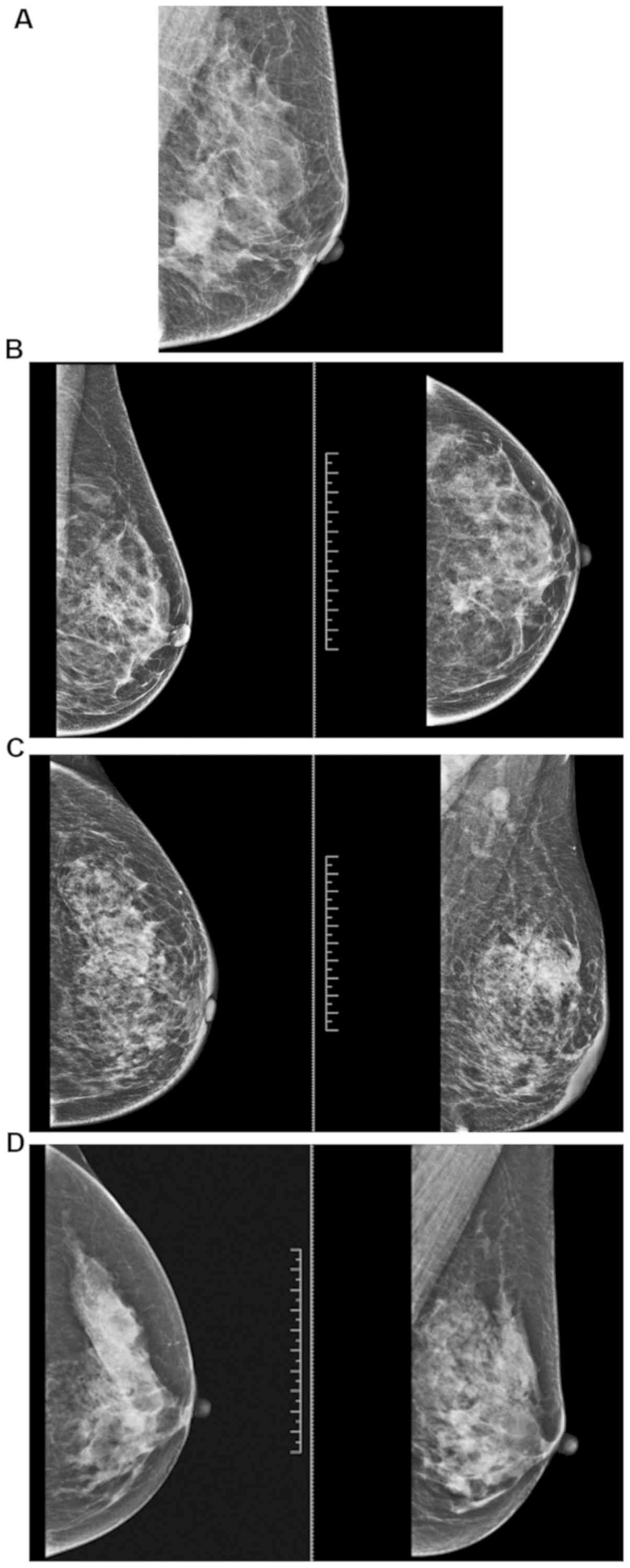
Introduction
Breast cancer tends to occur in the epithelial
tissue of the breast gland and is one of the most common female
malignant tumors (1). Breast cancer
has a great impact on women's physical and mental health, and in
severe cases, it can even be life-threatening. According to
statistics (2), the incidence of
breast cancer worldwide is on the rise year by year (3). Since most surgical patients are already
in the advanced stage of breast cancer when they visit the doctor,
even if the cancerous tissue is removed, breast cancer has a high
probability to metastasize (4).
Statistical data show that the postoperative recurrence rate of
breast cancer patients is high and the five-year survival rate is
low at this stage (5). Clinical
etiology of breast cancer is not completely clear, so early
diagnosis of breast cancer helps patients get timely treatment,
thus improving the survival rate of breast cancer patients
(6). According to relevant reports,
Luminal-A, Luminal-B, Her2-overexpressed and triple-negative breast
cancers are four different molecular types, and different molecular
types are more conducive for clinicians to choose the best
individualized treatment according to the characteristics of
different molecular sub-types (7).
However, molecular typing depends on relevant pathological tissues.
Although pathological tissue diagnosis is the gold standard for
clinical staging diagnosis of breast cancer, some patients are
still psychologically and physiologically unacceptable (8). With the continuous development and
innovation of medical diagnostic technology, imaging technology for
clinical diagnosis is also continuously upgraded, and the results
of diagnostic coincidence rate and pathological tissue diagnosis
are getting increasingly closer (9).
At present, molybdenum target imaging (10), breast ultrasound (11), magnetic resonance imaging (MRI)
(12), dynamic contrast-enhanced
magnetic resonance imaging (DCE-MRI) (13) and positron emission tomography (PET)
(14) are new imaging techniques
commonly used in clinical diagnosis. However, the breast molybdenum
target and other new imaging techniques of DCE-MRI have different
limitations (15). Therefore, this
study was performed to investigate the clinical efficacy of
molybdenum target, DCE-MRI and molybdenum target combined with
DCE-MRI in the diagnosis of breast cancer of different types.
Patients and methods
Data collection of the patients
From February 2015 to October 2017, 120 female
patients with breast cancer admitted to The First Affiliated
Hospital of Zhengzhou University (Zhengzhou, China) were diagnosed
with breast cancer through surgery or pathological biopsy, with the
age range of 28–67 years and the mean age of 47.46±4.54 years.
The inclusion and exclusion criteria were: i) Only
breast cancer patients admitted to The First Affiliated Hospital of
Zhengzhou University were included, and all tissue samples were
diagnosed as breast cancer after joint examination by general
surgery and pathology department (16). No radiotherapy and chemotherapy or
other treatment was given. ii) Pregnant women and patients with
allergic reaction, claustrophobia and other contraindications to
contrast media were excluded. Informed consent forms were signed in
advance by patients and their families.
The study was approved by the Ethics Committee of
the First Affiliated Hospital of Zhengzhou University.
Instruments and methods
GE Seno molybdenum target mammography machine
(purchased from Shenzhen Mercery Electronics Co., Ltd.) was used.
Automatic parameters were selected and X-ray exposure was
automatically adjusted according to the density of mammary glands.
All patients underwent imagings in double nipple caudal position
(CC position) and mediolateral oblique (MLO position), with breast
being moderately squeezed from left and right. DCE-MRI (Siemens)
scanning was performed in 3DT1-weighted sequence axial, with a
total of 6 phases (the first phase was plain scanning of the mask,
and the following 5 phases were enhanced scanning). Scan
parameters: TR/TE: 4.32/1.57 msec, double Angle (FA) 10°, FOV:
34×34 cm, matrix: 448×448, incentive number: 1 time, layer
thickness: 1 mm; there was no distance between scan, each scan
lasted 1 min and 7 sec, with a total scanning time of 7 min and 2
sec. The contrast enhancement agent gadolinium Gd-DTPA (purchased
from ACCDON Inc.) was injected with a dose of 0.1 mmol/kg and a
rate of 3.0 ml/sec through the elbow vein.
Significant image features of breast cancer with
different types of molybdenum target and DCE-MRI. The significant
image features of breast cancer with different types of molybdenum
target and DCE-MRI are shown in Figs.
1 and 2.
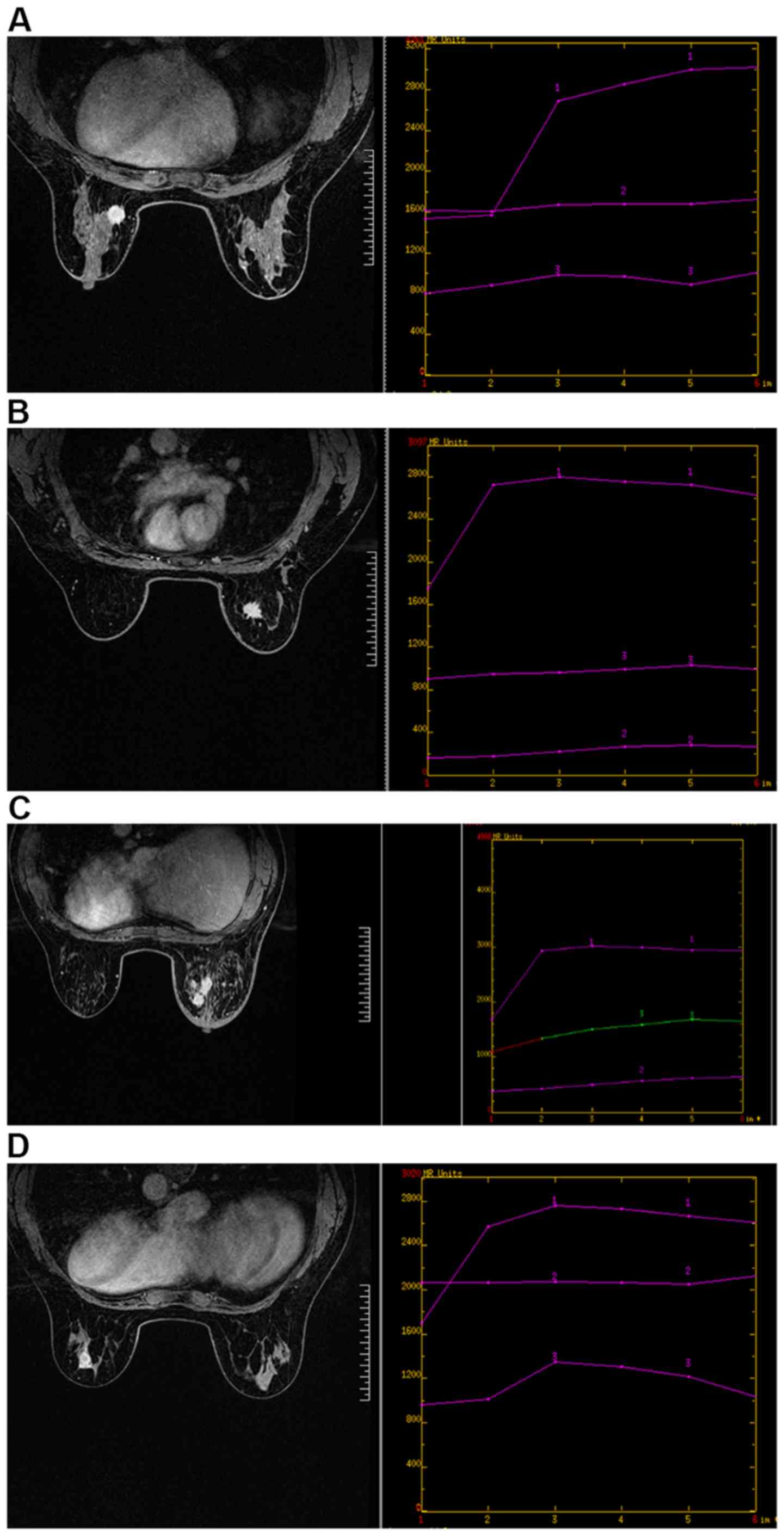 | Figure 2.DCE-MRI image features of different
types of breast cancer. (A) Luminal-A breast cancer. DCE-MRI shows
a larger mass with smooth edges, less calcification and structural
distortion. Irregular mass shadow can be seen at about 9 o'clock in
the left breast. The lesion margin is rough with visible bristles,
and the signal shadow is equal to T1 mixed with long T2. The
dispersion of DWI is limited and shows uneven enhancement, and the
time signal intensity curve shows an inflow pattern. (B) Luminal B
breast cancer. DCE-MRI shows most uneven enhancement in the lesion
area with burrs on the margin. Irregular mass shadow can be seen at
about 1 o'clock in the right breast, with multiple bristles on the
edge, showing equal T1 and long T2 signal shadow. The dispersion of
DWI is limited and shows uneven enhancement, and the time signal
intensity curve shows an outflow pattern. (C) Her2 overexpressed
breast cancer. DCE-MRI shows dynamic enhancement of the lesion
area. Multiple clumps and focal distribution lesions can be seen in
the upper and lower quadrant of the right breast, showing
equal/slightly longer T1 mixed with slightly longer T2 signals. DWI
has limited dispersion and unclear boundary. There are burrs and
uneven enhancement at the edge of the mass in the outer upper
quadrant of the right breast. The curve of time signal intensity is
plateau, and multiple lymph node shadows are seen in the right
axilla. (D) Triple-negative breast cancer. DCE-MRI shows ring
enhancement in the lesion area. Irregular mass shadow can be seen
at 5 o'clock in the left breast, showing mixed t1-long and t2-long
signal shadows. The non-uniform annular dispersion of DWI is
limited, and the non-uniform annular enhancement is enhanced. Burrs
are visible at the edges, and the time signal intensity curve
presents an outflow pattern. DCE-MRI, dynamic contrast-enhanced
magnetic resonance imaging. |
Statistical analysis
Application of SPSS 17.0 (Beijing Boyizhixun
Information Technology Co., Ltd.) software system was used for
statistical analysis and χ2 inspection for comparison of
accuracy rate of diagnosis. Enumeration data were expressed as [n
(%)]. P<0.05 was considered to indicate a statistically
significant difference.
Results
General data
General clinical data of the patients are shown in
Table I.
 | Table I.General clinical data of the
patients. |
Table I.
General clinical data of the
patients.
| Factors | [n (%)] |
|---|
| Age (years) |
|
<47.46 | 48 (40.00) |
|
≥47.46 | 72 (60.00) |
| Smoking |
| Yes | 84 (70.00) |
| No | 36 (30.00) |
| Alcohol
consumption |
| Yes | 65 (54.17) |
| No | 55 (45.83) |
| Menopausal
status |
|
Premenopause | 80 (66.67) |
|
Post-menopause | 40 (33.33) |
| Differentiated
degree |
| High | 51 (42.50) |
|
Middle | 30 (25.00) |
| Low | 39 (32.50) |
| Lymphatic
metastasis |
| Yes | 39 (32.50) |
| No | 81 (67.50) |
| Different
classification |
| Luminal-A
type | 50 (41.67) |
| Luminal-B
type | 31 (25.83) |
|
Her2-overexpressed type | 20 (16.67) |
|
Triple-negative type | 19 (15.83) |
Diagnostic efficacy of molybdenum target, DCE-MRI
and their combined detection in the diagnosis of different types of
breast cancer
Luminal-A breast cancer
The sensitivity, specificity and diagnostic
coincidence rates of Luminal-A breast cancer diagnosed by
molybdenum target were 84.00, 82.86 and 83.33%, respectively. The
sensitivity, specificity and diagnostic coincidence rates of
Luminal-A breast cancer diagnosed by DCE-MRI were 90.00, 88.57 and
89.17%, respectively. The sensitivity, specificity and diagnostic
coincidence rates of Luminal-A breast cancer diagnosed by
molybdenum target combined with DCE-MRI were 98.00, 88.57 and
92.50%, respectively. The sensitivity and diagnostic coincidence
rates of Luminal-A breast cancer diagnosed by molybdenum target
combined with DCE-MRI were significantly higher than those of
Luminal-A breast cancer diagnosed by molybdenum target or DCE-MRI
alone. There was no statistical difference in sensitivity and
diagnostic coincidence rates of Luminal-A breast cancer diagnosed
by molybdenum target or DCE-MRI alone (P>0.05) (Tables II and III).
 | Table II.Results of Luminal-A breast cancer
diagnosed by different method. |
Table II.
Results of Luminal-A breast cancer
diagnosed by different method.
|
|
| Pathological
results |
|
|---|
|
|
|
|
|
|---|
| Diagnosis
methods | Group | Luminal-A type | Non-luminal-A
type | Total |
|---|
| Molybdenum
target | Luminal-A type | 42 | 12 | 54 |
|
| Non-luminal-A
type | 8 | 58 | 66 |
|
| Total | 50 | 70 | 120 |
| DCE-MRI | Luminal-A type | 45 | 8 | 53 |
|
| Non-luminal-A
type | 5 | 62 | 67 |
|
| Total | 50 | 70 | 120 |
| Molybdenum target
combined | Luminal-A type | 49 | 8 | 57 |
| with DCE-MRI | Non-luminal-A
type | 1 | 62 | 63 |
|
| Total | 50 | 70 | 120 |
 | Table III.Diagnostic efficacy of molybdenum
target, DCE-MRI and their combined detection in the diagnosis of
Luminal-A breast cancer. |
Table III.
Diagnostic efficacy of molybdenum
target, DCE-MRI and their combined detection in the diagnosis of
Luminal-A breast cancer.
| Groups | Molybdenum target
diagnosis | DCE-MRI
diagnosis | Joint
diagnosis | χ2
value | P-value |
|---|
| Sensitivity | 84.00% (42/50) | 90.00% (45/50) | 98.00% (49/50) | 0.284 | 0.868 |
| Specificity | 82.86% (58/70) | 88.57% (62/70) | 88.57% (62/70) | 0.095 | 0.954 |
| Diagnostic
coincidence rate | 83.33%
(100/120) | 89.17%
(107/120) | 92.50%
(111/120) | 0.313 | 0.855 |
Luminal-B breast cancer
The sensitivity, specificity and diagnostic
coincidence rates of Luminal-B breast cancer diagnosed by
molybdenum target were 80.65, 82.02 and 81.67%, respectively. The
sensitivity, specificity and diagnostic coincidence rate of
Luminal-B breast cancer diagnosed by DCE-MRI were 87.10, 87.64 and
87.50%, respectively. The sensitivity, specificity and diagnostic
coincidence rates of Luminal-B breast cancer diagnosed by
molybdenum target combined with DCE-MRI were 96.77, 86.51 and
89.17%, respectively. The sensitivity and diagnostic coincidence
rate of Luminal-B breast cancer diagnosed by molybdenum target
combined with DCE-MRI were significantly higher than those of
Luminal-B breast cancer diagnosed by molybdenum target or DCE-MRI
alone. There was no statistical difference in sensitivity and
diagnostic coincidence rates of Luminal-B breast cancer diagnosed
by molybdenum target or DCE-MRI alone (P>0.05) (Tables IV and V).
 | Table IV.Results of Luminal-B breast cancer
diagnosed by different methods. |
Table IV.
Results of Luminal-B breast cancer
diagnosed by different methods.
|
|
| Pathological
results |
|
|---|
|
|
|
|
|
|---|
| Diagnosis
methods | Group | Luminal-B type | Non-luminal-B
type | Total |
|---|
| Molybdenum
target | Luminal-B type | 25 | 16 | 41 |
|
| Non-luminal-B
type | 6 | 73 | 79 |
|
| Total | 31 | 89 | 120 |
| DCE-MRI | Luminal-B type | 27 | 11 | 38 |
|
| Non-luminal-B
type | 4 | 78 | 82 |
|
| Total | 31 | 89 | 120 |
| Molybdenum target
combined | Luminal-B type | 30 | 12 | 42 |
| with DCE-MRI | Non-luminal-B
type | 1 | 77 | 78 |
|
| Total | 31 | 89 | 120 |
 | Table V.Diagnostic efficacy of molybdenum
target, DCE-MRI and their combined detection in the diagnosis of
Luminal-B breast cancer. |
Table V.
Diagnostic efficacy of molybdenum
target, DCE-MRI and their combined detection in the diagnosis of
Luminal-B breast cancer.
| Groups | Molybdenum target
diagnosis | DCE-MRI
diagnosis | Joint
diagnosis | χ2
value | P-value |
|---|
| Sensitivity | 80.65% (25/31) | 87.10% (27/31) | 96.77% (30/31) | 0.245 | 0.885 |
| Specificity | 82.02% (73/89) | 87.64% (78/89) | 86.51% (77/89) | 0.100 | 0.951 |
| Diagnostic
coincidence rate | 81.67%
(98/120) | 87.50%
(105/120) | 89.17%
(107/120) | 0.235 | 0.890 |
Her2-overexpressed breast cancer
The sensitivity, specificity and diagnostic
coincidence rates of Her2-overexpressed breast cancer diagnosed by
molybdenum target were 80.00, 87.00 and 85.83%, respectively. The
sensitivity and diagnostic coincidence rates of Her2-overexpressed
breast cancer diagnosed by DCE-MRI were 85.00, 88.00 and 87.50%,
respectively. The sensitivity, specificity and diagnostic
coincidence rates of Her2-overexpressed breast cancer diagnosed by
molybdenum target combined with DCE-MRI were 95.00, 88.00 and
89.17%, respectively. The sensitivity, specificity and diagnostic
coincidence rates of Her2-overexpressed breast cancer diagnosed by
molybdenum target combined with DCE-MRI were significantly higher
than those of Her2-overexpressed breast cancer diagnosed by
molybdenum target or DCE-MRI alone. There was no statistical
difference in sensitivity and diagnostic coincidence rates of
Her2-overexpressed breast cancer diagnosed by molybdenum target or
DCE-MRI alone (P>0.05) (Tables
VI and VII).
 | Table VI.Results of Her2-overexpressed breast
cancer diagnosed by different method. |
Table VI.
Results of Her2-overexpressed breast
cancer diagnosed by different method.
|
|
| Pathological
results |
|
|---|
|
|
|
|
|
|---|
| Diagnosis
methods | Group | Her2-overexpressed
type |
Non-Her2-overexpressed type | Total |
|---|
| Molybdenum
target | Her2-overexpressed
type | 16 | 12 | 28 |
|
|
Non-Her2-overexpressed type | 4 | 87 | 91 |
|
| Total | 20 | 100 | 120 |
| DCE-MRI | Her2-overexpressed
type | 17 | 12 | 29 |
|
|
Non-Her2-overexpressed type | 3 | 88 | 91 |
|
| Total | 20 | 100 | 120 |
| Molybdenum target
combined | Her2-overexpressed
type | 19 | 12 | 31 |
| with DCE-MRI |
Non-Her2-overexpressed type | 1 | 88 | 89 |
|
| Total | 20 | 100 | 120 |
 | Table VII.Diagnostic efficacy of molybdenum
target, DCE-MRI and their combined detection in the diagnosis of
Her2-overexpressed breast cancer. |
Table VII.
Diagnostic efficacy of molybdenum
target, DCE-MRI and their combined detection in the diagnosis of
Her2-overexpressed breast cancer.
| Groups | Molybdenum target
diagnosis | DCE-MRI
diagnosis | Joint
diagnosis | χ2
value | P-value |
|---|
| Sensitivity | 80.00% (16/20) | 85.00% (17/20) | 95.00% (19/20) | 0.143 | 0.931 |
| Specificity | 87.00%
(87/100) | 88.00%
(88/100) | 88.00%
(88/100) | 0.004 | 0.998 |
| Diagnostic
coincidence rate | 85.83%
(103/120) | 87.50%
(105/120) | 89.17%
(107/120) | 0.041 | 0.980 |
Triple-negative breast cancer
The sensitivity, specificity and diagnostic
coincidence rates of triple-negative breast cancer diagnosed by
molybdenum target were 78.94, 80.20 and 80.00%, respectively. The
sensitivity, specificity and diagnostic coincidence rates of
triple-negative breast cancer diagnosed by DCE-MRI were 84.21,
89.11 and 88.33%, respectively. The sensitivity, specificity and
diagnostic coincidence rates of triple-negative breast cancer
diagnosed by molybdenum target combined with DCE-MRI were 94.74,
88.12 and 89.17%, respectively. The sensitivity and diagnostic
coincidence rates of triple-negative breast cancer diagnosed by
molybdenum target combined with DCE-MRI were significantly higher
than those of triple-negative breast cancer diagnosed by molybdenum
target or DCE-MRI alone. There was no statistical difference in
sensitivity and diagnostic coincidence rates between the two groups
in the diagnosis of triple-negative breast cancer with molybdenum
target or DCE-MRI alone (P>0.05) (Tables VIII and IX).
 | Table VIII.Results of triple-negative breast
cancer diagnosed by different method. |
Table VIII.
Results of triple-negative breast
cancer diagnosed by different method.
|
|
| Pathological
results |
|
|---|
|
|
|
|
|
|---|
| Diagnosis
methods | Group | Triple-negative
breast cancer | Non-triple-negative
breast cancer | Total |
|---|
| Molybdenum
target | Triple-negative
breast cancer | 15 | 20 | 35 |
|
| Non-triple-negative
breast cancer | 4 | 81 | 85 |
|
| Total | 19 | 101 | 120 |
| DCE-MRI | Triple-negative
breast cancer | 16 | 11 | 27 |
|
| Non-triple-negative
breast cancer | 3 | 90 | 93 |
|
| Total | 19 | 101 | 120 |
| Molybdenum target
combined with DCE-MRI | Triple-negative
breast cancer | 18 | 12 | 30 |
|
| Non triple-negative
breast cancer | 1 | 89 | 90 |
|
| Total | 19 | 101 | 120 |
 | Table IX.Diagnostic efficacy of molybdenum
target, DCE-MRI and their combined detection in the diagnosis of
triple-negative breast cancer. |
Table IX.
Diagnostic efficacy of molybdenum
target, DCE-MRI and their combined detection in the diagnosis of
triple-negative breast cancer.
| Groups | Molybdenum target
diagnosis | DCE-MRI
diagnosis | Joint
diagnosis | χ2
value | P-value |
|---|
| Sensitivity | 78.94% (15/19) | 84.21% (16/19) | 94.74% (18/19) | 0.152 | 0.927 |
| Specificity | 80.20%
(81/101) | 89.11%
(90/101) | 88.12%
(89/101) | 0.307 | 0.858 |
| Diagnostic
coincidence rate | 80.00%
(96/120) | 88.33%
(106/120) | 89.17%
(107/120) | 0.393 | 0.822 |
Discussion
The morbidity and mortality of breast cancer are
increasing year by year (17). Since
the mechanism of breast cancer cannot be clearly explained at
present, the key to reduce morbidity and mortality is to accurately
diagnose the conditions of breast cancer patients and provide
corresponding treatment schemes (18). Medically, specific genotypes are made
according to the gene level of breast cancer patients, and
treatment plans are made according to different molecular genotypes
of breast cancer. Treatment plans and prognosis of breast cancer
patients with different molecular genotypes are greatly different
(19,20). In this study, molybdenum target,
DCE-MRI and molybdenum target combined with DCE-MRI were performed
on the patients, respectively, and the examination results were
compared with the pathological examination results of the patients,
in order to investigate the clinical efficacy of molybdenum target,
DCE-MRI and molybdenum target combined with DCE-MRI with different
types of breast cancer. First, we analyzed the diagnostic efficacy
of molybdenum target, DCE-MRI and their combined detection in the
diagnosis of Luminal-A and Luminal-B breast cancer, and found that
the sensitivity and diagnostic coincidence rates of Luminal-A and
Luminal-B breast cancer were significantly higher than those of
molybdenum target or DCE-MRI alone. There were no statistical
differences in sensitivity and diagnostic coincidence rates between
Luminal-A and Luminal-B breast cancer diagnosed by molybdenum
target or DCE-MRI alone. Luminal-A type is common in early breast
cancer with low recurrence rate (21), while Luminal-B type with high
histological grade is common in older breast cancer patients
(22).
It has been reported that there is a certain
correlation between histopathology, molecular biology and related
imaging features of tumors, indicating that breast cancer with
different molecular types has different imaging manifestations
(23). However, in the study of
Goffin et al (24) on breast
cancer diagnosed by molybdenum target combined with DCE-MRI, it was
found that the diagnostic coincidence rates of breast cancer
diagnosed by molybdenum target combined with DCE-MRI were
significantly higher than those of breast cancer diagnosed by
molybdenum target or DCE-MRI alone. This is similar to the research
results of this study, which to some extent supports our results.
Then, we analyzed the diagnostic efficacy of molybdenum target,
DCE-MRI, and molybdenum target combined with DCE-MRI in
Her2-overexpressed type and triple-negative breast cancer, and
found that the sensitivity and diagnostic coincidence rates of
Her2-overexpressed type and triple-negative breast cancer diagnosed
by molybdenum target combined with DCE-MRI were apparently higher
than those of diagnosis with molybdenum target or DCE-MRI alone.
There was no statistical difference in the sensitivity and
diagnostic coincidence rate between the two groups of
Her2-overexpressed breast cancer and triple-negative breast cancer
diagnosed by molybdenum target or DCE-MRI alone. It is reported
that although molybdenum target is widely used in the screening of
breast lesions, it shows poor sensitivity to small lesions located
at the edge of the breast, and the sensitivity of molybdenum target
to very dense breasts decreases by >40% (25). DCE-MRI, applied to the examination of
breast cancer, can better show the hemodynamic characteristics of
small breast cancer lesions, but it also has a certain rate of
missed diagnosis for small sand-like calcification lesions
(26). Clinical studies on tumor
lesions detected by DCE-MRI combined with molybdenum target showed
that DCE-MRI combined with molybdenum target could significantly
improve the clinical diagnosis rate (27). Kriege et al (28) also carried out DCE-MRI, molybdenum
target and DCE-MRI combined with molybdenum target detection for
breast cancer with different molecular types, and also found that
the diagnostic coincidence rates of DCE-MRI combined with
molybdenum target for breast cancer were significantly higher than
those of the single imaging detection.
In this study, due to the regional limitations of
the inclusion of research objects, the experimental results may be
biased to some extent. Therefore, we will continue to expand the
number of subjects in different regions for this research, and
conduct follow-up.
Collectively, the diagnostic efficacy of molybdenum
target combined with DCE-MRI in breast cancer with different
molecular types is better than that of imaging screening alone,
which is of great clinical significance in the development of
individualized comprehensive treatment for breast cancer patients
and is worthy of wide promotion in clinical practice.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH wrote the manuscript. YH and YZ collected and
analyzed the general data of patients. JC was responsible for the
analysis and discussion of the data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China). Patients who participated in this research had complete
clinical data. Informed consent forms were signed in advance by the
patients and their families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lantz PM and Booth KM: The social
construction of the breast cancer epidemic. Soc Sci Med.
46:907–918. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vallance JK, Courneya KS, Plotnikoff RC
and Mackey JR: Analyzing theoretical mechanisms of physical
activity behavior change in breast cancer survivors: Results from
the activity promotion (ACTION) trial. Ann Behav Med. 35:150–158.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeSantis CE, Bray F, Ferlay J,
Lortet-Tieulent J, Anderson BO and Jemal A: International variation
in female breast cancer incidence and mortality rates. Cancer
Epidemiol Biomarkers Prev. 24:1495–1506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kitamura T, Qian BZ, Soong D, Cassetta L,
Noy R, Sugano G, Kato Y, Li J and Pollard JW: CCL2-induced
chemokine cascade promotes breast cancer metastasis by enhancing
retention of metastasis-associated macrophages. J Exp Med.
212:1043–1059. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chee W, Lee Y, Im EO, Chee E, Tsai HM,
Nishigaki M, Yeo SA, Schapira MM and Mao JJ: A culturally tailored
Internet cancer support group for Asian American breast cancer
survivors: A randomized controlled pilot intervention study. J
Telemed Telecare. 23:618–626. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stuart-Harris R, Dahlstrom JE, Gupta R,
Zhang Y, Craft P and Shadbolt B: Recurrence in early breast cancer:
Analysis of data from 3,765 Australian women treated between 1997
and 2015. Breast. 44:153–159. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rouzier R, Perou CM, Symmans WF, Ibrahim
N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P,
et al: Breast cancer molecular subtypes respond differently to
preoperative chemotherapy. Clin Cancer Res. 11:5678–5685. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Edenfield J, Schammel C, Collins J,
Schammel D and Edenfield WJ: Metaplastic breast cancer: Molecular
typing and identification of potential targeted therapies at a
single institution. Clin Breast Cancer. 17:e1–e10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chou R, Cuevas C, Fu R, Devine B, Wasson
N, Ginsburg A, Zakher B, Pappas M, Graham E and Sullivan SD:
Imaging techniques for the diagnosis of hepatocellular carcinoma: A
systematic review and meta-analysis. Ann Intern Med. 162:697–711.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohuchi N, Suzuki A, Sobue T, Kawai M,
Yamamoto S, Zheng YF, Shiono YN, Saito H, Kuriyama S, Tohno E, et
al J-START investigator groups, : Sensitivity and specificity of
mammography and adjunctive ultrasonography to screen for breast
cancer in the Japan Strategic Anti-cancer Randomized Trial
(J-START): A randomised controlled trial. Lancet. 387:341–348.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brem RF, Lenihan MJ, Lieberman J and
Torrente J: Screening breast ultrasound: Past, present, and future.
AJR Am J Roentgenol. 204:234–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang Q, Luo Y and Zhang Q: Breast
ultrasound image segmentation: A survey. Int J CARS. 12:493–507.
2017. View Article : Google Scholar
|
|
13
|
Xia W, Yan Z and Gao X: Volume fractions
of DCE-MRI parameter as early predictor of histologic response in
soft tissue sarcoma: A feasibility study. Eur J Radiol. 95:228–235.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Perera M, Papa N, Christidis D, Wetherell
D, Hofman MS, Murphy DG, Bolton D and Lawrentschuk N: Sensitivity,
specificity, and predictors of positive 68Ga-prostate-specific
membrane antigen positron emission tomography in advanced prostate
cancer: a systematic review and meta-analysis. Eur Urol.
70:926–937. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao H, Zou L, Geng X and Zheng S:
Limitations of mammography in the diagnosis of breast diseases
compared with ultrasonography: A single-center retrospective
analysis of 274 cases. Eur J Med Res. 20:492015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mavaddat N, Michailidou K, Dennis J, Lush
M, Fachal L, Lee A, Tyrer JP, Chen TH, Wang Q, Bolla MK, et al
ABCTB Investigators; kConFab/AOCS Investigators; NBCS
Collaborators, : Polygenic risk scores for prediction of breast
cancer and breast cancer subtypes. Am J Hum Genet. 104:21–34. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Christiansen P, Bjerre K, Ejlertsen B,
Jensen MB, Rasmussen BB, Lænkholm AV, Kroman N, Ewertz M, Offersen
B, Toftdahl DB, et al Danish Breast Cancer Cooperative Group, :
Mortality rates among early-stage hormone receptor-positive breast
cancer patients: A population-based cohort study in Denmark. J Natl
Cancer Inst. 103:1363–1372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jafari SH, Saadatpour Z, Salmaninejad A,
Momeni F, Mokhtari M, Nahand JS, Rahmati M, Mirzaei H and Kianmehr
M: Breast cancer diagnosis: Imaging techniques and biochemical
markers. J Cell Physiol. 233:5200–5213. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Montes de Oca R, Gurard-Levin ZA, Berger
F, Rehman H, Martel E, Corpet A, de Koning L, Vassias I, Wilson LO,
Meseure D, et al: The histone chaperone HJURP is a new independent
prognostic marker for luminal A breast carcinoma. Mol Oncol.
9:657–674. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen L, Li CI, Tang MT, Porter P, Hill DA,
Wiggins CL and Cook LS: Reproductive factors and risk of luminal,
HER2-overexpressing, and triple-negative breast cancer among
multiethnic women. Cancer Epidemiol Biomarkers Prev. 25:1297–1304.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gallardo A, Garcia-Valdecasas B, Murata P,
Teran R, Lopez L, Barnadas A and Lerma E: Inverse relationship
between Ki67 and survival in early luminal breast cancer:
Confirmation in a multivariate analysis. Breast Cancer Res Treat.
167:31–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Adriaenssens E, Vanhecke E, Saule P,
Mougel A, Page A, Romon R, Nurcombe V, Le Bourhis X and Hondermarck
H: Nerve growth factor is a potential therapeutic target in breast
cancer. Cancer Res. 68:346–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Zhu Y, Burnside ES, Huang E, Drukker
K, Hoadley KA, Fan C, Conzen SD, Zuley M, Net JM, et al:
Quantitative MRI radiomics in the prediction of molecular
classifications of breast cancer subtypes in the TCGA/TCIA data
set. NPJ Breast Cancer. 2:22016. View Article : Google Scholar
|
|
24
|
Goffin J, Chappuis PO, Wong N and Foulkes
WD: Re: Magnetic resonance imaging and mammography in women with a
hereditary risk of breast cancer. J Natl Cancer Inst. 93:1754–1755.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shao H, Li B, Zhang X, Xiong Z, Liu Y and
Tang G: Comparison of the diagnostic efficiency for breast cancer
in Chinese women using mammography, ultrasound, MRI, and different
combinations of these imaging modalities. J Xray Sci Technol.
21:283–292. 2013.PubMed/NCBI
|
|
26
|
Shimauchi A, Jansen SA, Abe H, Jaskowiak
N, Schmidt RA and Newstead GM: Breast cancers not detected at MRI:
Review of false-negative lesions. AJR Am J Roentgenol.
194:1674–1679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bäuerle T, Bartling S, Berger M,
Schmitt-Gräff A, Hilbig H, Kauczor HU, Delorme S and Kiessling F:
Imaging anti-angiogenic treatment response with DCE-VCT, DCE-MRI
and DWI in an animal model of breast cancer bone metastasis. Eur J
Radiol. 73:280–287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kriege M, Brekelmans CT, Boetes C, Besnard
PE, Zonderland HM, Obdeijn IM, Manoliu RA, Kok T, Peterse H,
Tilanus-Linthorst MM, et al Magnetic Resonance Imaging Screening
Study Group, : Efficacy of MRI and mammography for breast-cancer
screening in women with a familial or genetic predisposition. N
Engl J Med. 351:427–437. 2004. View Article : Google Scholar : PubMed/NCBI
|