Introduction
Colon cancer is the most common type of tumor of the
gastrointestinal tract, and ranks as the third highest cause of
cancer-associated mortality worldwide (1). The etiology and pathogenesis of colon
cancer are complex and are associated with various factors, such as
diet- and lifestyle-associated genetic and epigenetic changes
(2). Recent advances in the
treatment of colon cancer have been reported, including surgery
combined with chemotherapy, radiofrequency ablation or targeted
therapy; however, the rate of postoperative recurrence remain at
~50%, leading to a poor overall survival (OS) for the patients with
colon cancer (3). Therefore, there
is an urgent need to identify novel biomarkers and potential
therapeutic targets for this deadly disease (4).
Long non-coding RNAs (lncRNAs), which are >200
nucleotides in length, have been reported to act as key regulators
of various biological processes; the aberrant expression of lncRNAs
are associated with several diseases, including cancer (5–9).
Accumulating evidence has suggested that lncRNAs could serve as
potential biomarkers for the early diagnosis, prognosis and
prediction of metastasis for various types of malignancy (10–15).
In recent years, with advances in bioinformatics and
interdisciplinary studies involving the development of a series of
computational methods and software tools for the analysis of
extensive biological data, numerous lncRNAs have been identified to
be dysregulated in colon cancer. For instance, by a bioinformatic
approach, a recent study classified Linc00659 as a novel oncogenic
lncRNA involved in the tumorigenesis of colon cancer by modulating
the progression of the cell cycle; downregulation of Linc00659
expression resulted in severe cell cycle arrest and enhanced the
apoptosis of colon cancer cells (16). Similarly, based on bioinformatics
analysis of The Cancer Genome Atlas (TCGA) and/or the Gene
Expression Omnibus datasets, as well as subsequent experimental
validation, metastasis-associated lung adenocarcinoma transcript 1
and small nuclear host gene 1 have been recently identified to be
oncogenic lncRNAs, which may serve as potential diagnostic and
therapeutic targets in colorectal cancer (CRC) (17–20).
These results suggest the potential clinical value of lncRNAs in
CRC; however, the lncRNAs associated with the prognosis and
survival of patients, as well as their biological roles, require
further investigation.
Therefore, the present study aimed to identify the
key lncRNAs associated with their prognostic and biological roles
using a comprehensive bioinformatics process. The gene expression
datasets downloaded from The Cancer Genome Atlas (TCGA) database,
which includes the corresponding survival and Tumor-Node-Metastasis
(TNM) stage (21) status of patients
with CRC, were utilized to construct a prognostic prediction
system.
Materials and methods
TCGA CRC data mining and
screening
The level 3 normalized lncRNA expression data of
CRC, CRC gene expression data and corresponding clinical data were
obtained from the TCGA database (https://cancergenome.nih.gov). The expression
profiling platform RNA-seqv2 was used. No further normalizations
were applied to the level 3 lncRNA expression profile data. A total
of 521 samples were obtained, of which 480 were CRC tissues and 41
were adjacent normal tissues. The lncRNA expression profile of
tumor and normal tissues was determined to screen for
differentially expressed lncRNAs using edgeR (http://www.bioconductor.org/packages/release/bioc/html/edgeR.html;
R software; version 3.4.2; Bell Laboratories) with thresholds of
|log2[fold-change (FC)]|>2.0 and adjusted P-value [false
discovery rate (FDR)]<0.05. A volcano plot was generated using
theplot function in R (https://www.rdocumentation.org/packages/graphics/versions/3.6.0/topics/pl;
R software; version 3.4.2; Bell Laboratories).
Survival analysis
Kaplan-Meier analysis followed by a log-rank test
was performed to assess the OS between low- and high-lncRNA
expression groups using the R package ‘survival’ (https://cran.r-project.org/web/packages/survival/index.html;
R software; version 3.4.2; Bell Laboratories). The Kruskal-Wallis
test was used to evaluate the association between tumor stage. The
staging system of colon cancer using UICC/AJCC (7th edition)
(21), and the lncRNAs that were
significantly associated with OS. Additionally, univariate and
multivariate Cox regression analyses were used to evaluate the
association between the expression levels of lncRNAs and the OS of
patients with CRC, and to identify independent prognostic values of
lncRNAs. P<0.05 was considered to indicate a statistically
significant difference.
Analysis of co-expressed
protein-coding genes (PCGs)
To determine the association between lncRNAs and
co-expressed PCGs, the Pearson correlation coefficients (r) of the
lncRNAs and PCGs were calculated using the cor.test function in R.
The PCGs with |r|>0.4 and P<0.001 were considered as
lncRNA-associated PCGs.
Functional and pathway enrichment
analyses
The identified co-expressed PCGs were further
investigated using clusterProfiler R package (http://www.bioconductor.org/packages/release/bioc/html/clusterProfiler.html;
R software; version 3.4.2; Bell Laboratories), including functional
Gene Ontology (GO) (22) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) (23) pathway enrichment analyses. P<0.05
was considered to indicate a statistically significant
difference.
Results
Identification of significantly
differentially expressed lncRNAs in CRC
In a preliminary screening, the data of 480 CRC and
41 adjacent normal colorectal mucosal tissues were extracted from
the symbol matrix. A total of 1,180 significant differentially
expressed lncRNAs were identified with |logFC|>2 and
FDR<0.05, of which 916 were upregulated and 264 were
downregulated. A volcano plot of the identified lncRNAs was
constructed (Fig. 1). The top 10
upregulated and downregulated lncRNAs are presented in Tables I and II.
 | Table I.Top 10 upregulated lncRNAs with
significantly different expression between tumor and normal tissues
in The Cancer Genome Atlas colon cancer data. |
Table I.
Top 10 upregulated lncRNAs with
significantly different expression between tumor and normal tissues
in The Cancer Genome Atlas colon cancer data.
| lncRNA | Ensembl_Gene_ID | logFC | P-value | FDR |
|---|
| PVT1 | ENSG00000249859 | 2.58 |
1.70×10−67 |
4.23×10−65 |
| CCAT1 | ENSG00000247844 | 4.47 |
1.68×10−59 |
2.51×10−57 |
| BLACAT1 |
ENSG00000281406 | 5.12 |
3.88×10−59 |
5.59×10−57 |
| LINC02163 |
ENSG00000251026 | 7.08 |
1.19×10−54 |
1.37×10−52 |
| CRNDE |
ENSG00000245694 | 4.58 |
1.30×10−52 |
1.31×10−50 |
| MAFG-AS1 |
ENSG00000265688 | 2.88 |
7.82×10−51 |
7.51×10−49 |
| RP5-884M6.1 |
ENSG00000228742 | 6.31 |
1.04×10−50 |
9.69×10−49 |
| CASC19 |
ENSG00000254166 | 4.51 |
3.30×10−50 |
3.02×10−48 |
| RP5-1120P11.1 |
ENSG00000237686 | 4.41 |
7.64×10−50 |
6.65×10−48 |
| AC007128.1 |
ENSG00000229970 | 4.48 |
2.64×10−48 |
2.17×10−46 |
 | Table II.Top 10 downregulated lncRNAs with
significantly different expression between tumor and normal tissues
in The Cancer Genome Atlas colon cancer data. |
Table II.
Top 10 downregulated lncRNAs with
significantly different expression between tumor and normal tissues
in The Cancer Genome Atlas colon cancer data.
| lncRNA |
Ensembl_Gene_ID | logFC | P-value | FDR |
|---|
|
XXbac-B476C20.9 |
ENSG00000225335 | −2.72 |
2.14×10−173 |
1.60×10−169 |
| CDKN2B-AS1 |
ENSG00000240498 | −5.21 |
9.05×10−140 |
3.39×10−136 |
| LINC01645 |
ENSG00000224968 | −4.60 |
6.90×10−127 |
1.72×10−123 |
| PP7080 |
ENSG00000188242 | −3.48 |
3.24×10−124 |
6.07×10−121 |
|
XXyac-YM21GA2.7 |
ENSG00000214888 | −5.69 |
7.06×10−120 |
1.06×10−116 |
| RP11-1090M7.1 |
ENSG00000265489 | −4.48 |
1.49×10−116 |
1.85×10−113 |
| RP11-396O20.2 |
ENSG00000254645 | −5.01 |
1.71×10−114 |
1.83×10−111 |
| AC007182.6 |
ENSG00000224721 | −4.67 |
4.65×10−108 |
4.35×10−105 |
| AC106869.2 |
ENSG00000226087 | −4.18 |
2.71×10−105 |
2.25×10−102 |
| LINC00682 |
ENSG00000245870 | −4.55 |
1.18×10−96 |
8.82×10−94 |
Analysis of significant differentially
expressed lncRNAs in CRC samples associated with OS and
pathological stages
To investigate the association between lncRNA
expression and OS, the expression profile of the 1,180 lncRNAs in
tumor samples were determined, of which 56 lncRNAs were associated
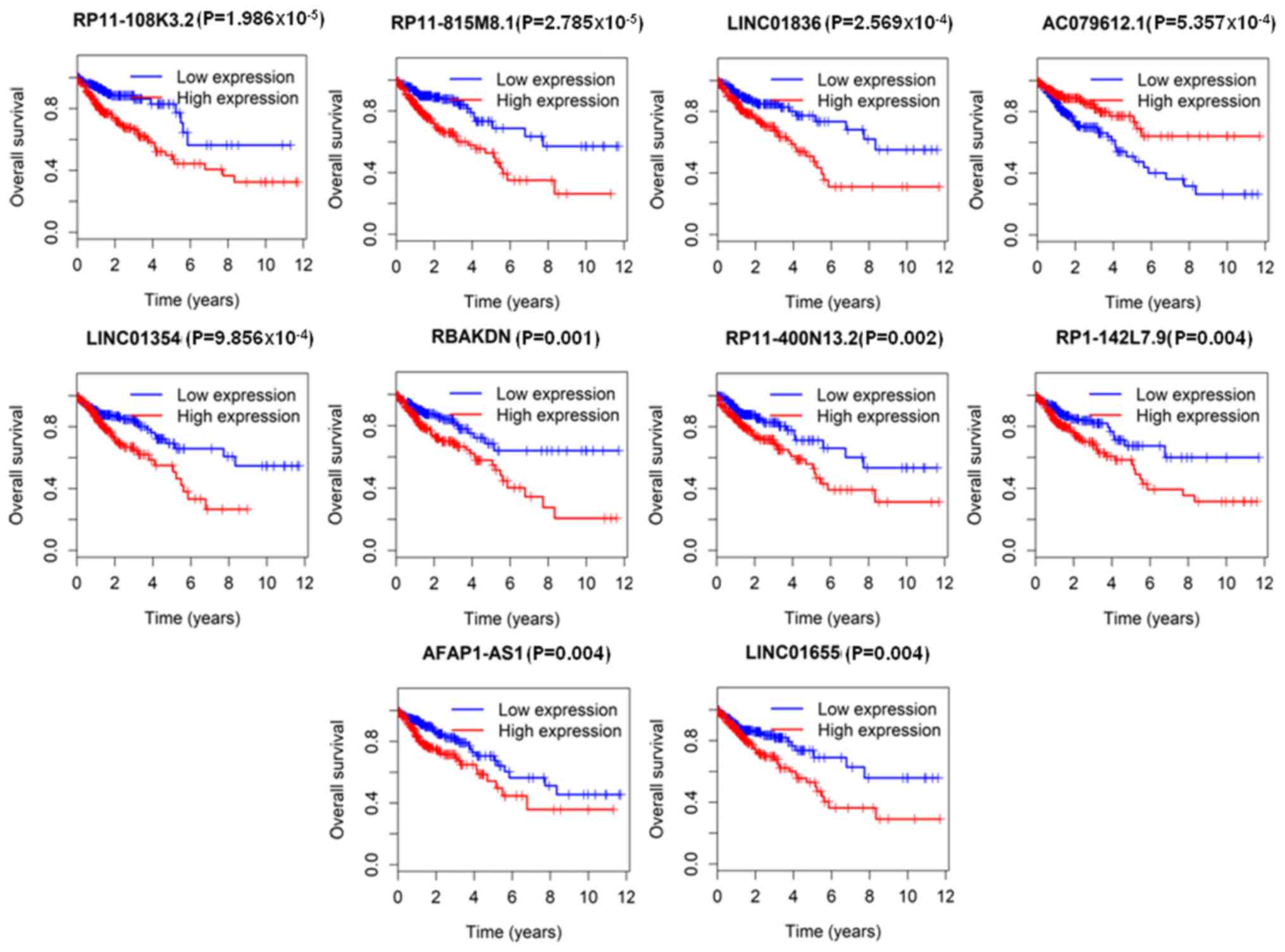
with OS, as determined by Kaplan-Meier analysis (Table III). The top 10 lncRNAs
significantly associated with OS (P<0.05) were RP11-108K3.2,
RP11-815M8.1, LINC01836, AC079612.1, LINC01354, RBAKDN,
RP11-400N13.2, RP1-142L7.9, AFAP1-AS1 and LINC01655 (Fig. 2).
 | Table III.lncRNAs significantly associated with
OS. |
Table III.
lncRNAs significantly associated with
OS.
| lncRNA |
Ensembl_Gene_ID | P-value |
|---|
| RP11-108K3.2 |
ENSG00000259306 |
1.98646×10−5 |
| RP11-815M8.1 |
ENSG00000238042 |
2.78454×10−5 |
| LINC01836 |
ENSG00000267530 | 0.000256940 |
| AC079612.1 |
ENSG00000196758 | 0.000535749 |
| LINC01354 |
ENSG00000231768 | 0.000985613 |
| RBAKDN |
ENSG00000273313 | 0.001143877 |
| RP11-400N13.2 |
ENSG00000228437 | 0.002268513 |
| RP1-142L7.9 |
ENSG00000270661 | 0.003700521 |
| AFAP1-AS1 |
ENSG00000272620 | 0.004000305 |
| LINC01655 |
ENSG00000227925 | 0.004380952 |
| RP11-10A14.5 |
ENSG00000248538 | 0.004693149 |
| RP11-384P7.7 |
ENSG00000260947 | 0.004845978 |
| RP11-434D9.2 |
ENSG00000249894 | 0.005561553 |
| RP11-742B18.1 |
ENSG00000249001 | 0.007363036 |
| CTC-327F10.4 |
ENSG00000251320 | 0.008786437 |
| AC064834.1 |
ENSG00000224099 | 0.00890189 |
| ARHGEF26-AS1 |
ENSG00000243069 | 0.008966797 |
| RP3-380B8.4 |
ENSG00000233064 | 0.009485845 |
| LINC01829 |
ENSG00000236780 | 0.009974597 |
| GAS1RR |
ENSG00000226237 | 0.013908392 |
| RP11-84A19.4 |
ENSG00000269967 | 0.014180465 |
| LINC02043 |
ENSG00000232233 | 0.016339686 |
| LINC00922 |
ENSG00000261742 | 0.016854765 |
| RP11-278L15.2 |
ENSG00000243885 | 0.017325027 |
| RP1-122P22.4 |
ENSG00000268628 | 0.018507105 |
| RP11-366L20.2 |
ENSG00000197301 | 0.019632443 |
| DUXAP8 |
ENSG00000206195 | 0.020361403 |
| CTB-181H17.1 |
ENSG00000272219 | 0.020685373 |
| MIR31HG |
ENSG00000171889 | 0.021365649 |
| AC012531.25 |
ENSG00000260597 | 0.023942649 |
| FOXD3-AS1 |
ENSG00000230798 | 0.024144909 |
| AC007128.1 |
ENSG00000229970 | 0.027145232 |
| DNAH17-AS1 |
ENSG00000267432 | 0.02735923 |
| LINC01833 |
ENSG00000259439 | 0.027941465 |
| RP11-429J17.5 |
ENSG00000254548 | 0.028089082 |
|
LL22NC03-N14H11.1 |
ENSG00000272872 | 0.031532618 |
| RP1-79C4.4 |
ENSG00000271811 | 0.031571197 |
| CTB-186G2.4 |
ENSG00000267375 | 0.031679272 |
| RP11-114H23.2 |
ENSG00000258088 | 0.033221364 |
| LINC00484 |
ENSG00000229694 | 0.035515205 |
| RP1-29C18.10 |
ENSG00000212939 | 0.035961207 |
| AC073326.3 |
ENSG00000228540 | 0.036298277 |
| RP11-126H7.4 |
ENSG00000204049 | 0.036449014 |
| CTD-2619J13.13 |
ENSG00000268307 | 0.037145687 |
| LINC01748 |
ENSG00000226476 | 0.038383393 |
| RP11-532F6.3 |
ENSG00000272463 | 0.042708274 |
| RP11-728G15.1 |
ENSG00000256008 | 0.043149989 |
| LINC01060 |
ENSG00000249378 | 0.045508319 |
| KCNQ1OT1 |
ENSG00000269821 | 0.045529181 |
| LINC01996 |
ENSG00000261863 | 0.046591159 |
| ELFN1-AS1 |
ENSG00000236081 | 0.046625182 |
| HOTAIR |
ENSG00000228630 | 0.047908786 |
| LINC00461 |
ENSG00000245526 | 0.04796558 |
| CTD-2600O9.1 |
ENSG00000187185 | 0.048650792 |
| LEF1-AS1 |
ENSG00000232021 | 0.049375626 |
| FLJ16779 |
ENSG00000275620 | 0.049920309 |
The association between clinical stages (UICC/AJCC
7th Edition) (21) and the 56
lncRNAs associated with OS was determined via a Kruskal-Wallis
test. The results demonstrated that 7 lncRNAs were identified as
key lncRNAs associated with the Tumor-Node-Metastasis (TNM) stages
of colon cancer, including DNAH17-AS1, RP11-429J17.5,
RP11-742B18.1, RP11-400N13.2, LL22NC03-N14H11.1, LINC01836 and
HOTAIR (Table IV; Fig. 3). The detailed information of the
patients at each TNM stage is presented in Table SI. Notably, these 7 lncRNAs were
upregulated in colon cancer tissues compared with adjacent normal
tissues, suggesting that they may serve a tumorigenic role in the
initiation and progression of CRC.
 | Table IV.lncRNAs significantly associated with
tumor clinical stage. |
Table IV.
lncRNAs significantly associated with
tumor clinical stage.
| lncRNA |
Ensembl_Gene_ID | P-value |
|---|
| FLJ16779 |
ENSG00000275620 | 0.049920309 |
| DNAH17-AS1 |
ENSG00000267432 |
6.42×10−5 |
| RP11-429J17.5 |
ENSG00000254548 | 0.000628536 |
| RP11-742B18.1 |
ENSG00000249001 | 0.00141941 |
| RP11-400N13.2 |
ENSG00000228437 | 0.003179863 |
|
LL22NC03-N14H11.1 |
ENSG00000272872 | 0.005376623 |
| LINC01836 |
ENSG00000267530 | 0.011461646 |
| HOTAIR |
ENSG00000228630 | 0.013000989 |
Identification of independent
prognostic lncRNAs in CRC
In order to detect potential independent prognostic
lncRNAs in patients with CRC, univariate and multivariate Cox
regression analyses of these 7 lncRNAs associated with TNM stage
were performed. The association between the expression levels of
lncRNAs and the OS of patients with colon cancer was explored using
the R package ‘survival’; 2 lncRNAs, DNAH17-AS1 and RP11-400N13.2,
were identified to be independent prognostic factors for OS in
patients with CRC (P<0.05; Tables
V and VI).
 | Table V.Cox regression analyses of the
association between DNAH17-AS1 and patient clinicopathological
characteristics. |
Table V.
Cox regression analyses of the
association between DNAH17-AS1 and patient clinicopathological
characteristics.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Characteristic | P-value | HR | HR (lower
0.95) | HR (upper
0.95) | P-value | HR | HR (lower
0.95) | HR (upper
0.95) |
|---|
| Age, years |
1.54×10−2 | 1.024705 | 1.00467006 | 1.045139527 |
8.62×10−5 | 1.043293 | 1.021452741 | 1.065600322 |
| Sex |
8.11×10−1 | 1.055993 | 0.675320904 | 1.651244946 |
5.15×10−1 | 0.859753 | 0.545233986 | 1.355701712 |
| Stage |
4.32×10−10 | 2.260538 | 1.749817646 | 2.92032165 |
3.09×10−1 | 1.481753 | 0.693752964 | 3.164805106 |
| T |
5.85×10−6 | 2.842169 | 1.809061684 | 4.465257511 |
4.32×10−2 | 1.753715 | 1.017244601 | 3.023378521 |
| M |
6.75×10−10 | 4.348202 | 2.726493071 | 6.934496948 |
3.83×10−1 | 1.584756 | 0.562533031 | 4.464537953 |
| N |
1.29×10−7 | 2.014039 | 1.553071957 | 2.611824311 |
2.84×10−1 | 1.281519 | 0.813788046 | 2.01808327 |
| DNAH17-AS1 |
1.17×10−3 | 56.08087 | 4.924770723 | 638.6214937 |
1.61×10−2 | 29.59342 | 1.872773849 | 467.6327355 |
 | Table VI.Cox regression analyses of the
association between RP11-400N13.2 and patient clinicopathological
characteristics. |
Table VI.
Cox regression analyses of the
association between RP11-400N13.2 and patient clinicopathological
characteristics.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Characteristic | P-value | HR | HR (lower
0.95) | HR (upper
0.95) | P-value | HR | HR (lower
0.95) | HR (upper
0.95) |
|---|
| Age, years |
1.54×10−2 | 1.024705 | 1.00467006 | 1.045139527 |
3.70×10−4 | 1.03741 | 1.016647195 | 1.058596272 |
| Sex |
8.11×10−1 | 1.055993 | 0.675320904 | 1.651244946 |
7.37×10−1 | 0.925432 | 0.588464244 | 1.455355124 |
| Stage |
4.32×10−10 | 2.260538 | 1.749817646 | 2.92032165 |
2.61×10−1 | 1.54623 | 0.722569696 | 3.308782317 |
| T |
5.85×10−6 | 2.842169 | 1.809061684 | 4.465257511 |
3.02×10−2 | 1.842378 | 1.059948791 | 3.202377507 |
| M |
6.75×10−10 | 4.348202 | 2.726493071 | 6.934496948 |
3.49×10−1 | 1.628719 | 0.586703577 | 4.521408077 |
| N |
1.29×10−7 | 2.014039 | 1.553071957 | 2.611824311 |
4.82×10−1 | 1.181526 | 0.741699234 | 1.882169148 |
| RP11-400N13.2 |
2.49×10−4 | 1.14629 | 1.065532018 | 1.233169628 |
6.46×10−3 | 1.117075 | 1.031523289 | 1.209722556 |
Analyses of PCGs co-expressed with
lncRNAs DNAH17-AS1 and RP11-400N13.2
Analysis of the PCGs co-expressed with DNAH17-AS1
and RP11-400N13.2 was conducted using the cor.test function with
thresholds of |r|>0.4 and P<0.001. The results revealed that
1,048 PCGs were co-expressed with DNAH17-AS1 (Fig. 4A). Due to a large number of PCGs in
DNAH17-AS1, only the top 100 genes with a lower P-value were
presented in Fig. 4A. A total of 126
PCGs co-expressed with RP11-400N13.2 (Fig. 4B). The top 10 significant PCGs that
were identified to be co-expressed with the 2 lncRNAs are listed in
Table VII.
 | Table VII.Co-expression analyses between
DNAH17-AS1 and RP11-400N13.2 and the top 10 significant
protein-coding genes. |
Table VII.
Co-expression analyses between
DNAH17-AS1 and RP11-400N13.2 and the top 10 significant
protein-coding genes.
| A, Genes
co-expressed with DNAH17-AS1 |
|---|
|
|---|
| Co-expressed
gene |
Ensembl_Gene_ID | ra | P-value |
|---|
| DNAH17 |
ENSG00000187775 | 0.656 |
2.73×10−65 |
| SSPO |
ENSG00000197558 | 0.564 |
4.89×10−45 |
| TPTE |
ENSG00000274391 | 0.505 |
4.31×10−35 |
| DPPA2 |
ENSG00000163530 | 0.473 |
1.96×10−30 |
| GPR179 |
ENSG00000277399 | 0.473 |
2.22×10−30 |
| RP11-505K9.4 |
ENSG00000260300 | 0.454 |
8.07×10−28 |
| CFAP74 |
ENSG00000142609 | 0.453 |
1.15×10−27 |
| POLG2 |
ENSG00000256525 | 0.451 |
1.99×10−27 |
| KIF6 |
ENSG00000164627 | 0.449 |
3.41×10−27 |
| STKLD1 |
ENSG00000198870 | 0.445 |
9.44×10−27 |
|
| B, Genes
co-expressed with RP11-400N13.2 |
|
| Co-expressed
gene |
Ensembl_Gene_ID | ra | P-value |
|
| RP11-371E8.4 |
ENSG00000259066 | 0.439 |
5.20×10−26 |
| RP11-507M3.1 |
ENSG00000276087 | 0.438 |
8.64×10−26 |
| HIST3H3 |
ENSG00000168148 | 0.428 |
1.26×10−24 |
| RP4-809F18.1 |
ENSG00000255595 | 0.428 |
1.34×10−24 |
| OPRPN |
ENSG00000171199 | 0.426 |
2.51×10−24 |
| RP11-385D13.1 |
ENSG00000251537 | 0.423 |
4.79×10−24 |
| OR52B2 |
ENSG00000255307 | 0.422 |
5.87×10−24 |
| OR52B6 |
ENSG00000187747 | 0.422 |
7.24×10−24 |
| OR2AG2 |
ENSG00000188124 | 0.401 |
1.44×10−21 |
| BDNF |
ENSG00000176697 | 0.397 |
3.93×10−21 |
GO and KEGG enrichment analyses of the
PCGs co-expressed with DNAH17-AS1 and RP11-400N13.2
To further investigate the potential roles of the
two independent prognostic lncRNAs identified in the present study,
functional enrichment analyses for their co-expressed PCGs were
performed using the clusterProfiler R package. The results
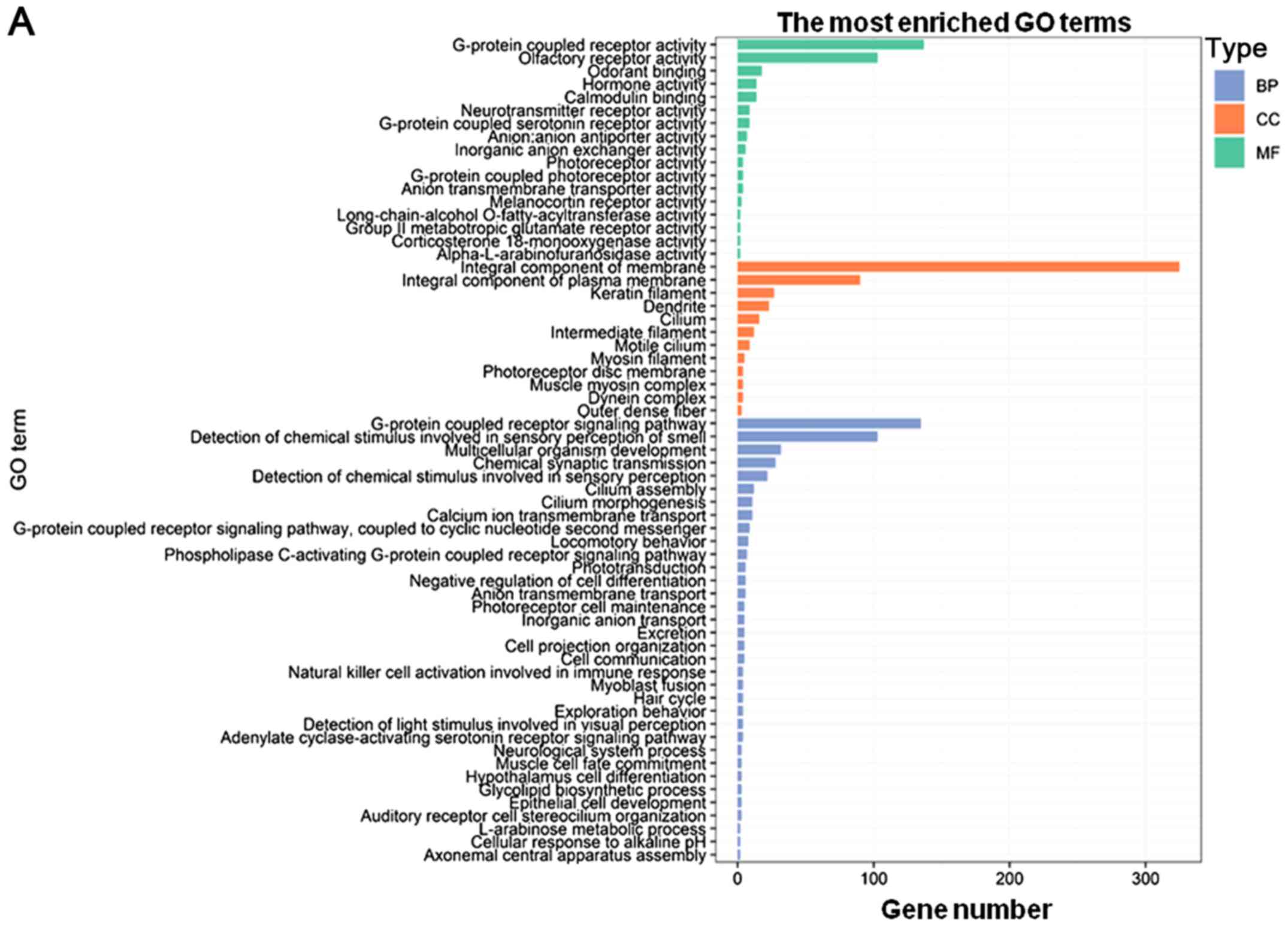
indicated that the PCGs co-expressed with DNAH17-AS1 were mainly
enriched in ‘G-protein coupled receptor signaling pathway’,
‘detection of chemical stimulus involved in sensory perception of
smell’, ‘integral component of membrane’, ‘integral component of
plasma membrane’, ‘G-protein coupled receptor activity’ and
‘olfactory receptor activity’. Collectively, these PCGs were
associated with G-protein coupling and cell membrane function
(Fig. 5A). Additionally, the PCGs
co-expressed with RP11-400N13.2 were mainly enriched in ‘G-protein
coupled receptor signaling pathway’, ‘G-protein coupled receptor
activity’, ‘spermatogenesis’, ‘negative regulation of endopeptidase
activity’ and ‘endopeptidase inhibitor activity’, which are
involved in G-protein coupling and endopeptidase function (Fig. 5B). Functional enrichment analysis
revealed a high level of involvement of the associated PCGs
inG-protein coupling, which suggested a crucial biological function
of DNAH17-AS1 and RP11-400N13.2. The detailed information of the
genes with G-protein-associated functions co-expressed with
DNAH17-AS1 and RP11-400N13.2 is presented in Table SII. A total of 3 of these genes,
5′-hydroxytryptamine receptor 6, melanocortin 5 receptor and
prokineticin receptor 2, were significantly associated with OS
(P<0.05; Fig. S1).
KEGG pathway enrichment analysis of the PCGs
co-expressed with the two independent prognostic lncRNAs was
performed using clusterProfiler package in R with a threshold of
P<0.05. The results revealed that the PCGs co-expressed with
DNAH17-AS1 were involved in seven pathways, including ‘olfactory
transduction’, ‘neuroactive ligand-receptor interaction’,
‘phototransduction’, ‘nicotine addiction’, ‘cocaine addiction’,
‘collecting duct acid secretion’ and ‘signaling pathways regulating
pluripotency of stem cells’ (Fig.
6). This result provided novel insights into the potential
associations between these pathways and CRC, which warrant further
investigation. Of note, P>0.05 was reported for the enriched
pathways of the PCGs co-expressed with RP11-400N13.2.
Discussion
In the present study, in silico analysis
revealed 1,180 significantly differentially expressed lncRNAs that
were associated with colorectal cancer, of which 56 and 7 genes
were significantly associated with OS and TNM stage, respectively.
Subsequent univariate and multivariate Cox regression analyses
indicated that 2 of the 7 lncRNAs, DNAH17-AS1 and RP11-400N13.2,
may be independent prognostic lncRNAs for the OS of patients with
colorectal cancer.
To the best of our knowledge, lncRNAs DNAH17-AS1 and
RP11-400N13.2 have not been previously associated with colon
cancer; however, a missense variant p.R3953Y of DNAH17, the related
protein of DNAH17-AS1, was reported in undifferentiated embryonal
sarcoma of the liver in a child (24). Additionally, p.R3953 of DNAH17
exhibited a high level of conservation among a variety of species,
suggesting that this allele may be an important locus associated
with protein function (24). A
recent study revealed the mutational profile and a distinct
mutation signature of T:A>A:T transversion in early-stage
hepatocellular carcinoma (HCC) with hepatitis B virus (HBV)
infection; thus, as a key gene of the mutational profile, DNAH17
was proposed to serve an important role in the HBV-mediated
transformation of liver cells (25).
Additionally, the hypomethylation status of DNAH17 has been
reported in HCC, which is associated with several clinical
characteristics and may serve as a potential biomarker of tumor
thrombosis in patients with HCC (26). In the present study, the lncRNA
expression level of DNAH17-AS1 in CRC samples was analyzed and
compared with that in normal samples; however, the expression level
and the methylation status of DNAH17 were not analyzed. Although
the expression level of DNAH17, as well as its methylation status
in CRC samples, may be informative to determine the role of DNAH17
in CRC, this was beyond the scope of the present study. Therefore,
relevant studies will be performed in the future.
A limited number of studies have investigated
RP11-400N13.2; however, other RP11 family members have been
frequently reported to be dysregulated in CRC. RP11-708H21.4, an
RP11 family lncRNA located in the 17q21 gene desert region, was
proposed to serve a suppressive role in the tumorigenesis of
colorectal cancer and act as a novel powerful diagnostic biomarker,
as well as a therapeutic target for the treatment of CRC (27). The expression levels of
RP11-462C24.1, another member of the RP11 family, were determined
to be significantly correlated with distant metastasis in patients
with CRC, and may serve as a potential prognostic marker for such
patients (28). Additionally, the
dysregulation of RP11 family members has been reported to be
involved in other types of cancer. For instance, a recent study
revealed that overexpression of lncRNA RP11-190D6.2 inhibited the
proliferation, migration and invasion of epithelial ovarian cancer
(EOC) cells and may be considered a novel biomarker and therapeutic
target for EOC (29). Furthermore,
lncRNA RP11-436H11.5 was identified to function as a competing
endogenous RNA to promote the proliferation and invasion of renal
cell carcinoma (RCC) cells, which suggests that RP11-436H11.5 may
be a potential therapeutic target to suppress RCC tumorigenesis
(30). Collectively, the RP11 family
of lncRNAs serve important roles in carcinogenesis and may be used
as potential diagnostic and prognostic biomarkers for various types
of cancer.
Following the identification of two independent
prognostic lncRNAs in colorectal cancer, the co-expressed PCGs were
analyzed, and stepwise GO and KEGG enrichment analyses were
conducted to determine the potential biological functions of these
lncRNAs associated with CRC and the signaling pathways involved.
The results of the functional enrichment analysis of DNAH17-AS1 and
RP11-400N13.2 differed; however, these lncRNAs were determined to
possess similar G-protein coupling-associated functions. G-protein
coupled receptors have been previously reported to be associated
with CRC tumorigenesis (31–34). For example, the G-protein coupled
receptor GPR55 may promote tumor progression by acting as an
pro-oncogenic factor in CRC (31).
In addition, GPR55 has been proposed to be involved in the
migration of CRC cells and may serve as a potential target for the
prevention of metastasis (32). On
the contrary, orexin receptor type 1 and cholecystokinin A
receptor, which belong to family A of the G-protein coupled
receptors, serve opposing roles in the regulation of HT-29 CRC cell
migration, but have also been reported to be involved in the
pathogenesis of CRC metastasis (33). Furthermore, a recent study revealed
that GPR109A, a G-protein coupled receptor for short-chain fatty
acids, was silenced in CRC cells (34). In addition, the host immune system
may employ interferon γ to counteract methylation-mediated
silencing of GPR109A as a mechanism to suppress tumor development
(34). Therefore, G-protein coupled
receptors maybe associated with the carcinogenesis and metastasis
of CRC; the roles of DNAH17-AS1 and RP11-400N13.2 in CRC, which may
be mediated by these receptors, require further investigation.
In the present study, DNAH17-AS1 and RP11-400N13.2
were identified as potential independent prognostic lncRNAs for OS
in patients with CRC. Further bioinformatics analyses revealed that
these 2 lncRNAs may serve a pro-oncogenic role in CRC via G-protein
coupling-related functions. Therefore, DNAH17-AS1 and RP11-400N13.2
may serve as prognostic biomarkers for CRC in the future. The
detailed methodology of the present study is presented in Fig. S2.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural
Science Foundation of China (grant no. 81572416), the National Key
Technologies R&D Program of China (grant no. 2016YFC1303200),
and the Tianjin Medical University Cancer Institute and Hospital
Cancer Translational Medicine Seed Funds (grant no. 1701-1).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL designed and supervised the study and finalized
the manuscript. WZ and BP made substantial contributions to the
study design, performed the bioinformatics analysis and drafted the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
lncRNAs
|
long non-coding RNAs
|
|
CRC
|
colorectal cancer
|
|
OS
|
overall survival
|
|
PCGs
|
protein-coding genes
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ghareeb AE, Moawed FSM, Ghareeb DA and
Kandil EI: Potential prophylactic effect of berberine against rat
colon carcinoma induce by 1,2-dimethyl hydrazine. Asian Pac J
Cancer Prev. 19:1685–1690. 2018.PubMed/NCBI
|
|
3
|
Sanoff HK, Sargent DJ, Campbell ME, Morton
RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC
and Goldberg RM: Five-year data and prognostic factor analysis of
oxaliplatin and irinotecan combinations for advanced colorectal
cancer: N9741. J Clin Oncol. 26:5721–5727. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong Z, Zheng L, Liu W and Wang C:
Association of mRNA expression of TP53 and the TP53 codon 72
Arg/Pro gene polymorphism with colorectal cancer risk in Asian
population: A bioinformatics analysis and meta-analysis. Cancer
Manag Res. 10:1341–1349. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xue W, Li J, Wang F, Han P, Liu Y and Cui
B: A long non-coding RNA expression signature to predict survival
of patients with colon adenocarcinoma. Oncotarget. 8:101298–101308.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dykes IM and Emanueli C: Transcriptional
and posttranscriptional gene regulation by long non-coding RNA.
Genomics Proteomics Bioinformatics. 15:177–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iyer MK, Niknafs YS, Malik R, Singhal U,
Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et
al: The landscape of long non-coding RNAs in the human
transcriptome. Nat Genet. 47:199–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prensner JR and Chinnaiyan AM: The
emergence of lncRNAs in cancer biology. Cancer Discov. 1:391–407.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou M, Wang X, Li J, Hao D, Wang Z, Shi
H, Han L, Zhou H and Sun J: Prioritizing candidate disease-related
long non-coding RNAs by walking on the heterogeneous lncRNA and
disease network. Mol Biosyst. 11:760–769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lou Y, Jiang H, Cui Z, Wang X, Wang L and
Han Y: Gene microarray analysis of lncRNA and mRNA expression
profiles in patients with high-grade ovarian serous cancer. Int J
Mol Med. 42:91–104. 2018.PubMed/NCBI
|
|
11
|
Jin B, Jin H, Wu HB, Xu JJ and Li B: Long
non-coding RNA SNHG15 promotes CDK14 expression via miR-486 to
accelerate non-small cell lung cancer cells progression and
metastasis. J Cell Physiol. 233:7164–7172. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li S, Zhou J, Wang Z, Wang P, Gao X and
Wang Y: Long noncoding RNA GAS5 suppresses triple negative breast
cancer progression through inhibition of proliferation and invasion
by competitively binding miR-196a-5p. Biomed Pharmacother.
104:451–457. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Z, Yan Y, Cao S and Chen Y: Long
non-coding RNA SNHG14 contributes to gastric cancer development
through targeting miR-145/SOX9 axis. J Cell Biochem. 119:6905–6913.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan H, Lv P, Mu T, Zhao X, Liu Y, Feng Y,
Lv J, Liu M and Tang H: lncRNA n335586/miR-924/CKMT1A axis
contributes to cell migration and invasion in hepatocellular
carcinoma cells. Cancer Lett. 429:89–99. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye XT, Huang H, Huang WP and Hu WL: lncRNA
THOR promotes human renal cell carcinoma cell growth. Biochem
Biophys Res Commun. 501:661–667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsai KW, Lo YH, Liu H, Yeh CY, Chen YZ,
Hsu CW, Chen WS and Wang JH: Linc00659, a long noncoding RNA, acts
as novel oncogene in regulating cancer cell growth in colorectal
cancer. Mol Cancer. 17:722018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu Q, Meng WY, Jie Y and Zhao H: lncRNA
MALAT1 induces colon cancer development by regulating
miR-129-5p/HMGB1 axis. J Cell Physiol. 233:6750–6757. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang H, Wang S, Kang YJ, Wang C, Xu Y,
Zhang Y and Jiang Z: Long non-coding RNA SNHG1 predicts a poor
prognosis and promotes colon cancer tumorigenesis. Oncol Rep.
40:261–271. 2018.PubMed/NCBI
|
|
19
|
Xu Y, Zhang X, Hu X, Zhou W, Zhang P,
Zhang J, Yang S and Liu Y: The effects of lncRNA MALAT1 on
proliferation, invasion and migration in colorectal cancer through
regulating SOX9. Mol Med. 24:522018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu M, Chen X, Lin K, Zeng K, Liu X, Pan B,
Xu X, Xu T, Hu X, Sun L, et al: The long noncoding RNA SNHG1
regulates colorectal cancer cell growth through interactions with
EZH2 and miR-154-5p. Mol Cancer. 17:1412018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edge SB, Byrd DR, Compton CC, Fritz AG
Greene, F and Trotti A: AJCC Cancer Staging Manual. 7th.
Springer-Verlag; New York, NY: 2010
|
|
22
|
Gene Ontology Consortium: The gene
ontology (GO) project in 2006. Nucleic Acids Res. 34:D322–D326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Altermann E and Klaenhammer TR:
Pathwayvoyager: Pathway mapping using the Kyoto encyclopedia of
genes and genomes (KEGG) database. BMC Genomics. 6:602005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim JH, Sio CA, Park H, Kim H, Shin HD and
Jung K: Undifferentiated embryonal sarcoma of the liver in a child:
A whole exome sequencing analysis. Dig Liver Dis. 49:944–946. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhan H, Jiang J, Sun Q, Ke A, Hu J, Hu Z,
Zhu K, Luo C, Ren N, Fan J, et al: Whole-exome sequencing-based
mutational profiling of hepatitis B virus-related early-stage
hepatocellular carcinoma. Gastroenterol Res Pract 2017.
20293152017.
|
|
26
|
Fan X, Guo H, Dai B, He L, Zhou D and Lin
H: The association between methylation patterns of DNAH17 and
clinicopathological factors in hepatocellular carcinoma. Cancer
Med. 8:337–350. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun L, Jiang C, Xu C, Xue H, Zhou H, Gu L,
Liu Y and Xu Q: Down-regulation of long non-coding RNA
RP11-708H21.4 is associated with poor prognosis of colorectal
cancer and promotes tumorigenesis through regulating AKT/mTOR
pathway. Oncotarget. 8:27929–27942. 2017.PubMed/NCBI
|
|
28
|
Shi D, Zheng H, Zhuo C, Peng J, Li D, Xu
Y, Li X, Cai G and Cai S: Low expression of novel lncRNA
RP11-462C24.1 suggests a biomarker of poor prognosis in colorectal
cancer. Med Oncol. 31:312014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tong W, Yang L, Yu Q, Yao J and He A: A
new tumor suppressor lncRNA RP11-190D6.2 inhibits the
proliferation, migration and invasion of epithelial ovarian cancer
cells. Onco Targets Ther. 10:1227–1235. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang KF, Jin W, Song Y and Fei X: lncRNA
RP11-436H11.5, functioning as a competitive endogenous RNA,
upregulates BCL-W expression by sponging miR-335-5p and promotes
proliferation and invasion in renal cell carcinoma. Mol Cancer.
16:1662017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hasenoehrl C, Feuersinger D, Sturm EM,
Bärnthaler T, Heitzer E, Graf R, Grill M, Pichler M, Beck S,
Butcher L, et al: G protein-coupled receptor GPR55 promotes
colorectal cancer and has opposing effects to cannabinoid receptor
1. Int J Cancer. 142:121–132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kargl J, Andersen L, Hasenöhrl C,
Feuersinger D, Stančić A, Fauland A, Magnes C, El-Heliebi A, Lax S,
Uranitsch S, et al: GPR55 promotes migration and adhesion of colon
cancer cells indicating a role in metastasis. Br J Pharmacol.
173:142–154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bai B, Chen X, Zhang R, Wang X, Jiang Y,
Li D, Wang Z and Chen J: Dual-agonist occupancy of orexin receptor
1 and cholecystokinin A receptor heterodimers decreases
G-protein-dependent signaling and migration in the human colon
cancer cell line HT-29. Biochim Biophys Acta Mol Cell Res 1864.
1153–1164. 2017. View Article : Google Scholar
|
|
34
|
Bardhan K, Paschall AV, Yang D, Chen MR,
Simon PS, Bhutia YD, Martin PM, Thangaraju M, Browning DD,
Ganapathy V, et al: IFNγ Induces DNA methylation-silenced GPR109A
expression via pSTAT1/p300 and H3K18 acetylation in colon cancer.
Cancer Immunol Res. 3:795–805. 2015. View Article : Google Scholar : PubMed/NCBI
|