Introduction
Pain associated with cancer is a serious problem for
patients, significantly affecting their quality of life and is
difficult to treat. A considerable proportion of patients suffer
from refractory cancer pain. Refractory pain is associated with the
growth and metastasis of tumors (1,2),
therefore, pain relief may be beneficial for improving the
patient's quality of life while also supplementing cancer therapy.
The World Health Organization has proposed that a three-step
analgesic ladder is effective for treating cancer pain (3). For neuropathic pain in advanced cancer,
the first-line therapeutic agent is typically an opioid, such as
morphine (4–7), however, pain may not be fully relieved
for many patients. In these cases, the combination of
pharmacological agents may be necessary to relieve the refractory
pain. The combination of morphine and ketamine has been considered
safe and efficacious in numerous patients (8–13).
Clinically, patients with refractory cancer pain report improved
pain relief with fewer side effects when morphine and a low dose of
ketamine were used compared with morphine alone.
A number of patients experience cancer pain driven
by nociception and neuropathy (14)
and neuropathic cancer pain driven by nociception is considered to
be resistant to opioids (15–17). For
neuropathic pain, peripheral and central sensitization are the
primary causes. Ketamine is an N-methyl-D aspartate (NMDA)
antagonist and is also a non-opioid anesthetic agent. The primary
clinical application of ketamine is for short diagnostic or
surgical procedures (17). NMDA
receptors are activated by the excitatory neurotransmitter
glutamate in the spinal dorsal horn, which results in sensitization
of the central nervous system (18–21).
Therefore, attenuating this sensitization may underlie the
analgesic effects of ketamine (19–21).
Similarly, NMDA antagonism may be involved in the ketamine-mediated
attenuation of acquired opioid tolerance (20–22).
However, in laboratory animals and humans, morphine
has been reported to produce numerous immunomodulatory effects
in vivo and in vitro. As a potent immunomodulator,
morphine has inhibitory and stimulatory effects on immune function,
including altering the expression of CD4+ and
CD8+ T cells, reducing the activity of natural killer
cells (23–25), altering the balance between Th1 and
Th2 cells (26,27), increasing the production of
interleukin (IL)-12 by peritoneal macrophages (28), increasing the production of IL-6 and
tumor necrosis factor when induced by lipopolysaccharides, and
increasing the activity of nuclear factor-κB (NF-κB) in macrophages
and peripheral blood mononuclear cells (PBMCs) (29,30). In
particular, in activated T cells, morphine has been shown to
enhance the expression of IL-4 and suppress the expression of
interferon (IFN)-γ and IL-2 (31–35). For
patients with cancer and an impaired immune system, the
immunomodulatory effects of morphine may cause unwanted side
effects during pain treatment. However, there are few reports
regarding the effects of morphine on T cells in patients who suffer
from refractory cancer pain. Similarly, there are few reports
regarding the effect of ketamine on immune cells, particularly in T
cells. Therefore, the aim of the present study was to assess the
direct effect of morphine and a low dose of ketamine on the T cells
of patients with refractory cancer pain in vitro.
Materials and methods
T cell culture, T cell activation and
reagents
The entire protocol (SDTHEC201504001) was approved
by the Ethics Committee of Shandong Cancer Hospital and Institute
(Jinan, China; Chairperson Dr Jinming Yu) and conducted according
to the principles of the Helsinki Declaration. Informed, written
consent was obtained from all patients with cancer pain, and the
patients were involved in the study for >3 months.
Blood was withdrawn following venipuncture.
Peripheral blood mononuclear cells (PBMCs) were isolated using the
Ficoll-Hypaque density gradient method according to the
manufacturer's protocol (Cedarlane). The T cells were isolated by
positive selection using anti-CD3 beads (Miltenyi Biotec, Inc.)
according to the manufacturer's protocol, and confirmed by
fluorescence-associated cell sorting (85% purity). A final
concentration of 1×105 cells/ml were plated into 96-well
plates, and the T cells were cultivated at 37°C and 5%
CO2 in RPMI 1640 medium supplemented with 10% fetal calf
serum, 100 U/ml penicillin and 100 mg/ml streptomycin (all from
Gibco; Thermo Fisher Scientific, Inc.).
The T cells were treated with either vehicle (normal
saline), 200 ng/ml of morphine (Humanwell Healthcare (Group) Co.,
Ltd.), or 200 ng/ml of morphine + 100 ng/ml ketamine (pure
hydrochloride salt without preservatives, Sigma-Aldrich; Merck
KGaA) for 24 h at 37°C. By determining the plasma concentration of
morphine and ketamine in patients with refractory cancer pain in
the clinic, and referring to other human and animal experiments
using morphine and ketamine, a morphine concentration of 200 ng/ml
and ketamine concentration of 100 ng/ml were established (36–38).
These concentrations are approximately equal to the doses of
morphine and ketamine commonly used in patients with refractory
cancer pain in the clinic. Subsequently, the cells were stimulated
with anti-CD3 and anti-CD28 (R&D Systems, Inc.;
mouse-anti-human, 5 mg/ml) for 24 h at 37°C. The groups were
designated as the control group, morphine group and morphine +
ketamine group. To activate T cells, it is necessary for the
antigen-specific T cell receptor and a second co-receptor, such as
CD28, to be sufficiently occupied (39). In the present study, anti-CD3 and
anti-CD28 stimulation were used to investigate the effects of
morphine and ketamine on the T cells.
Flow cytometric analysis of
CD3+ T cells
Following experimental treatment, the T cells
(1×105/ml) were harvested by centrifugation for 5 min at
300 × g room temperature, washed in phosphate-buffered saline and
subsequently incubated with trichromatism monoclonal antibody
reagent [CD4 fluorescein isothiocyanate/CD8 phycoerythrin/CD3 PerCP
(BD Biosciences), 0.5 mg/ml] according to the manufacturer's
protocol. Subsequently, the quantity and percentage of CD3/CD4/CD8T
cells were measured using a flow cytometer (FACSCalibur; BD
Biosciences).
Supernatant cytokine protein
analysis
Cytokine-specific ELISA kits (R&D Systems, Inc.)
were used to measure the protein concentrations of IL-2 and IFN-γ
according to the manufacturer's protocol. The experiments were
repeated three times.
Reverse transcription-quantitative PCR
analysis
Total RNA was extracted from the T cells by lysis
using guanidinium isothiocyanate and phenol acid extraction. A
total of 1 µg RNA, 0.5 µl RNase H minus (Promega Corporation) and 1
µl Moloney murine leukemia virus reverse transcriptase were used
for cDNA synthesis with the following thermocycling conditions:
95°C 5 min; 95°C 15 sec, 60°C 35 sec, 40 cycles; 72°C 5 min; 4°C
terminal. For each PCR, 2 µl cDNA was used and the primers were
obtained from BD Biosciences. The primer sequences were as follows:
IL-2 forward, 5′-GAATGGAATTAATAATTACAAGAATCCC-3′ and reverse,
5′-TGTTTCAGATCCCTTTAGTTCCAG-3′; and IFN-γ forward,
5′-TCGGTAACTGACTTGAATGTCCA-3′ and reverse,
5′-TCCTTTTTCGCTTCCCTGTTTT-3′. The Light Cycler-Fast Start DNA
Master SYBR Green I kit (Roche Diagnostics) was used for the
RT-qPCR analysis of IL-2, and the Revert Aid™ First Strand cDNA
Synthesis kit (Roche Diagnostics) was used for IFN-γ, according to
the manufacturer protocols. The quantitative analysis of original
template was performed by the change of fluorescence of the
amplification product.
Western blot analysis for activated
p65 NF-κB
The T cells were lysed with RIPA protein lysis
buffer (100 µg/ml Nonidet P-40, 150 mol/l NaCl and 50 mmol/l
Tris-HCl supplemented with a protease inhibitor mixture). Following
one freeze/thaw cycle, the lysates were centrifuged for 15 min at
12,000 g (2–8°C). A BCA protein concentration kit was used to
determine protein concentration. Following electrophoresis using
10% SDS-PAGE, the resolved protein samples were transferred onto an
ECL nitrocellulose membrane (EMD Millipore) by electroblotting. 5%
(w/v) of skimmed milk powder was used for blocking for 1 h at room
temperature. Subsequently, the nitrocellulose membranes were
incubated overnight at 4°C with primary antibody [rabbit anti-NF-κB
p65 antibody (cat. no., ab16502); dilution, 1:2,000; Abcam/rabbit
anti-β-actin (cat. no., ab8227); dilution, 1:2,000; Abcam], and
then incubated with the secondary goat anti-Rabbit horseradish
peroxidase antibody (cat. no., ab205718; 1:2,000; Abcam) at room
temperature for 2 h. Protein concentration was determined by ECL
western blotting detection kit (cat. no., ab65623; Abcam). Image J
version 1.8.0 (National Institutes of Health) was used for
quantification of blots.
Statistical analysis
All variables were assessed in triplicate and all
experiments were repeated at least three times. All data are
expressed as the mean ± standard deviation. Statistical comparisons
between experimental groups were performed, where appropriate, with
SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). Statistical
significance was analyzed using a paired t-test or one-way ANOVA
followed by a non-parametric Student-Newman-Keuls test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
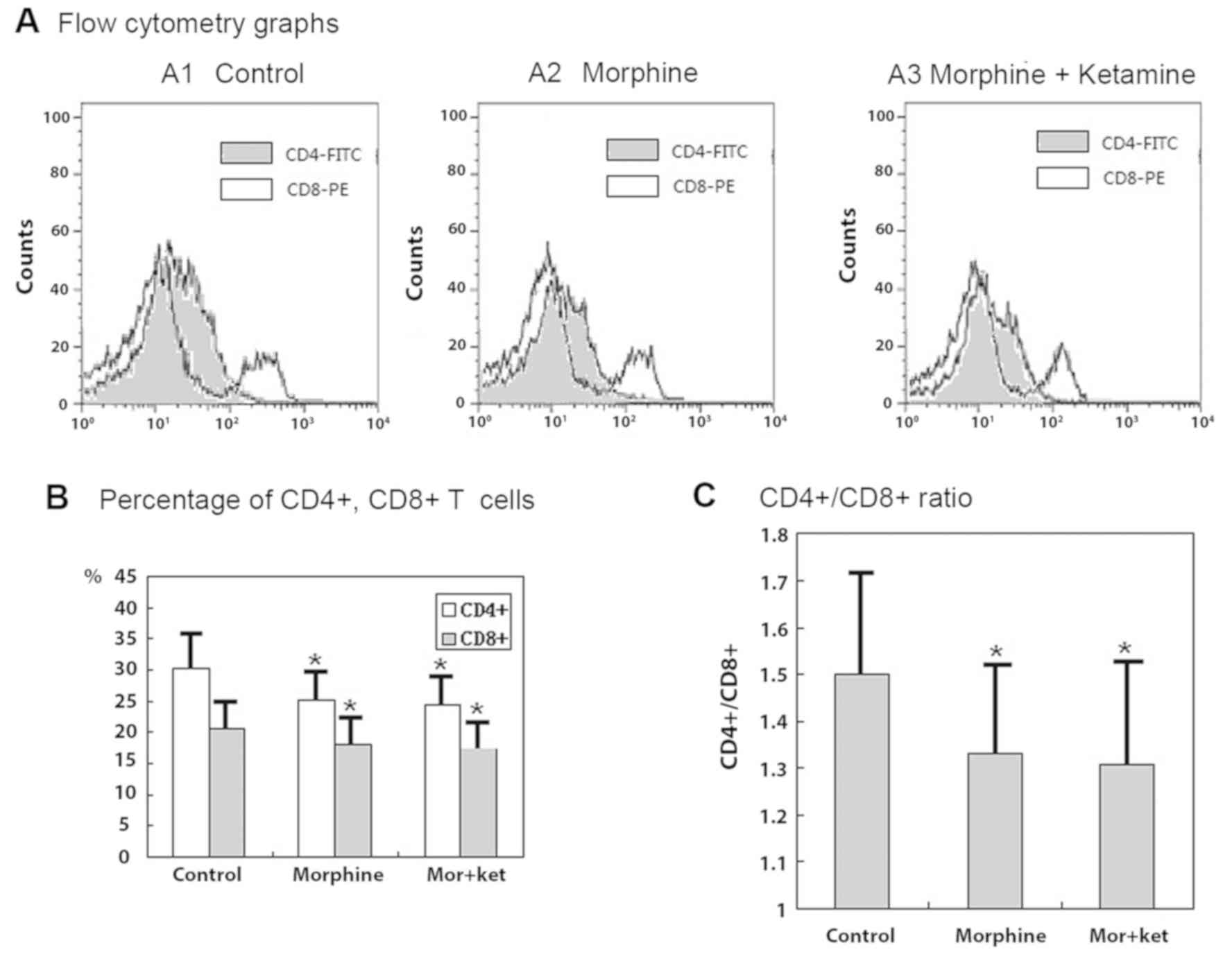
Flow cytometric analysis of
CD3+ T cells
The counts of T cells expressing CD4 and CD8 were
significantly decreased when the cells were treated with morphine
and when treated with morphine + ketamine. The decrease in the
count of CD4+ T cells was larger than that of
CD8+ T cells, therefore, the ratio of
CD4+/CD8+ was also significantly decreased by
morphine and morphine + ketamine treatments (Fig. 1A-C; all P<0.05). However, the
decreases in the CD4+ and CD8+ T cell counts
and the CD4+/CD8+ ratio did not differ
significantly when the cells were treated with morphine compared
with those when the cells were treated with morphine +
ketamine.
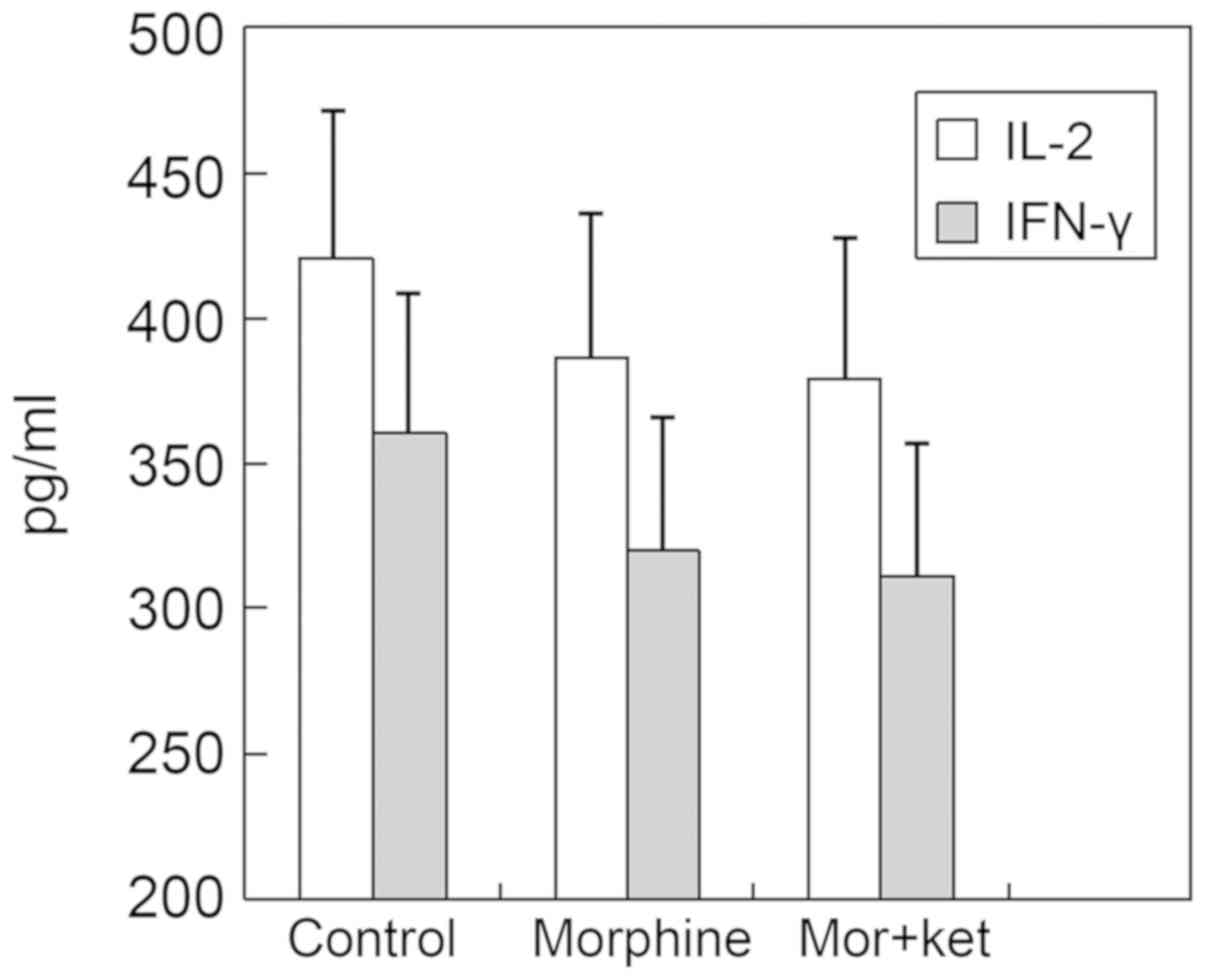
Supernatant cytokine protein analysis
and RT-qPCR analysis
There were no significant differences in the
concentrations of IL-2 and IFN-γ in the supernatants of the
morphine group and the morphine + ketamine group compared with
those in the control group (Fig. 2).
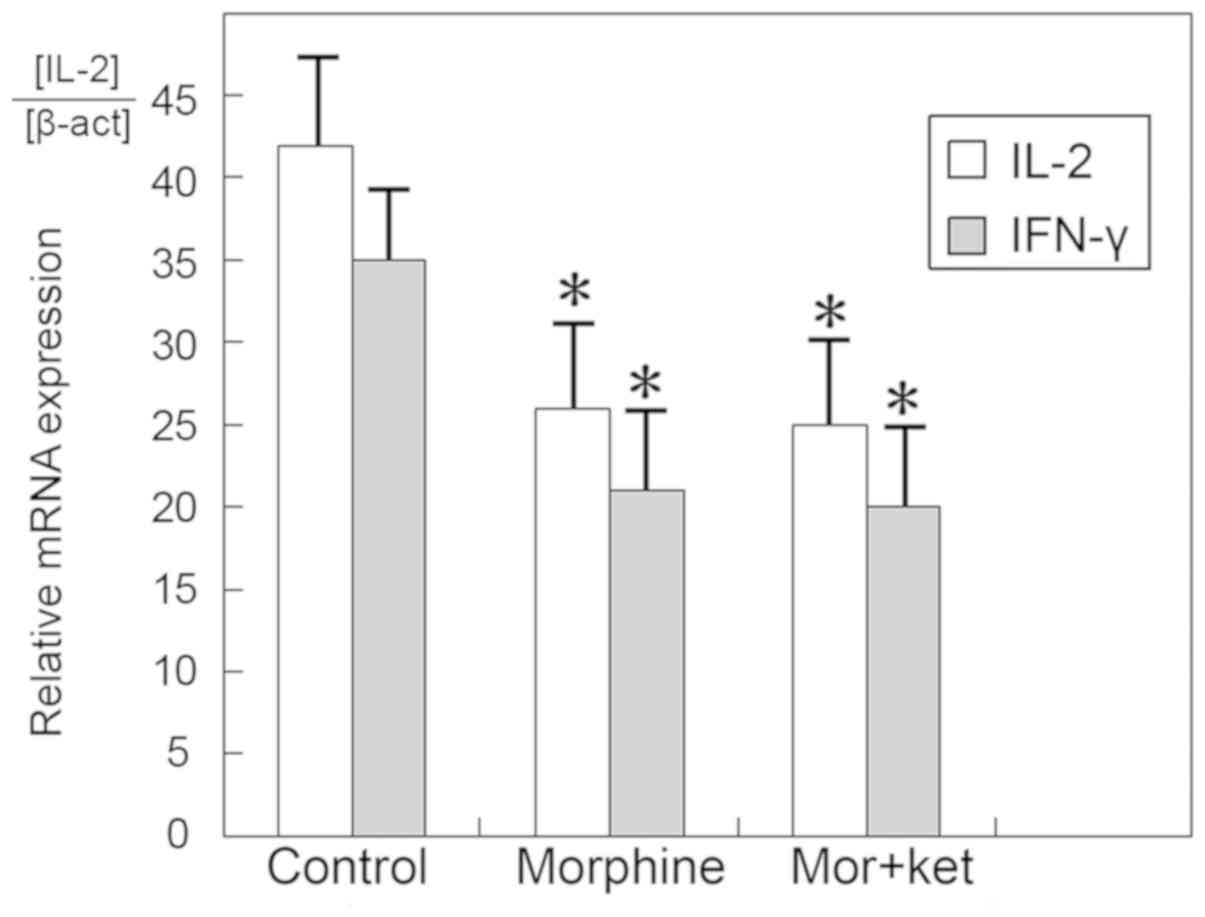
However, the mRNA expression levels of IL-2 and IFN-γ were
significantly decreased in the morphine group and the morphine +
ketamine group compared with those in the control group (Fig. 3; P<0.05). The results of the
morphine group did not differ significantly from those of the
morphine + ketamine group.
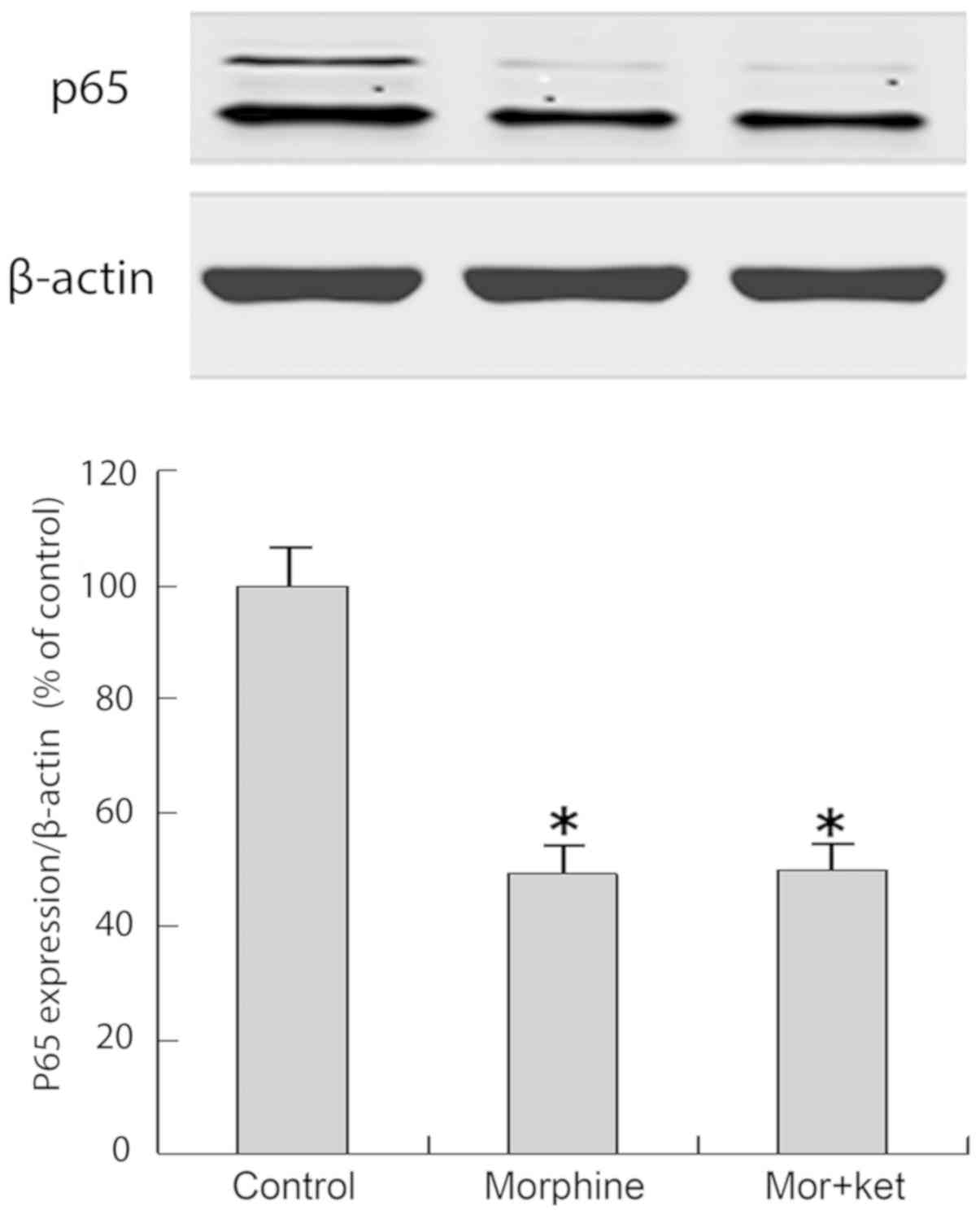
Western blot analysis of activated p65
NF-κB
The morphine and morphine + ketamine treatments
decreased the quantity of activated p65 NF-κB entering the nuclei
of T cells compared with that in the control group (Fig. 4; P<0.05). The results in the
morphine group did not differ significantly from those in the
morphine + ketamine group.
Discussion
Patients with refractory cancer pain suffer from
pain and consequent stress on a daily basis. Those individuals
experiencing severe pain, stress or who become addicted to opioids
are generally immunosuppressed resulting from activation of the
neuroendocrine-immune network and the potent effect of neuroactive
ligands on cells of the immune system (40–45).
Opioids have been demonstrated to modulate immune responses, and
opioid receptors are expressed on immune cells (41). In vivo and in vitro,
the therapeutic and chronic use of morphine affects the
physiological function of the immune system, including innate and
adaptive immunity (46,47).
T cells and the various subpopulations serve
important roles in cell-mediated immunity. The ratios of the
subpopulations of T cells are stably maintained to ensure an
effective immune response. In the present study, CD4+
and CD8+ T cells, and the ratio of
CD4+/CD8+ T cells were all decreased by
morphine, which suggested an attenuation of the immune
response.
IL-2 is a pivotal cytokine for T cell function, and
a previous study demonstrated that chronic treatment with morphine
can inhibit the mRNA expression and secretion of IL-2 (48). Similarly, in the present study on
patients with refractory cancer pain, the mRNA expression levels of
IL-2 and IL-2 were downregulated by morphine treatment. A previous
study showed that the expression of IL-2 was regulated almost
entirely at the transcriptional level and that the essential
elements lie within 300 bp upstream of the start codon (49), which can be bound by several
inducible transcription factors including NF-κB. It has been
hypothesized that the production of IL-2 is inhibited with chronic
morphine treatment at the transcriptional level and through
epigenetic mechanisms (31,34,35,50,51).
As a cytokine modulating all phases of immune
processes, IFN-γ is produced in large quantities in activated T
cells (52) and serves an important
role in host resistance to infection (53). Previous studies have demonstrated
that chronic morphine treatment inhibited IFN-γ protein synthesis
(32,54), similar to the results of the present
study. Furthermore, the results of the present study demonstrated
that the mRNA expression levels of IFN-γ were downregulated by
morphine, suggesting that morphine modulated IFN-γ by altering the
gene expression and protein synthesis of IFN-γ. It is hypothesized
that transcription of the IFN-γ gene is coordinated by the
transcription factor NF-κB (55–57).
As a central regulator of the immune response
(58), NF-κB regulates the
transcription of various genes, including a number of cytokines and
genes encoding immunoreceptors (59,60), and
it is sequestered in the cytoplasm in the majority of cell types
(61). NF-κB is also vital for
regulating the activation, proliferation and differentiation of T
cells (62). Roy et al
(30) demonstrated that morphine
prevented the binding of NF-κB to its consensus DNA sequence in
macrophages and activated T cells of patients who had received
chronic morphine treatment.
In vivo, the pathways regulating the
immunomodulatory effects of opioids transduced to the immune
effector cells primarily include the following: i) Indirectly via
the central nervous system, for example, by activation of the
hypothalamic-pituitary-adrenal axis (63,64); ii)
directly via atypical opioid receptors (25); and iii) directly via the classic µ-
(preferential), δ- and κ-opioid receptor subtypes present on immune
cells (65,66) that belong to the Gi
protein-coupled receptor superfamily (67). Additionally, it has been shown that
the activation of NF-κB was inhibited by elevated cAMP in T cells
at the molecular level (68–70). Therefore, morphine may bind to
µ-opioid receptors and activate Gi protein-coupled
receptors, resulting in the intracellular accumulation of cAMP, and
thus leading to decreased NF-κB activation and the inhibition of
IL-2 and IFN-γ transcription.
Ketamine is often administered epidurally or
intravenously in combination with an opioid for the treatment of
chronic cancer pain (71,72). For the clinical treatment of
refractory cancer pain, ketamine is often used as an adjuvant
analgesic; as ketamine alone is not used for refractory cancer
pain, a group treated with ketamine only was not included in the
present study. However, ketamine also affects immune cells. It has
been suggested that ketamine inhibits the production and function
of dendritic cells (73). Treatment
with ketamine alone has also been shown to increase the ratios of
Th1/Th2 and IFN-γ/IL-4 (74).
However, in the present study, ketamine did not induce significant
effects in any of the measurements, including CD4+ T
cells, CD8+ T cells, the CD4+/CD8+
ratio, concentrations of IL-2 and IFN-γ in the supernatant, mRNA
expression levels of IL-2 and IFN-γ and the activation of NF-κB. A
possible explanation for the lack of effect of ketamine was the
dose used, which may have been too low. In clinical use, the dose
of ketamine used is variable. The epidural administration of
ketamine is typically ~0.4 mg/ml (~1.5 mM) (75–80),
whereas intrathecal administration is typically <25 mg/ml (~93
mM) (81–83). Another study demonstrated that the
infusion dose of ketamine for cancer-associated treatment was
0.084–0.6 mg/kg/h (20). In the
present study, a dose of 100 ng/ml of ketamine was considered to be
ineffective.
In conclusion, in vitro treatment with
morphine alone and in conjunction with ketamine inhibited the
immune functions of patients with refractory cancer pain. Treatment
decreased the counts of CD4+ and CD8+ T
cells, CD4+/CD8+ ratio, concentrations of
IL-2 and IFN-γ in the supernatant, mRNA expression levels of IL-2
and IFN-γ and the activation of NF-κB, but the effect of morphine
in conjunction with ketamine did not differ significantly from the
effect of morphine alone. The present study may provide evidence
for clinicians to offer more relief of pain and less suppression of
immune function to patients with refractory cancer pain.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Natural Science Foundation (Shandong, China; grant no.
ZR2011HM039).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
NBZ, KGW, ZJF designed the study and completed the
organization and writing. NBZ conducted the experiments and
analyzed the data.
Ethics approval and consent to
participate
The entire protocol (SDTHEC201504001) was approved
by the Ethics Committee of Shandong Cancer Hospital and Institute
(Jinan, China; chairperson Dr Jinming Yu) and was conducted
according to the principles of the Helsinki Declaration. Informed,
written consent was obtained from patients with cancer pain.
Patient consent for publication
The patients consented to publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Page GG, Ben-Eliyahu S, Yirmiya R and
Liebeskind JC: Morphine attenuates surgery-induced enhancement of
metastatic colonization in rats. Pain. 54:21–28. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sasamura T, Nakamura S, Iida Y, Fuji H,
Murata J, Saiki I, Nojima H and Kuraishi Y: Morphine analgesia
suppresses tumor growth and metastasis in a mouse model of cancer
pain produced by orthotopic tumor inoculation. Eur J Pharmacol.
441:185–191. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zech DF, Grond S, Lynch J, Hertel D and
Lehmann KA: Validation of World Health Organization guidelines for
cancer pain relief: A 10-year prospective study. Pain. 63:65–76.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Collins JJ, Grier HE, Kinney HC and Berde
CB: Control of severe pain in children with terminal malignancy. J
Pediatr. 126:653–657. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hurwitz CA, Duncan J and Wolfe J: Caring
for the child with cancer at the close of life: ‘There are people
who make it and I'm hoping I'm one of them.’. JAMA. 292:2141–2149.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jalmsell L, Kreicbergs U, Onelöv E,
Steineck G and Henter JI: Symptoms affecting children with
malignancies during the last month of life: A nationwide follow-up.
Pediatrics. 117:1314–1320. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wolfe J, Grier HE, Klar N, Levin SB,
Ellenbogen JM, Salem-Schatz S, Emanuel EJ and Weeks JC: Symptoms
and suffering at the end of life in children with cancer. N Engl J
Med. 342:326–333. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Finkel JC, Pestieau SR and Quezado ZM:
Ketamine as an adjuvant for treatment of cancer pain in children
and adolescents. J Pain. 8:515–521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mercadante S, Lodi F, Sapio M, Calligara M
and Serretta R: Longterm ketamine subcutaneous continuous infusion
in neuropathic cancer pain. J Pain Symptom Manage. 10:564–568.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elsewaisy O, Slon B and Monagle J:
Analgesic effect of subanesthetic intravenous ketamine in
refractory neuropathic pain: A case report. Pain Med. 11:946–950.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Webster LR and Walker MJ: Safety and
efficacy of prolonged outpatient ketamine infusions for neuropathic
pain. Am J Ther. 13:300–305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Visser E and Schug SA: The role of
ketamine in pain management. Biomed Pharmacother. 60:341–348. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Subramaniam K, Subramaniam B and
Steinbrook RA: Ketamine as adjuvant analgesic to opioids: A
quantitative and qualitative systematic review. Anesth Analg.
99:482–495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grond S, Radbruch L, Meuser T, Sabatowski
R, Loick G and Lehmann KA: Assessment and treatment of neuropathic
cancer pain following WHO guidelines. Pain. 79:15–20. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cherny NI, Thaler HT, Friedlander-Klar H,
Lapin J, Foley KM, Houde R and Portenoy RK: Opioid responsiveness
of cancer pain syndromes caused by neuropathic or nociceptive
mechanisms: A combined analysis of controlled, single-dose studies.
Neurology. 44:857–861. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martin LA and Hagen NA: Neuropathic pain
in cancer patients: Mechanisms, syndromes, and clinical
controversies. J Pain Symptom Manage. 14:99–117. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Enarson MC, Hays H and Woodroffe MA:
Clinical experience with oral ketamine. J Pain Symptom Manage.
17:384–386. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coggeshall RE and Carlton SM: Receptor
localization in the mammalian dorsal horn and primary afferent
neurons. Brain Res Brain Res Rev. 24:28–66. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Persson J, Axelsson G, Hallin RG and
Gustafsson LL: Beneficial effects of ketamine in a chronic pain
state with allodynia, possibly due to central sensitization. Pain.
60:217–222. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okon T: Ketamine: An introduction for the
pain and palliative medicine physician. Pain Physician. 10:493–500.
2007.PubMed/NCBI
|
|
21
|
Grande LA, O'Donnell BR, Fitzgibbon DR and
Terman GW: Ultra-low dose ketamine and memantine treatment for pain
in an opioid-tolerant oncology patient. Anesth Analg.
107:1380–1383. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimoyama N, Shimoyama M, Inturrisi CE and
Elliott KJ: Ketamine attenuates and reverses morphine tolerance in
rodents. Anesthesiology. 85:1357–1366. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weber RJ and Pert A: The periaqueductal
gray matter mediates opiate-induced immunosuppression. Science.
245:188–190. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gavériaux-Ruff C, Matthes HW, Peluso J and
Kieffer BL: Abolition of morphine-immunosuppression in mice lacking
the mu-opioid receptor gene. Proc Natl Acad Sci USA. 95:6326–6330.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roy S, Barke RA and Loh HH: MU-opioid
receptor-knockout mice: Role of mu-opioid receptor in morphine
mediated immune functions. Brain Res Mol Brain Res. 61:190–194.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roy S, Charboneau RG and Barke RA:
Morphine synergizes with lipopolysaccharide in a chronic
endotoxemia model. J Neuroimmunol. 95:107–114. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sacerdote P, Gaspani L and Panerai AE: The
opioid antagonist naloxone induces a shift from type 2 to type 1
cytokine pattern in normal and skin-grafted mice. Ann NY Acad Sci.
917:755–763. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peng X, Mosser DM, Adler MW, Rogers TJ,
Meissler JJ Jr and Eisenstein TK: Morphine enhances interleukin-12
and the production of other pro-inflammatory cytokines in mouse
peritoneal macrophages. J Leukoc Biol. 68:723–728. 2000.PubMed/NCBI
|
|
29
|
Chao CC, Molitor TW, Close K, Hu S and
Peterson PK: Morphine inhibits the release of tumor necrosis factor
in human peripheral blood mononuclear cell cultures. Int J
Immunopharmacol. 15:447–453. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roy S, Cain KJ, Chapin RB, Charboneau RG
and Barke RA: Morphine modulates NF kappa B activation in
macrophages. Biochem Biophys Res Commun. 245:392–396. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roy S, Chapin RB, Cain KJ, Charboneau RG,
Ramakrishnan S and Barke RA: Morphine inhibits transcriptional
activation of IL-2 in mouse thymocytes. Cell Immunol. 179:1–9.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Roy S, Balasubramanian S, Sumandeep S,
Charboneau R, Wang J, Melnyk D, Beilman GJ, Vatassery R and Barke
RA: Morphine directs T cells toward T(H2) differentiation. Surgery.
130:304–309. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roy S, Wang J, Charboneau R, Loh HH and
Barke RA: Morphine induces CD4+ T cell IL-4 expression through an
adenylyl cyclase mechanism independent of the protein kinase A
pathway. J Immunol. 175:6361–6367. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang J, Barke RA, Charboneau R, Loh HH and
Roy S: Morphine negatively regulates interferon-gamma promoter
activity in activated murine T cells through two distinct cyclic
AMP-dependent pathways. J Biol Chem. 278:37622–37631. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang J, Barke RA and Roy S:
Transcriptional and epigenetic regulation of interleukin-2 gene in
activated T cells by morphine. J Biol Chem. 282:7164–7171. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pacifici GM: Metabolism and
pharmacokinetics of morphine in neonates: A review. Clinics (Sao
Paulo). 71:474–480. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Heiskanen T, Langel K, Gunnar T, Lillsunde
P and Kalso EA: Opioid concentrations in oral fluid and plasma in
cancer patients with pain. J Pain Symptom Manage. 50:524–532. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Khalili-Mahani N, Martini CH, Olofsen E,
Dahan A and Niesters M: Effect of subanaesthetic ketamine on plasma
and saliva cortisol secretion. Br J Anaesth. 115:68–75. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang J, Salojin KV, Gao JX, Cameron MJ,
Bergerot I and Delovitch TL: p38 mitogen-activated protein kinase
mediates signal integration of TCR/CD28 costimulation in primary
murine T cells. Immunol. 162:3819–3829. 1999.
|
|
40
|
Rouveix B: Opiates and immune function:
Consequences on infectious diseases with special reference to AIDS.
Therapie. 47:503–512. 1992.PubMed/NCBI
|
|
41
|
Sibinga NE and Goldstein A: Opioid
peptides and opioid receptors in cells of the immune system. Annu
Rev Immunol. 6:219–249. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Weigent DA and Blalock JE: Interactions
between the neuroendocrine and immune systems: Common hormones and
receptors. Immunol Rev. 100:79–108. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nair MP, Schwartz SA, Polasani R, Hou J,
Sweet A and Chadha KC: Immunoregulatory effects of morphine on
human lymphocytes. Clin Diagn Lab Immunol. 4:127–132.
1997.PubMed/NCBI
|
|
44
|
Kulkarni-Narla A, Walcheck B and Brown DR:
Opioid receptors on bone marrow neutrophils modulate chemotaxis and
CD11b/CD18 expression. Eur J Pharmacol. 414:289–294. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
McCarthy L, Wetzel M, Sliker JK,
Eisenstein TK and Rogers TJ: Opioids, opioid receptors, and the
immune response. Drug Alcohol Depend. 62:111–123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Roy S and Loh HH: Effects of opioids on
the immune system. Neurochem Res. 21:1375–1386. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bryant HU, Bernton EW and Holaday JW:
Immunosuppressive effects of chronic morphine treatment in mice.
Life Sci. 41:1731–1738. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang J, Charboneau R, Balasubramanian S,
Barke RA, Loh HH and Roy S: Morphine modulates lymph node-derived T
lymphocyte function: Role of caspase-3, −8 and nitric oxide. J
Leukocyte Biol. 70:527–536. 2001.PubMed/NCBI
|
|
49
|
Jain J, Loh C and Rao A: Transcriptional
regulation of the IL-2 gene. Curr Opin Immunol. 7:333–342. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang JH, Barke RA and Roy S:
Transcriptional and epigenetic regulation of interleukin-2 gene in
activated T cells by morphine. J Biol Chem. 282:7164–7171. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Thomas PT, House RV and Bhargava HN:
Direct cellular immunomodulation produced by diacetylmorphine
(heroin) or methadone. Gen Pharmacol. 26:123–130. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Young HA and Hardy KJ: Interferon-gamma:
Producer cells, activation stimuli, and molecular genetic
regulation. Pharmacol Ther. 45:137–151. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Reuben JM, Lee BN, Paul M, Kline MW, Cron
SG, Abramson S, Lewis D, Kozinetz CA and Shearer WT: Magnitude of
IFN-gamma production in HIV-1-infected children is associated with
virus suppression. J Allergy Clin Immunol. 110:255–261. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Beyers AD, van Rie A, Adams J, Fenhalls G,
Gie R and Beyers N: Signals that regulate the host response to
Mycobacterium tuberculosis. Novartis Found Symp. 217:145–159. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sweetser MT, Hoey T, Sun YL, Weaver WM,
Price GA and Wilson CB: The roles of nuclear factor of activated T
cells and ying-yang 1 in activation-induced expression of the
interferon-gamma promoter in T cells. J Biol Chem. 273:34775–34783.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cippitelli M, Ye J, Viggiano V, Sica A,
Ghosh P, Gulino A, Santoni A and Young HA: Retinoic acid-induced
transcriptional modulation of the human interferon-gamma promoter.
J Biol Chem. 271:26783–26793. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kaminuma O, Elly C, Tanaka Y, Mori A, Liu
YC, Altman A and Miyatake S: Vav-induced activation of the human
IFN-gamma gene promoter is mediated by upregulation of AP-1
activity. FEBS Lett. 514:153–158. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sen R and Baltimore D: Multiple nuclear
factors interact with the immunoglobulin enhancer sequences. Cell.
46:705–716. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pahl HL: Activators and target genes of
Rel/NF-kappaB transcription factors. Oncogene. 18:6853–6866. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pannen BH and Robotham JL: The acute-phase
response. New Horiz. 3:183–197. 1995.PubMed/NCBI
|
|
61
|
Baeuerle PA and Baltimore D: Activation of
DNA-binding activity in an apparently cytoplasmic precursor of the
NF-kappa B transcription factor. Cell. 53:211–217. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ghosh S, May MJ and Kopp EB: NF-kappa B
and Rel proteins: Evolutionarily conserved mediators of immune
responses. Annu Rev Immunol. 16:225–260. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cabot PJ, Carter L, Schafer M and Stein C:
Methionine-enkephalin-and Dynorphin A-release from immune cells and
control of inflammatory pain. Pain. 93:207–212. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Roy S, Wang JH, Balasubramanian S,
Sumandeep, Charboneau R, Barke R and Loh HH: Role of
hypothalamic-pituitary axis in morphine-induced alteration in
thymic cell distribution using mu-opioid receptor knockout mice. J
Neuroimmunol. 116:147–155. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kieffer BL: Recent advances in molecular
recognition and signal transduction of active peptides: Receptors
for opioid peptides. Cell Mol Neurobiol. 15:615–635. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Pol O and Puig MM: Expression of opioid
receptors during peripheral inflammation. Curr Top Med Chem.
4:51–61. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Rogers TJ and Peterson PK: Opioid G
protein-coupled receptors: Signals at the crossroads of
inflammation. Trends Immunol. 24:116–121. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Haraguchi S, Good RA and Day NK:
Immunosuppressive retroviral peptides: cAMP and cytokine patterns.
Immunol Today. 16:595–603. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jimenez JL, Punzon C, Navarro J,
Munoz-Fernandez MA and Fresno MJ: Phosphodiesterase 4 inhibitors
prevent cytokine secretion by T lymphocytes by inhibiting nuclear
factor-kappaB and nuclear factor of activated T cells activation. J
Pharmacol Exp Ther. 299:753–759. 2001.PubMed/NCBI
|
|
70
|
Chen D and Rothenberg EV: Interleukin 2
transcription factors as molecular targets of cAMP inhibition:
Delayed inhibition kinetics and combinatorial transcription roles.
J Exp Med. 179:931–942. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Walker SM, Goudas LC, Cousins MJ and Carr
DB: Combination spinal analgesic chemotherapy: A systematic review.
Anesth Analg. 95:674–715. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hocking G and Cousins MJ: Ketamine in
chronic pain management: An evidence-based review. Anesth Analg.
97:1730–1739. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ohta N, Ohashi Y and Fujino Y: Ketamine
inhibits maturation of bone marrow-derived dendritic cells and
priming of the Th1-type immune response. Anesth Analg. 109:793–800.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gao M, Jin W, Qian Y, Ji L, Feng G and Sun
J: Effect of N-methyl-D-aspartate receptor antagonist on T helper
cell differentiation induced by phorbol-myristate-acetate and
ionomycin. Cytokine. 56:458–465. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chia YY, Liu K, Liu YC, Chang HC and Wong
CS: Adding ketamine in a multimodal patient-controlled epidural
regimen reduces postoperative pain and analgesic consumption.
Anesth Analg. 86:1245–1249. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tan PH, Kuo MC, Kao PF, Chia YY and Liu K:
Patient-controlled epidural analgesia with morphine or morphine
plus ketamine for postoperative pain relief. Eur J Anaesthesiol.
16:820–825. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Choudhuri AH, Dharmani P, Kumarl N and
Prakash A: Comparison of caudal epidural bupivacaine with
bupivacaine plus tramadol and bupivacaine plus ketamine for
postoperative analgesia in children. Anaesth Intensive Care.
36:174–179. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Gunes Y, Secen M, Ozcengiz D, Gündüz M,
Balcioglu O and Isik G: Comparison of caudal ropivacaine,
ropivacaine plus ketamine and ropivacaine plus tramadol
administration for postoperative analgesia in children. Paediatr
Anaesth. 14:557–563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ozbek H, Bilen A, Ozcengiz D, Gunes Y,
Ozalevli M and Akman H: The comparison of caudal ketamine,
alfentanil and ketamine plus alfentanil administration for
postoperative analgesia in children. Paediatr Anaesth. 12:610–616.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Gunduz M, Ozalevli M, Ozbek H and Ozcengiz
D: Comparison of caudal ketamine with lidocaine or tramadol
administration for postoperative analgesia of hypospadias surgery
in children. Paediatr Anaesth. 16:158–163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Vranken JH, Troost D, Wegener JT, Kruis MR
and van der Vegt MH: Neuropathological findings after continuous
intrathecal administration of S(+)-ketamine for the management of
neuropathic cancer pain. Pain. 117:231–235. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sator-Katzenschlager S, Deusch E, Maier P,
Spacek A and Kress HG: The long-term antinociceptive effect of
intrathecal S(+)-ketamine in a patient with established morphine
tolerance. Anesth Analg. 93:1032–1034. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Benrath J, Scharbert G, Gustorff B, Adams
HA and Kress HG: Longterm intrathecal S(+)-ketamine in a patient
with cancer-related neuropathic pain. Br J Anaesth. 95:247–279.
2005. View Article : Google Scholar : PubMed/NCBI
|