Introduction
Colorectal cancer (CRC) is one of the most common
malignancies of the digestive system and has a worldwide incidence
of >1.3 million cases/year (1).
Previous studies demonstrated that ~25% of patients were diagnosed
at an advanced stage of the disease, and ~50% of patients suffered
from liver metastasis, which significantly increased the mortality
rates for CRC (2). This highlights
the need to identify effective therapeutic agents to treat this
malignant disease.
In the past decades, 5-fluorouracil (5-FU) has been
a mainstay of CRC treatment, and several clinical trials have
revealed that chemotherapy regimens consisting of 5-FU in
combination with other cytotoxic agents, such as irinotecan and
oxaliplatin, significantly improved the survival of patients with
advanced CRC (3–5). However, 5-FU-based chemotherapy is not
always effective and the disease may progress, due to the
development of chemo-resistance (6).
Therefore, it may be beneficial to personalize treatment based on
the individual molecular characteristics of the tumor. Development
of novel targets to increase the sensitivity of tumors to 5-FU may
enhance the cytotoxic effects of 5-FU on tumor cells and decrease
the adverse effects of chemotherapy on the immune system.
Furthermore, the reduction in treatment costs would have economic
benefits.
It has been demonstrated that microRNAs
(miRNAs/miRs) play a vital role in the regulation of genes exerting
antitumor effects, including genes driving cell apoptosis,
inhibiting cell proliferation and regulating drug efflux mechanisms
(7,8). miRNAs are a class of small, endogenous,
non-coding, regulatory RNA molecules. These small RNAs are 18–24
nucleotides in length and are involved in the regulation of the
expression of two-thirds of all human genes through binding to mRNA
3′-untranslated regions (3′UTRs) (9–11). It
was previously demonstrated that miRNA-mediated gene regulation is
an important process in the occurrence, pathogenesis and
progression of several diseases, including gastrointestinal cancer,
immune-related diseases and neurodegenerative diseases (10). miRNAs may act as oncogenes or tumor
suppressors in tumor occurrence and development. miRNAs may act as
oncogenes, referred to as ‘oncomirs’, by inhibiting the effect of
tumor suppressor genes or regulating cell apoptosis, promoting the
occurrence and development of tumors (8). The identification of miRNAs implicated
in the response to antitumor therapy has revealed novel therapeutic
approaches to reverse drug resistance (8). For example, overexpression of miR-216b
sensitized non-small cell lung cancer cells to cisplatin-induced
apoptosis (12). miR-30a
overexpression inhibited chemoresistance-associated autophagy in
gastric cancer cells (13).
Furthermore, overexpression of miR-22 increased the sensitivity of
osteosarcoma cells to cisplatin treatment (14). Although several studies have reported
that miRNAs can regulate chemosenstivity of CRC cells (7,10,15–19),
the molecular mechanism(s) underlying the role of miRNAs in the
chemoresistance of CRC has not been fully elucidated.
The present study aimed to identify candidate miRNAs
that regulate the susceptibility of CRC cells to 5-FU treatment.
Additionally, the downstream targets of the candidate miRNAs
associated with the 5-FU response were also investigated. The
results obtained in the present study may provide a novel
therapeutic strategy to overcome 5-FU resistance in CRC.
Materials and methods
Cell culture and materials
CRC HCT116 and HT29 cell lines were purchased from
the Type Culture Collection of the Chinese Academy of Sciences.
Cells were grown in high glucose Dulbecco's Modified Eagle Medium
(DMEM) (Thermo Fisher Scientific, Inc.) containing 10% fetal bovine
serum (Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin at 37°C in a humidified atmosphere
containing 5% CO2. The 5-FU-resistant colorectal
carcinoma cell lines HCT116-Res and HT29-Res were established as
previously described (20). Briefly,
the CRC cells were treated with 1 µg/ml of 5-FU (Sigma-Aldrich;
Merck KGaA) at 37°C with 5% CO2 for 24 h. The spent
medium was replaced by fresh culture medium, including DMEM
containing 5% fetal bovine serum and 1% penicillin/streptomycin.
Upon reaching a confluence of 90%, cells were treated with 2.5
µg/ml 5-FU at 37°C with 5% CO2 for 24 h. This process
was repeated using 5 and 10 µg/ml 5-FU. The 5-FU-resistant cell
lines HCT116-Res and HT29-Res were therefore established by
exposure to gradually increasing concentrations of 5-FU (1, 2.5, 5
and 10 µg/ml).
Cell viability
Cell viability was quantified with a Cell Counting
Kit-8 assay (CCK-8; Dojindo Molecular Technologies, Inc.). Cells
were plated into 96-well plates at a density of 3×103
cells/well with 100 µl DMEM containing 0, 5, 10 and 20 µg/ml of
5-Fu at 37°C with 5% CO2 for 48 h. Subsequently, 10 µl
CCK-8 solution was added into each well, followed by incubation for
2 h at 37°C. The absorbance (A) was read at a wavelength of 450 nm
using an absorbance microplate reader (BioTek Instruments, Inc.).
The cell viability was expressed as the relative percentage
compared with the mean absorbency of the untreated CRC cell lines
(control cells). The cell viability percentage was calculated using
the following equation: Relative cell viability (% of
control)=[(Asample-Ablank)/(Acontrol-Ablank)]
×100. The assay was repeated at least three times.
Transfection of miRNA mimic and
siRNA
The miR-361 mimic (sequence
5′-ACCCCUGGAGAUUCUGAUAAUU-3′), miR-361 mimic negative control (NC)
(sequence 5′-UUCUCCGAACGUGUCACGUTT-3′), Forkhead box M1 (FOXM1)
small interfering (si)RNA (siFOXM1 sequence: Sense:
5′-GGACCACUUUUCCCUACUUUDTDT-3′, antisense:
5′-AAAGUAGGGAAAGUGGUCCDTDT-3′) and siRNA NC (sequence
5′-UUCUCCGAACGUGUCACGUTT-3′, antisense:
5′-ACGUGACACGUUCGGAGAATT-3′) were designed and synthesized by
Shanghai GenePharma Co., Ltd. A total of 2×105
HCT116-Res and HT29-Res cells were seeded in 6-well plates. On
reaching ~30% confluence, the cells were transfected with miR-361
mimic or miR NC (both 100 nM) using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. siFOXM1 or siRNA NC (both 100 nM) was
transfected into HCT116-Res cells or HT29-Res cells by using
Lipofectamine® 3000, according to the manufacturer's
protocol. RNA extraction and reverse-transcription quantitative PCR
(RT-qPCR) experiments were conducted 24 h after transfection, and
western blot analysis, colony formation, apoptosis and luciferase
assays were carried out 48 h after the transfection.
RNA extraction and RT-qPCR
Total RNA was isolated using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. miRNA was obtained using an miRcute
miRNA extraction kit (Tiangen Biotech Co. Ltd.). RNA and miRNA were
reverse transcribed into cDNA using a GoScript™ RT reagent kit
(Promega Corporation) according to the manufacturer's protocol.
qPCR analysis was subsequently performed using SYBR®
Green Premix Ex Taq™ II (Takara Biotechnology Co., Ltd.) and an ABI
Prism 7900 Sequence Detection system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). Literature review indicated that several
miRNAs have been reported to be associated with chemoresistance
(7,10,15–19), and
were therefore utilized as candidate miRNAs in this study, and
their role in 5-Fu resistance was confirmed. The expression of the
miRNA candidates by qRCR were identified. The thermocycling
conditions were as follow: 95°C for 30 sec, followed by 40 cycles
at 95°C for 5 sec and 60°C for 30 sec and a final melt curve stage
which was 95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec. The
following primer pairs were used for qPCR: FOXM1 forward,
5′-CGTCGGCCACTGATTCTCAAA-3′ and reverse,
5′-GGCAGGGGATCTCTTAGGTTC-3′; β-actin forward,
5′-AGAGCTACGAGCTGCCTGAC-3′ and reverse, 5′-AGCACTGTGTTGGCGTACAG-3′;
U6 forward, 5′-CTCGCTTCGGCAGCACA-3′, and reverse,
5′-AACGCTTCACGAATTTGCGT-3′; miR-361-5p loop reverse primer,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGTACCCC-3′, forward primer,
5′-ACACTCCAGCTGGGTTATCAGAATCTCCA-3′; miR-21 loop reverse primer,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCAACAT-3′, forward primer,
5′-ACACTCCAGCTGGGUAGCUUAUCAGACUG-3′; miR-224 loop reverse primer,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCTAAACG-3′, forward primer,
5′-ACACTCCAGCTGGGUCAAGUCACUAGUGGUUC-3′; miR-134 loop reverse
primer, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCTCCCC-3′, forward
primer, 5′-ACACTCCAGCTGGGUGUGACUGGUUGACC-3′; miR-29a loop reverse
primer, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTAACCGA-3′, forward
primer, 5′-ACACTCCAGCTGGGUAGCACCAUCUGAAA-3′; miR-29c loop reverse
primer, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTAACCGA-3′, forward
primer, 5′-ACACTCCAGCTGGGUAGCACCAUUUGAAA-3′; miR-149 loop reverse
primer, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGGGAGTG-3′, forward
primer, 5′-ACACTCCAGCTGGGUCUGGCUCCGUGUCUU-3′; miR-200c loop reverse
primer, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCCATCA-3′, forward
primer, 5′-ACACTCCAGCTGGGUAAUACUGCCGGGUAA-3′; miR-34a loop reverse
primer, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGACAACCA-3′, forward
primer, 5′-ACACTCCAGCTGGGUGGCAGUGUCUUAGC-3′ and the QPCR reverse
primer for all miRNA candidates was 5′-TGGTGTCGTGGAGTCG-3′. β-actin
and U6 were used for mRNA and miRNA normalization, respectively.
Relative quantification of gene expression levels were expressed as
fold-change using the 2−ΔΔCq method (21). The relative fold change of miRNAs
>1.5 was considered as upregulated miRNAs, while <-1.5 as
downregulated miRNAs.
Western blotting
Total protein of HCT116, HT29, HCT116-Res, HT29-Res
cells was extracted using radioimmunoprecipitation assay buffer
(Beyotime Institute of Biotechnology) and a protease inhibitor
cocktail (Roche Diagnostics GmbH) according to the manufacturer's
protocol. Total protein was quantified using a Pierce™ BCA Protein
Assay kit (Thermo Fisher Scientific, Inc.). The cell lysate
supernatant was mixed with 6X SDS loading buffer (Beyotime
Institute of Biotechnology) and boiled at 100°C for 10 min.
Subsequently, 30 µg protein/lane was separated via 10% SDS-PAGE and
transferred onto PVDF membranes (Merck KGaA). The membrane was
blocked for 1 h at room temperature using 5% non-fat milk dissolved
in Tris buffered saline with 0.1% Tween (TBS-T). The membrane was
incubated with the following primary antibodies overnight at 4°C:
Anti-FOXM1 (1:1,000; cat. no. ab180710 Abcam) and β-actin (1:1,000;
cat. no. AA132 Beyotime Institute of Biotechnology), Anti-ABCC5
(1:1,000; cat. no. ab180724; Abcam), Anti-ABCC10 (1:1,000; cat. no.
ab91451; Abcam). Membranes were subsequently washed in TBS-T three
times and incubated with horseradish peroxidase (HRP)-conjugated
secondary antibodies (1:2,000; cat. no. A0208 for anti-rabbit and
cat. no. A0216 for anti-mouse; Beyotime Institute of Biotechnology)
for 1 h at room temperature. The protein bands were visualized
using Millipore Immobilon Western Chemiluminescent HRP substrate
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Each reaction was performed in triplicate.
Luciferase assay
Wild-type FOXM1-3′UTR containing a miR-361-5p
targeted region or mutant-form 3′UTR was inserted into a pmirGlO
Dual-luciferase miRNA Target Expression Vector (cat. no. E1330;
Promega Corporation). The HCT116-Res or HT29-Res cells
(2×105) were seeded into 24-well plate to reached 70%
confluence. Then HCT116-Res or HT29-Res cells were transfected with
2 µg Wild-type FOXM1-3′UTR or mutant-form 3′UTR and 100 nM
miR-361-5p mimics using Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) Cells were analyzed for luciferase
activity was measured 48 h following transfection using the
Dual-Glo® Luciferase Assay System (cat. no. E2920;
Promega Corporation), according to the manufacturer's protocol.
Firefly luciferase activity was normalized to Renilla
luciferase activity.
Colony formation assay
5-FU-resistant HCT116 or HT29 cells transfected with
FOXM1 siRNA, miR-361 mimic or respective NCs were plated in 6-well
plates (500 cells/well) and incubated at 37°C for 14 days to allow
colony formation. The cell medium was subsequently removed and
cells were washed using PBS. Cells were fixed with 4%
paraformaldehyde for 10 min at room temperature. The cells were
stained with crystal violet kit (cat. no. C0121; Beyotime Institute
of Biotechnology) for 15 min at room temperature, according to the
manufacturer's protocols. The colonies were washed, imaged by Sony
camera (NEX-3N; SONY Corp.) and counted using ImageJ software.
Apoptosis assay
Flow cytometry was performed to detect cell
apoptosis by Annexin V and propidium iodide (PI) double staining.
5-FU resistant HCT116 and HT29 cells transfected with the miR-361
mimic and NC were harvested using non-EDTA trypsinase and stained
with Annexin V-FITC kit (Miltenyi Biotec, Inc.), according to the
manufacturer's protocol. Subsequently, stained cells were detected
using a flow cytometer (FACSCanto II; BD Biosciences) and analyzed
by BD FACSDiva 8.0.1 software (BD Biosciences). Additionally, a
Caspase-Glo 3/7 kit (cat. no. G8090; Promega Corporation) was used
to measure caspase 3/7 activity according to the manufacturer's
protocols.
miRNA targets prediction
MicroRNA.org (http://www.microrna.org) is a comprehensive resource
of microRNA target predictions and expression profiles (22). Target predictions are based on a
development of the miRanda algorithm. The algorithm provided a
machine learning method named mirSVR to rank microRNA target sites
by a downregulation score. By using this online prediction software
(August 2010 Release) (23,24), it was found that miR-361 binds to the
3′UTR region of FOXM1 with highest mirSVR score among the predicted
targets, and FOXM1 was therefore selected for further study.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software (version 6.0; GraphPad Software, Inc.). Data are
presented as the mean ± standard deviation. Differences between
treated and untreated control cells were assessed using the
unpaired Student's t-test. The Bonferroni test was used to evaluate
differences between multiple groups following two-way ANOVA
analysis. All experiments were performed at least three times.
P<0.05 was used to indicate a statistically significant
difference.
Results
Expression level of miR-361 is
decreased in 5-FU-resistant HCT116 and HT29 cells
5-FU-resistant HCT116 and HT29 cells were
successfully established in the current study using a methodology
described in a previous study (25).
The resistant and parental cells were treated with 5, 10 and 20
µg/ml 5-FU for 48 h. The viability of parental HCT116 and HT29
cells was significantly decreased compared with the resistant cells
(P<0.01; Fig. 1A). The cell
viability of resistant HCT116 and HT29 cells at 5 and 10 µg/ml of
5-FU was not significantly inhibited (P>0.05); however,
inhibition was observed in resistant cells in the presence of 20
µg/ml 5-FU (P<0.01), suggesting that the resistant cells in the
present study exhibited optimal survival in 5 and 10 µg/ml
5-FU.
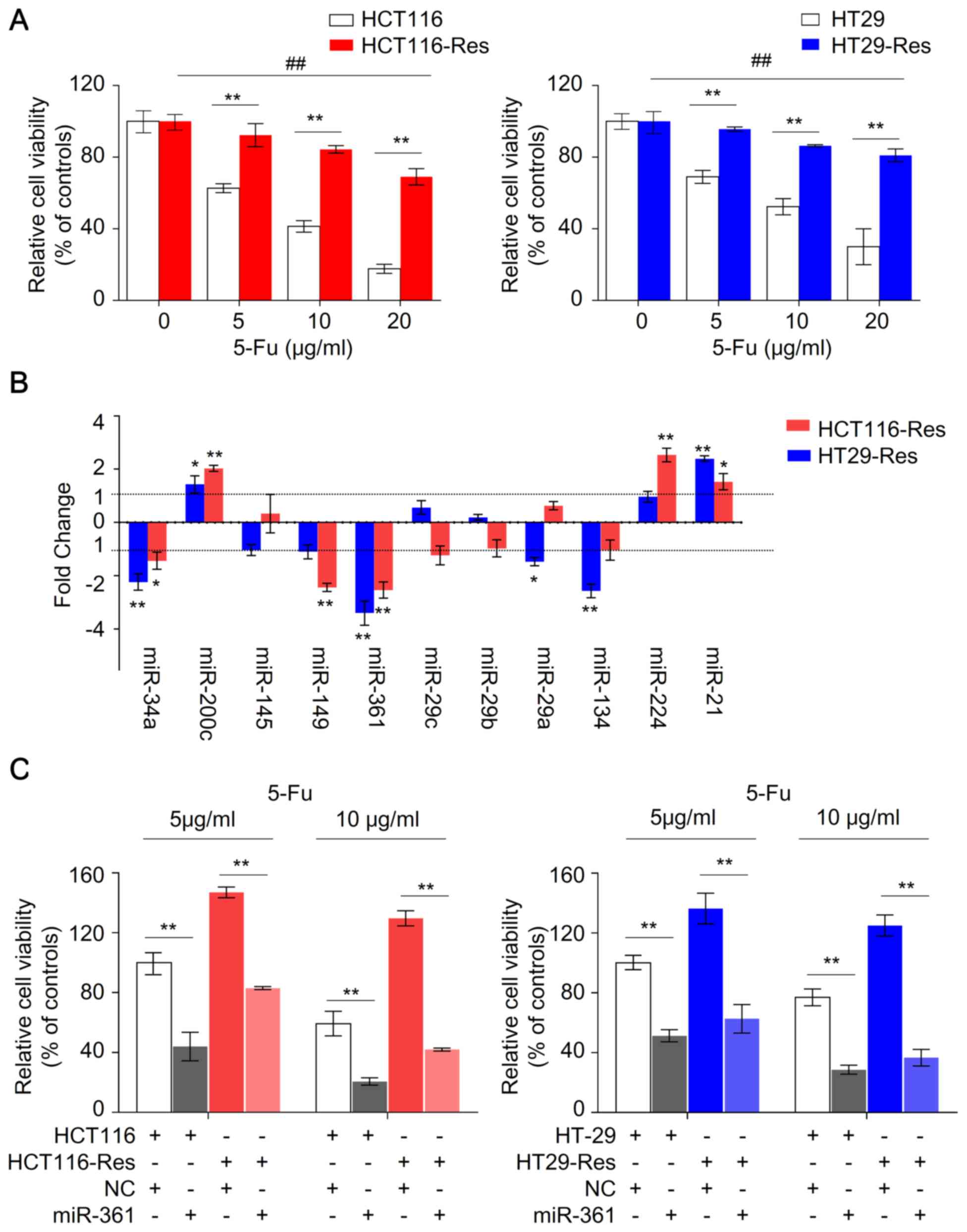 | Figure 1.miR-361 is upregulated in
5-FU-resistant HCT116 and HT29 cells. (A) Parental and
5-FU-resistant HCT116 and HT29 cells were treated with 5-FU at the
indicated concentrations for 48 h and subjected to a CCK-8 assay.
Cell viability was presented as the relative value normalized to
the 0 µg/ml group (n=6; **P<0.01, parental vs. resistant cells;
##P<0.01, the viability of resistant cells at 20
µg/ml vs. 0 µg/ml). (B) Relative differential expression of
candidate miRNAs between parental and 5-FU resistant HCT116 and
HT29 cells (n=3; *P<0.05, **P<0.01, vs. parental cells). (C)
Overexpression of miR-361 sensitizes parental and resistant HCT116
and HT29 to 5-FU. The parental and 5-FU-resistant HCT116 and HT29
cells were transfected with an miR-361 mimic or NC, exposed to 5-FU
at the indicated concentrations for 48 h and subjected to a CCK-8
assay (n=6; **P<0.01). miR/miRNA, microRNA; 5-FU,
5-fluorouracil; CCK-8, Cell Counting Kit-8; NC, negative control;
Res, resistant. |
To identify novel miRNAs associated with
chemoresistance in CRC, the expression levels of several candidate
miRNAs in parental and resistant HCT116 and HT29 cells were
measured by qPCR (Fig. 1B). miR-21,
miR-224 and miR-200c were upregulated, whereas miR-34a, miR-361
miR-134 were downregulated in resistant cells compared with
parental cells. Among these miRNAs, the expression level of miR-361
was the most markedly altered, with ~3.5-fold change in resistant
HCT116 and HT29 cells compared with parental cells, suggesting that
that miR-361 is a potential regulator of 5-FU chemosensitivity in
CRC.
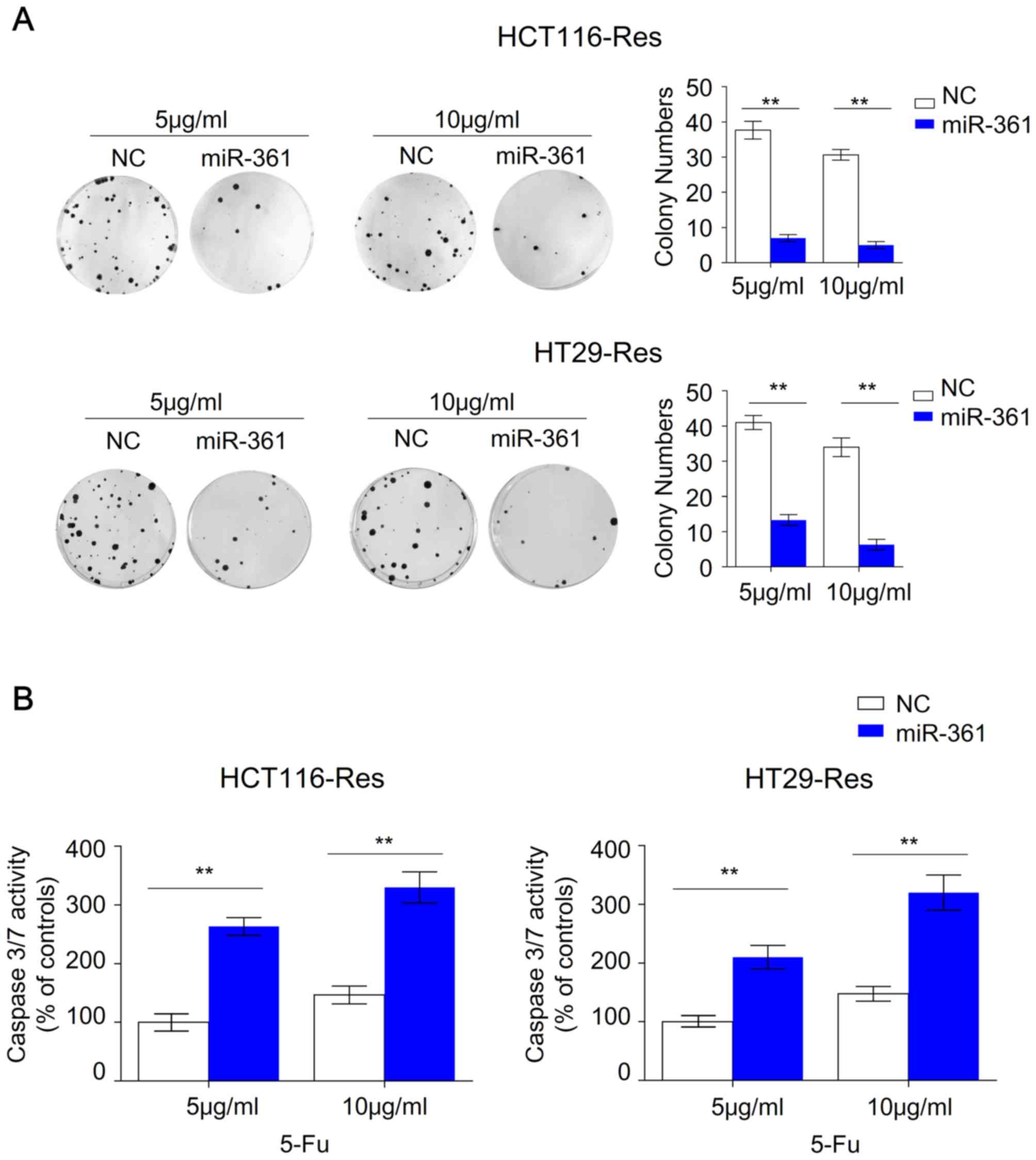
Overexpression of miR-361 sensitizes
resistant CRC cells to 5-FU, inhibits colony formation and induces
apoptosis
To investigate whether miR-361 modulates sensitivity
to 5-FU in CRC, parental and resistant cells were transfected with
miR-361 mimic or NC, and exposed to 5 or 10 µg/ml 5-FU.
Overexpression of miR-361 enhanced the susceptibility of parental
HCT116 and HT29 cells to 5-FU and increased 5-FU sensitivity in
resistant cells, suggesting that miR-361 may sensitize CRC cells to
5-FU (Fig. 1C). Colony formation and
apoptosis assays were subsequently performed to further investigate
this effect.
Overexpression of miR-361 in resistant HCT116 and
HT29 cells significantly reduced colony numbers at 5 or 10 µg/ml
5-FU, suggesting that miR-361 significantly impaired the ability of
resistant cells to grow under 5-FU treatment (Fig. 2A). Previous studies revealed that
5-FU-resistant CRC cells are able to survive under 5-FU treatment
with decreased apoptosis compared with parental cells (26–29). The
present study subsequently investigated whether the inhibited cell
viability and colony formation of the resistant cells was caused by
miR-361-induced apoptosis. Overexpression of miR-361 in resistant
HCT116 and HT29 cells significantly increased caspase 3/7 activity
compared with the NC, suggesting that apoptotic signaling pathways
were activated (Fig. 2B).
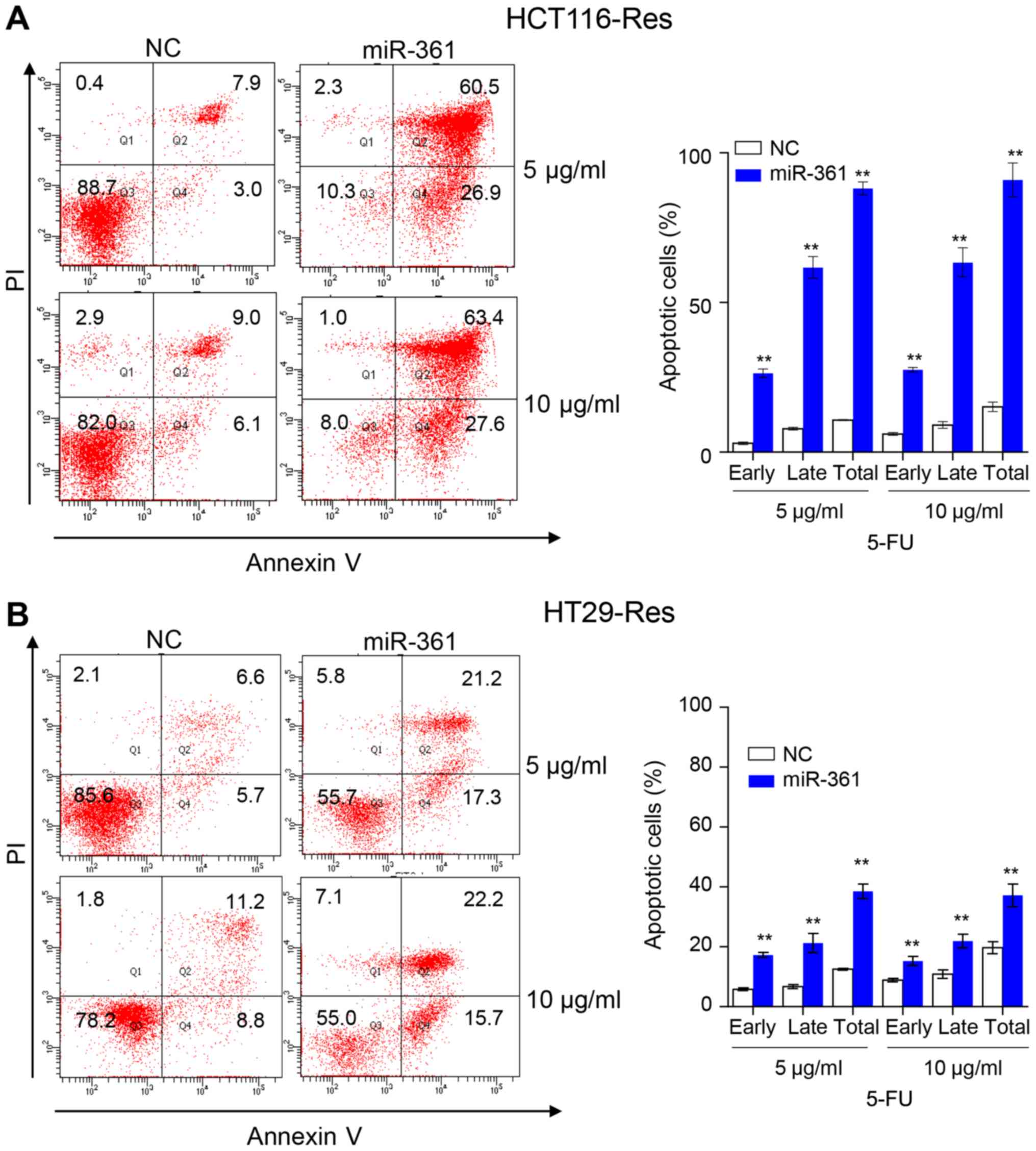
Furthermore, an Annexin V/PI assay revealed that miR-361
overexpression induced apoptosis in resistant HCT116 and HT29
compared with the NC (Fig. 3).
Resistant HCT116 cells overexpressing miR-361 exhibited a higher
percentage of early apoptotic cells compared with NC cells
(26.4±1.4% vs. 2.9±0.4% at 5 µg/ml 5-FU, P<0.001; 27.6±0.8% vs.
6.1±0.5% at 10 µg/ml 5-FU, P<0.001) or a higher percentage of
late apoptotic cells compared with NC cells (61.7±3.6% vs. 7.8±0.5%
at 5 µg/ml 5-FU, P<0.001; 63.4±4.9% vs. 9.1±1.1% at 10 µg/ml
5-FU, P<0.001). Similarly, resistant HT29 cells overexpressing
miR-361 exhibited a higher percentage of early apoptotic cells
compared with NC cells (17.3±0.8% vs. 5.8±0.5% at 5 µg/ml 5-FU,
P<0.001; 15.3±1.5% vs. 8.9±0.6% at 10 µg/ml 5-FU, P<0.001) or
a higher percentage of late apoptotic cells compared with NC cells
(21.3±3.2% vs. 6.7±0.7% at 5 µg/ml 5-FU, P<0.001; 21.9±2.3% vs.
10.9±1.4% at 10 µg/ml 5-FU, P<0.001).
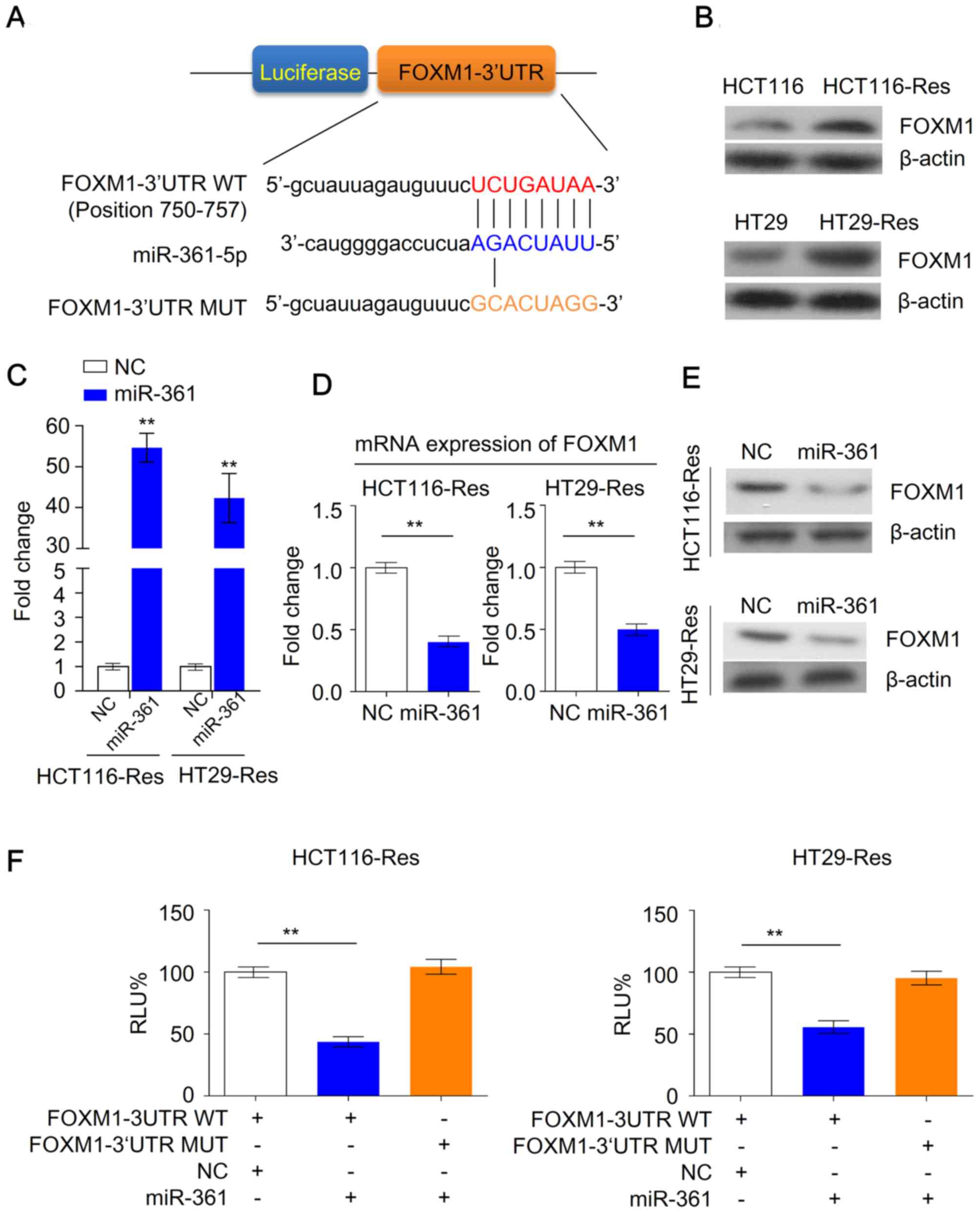
miR-361 is a negative regulator of
FOXM1 expression in 5-FU-resistant cells
As miR-361 may serve an important role in the
regulation of chemosensitivity in CRC cells, the present study
investigated the potential targets of miR-361 in resistant cells.
Despite the complex interactions between miRNAs and mRNAs, several
algorithms are available for exploring such interactions. By using
microRNA algorithms (www.microrna.org), FOXM1 was predicted as a potential
target of miR-361 (Fig. 4A).
Furthermore, western blotting demonstrated that FOXM1 was
upregulated in resistant cells compared with parental cells
(Fig. 4B). As miR-361 was previously
revealed to be downregulated in resistant cells (Fig. 1B), this suggested that FOXM1 may be a
potential target of miR-361.
To further investigate the effects of miR-361 on
FOXM1 expression in resistant HCT116 or HT29 cells, miR-361 was
overexpressed in CRC cells (Fig.
4C). FOXM1 mRNA and protein levels were significantly decreased
following overexpression of miR-361 compared with NC cells,
suggesting that FOXM1 may be a direct target of miR-361 in
resistant cancer cells (Fig. 4D and
E). Luciferase reporter assays were performed to further
investigate whether miR-361 regulates the expression of FOXM1 in
resistant cells in a direct or indirect manner. miR-361 binding
sites of FOXM1 were cloned into luciferase reporter plasmids to
establish a wild type FOXM1 3′UTR reporter plasmid. Mutated miR-361
binding sites were cloned to establish a mutant FOXM1 3′UTR
reporter plasmid. Resistant HCT116 and HT29 cells were transiently
transfected with these reporter constructs along with the NC or the
miR-361 mimic. Overexpression of miR-361 reduced the luciferase
activity in cells transfected with the wild type FOXM1 3′UTR
reporter plasmid; however, no decreased activity was observed in
cells transfected the mutated FOXM1 3′UTR reporter plasmid
(Fig. 4F). Taken together, these
data suggested that miR-361 suppressed FOXM1 expression through
direct binding to the putative 3′UTR binding site of FOXM1
mRNA.
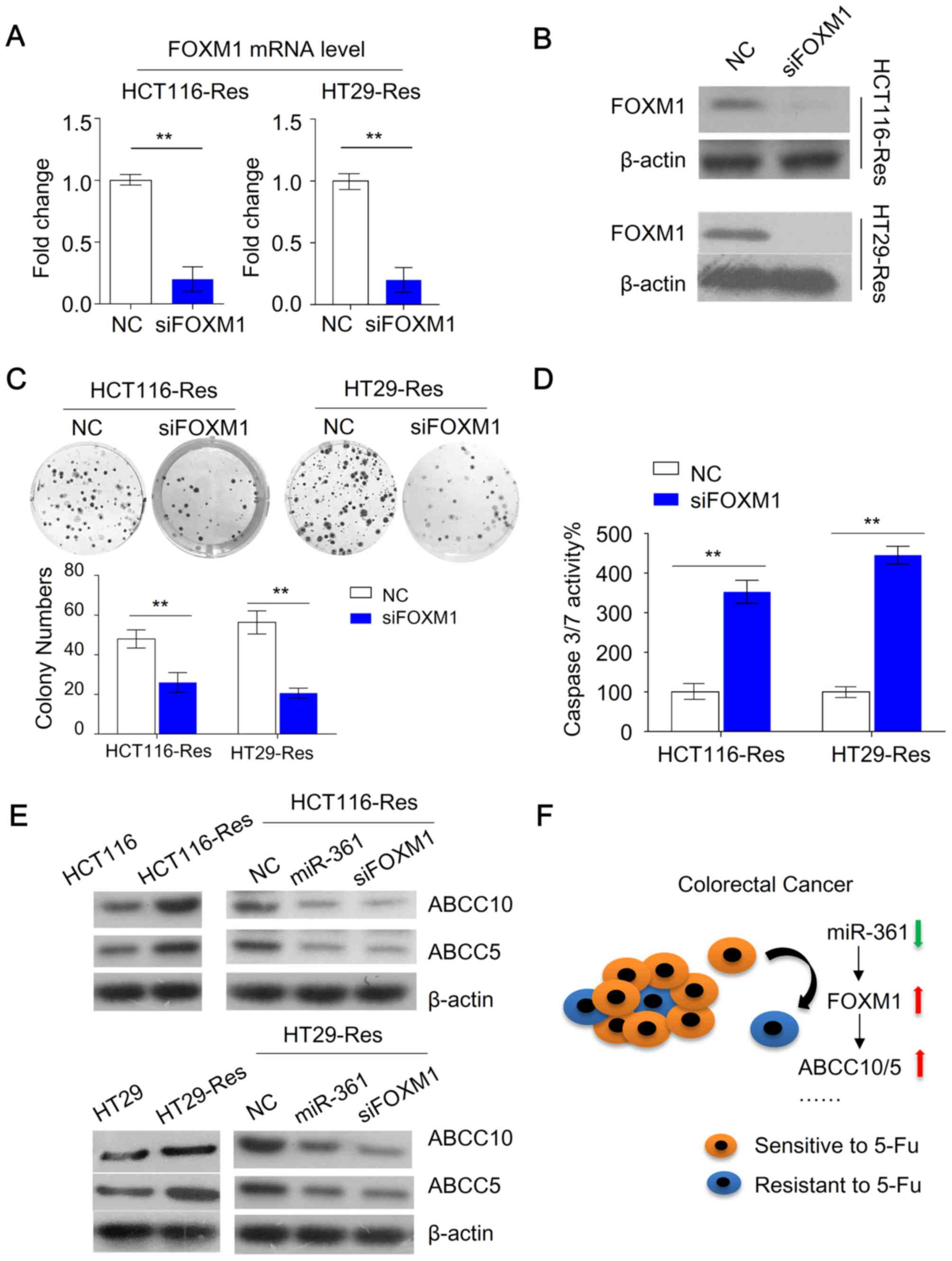
Targeting FOXM1 improves the
cytotoxicity of 5-FU in resistant CRC cells
The current study investigated whether changes in
FOXM1 expression modulated the chemosensitivity of resistant CRC
cells. Knockdown of FOXM1 in resistant HCT116 cells was achieved
using FOXM1 siRNA (Fig. 5A and B).
The cells were subsequently subjected to colony formation and
caspase 3/7 activity assays. FOXM1 knockdown inhibited colony
formation compared with NC cells (Fig.
5C). Furthermore, FOXM1 knockdown increased caspase 3/7
activity compared with NC cells (Fig.
5D). Recent studies demonstrated that FOXM1 promotes
chemoresistance by upregulating ATP binding cassette subfamily C
(ABCC) 10 (30) and ABCC5 (31), which mediate drug efflux leading to
acquired drug resistance. The present study investigated whether
inhibition of FOXM1 expression affected ABCC10 and ABCC5
expression. Decreased FOXM1 expression resulted in a downregulation
of ABCC10 and ABCC5 compared with the NC (Fig. 5E). Furthermore, resistant HCT116
cells overexpressing miR-361 exhibited reduced expression of ABCC10
and ABCC5 compared with the NC (Fig.
5E). Collectively, these data suggested that miR-361 exerted
functions in CRC, at least in part, through inhibition of FOXM1,
and its downstream targets ABCC10 and ABCC5 (Fig. 5F).
Discussion
CRC is a leading cause of mortality worldwide
(1). Patients diagnosed at an early
stage typically have a good prognosis; however, a number of
patients present with liver metastasis at initial diagnosis, and
the prognosis of such patients is usually poor (2). 5-FU-based chemotherapy is a commonly
used treatment strategy for patients with advanced CRC.
Chemoresistance is associated with the recurrence of CRC during
clinical therapy with 5-FU (3–6). The
molecular mechanisms underlying chemoresistance in CRC remain
largely unknown. It was previously demonstrated that miRNAs
regulate diverse biological processes in cancer cells and may serve
an important role in chemosenstivity (32). The present study demonstrated that
miR-361 is a novel regulator of chemosensitivity in CRC.
Furthermore, modulation of miR-361 expression increased the
chemosensitivity of resistant CRC cells. Additionally, the present
study revealed that miR-361 functions as a chemosensitizer through
the FOXM1-ABCC10/5 signaling pathway.
Numerous studies reported aberrant serum or tissue
miRNA levels in patients with CRC, and these dysregulated
expression profiles are often associated with aggressive clinical
phenotypes, drug resistance or poor prognosis (18,33). The
current study established in vitro 5-FU-resistant CRC cells
to identify 5-FU-associated miRNAs. Several candidate miRNAs, which
may function as tumor suppressors or oncogenes, were identified.
miR-21, miR-224 and miR-200c were upregulated, whereas miR-34a,
miR-361 and miR-134 were downregulated in the resistant cells.
Among these miRNAs, the expression levels of miR-361 were the most
significantly changed. miR-361 is a tumor suppressor and inhibits
cell proliferation and invasion of several types of cancer cells,
such as gastric cancer, breast cancer and thyroid cancer (34–36). To
the best of our knowledge, the present study is the first to
demonstrate that miR-361 restores 5-FU sensitivity in resistant CRC
cells, by inhibiting cell viability, colony formation and inducing
cell apoptosis, suggesting that miR-361 may serve as a promising
therapeutic target to enhance chemosensitivity to 5-FU in CRC.
It has been reported that miRNAs may serve as
potential therapeutic agents due to their ability to regulate the
expression of multiple target genes (10). While the identification of downstream
targets of miRNAs regulating 5-FU sensitivity is challenging, the
present study used bioinformatics prediction and several in
vitro approaches to identify FOXM1 as an important direct
target of miR-361. Previous studies reported that FOXM1 was
frequently upregulated in colorectal cancer tissues and serves as a
prognostic marker in CRC (9,37). Moreover, overexpression of FOXM1 in
CRC cells was shown to correlate with 5-FU resistance (19,30,38).
Therefore, 5-FU-based chemotherapy may not be appropriate for
patients with CRC with upregulated FOXM1 expression. Moreover,
ABCC5 and ABCC10, revealed as downstream targets of FOXM1 in the
present study, have been previously suggested as promising
therapeutic agents in patients with resistant cancer, such as
breast cancer and pancreatic cancer (39–42).
The present study had a number of limitations. The
association between miR-361 expression and its target genes FOXM1,
ABCC5 and ABCC10 was not evaluated in tissues from 5-FU resistance
patients with CRC or animal models with 5-FU resistant xenograft.
Future experiments to validate the interaction between miR-361 and
its target genes in a large cohort of patients with CRC with
complete chemoresponse information are required. Moreover, an
miR-361-expressing xenograft animal model to investigate the
regulatory effect of miR-361 on its target genes is required.
In summary, the current study investigated the
association between miR-361 and FOXM1-ABCC5/10 in 5-FU resistance
in CRC. miR-361 may regulate chemosensitivity to 5-FU by targeting
FOXM1-ABCC5/10. miR-361-based therapy may serve as a potential
strategy to enhance 5-FU sensitivity in patients with resistant
CRC. Furthermore, the addition of FOXM1 or ABCC5/10 inhibitors to a
chemotherapeutic regimen may substantially reduce the required 5-FU
dosage in patients with CRC. The results obtained in the current
study may provide novel targets for the treatment of patients with
advanced or chemoresistant CRC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JS conceived and designed the study and participated
in data analysis. LZ performed the experiment and drafted the
manuscript. BL and BZ performed the data analysis and
interpretation. HZ performed the statistical analysis.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
R, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Cutsem E, Cervantes A, Nordlinger B
and Arnold D; ESMO Guidelines Working Group, : Metastatic
colorectal cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 25 (Suppl 3):iii1–iii9. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Montagnani F, Chiriatti A, Turrisi G,
Francini G and Fiorentini G: A systematic review of FOLFOXIRI
chemotherapy for the first-line treatment of metastatic colorectal
cancer: Improved efficacy at the cost of increased toxicity.
Colorectal Dis. 13:846–852. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cersosimo RJ: Management of advanced
colorectal cancer, Part 1. Am J Health Syst Pharm. 70:395–406.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cersosimo RJ: Management of advanced
colorectal cancer, Part 2. Am J Health Syst Pharm. 70:491–506.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mohelnikova-Duchonova B, Melichar B and
Soucek P: FOLFOX/FOLFIRI pharmacogenetics: The call for a
personalized approach in colorectal cancer therapy. World J
Gastroenterol. 20:10316–10330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu HW and Cho WC: The emerging role of
miRNAs in combined cancer therapy. Expert Opin Biol Ther.
15:923–925. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Y and Srivastava D: A developmental
view of microRNA function. Trends Biochem Sci. 32:189–197. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weng W, Okugawa Y, Toden S, Toiyama Y,
Kusunoki M and Goel A: FOXM1 and FOXQ1 Are promising prognostic
biomarkers and novel targets of Tumor-Suppressive miR-342 in human
colorectal cancer. Clin Cancer Res. 22:4947–4957. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weng W, Feng J, Qin H, Ma Y and Goel A: An
update on miRNAs as biological and clinical determinants in
colorectal cancer: A bench-to-bedside approach. Future Oncol.
11:1791–1808. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Irwandi RA and Vacharaksa A: The role of
microRNA in periodontal tissue: A review of the literature. Arch
Oral Biol. 72:66–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang G, Pan J, Ye Z, Fang B, Cheng W and
Cao Z: Overexpression of miR-216b sensitizes NSCLC cells to
cisplatin-induced apoptosis by targeting c-Jun. Oncotarget.
8:104206–104215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du X, Liu B, Luan X, Cui Q and Li L:
miR-30 decreases multidrug resistance in human gastric cancer cells
by modulating cell autophagy. Exp Ther Med. 15:599–605.
2018.PubMed/NCBI
|
|
14
|
Zhou X, Natino D, Zhai X, Gao Z and He X:
MicroRNA22 inhibits the proliferation and migration, and increases
the cisplatin sensitivity, of osteosarcoma cells. Mol Med Rep.
17:7209–7217. 2018.PubMed/NCBI
|
|
15
|
Salendo J, Spitzner M, Kramer F, Zhang X,
Jo P, Wolff HA, Kitz J, Kaulfuß S, Beißbarth T, Dobbelstein M, et
al: Identification of a microRNA expression signature for
chemoradiosensitivity of colorectal cancer cells, involving
miRNAs-320a,-224,-132 and let7g. Radiothe Oncol. 208:451–457. 2013.
View Article : Google Scholar
|
|
16
|
Ye Q, Su L, Chen D, Zheng W and Liu Y:
Astragaloside IV induced miR-134 expression reduces EMT and
Increases chemotherapeutic sensitivity by suppressing CREB1
signaling in colorectal cancer cell line SW-480. Cell Physiol
Biochem. 43:1617–1626. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tokarz P and Blasiak J: The role of
microRNA in metastatic colorectal cancer and its significance in
cancer prognosis and treatment. Acta Biochim Pol. 59:467–474. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li XJ, Ren ZJ and Tang JH: MicroRNA-34a: A
potential therapeutic target in human cancer. Cell Death Dis.
5:e13272014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Xie T, Mao X, Xue L, Chu X and Chen
L: MicroRNA-149 increases the sensitivity of colorectal cancer
cells to 5-Fluorouracil by targeting forkhead box transcription
factor FOXM1. Cell Physiol Biochem. 39:617–629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dabkeviciene D, Jonusiene V, Zitkute V,
Zalyte E, Grigaitis P, Kirveliene V and Sasnauskiene A: The role of
interleukin-8 (CXCL8) and CXCR2 in acquired chemoresistance of
human colorectal carcinoma cells HCT116. Med Oncol. 32:2582015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Betel D, Wilson M, Gabow A, Marks DS and
Sander C: The microRNA.org resource:targets and expression. Nucleic
Acids Res. 36((Database Issue)): D149–D153. 2008.PubMed/NCBI
|
|
23
|
Betel D, Koppal A, Agius P, Sander C and
Leslie C: Comprehensive modeling of microRNA targets predicts
functional non-conserved and non-canonical sites. Genome Biol.
11:R902010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human MicroRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boyer J, McLean EG, Aroori S, Wilson P,
McCulla A, Carey PD, Longley DB and Johnston PG: Characterization
of p53 wild-type and null isogenic colorectal cancer cell lines
resistant to 5-fluorouracil, oxaliplatin, and irinotecan. Clin
Cancer Res. 10:2158–2167. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yao Z, Bhandari A, Wang Y, Pan Y, Yang F,
Chen R, Xia E and Wang O: Dihydroartemisinin potentiates antitumor
activity of 5-fluorouracil against a resistant colorectal cancer
cell line. Biochem Biophys Res Commun. 501:636–642. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lai Z, Yan Z, Chen W, Peng J, Feng J, Li
Q, Jin Y and Lin J: Hedyotis diffusa Willd suppresses metastasis in
5-fluorouracil-resistant colorectal cancer cells by regulating the
TGF-β signaling pathway. Mol Med Rep. 16:7752–7758. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu B, Liu Y, Zhao L, Pan Y, Shan Y, Li Y
and Jia L: Upregulation of microRNA-135b and microRNA-182 promotes
chemoresistance of colorectal cancer by targeting ST6GALNAC2 via
PI3K/AKT pathway. Mol Carcinog. 56:2669–2680. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao C, Zhao Q, Zhang C, Wang G, Yao Y,
Huang X, Zhan F, Zhu Y, Shi J, Chen J, et al: miR-15b-5p
resensitizes colon cancer cells to 5-fluorouracil by promoting
apoptosis via the NF-KB/XIAP axis. Sci Rep. 7:41942017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie T, Geng J, Wang Y, Wang L, Huang M,
Chen J, Zhang K, Xue L, Liu X, Mao X, et al: FOXM1 evokes
5-fluorouracil resistance in colorectal cancer depending on ABCC10.
Oncotarget. 8:8574–8589. 2017.PubMed/NCBI
|
|
31
|
Hou Y, Zhu Q, Li Z, Peng Y, Yu X, Yuan B,
Liu Y, Liu Y, Yin L, Peng Y, et al: The FOXM1-ABCC5 axis
contributes to paclitaxel resistance in nasopharyngeal carcinoma
cells. Cell Death Dis. 8:e26592017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hollis M, Nair K, Vyas A, Chaturvedi LS,
Gambhir S and Vyas D: MicroRNAs potential utility in colon cancer:
Early detection, prognosis, and chemosensitivity. World J
Gastroenterol. 21:8284–8292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garzon R, Marcucci G and Croce CM:
Targeting microRNAs in cancer: Rationale, strategies and
challenges. Nat Rev Drug Discov. 9:775–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tian L, Zhao Z, Xie L and Zhu J:
MiR-361-5p inhibits the mobility of gastric cancer cells through
suppressing epithelial-mesenchymal transition via the Wnt/β-catenin
pathway. Gene. 675:102–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han J, Yu J, Dai Y, Li J, Guo M, Song J
and Zhou X: Overexpression of miR-361-5p in triple-negative breast
cancer (TNBC) inhibits migration and invasion by targeting RQCD1
and inhibiting the EGFR/PI3K/Akt pathway. Bosn J Basic Med Sci.
19:52–59. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li R, Dong B, Wang Z, Jiang T and Chen G:
MicroRNA-361-5p inhibits papillary thyroid carcinoma progression by
targeting ROCK1. Biomed Pharmacother. 102:988–995. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li L, Wu D, Yu Q, Li L and Wu P:
Prognostic value of FOXM1 in solid tumors: A systematic review and
meta-analysis. Oncotarget. 8:32298–32308. 2017.PubMed/NCBI
|
|
38
|
Cao S, Lin L, Xia X and Wu H: MicroRNA-761
promotes the sensitivity of colorectal cancer cells to
5-Fluorouracil through targeting FOXM1. Oncotarget. 9:321–331.
2017.PubMed/NCBI
|
|
39
|
Anreddy N, Patel A, Sodani K, Kathawala
RJ, Chen EP, Wurpel JN and Chen ZS: PD173074, a selective FGFR
inhibitor, reverses MRP7 (ABCC10)-mediated MDR. Acta Pharm Sin B.
4:202–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun YL, Chen JJ, Kumar P, Chen K, Sodani
K, Patel A, Chen YL, Chen SD, Jiang WQ and Chen ZS: Reversal of
MRP7 (ABCC10)-mediated multidrug resistance by tariquidar. PLoS
One. 8:e555762013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu Y, Yu F, Jiao Y, Feng J, Tang W, Yao
H, Gong C, Chen J, Su F, Zhang Y and Song E: Reduced miR-128 in
breast tumor-initiating cells induces chemotherapeutic resistance
via Bmi-1 and ABCC5. Clin Cancer Res. 17:7105–7115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hagmann W, Faissner R, Schnölzer M, Löhr M
and Jesnowski R: Membrane drug transporters and chemoresistance in
human pancreatic carcinoma. Cancers (Basel). 3:106–125. 2010.
View Article : Google Scholar : PubMed/NCBI
|