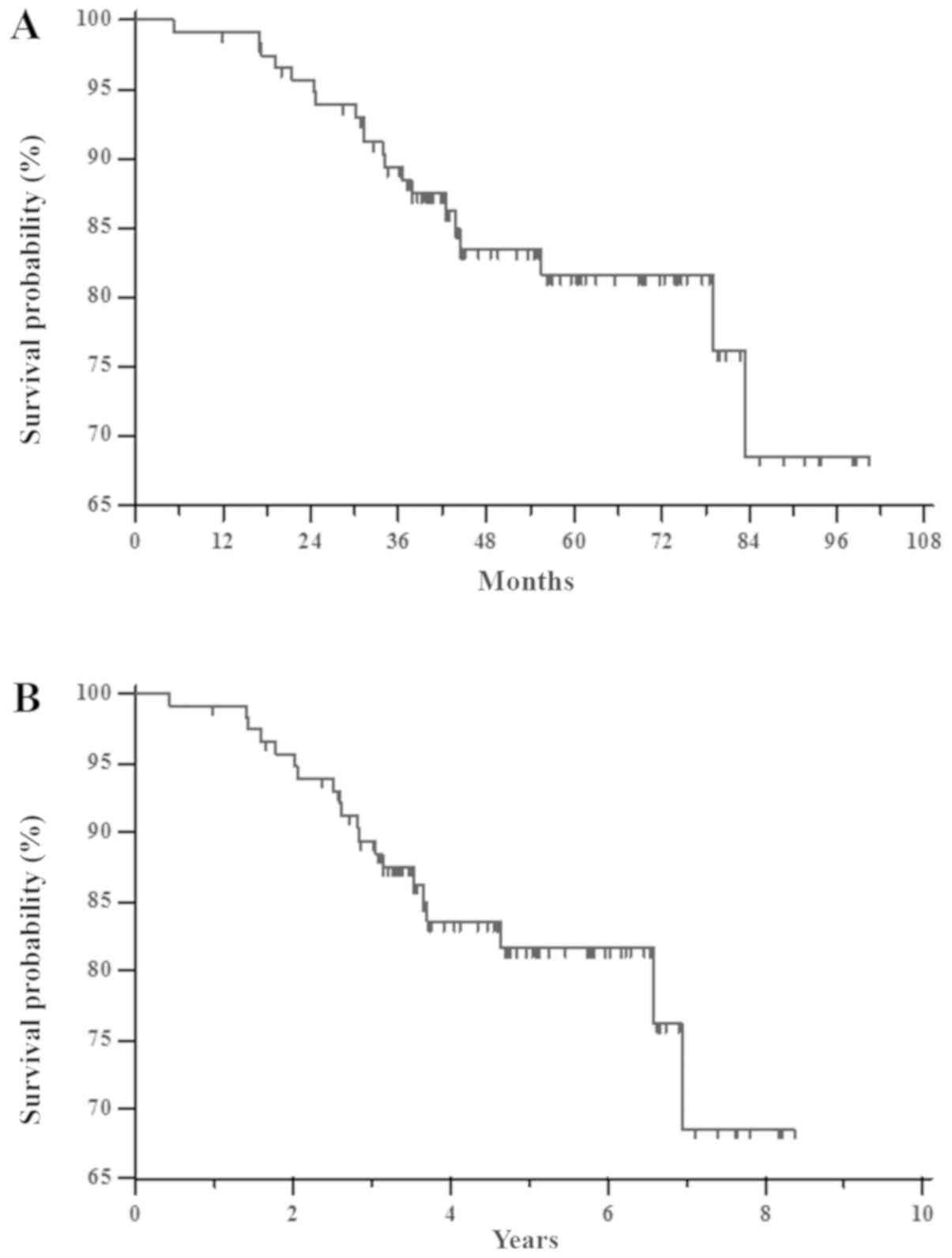
The RFS mean for all groups (117 patients) was 85
months (SE=3; 95% CI 79–91). The median was not reached and the
mean follow-up time was 55 months (median 52 months; range 11–100
months). In this time, about twenty patients (17%) experienced
tumor recurrence (Fig. 3A). The 1–7
year RFS rates were 99.1, 95.7, 89.4, 83.5, 81.7, 81.7 and 68.6%,
respectively (Fig. 3B).
At univariate analysis, pathological response was
significantly associated with receptor-based subtype (P=0.0001),
menopausal status (P=0.0368) and T-stage (P=0.0007). On the
contrary, obesity (P=0.6128), histological subtype (P=0.3238), Ki67
status (P=0.1747), N-stage (P=0.8914) and age (P=0.0730) did not
seem to correlate with pathological response in this patients'
series (Table V).
 | Table V.Correlation between pathological
responses and clinical and pathological features. |
Table V.
Correlation between pathological
responses and clinical and pathological features.
| Clinical and
pathological features | pCR breast and
axilla number | Not-pCR | P-value |
|---|
| Histological
subtype |
|
|
|
|
Ductal | 33 | 72 |
|
|
Lobular | 2 | 5 | 0.3238 |
|
Mixed | 0 | 5 |
|
| Receptor-based
subtype |
|
|
|
|
ER-positive (ER+; HER2-) | 10 | 59 | 0.0001 |
|
Triple-negative (ER-; PR-;
HER2-) | 7 | 8 |
|
|
HER2+ | 18 | 15 |
|
| Ki67 status |
|
|
|
| Low
(<14%) | 6 | 18 |
|
|
Intermediate (14–30%) | 13 | 39 | 0.1747 |
| High
(>30%) | 16 | 25 |
|
| Menopausal
status |
|
|
|
|
Premenopausal | 21 | 32 | 0.0368 |
|
Postmenopausal | 14 | 50 |
|
| T-stage |
|
|
|
| T1 | 8 | 3 |
|
| T2 | 10 | 39 |
|
| T3 | 1 | 14 | 0.0007 |
| T4 | 16 | 26 |
|
| N-stage |
|
|
|
| N0 | 4 | 14 |
|
| N1 | 8 | 18 | 0.8914 |
|
N2/N3 | 20 | 44 |
|
| NX | 3 | 6 |
|
| BMI |
|
|
|
|
Overweight | 15 | 41 | 0.6128 |
|
Normal | 20 | 41 |
|
| Age |
|
|
|
|
>50 | 14 | 48 | 0.0730 |
|
<50 | 21 | 34 |
|
At multivariate analysis by using linear multiple
regression and including receptor status, menopausal status and
T-stage, the model was not significant for menopausal status
(P=0.062).
However, by using the multiple logistic regression,
and including age, pCR was significantly associated with ER+
HER2neg (P=0.006), T2 (P=0.043), and T3 (P=0.018).
Histological heterogeneity of breast
cancer
Fourteen tumor histotypes changed: 5 mixed
ductal/lobular tumors became ductal (4 cases) and lobular (1 case),
8 ductal carcinomas became lobular (7 cases) and ductal hyperplasia
(1 case), one lobular carcinoma became ductal. ER/PgR and HER2
status changed in 15 out of 117 patients (12%). In particular, 5
patients became HER2 positive and 5 patients became negative
(respectively about 4%). ER/PgR status changed in 7 patients (6%)
with 5 patients becoming hormone-responsive.
Discussion
In this retrospective review of clinical practice
treatment with NAC of BC patients, we obtained a high radiological
and pathological response rate. The association of an
anthracycline-based regimen plus an anti-HER2 therapy was
significantly associated with high pCR rate. Moreover, clinical and
pathological response was independent from proliferation rate as
expressed by Ki-67 status, reflecting an association to still
unspecified molecular features more than to the simple
proliferation rate of the neoplasm as previously reported. In this
series, pCR does not significantly correlate with both RFS and OS,
differently from most published studies. A meta-analysis of
randomized clinical trials for resectable BC comparing the survival
benefit of NAC vs. postoperative chemotherapy showed that NAC
cycles, the total number of chemotherapy cycles, administration of
tamoxifen, administration of adjuvant chemotherapy, or type of NAC
regimen, did not influence OS. The pooled HR estimate for RFS was
influenced by anthracycline-containing regimens. Patients with a
pCR had superior survival outcomes compared with patients who had
residual disease (5). However, we
found a significant correlation between the pathological response
and the RFS when compared to stable or progressive disease. In the
present study, an anthracycline-based regimen plus the anti-HER2
agent trastuzumab showed better results in term of pCR.
Interestingly, when treating HER2 positive tumours the best
response is given by trastuzumab and pertuzumab combined with
docetaxel. The responses observed in this subset of patients is
online with previously published trials (13,14).
Moreover, the synergism between pertuzumab and trastuzumab is not
surprising if we consider that this kind of tumours are likely
driven by an overexpression and hyperactivation of peptide growth
factor receptors and, therefore, are more likely responsive to the
block of these pathways. HER2-positive breast cancers are more
aggressive than other types of BC and anti-HER2 agents bind
different domains of HER2 receptor inducing a dual blockade of the
receptor and consequent inhibition of the downstream signaling
processes associated with tumor growth and progression. The
concomitant use of the two antibodies can have synergistic effects
based upon the different biological effects triggered by the two
different weapons: Trastuzumab activates immunological effects
(i.e.: ADCC) and blocks receptor activation and pertuzumab inhibits
receptor dimerization thus specifically blocking the different
signal transduction activated by the receptors overcoming
resistance to trastuzumab due to HER2 truncations (15). However, it is still not clear and
defined how to predict the response of BC to NAC regimens and how
to select the best combination in patients. Molecular research on
genetic characteristics of BC could be useful in the future to
predict the best choice in this subset of patients as already
experienced some years ago for taxanes (16). In the present study we recorded also
a change in some cases of the pathological molecular features of BC
after the treatment of the patients with NAC. In fact, HER2 status
changed in 8% of cases. ER/PgR status changed in 6% of the
patients. These data are similar to previously reported similar
series (17). The difference between
IHC from preliminary biopsy and definitive surgical specimens may
reflect not only different kind of samples but also a change in
tumor itself conditioned by NAC.
Obesity does not seem to correlate with pathological
response in this patients' series. Previous studies assessed the
value of obesity in patients treated with NAC. Obesity seems to
have a negative impact on survival that is independent from
chemotherapy dosing (18–21).
Although the reduced number of patients and the
retrospective analysis limited the value of this study, the data
reported reflect the clinical practice of a single institution and
suggest evaluating T-stage, menopausal status and receptor status
in patients with inoperable BC treated with NAC to predict the
pathological response. Despite the increased knowledge of
distinctive clinical and pathological parameters and insights into
genetic variability of BC, a standardized model predictive of
response to NAC is not presently available. A tailored evaluation
of presumptive better chemotherapeutic regimens for each tumor
subtype is desirable in the next future.
Acknowledgements
Not applicable.
Funding
This article was supported by a grant from POR
CAMPANIA FESR 2014/2020
Availability of data and materials
All data generated and/or analyzed during the
present study are included in this published article.
Authors' contributions
SDP, MC, AL, LM, PS, GC, EC, OF, MA, PI and RA
designed the study and collected the data. RA and MC wrote the
paper. GG, EL and AA performed the statistical analysis.
Ethics approval and consent to
participate
All patients provided informed consent and Local
Ethical Committee of San Giovanni di Dio Hospital of Frattamaggiore
gave formal approval to this retrospective study (approval no. 1250
on 20th February 2018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thompson AM and Moulder-Thompson SL:
Neoadjuvant treatment of breast cancer. Ann Oncol. 23 (Suppl
10):x231–x236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Untch M, Konecny GE, Paepke S and von
Minckwitz G: Current and future role of neoadjuvant therapy for
breast cancer. Breast. 23:526–537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rubovszky G and Horváth Z: Recent advances
in the neoadjuvant treatment of breast cancer. J Breast Cancer.
20:119–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen Y, Shi XE, Tian JH, Yang XJ, Wang YF
and Yang KH: Survival benefit of neoadjuvant chemotherapy for
resectable breast cancer: A meta-analysis. Medicine (Baltimore).
97:e106342018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Long-term outcomes for neoadjuvant
versus adjuvant chemotherapy in early breast cancer: Meta-analysis
of individual patient data from ten randomised trials. Lancet
Oncol. 19:27–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaufmann M, Hortobagyi GN, Goldhirsch A,
Scholl S, Makris A, Valagussa P, Blohmer JU, Eiermann W, Jackesz R,
Jonat W, et al: Recommendations from an international expert panel
on the use of neoadjuvant (primary) systemic treatment of operable
breast cancer: An update. J Clin Oncol. 24:1940–1949. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang L, Zhong X, Pu T, Qiu Y, Ye F and Bu
H: Clinical significance and prognostic value of receptor
conversion in hormone receptor positive breast cancers after
neoadjuvant chemotherapy. World J Surg Oncol. 16:512018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoshida A, Hayashi N, Suzuki K, Takimoto
M, Nakamura S and Yamauchi H: Change in HER2 status after
neoadjuvant chemotherapy and the prognostic impact in patients with
primary breast cancer. J Surg Oncol. 116:1021–1028. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Straver ME, Rutgers EJ, Rodenhuis S, Linn
SC, Loo CE, Wesseling J, Russell NS, Oldenburg HS, Antonini N and
Vrancken Peeters MT: The relevance of breast cancer subtypes in the
outcome of neoadjuvant chemotherapy. Ann Surg Oncol. 17:2411–2418.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Curigliano G, Burstein HJ, P Winer E,
Gnant M, Dubsky P, Loibl S, Colleoni M, Regan MM, Piccart-Gebhart
M, Senn HJ, et al: De-escalating and escalating treatments for
early-stage breast cancer: The St. Gallen International expert
consensus conference on the primary therapy of early breast cancer
2017. Ann Oncol. 28:1700–1712. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Debiasi M, Polanczyk CA, Ziegelmann P,
Barrios C, Cao H, Dignam JJ, Goss P, Bychkovsky B, Finkelstein DM,
Guindalini RS, et al: Efficacy of anti-HER2 agents in combination
with adjuvant or neoadjuvant chemotherapy for early and locally
advanced HER2-positive breast cancer patients: A network
meta-analysis. Front Oncol. 8:1562018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Swain SM, Ewer MS, Viale G, Delaloge S,
Ferrero JM, Verrill M, Colomer R, Vieira C, Werner TL, Douthwaite
H, et al: Pertuzumab, trastuzumab, and standard anthracycline- and
taxane-based chemotherapy for the neoadjuvant treatment of patients
with HER2-positive localized breast cancer (BERENICE): A phase II,
open-label, multicenter, multinational cardiac safety study. Ann
Oncol. 29:646–653. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gleeson JP, Keegan NM and Morris PG:
Adding pertuzumab to trastuzumab and taxanes in HER2 positive
breast cancer. Expert Opin Biol Ther. 18:251–262. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nami B, Maadi H and Wang Z: Mechanisms
underlying the action and synergism of trastuzumab and pertuzumab
in targeting HER2-positive breast cancer. Cancers (Basel). 10(pii):
E3422018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang JC, Wooten EC, Tsimelzon A,
Hilsenbeck SG, Gutierrez MC, Elledge R, Mohsin S, Osborne CK,
Chamness GC, Allred DC and O'Connell P: Gene expression profiling
for the prediction of therapeutic response to docetaxel in patients
with breast cancer. Lancet. 362:362–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burcombe RJ, Makris A, Richman PI, Daley
FM, Noble S, Pittam M, Wright D, Allen SA, Dove J and Wilson GD:
Evaluation of ER, PgR, HER-2 and Ki-67 as predictors of response to
neoadjuvant anthracycline chemotherapy for operable breast cancer.
Br J Cancer. 92:147–155. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu YL, Connolly EP and Kalinsky K:
Obesity's impact on survival is independent of dose adjustments in
neoadjuvant chemotherapy in women with breast cancer. Breast Cancer
Res Treat. 168:2852018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu YL, Saraf A, Catanese B, Lee SM, Zhang
Y, Connolly EP and Kalinsky K: Obesity and survival in the
neoadjuvant breast cancer setting: Role of tumor subtype in an
ethnically diverse population. Breast Cancer Res Treat.
167:277–288. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karatas F, Erdem GU, Sahin S, Aytekin A,
Yuce D, Sever AR, Babacan T, Ates O, Ozisik Y and Altundag K:
Obesity is an independent prognostic factor of decreased
pathological complete response to neoadjuvant chemotherapy in
breast cancer patients. Breast. 32:237–244. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fontanella C, Lederer B, Gade S, Vanoppen
M, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Gerber B, Hanusch
C, et al: Impact of body mass index on neoadjuvant treatment
outcome: A pooled analysis of eight prospective neoadjuvant breast
cancer trials. Breast Cancer Res Treat. 150:127–139. 2015.
View Article : Google Scholar : PubMed/NCBI
|