Introduction
Renal cell carcinoma (RCC), which originates from
renal parenchyma, is the most common malignant kidney tumor in
adults (87% of all renal malignancies) (1). An estimated 65,340 new cases of kidney
and renal pelvis cancers will be diagnosed in the US in 2018
(42,680 in men and 22,660 in women), of which 14,970 will be fatal
(2). Among the World Health
Organization histological RCC subtypes, clear cell RCC (ccRCC) is
the most common subtype (80–90%) (3). Because of the high aggressiveness of
ccRCC, approximately 30% of patients show metastasis at the time of
diagnosis, and the prognosis is typically very poor (4). Thus, studies of the underlying
molecular characteristics of ccRCC are necessary to develop new
biomarkers and therapeutic strategies.
MicroRNAs (miRNAs), which are short non-coding RNA
molecules, regulate gene expression by cleaving transcribed target
mRNAs and/or repressing protein translation. miRNA biogenesis is a
well-organized process, involving ‘miRNA machinery’ (5) and is regulated by various miRNA
biogenesis components including the RNase III endonuclease DROSHA
(RNASEN), a double-stranded RNA binding protein known as DiGeroge
syndrome critical region gene 8 (DGCR8), the RNase III ribonuclease
DICER, and a component of the RNA-induced silencing complex known
as Argonaute 2 (AGO2) (6).
Accumulating evidence reveals that dysregulated miRNA biogenesis
components have pathophysiological importance in various human
cancers (7–11). Furthermore, Dicer has been reported
to be down-regulated in and to suppress the malignant phenotype of
ccRCC (12,13). Interestingly, significant
associations were observed between haplotypes/diplotypes of the
DROSHA SNPs (rs6877842 and rs10719) and ccRCC recurrence (14). However, there is still very little
published study on the mRNA levels of other miRNA biogenesis
components, such as DROSHA, DGCR8, and AGO2, and
their clinicopathological associations in ccRCC.
In the present study, we investigated the mRNA
levels of four important miRNA biogenesis components using mRNA
expression data from The Cancer Genome Atlas (TCGA) KIRC cohorts
and a Korean ccRCC dataset and evaluated the clinical relevance of
the altered expression in both cohorts.
Materials and methods
Gene expression databases and cluster
analysis
The gene expression RNA-Seq dataset (level 3) and
clinical data for TCGA Kidney Clear Cell Carcinoma (TCGA KIRC)
cohort were downloaded from the UCSC Xena database (xena.ucsc.edu, dataset ID is
TCGA.KIRC.sampleMap/HiSeqV2). Nearly all data (496 patients) in
TCGA KIRC cohort were described in detail previously (15). The four miRNA component genes present
in TCGA KIRC cohort were DICER1, DROSHA (RNASEN),
DGCR8, and AGO2 (EIF2C2). Comprehensive
genetic information was available for 537 patients with ccRCC in
TCGA KIRC cohort. Among the total data, we selected 72 paired data
sets with mRNA level data for analysis of miRNA biogenesis-related
genes (6) and the four miRNA
biogenesis components in ccRCC tissues and adjacent non-neoplastic
surround tissues (ST) from patients with ccRCC. Clinicopathological
parameters of TCGA KIRC was downloaded from the UCSC Xena database
(xena.ucsc.edu, dataset ID is
TCGA.KIRC.sampleMap/KIRC_clinicalMatrix). This study meets the
publication guidelines provided by TCGA. Cluster analysis was
performed using Cluster 3.0 to classify the samples into
statistically similar groups, and the resulting heatmaps were
visualized in TreeView 1.6 (www.eisenlab.org/eisen).
Patients and tissues
Fifty-three patients diagnosed with ccRCC were
included in this retrospective study. ccRCC tissues and adjacent
non-neoplastic tissues were obtained from patients undergoing
surgery in Dongsan Medical Center (Daegu, Korea) between April 2008
and November 2013. The clinicopathological characteristics and
oncological outcome data were retrospectively collected from the
Colorectal Cancer Database in the Department of Colorectal Cancer
Surgery, Dongsan Medical Center (Daegu, Korea) and the Pathological
Diagnosis Database in the Department of Pathology, Dongsan Medical
Center (Daegu, Korea), respectively. Enrolled patients with ccRCC
were classified according to the American Joint Committee on Cancer
Tumor-Node-Metastasis (TNM) staging criteria (16). After surgery, tissue samples were
immediately frozen in liquid nitrogen and stored at −196°C until
RNA isolation. Tissue samples were provided by the Keimyung Human
Bio-resource Bank, Korea.
RNA isolation and reverse
transcription-quantitative (RT-q) PCR
Total cellular RNA was extracted from tissues using
TRIzol reagent (Molecular Research Center, Inc., Cincinnati, OH,
USA). RNAs were quantified using a NanoDrop 1000 (Thermo
Scientific, Waltham, MA, USA). Each cDNA was synthesized from 1 µg
of total RNA using Moloney murine leukemia virus reverse
transcriptase (Promega Corporation, Madison, WI, USA) according to
the manufacturer's protocol. Using the specific primer pairs shown
in Table I and SYBR Green Premix
(Toyobo, Japan), qPCR was performed on a LightCycler®
480 real-time PCR system (Roche Diagnostics GmbH, Mannheim,
Germany). The following thermocycling conditions were maintained:
Pre-incubation, 5 min at 95°C; amplification, 45 cycles with 10 sec
at 95°C, 10 sec at 55°C, and 15 sec at 72°C; melting curve,
gradually from 65°C to 95°C; cooling, 10 min at 37°C. β-Actin was
used as a housekeeping gene for normalization, and a no-template
sample was used as a negative control. qPCR data were analyzed
using the 2−∆∆Cq method (17) in Microsoft Excel (Microsoft
Corporation, Redmond, WA., USA). Three replicates were evaluated
and showed similar results.
 | Table I.Primer sequences of microRNA
machinery components used in quantitative PCR. |
Table I.
Primer sequences of microRNA
machinery components used in quantitative PCR.
| Primer | Sequence
(5′-3′) |
|---|
| DICER1 |
|
|
Forward |
TTAACCTTTTGGTGTTTGATGAGTGT |
|
Reverse |
AGGACATGATGGACAATT |
| DROSHA |
|
|
Forward |
CTGTCGATGCACCAGATT |
|
Reverse |
TGCATAACTCAACTGTGCAGG |
| DGCR8 |
|
|
Forward |
CAAGCAGGAGACATCGGACAAG |
|
Reverse |
CACAATGGACATCTTGGGCTTC |
| AGO2 |
|
|
Forward |
TCATGGTCAAAGATGAGATGACAGA |
|
Reverse |
TTTATTCCTGCCCCCGTAGA |
| β-actin |
|
|
Forward |
CAGCCATGTACGTTGCTATCCAGG |
|
Reverse |
AGGTCCAGACGCAGGATGGCATG |
Statistical analysis
Statistical analysis was performed with SPSS
software (version 22.0; IBM SPSS, Armonk, NY, USA). Differences
between groups were statistically analyzed by using paired t-test.
Co-expression of mRNA levels of various miRNA biogenesis components
in TCGA KIRC cohort was searched using cBioPortal (cbioportal.org). The association between
inter-individual mRNA levels of miRNA biogenesis components in
Korean ccRCC was assessed using the Pearson's correlation
coefficient analysis for continuous variables. Clinicopathological
associations with the mRNA levels of various miRNA biogenesis
components in both TCGA KIRC cohort and Korean ccRCC were analyzed
using the linear-by-linear association model, Pearson's Chi-square
test, and Fisher's exact test for categorical variables. The mean
value was used as a cut-off value (low and high) for categorical
variables. P<0.05 was considered to indicate a
statistically significant difference.
Results
Altered expression levels of miRNA
biogenesis-related genes in TCGA KIRC cohorts
To investigate whether the miRNA biogenesis-related
genes (6) are dysregulated in ccRCC
tissues, we re-analyzed the sorted mRNA expression values from raw
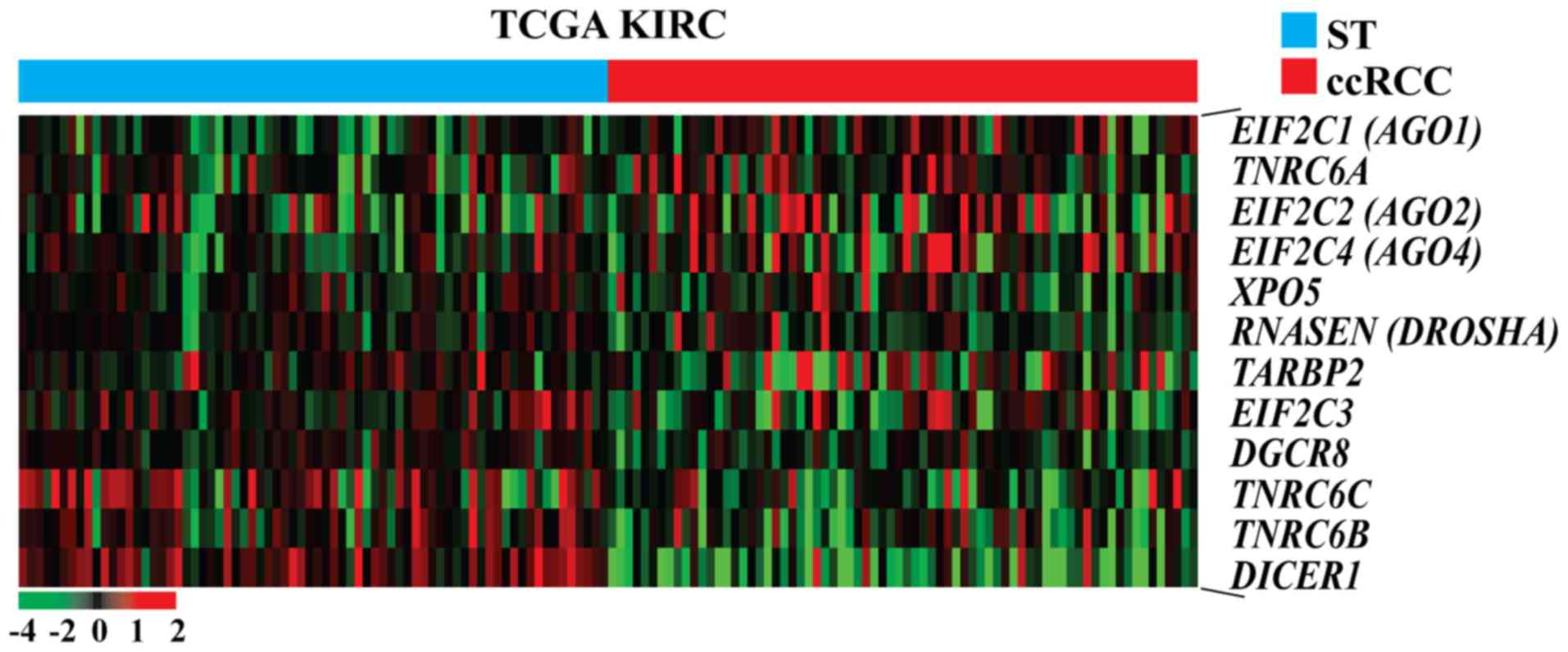
data of TCGA KIRC cohort using paired t-test. Hierarchical
clustering showed that various miRNA biogenesis-related genes were
dysregulated in carcinomatous tissues compared with ST of patients
with ccRCC (Fig. 1). To evaluate the
significance of altered mRNA levels between ccRCC and ST, paired
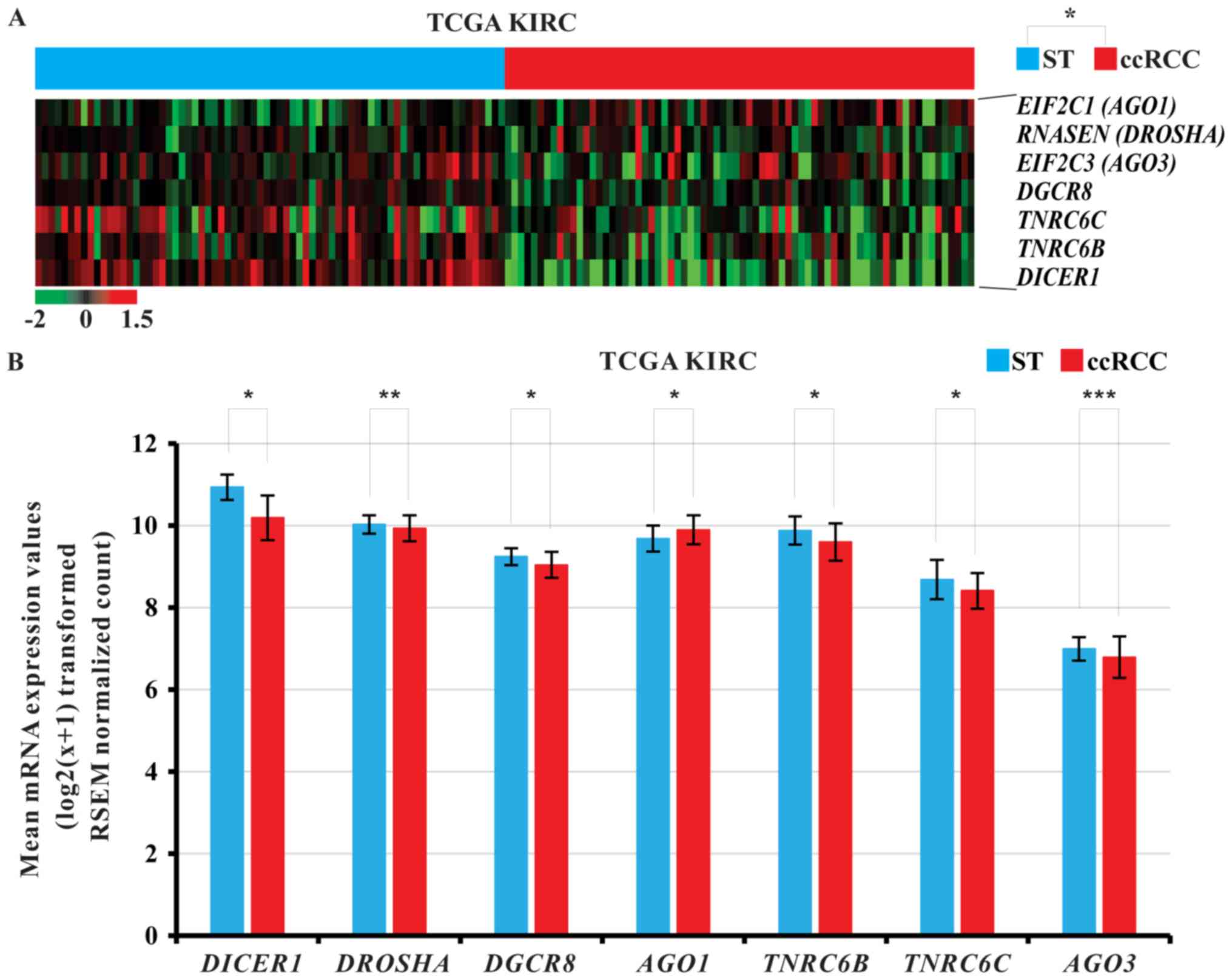
t-test was performed. Hierarchical clustering revealed that various
miRNA biogenesis-related genes were significantly dysregulated in
ccRCC tissues compared with ST of the 72 patients with ccRCC
(P<0.05; Fig. 2A). Among the
various miRNA biogenesis-related genes, AGO1 was
significantly upregulated in ccRCC tissues of TCGA KIRC cohort,
while DICER1, DROSHA, DGCR8, trinucleotide repeat containing
6B (TNRC6B), trinucleotide repeat containing 6C
(TNRC6C), and AGO3 were significantly downregulated
(paired t-test, P<0.05; Fig.
2B).
Altered mRNA levels of four important
miRNA biogenesis components, DICER1, DROSHA, DGCR8, and AGO2, in
the Korean ccRCC cohort
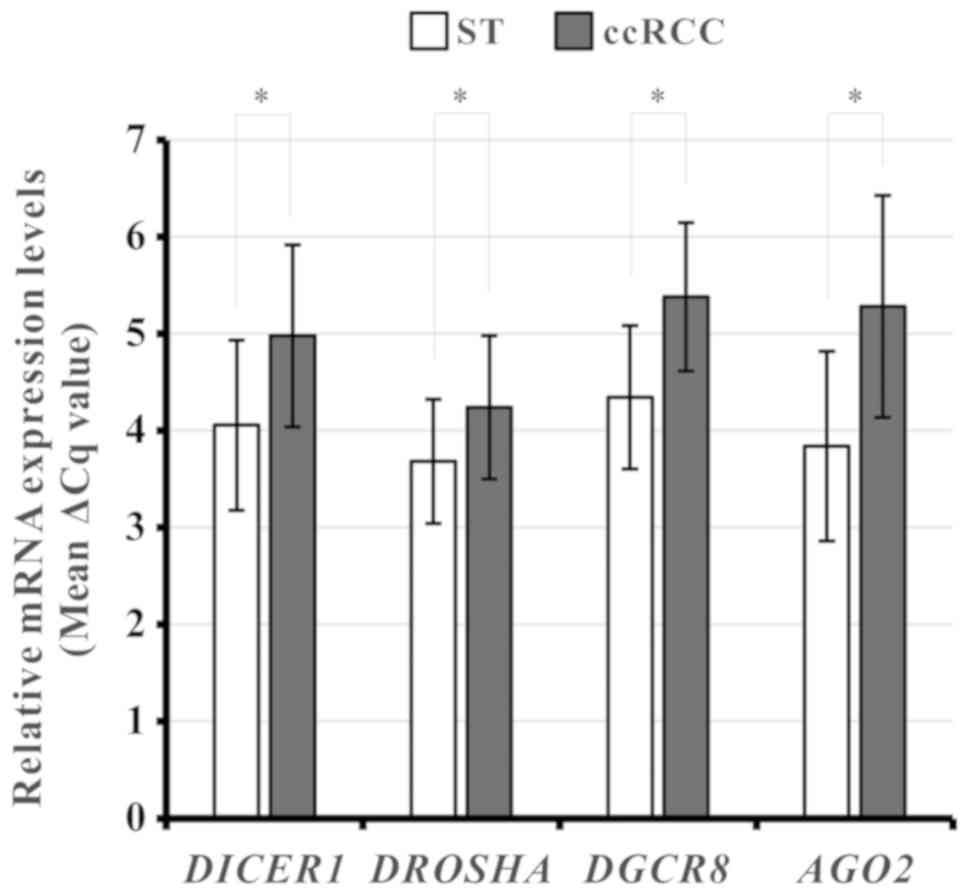
To investigate whether DICER1, DROSHA, DGCR8,
and/or AGO2 were dysregulated in the Korean ccRCC cohort, we
measured the mRNA levels of the four miRNA biogenesis components by
qPCR in 53-paired ccRCC and ST specimens from the Korean cohort.
After excluding unqualified qPCR results, we analyzed the data. The
mRNA levels of all four components were significantly downregulated
in ccRCC tissues compared with the corresponding ST (paired t-test,
DICER1: P<0.001; DROSHA: P<0.001; DGCR8:
P<0.001; AGO2: P<0.001; Fig. 3).
Correlations between inter-individual
mRNA levels of miRNA biogenesis components in the two cohorts
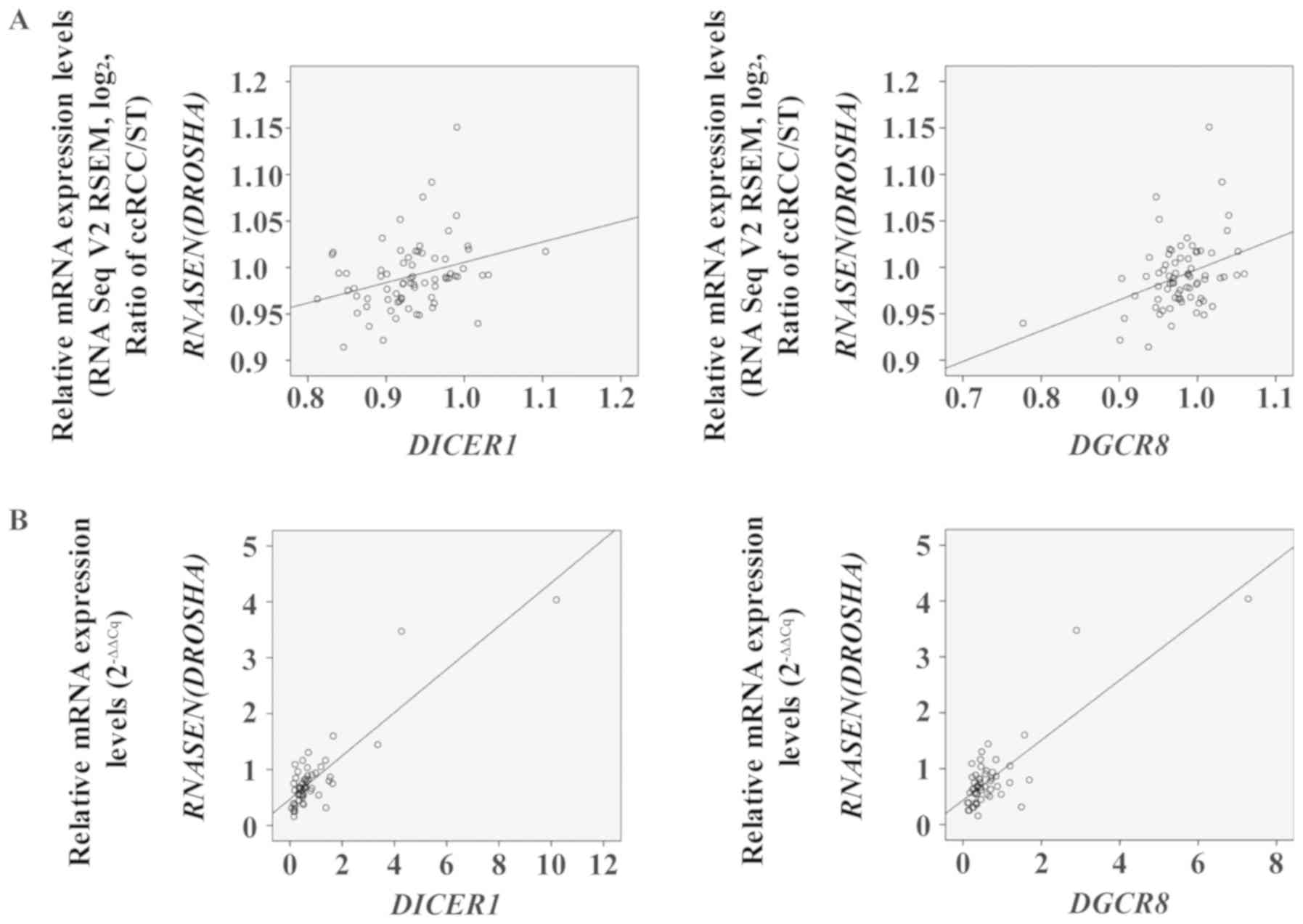
To evaluate the associations between the mRNA levels
of each miRNA biogenesis component in ccRCC specimens, Pearson's
correlation coefficient analysis was performed in both TCGA KIRC
cohort and the Korean ccRCC cohort. Prior to statistical analysis,
significantly altered mRNA levels of miRNA biogenesis components
were sorted from both cohorts (Table
II). We found significant correlations between the mRNA levels
of specific miRNA biogenesis components in both cohorts (Table II). Notably, significant
correlations were observed not only between the mRNA levels of
DICER1 and DROSHA, but also between those of
DROSHA and DGCR8 in both cohorts (Fig. 4; Table
II).
 | Table II.Pearson's correlation analysis
between individual components of microRNA biogenesis. |
Table II.
Pearson's correlation analysis
between individual components of microRNA biogenesis.
| A, TCGA KIRC
cohort |
|---|
|
|---|
| Correlations
between components | Pearson's
correlation coefficient value | P-value |
|---|
| DICER1 and
DROSHA | 0.311 | 0.008 |
| DICER1 and
AGO1 | 0.265 | 0.024 |
| DICER1 and
TNRC6B | 0.609 | <0.001 |
| DICER1 and
TNRC6C | 0.452 | <0.001 |
| DICER1 and
AGO3 | 0.543 | <0.001 |
| DROSHA and
DGCR8 | 0.364 | 0.002 |
| DROSHA and
AGO1 | 0.258 | 0.029 |
| DROSHA and
TNRC6B | 0.599 | <0.001 |
| DROSHA and
TNRC6C | 0.316 | 0.007 |
| DROSHA and
AGO3 | 0.267 | 0.023 |
| DGCR8 and
TNRC6B | 0.350 | 0.003 |
| DGCR8 and
TNRC6C | 0.336 | 0.004 |
| AGO1 and
TNRC6B | 0.270 | 0.022 |
| AGO1 and
AGO3 | 0.529 | <0.001 |
| TNRC6B and
TNRC6C | 0.558 | <0.001 |
| TNRC6B and
AGO3 | 0.365 | 0.002 |
| DICER1 and
DGCR8 | 0.168 | 0.157 |
| DGCR8 and
AGO1 | 0.095 | 0.428 |
| DGCR8 and
AGO3 | 0.065 | 0.587 |
| AGO1 and
TNRC6C | 0.162 | 0.175 |
| TNRC6C and
AGO3 | 0.025 | 0.836 |
|
| B, Korean ccRCC
cohort |
|
| Correlations
between components | Pearson's
correlation coefficient value | P-value |
|
| DICER1 and
DROSHA | 0.877 | <0.001 |
| DICER1 and
DGCR8 | 0.954 | <0.001 |
| DICER1 and
AGO2 | 0.774 | <0.001 |
| DROSHA and
DGCR8 | 0.840 | <0.001 |
| DROSHA and
AGO2 | 0.641 | <0.001 |
| DGCR8 and
AGO2 | 0.837 | <0.001 |
Relationship between mRNA levels of
the four miRNA biogenesis components and their clinicopathological
parameters in both cohorts
To investigate the clinicopathological effects of
altered mRNA levels of the selected four miRNA biogenesis
components in both cohorts, the correlations between mRNA levels of
the miRNA biogenesis components and clinicopathological parameters
were statistically evaluated. For TCGA KIRC cohort, downloaded data
were processed as the ccRCC to ST ratio. Seventy-two patients with
ccRCC, whose clinicopathological data were available, were
classified according to each clinicopathological characteristic
(Table III). Statistical analysis
of the TCGA KIRC cohort data revealed that mRNA levels of
DICER1 and DGCR8 were significantly associated with
histologic grade, respectively (P=0.025, P=0.044; Table III). Moreover, the mRNA levels of
DGCR8 were significantly associated with sex (P=0.024;
Table III). Prior to statistical
analysis of the Korean ccRCC cohort, raw qPCR data were processed
using the 2−∆∆Cq method (17). Fifty-three patients with ccRCC were
classified according to each clinicopathological parameter
(Table IV). Altered mRNA levels of
the analyzed components were not significantly associated with any
clinical parameters, including sex, age, T stage, Fuhrman grade/The
International Society of Urological Pathology (ISUP) grade,
lymphovascular invasion, and peri-renal fat invasion (Table IV).
 | Table III.DICER1, DROSHA and
DGCR8 mRNA expression levels in relation to
clinicopathological parameters of TCGA KIRC. |
Table III.
DICER1, DROSHA and
DGCR8 mRNA expression levels in relation to
clinicopathological parameters of TCGA KIRC.
|
| DICER1 | DROSHA | DGCR8 |
|---|
|
|
|
|
|
|---|
| Parameters | Low | High | P-value | Low | High | P-value | Low | High | P-value |
|---|
| Sex |
|
| 0.293a |
|
| 0.836a |
|
| 0.024a |
|
Male | 28 | 24 |
| 30 | 22 |
| 31 | 21 |
|
|
Female | 8 | 12 |
| 11 | 9 |
| 6 | 14 |
|
| Age, years |
|
| 0.477b |
|
| 0.640a |
|
| 0.057b |
|
<60 | 22 | 18 |
| 21 | 18 |
| 25 | 15 |
|
|
≥60 | 14 | 18 |
| 19 | 13 |
| 12 | 20 |
|
| T stage |
|
| 0.803c |
|
| 0.916c |
|
| 0.983c |
|
1a,1b | 13 | 14 |
| 16 | 11 |
| 13 | 14 |
|
| 2 | 9 | 5 |
| 7 | 7 |
| 9 | 5 |
|
|
3a,3b | 13 | 16 |
| 17 | 12 |
| 14 | 15 |
|
| 4 | 1 | 1 |
| 1 | 1 |
| 1 | 1 |
|
| M stage |
|
| 0.181a |
|
| 0.524a |
|
| 0.683a |
| No
metastasis | 24 | 29 |
| 29 | 24 |
| 28 | 25 |
|
|
Metastasis | 12 | 7 |
| 12 | 7 |
| 9 | 10 |
|
| Pathologic
stage |
|
| 0.682a |
|
| 0.570a |
|
| 0.754a |
| Stage
I | 11 | 14 |
| 12 | 13 |
| 11 | 14 |
|
| Stage
II | 6 | 5 |
| 8 | 3 |
| 6 | 5 |
|
| Stage
III | 7 | 9 |
| 9 | 7 |
| 8 | 8 |
|
| Stage
IV | 12 | 8 |
| 12 | 8 |
| 12 | 8 |
|
| Histologic
grade |
|
| 0.025c |
|
| 0.169c |
|
| 0.044c |
| G1 | 0 | 1 |
| 1 | 0 |
| 0 | 1 |
|
| G2 | 10 | 18 |
| 12 | 16 |
| 10 | 18 |
|
| G3 | 16 | 12 |
| 18 | 10 |
| 18 | 10 |
|
| G4 | 10 | 5 |
| 10 | 5 |
| 9 | 6 |
|
 | Table IV.DICER1, DROSHA, DGCR8, and
AGO2 mRNA expression levels in relation to
clinicopathological parameters of Korean clear cell renal cell
carcinoma. |
Table IV.
DICER1, DROSHA, DGCR8, and
AGO2 mRNA expression levels in relation to
clinicopathological parameters of Korean clear cell renal cell
carcinoma.
|
| DICER1 | DROSHA | DGCR8 | AGO2 |
|---|
|
|
|
|
|
|
|---|
| Parameters | Low | High | P-value | Low | High | P-value | Low | High | P-value | Low | High | P-value |
|---|
| Sex |
|
| 0.560a |
|
| 0.077b |
|
| 0.810a |
|
| 0.728b |
|
Male | 20 | 7 |
| 15 | 12 |
| 20 | 7 |
| 21 | 6 |
|
|
Female | 21 | 5 |
| 21 | 5 |
| 20 | 6 |
| 22 | 4 |
|
| Age, years |
|
| 0.941a |
|
| 0.168a |
|
| 0.379a |
|
| 0.293b |
|
<60 | 21 | 6 |
| 16 | 11 |
| 19 | 8 |
| 20 | 7 |
|
|
≥60 | 20 | 6 |
| 20 | 6 |
| 21 | 5 |
| 23 | 3 |
|
| T stage |
|
| 0.589c |
|
| 0.878c |
|
| 0.342c |
|
| 0.677c |
|
1A,1B | 22 | 6 |
| 18 | 10 |
| 22 | 6 |
| 23 | 5 |
|
|
2A,2B | 7 | 1 |
| 7 | 1 |
| 7 | 1 |
| 7 | 1 |
|
|
3A,3B | 12 | 5 |
| 11 | 6 |
| 11 | 6 |
| 13 | 4 |
|
| Fuhrman grade/ISUP
grade |
|
| 0.250c |
|
| 0.280c |
|
| 0.105c |
|
| 0.305c |
| 1 | 2 | 0 |
| 1 | 1 |
| 2 | 0 |
| 2 | 0 |
|
| 2 | 16 | 1 |
| 15 | 12 |
| 16 | 1 |
| 16 | 1 |
|
| 3 | 15 | 10 |
| 14 | 11 |
| 15 | 10 |
| 17 | 8 |
|
| 4 | 8 | 1 |
| 6 | 3 |
| 7 | 2 |
| 8 | 1 |
|
| Lymphovascular
invasion |
|
| 0.183b |
|
| 0.667b |
|
| 0.053b |
|
| 0.604b |
|
(−) | 37 | 9 |
| 32 | 14 |
| 37 | 9 |
| 38 | 8 |
|
|
(+) | 4 | 3 |
| 4 | 3 |
| 3 | 4 |
| 5 | 2 |
|
| Peri-renal fat
invasion |
|
| 0.423b |
|
| 0.471b |
|
| 1.000b |
|
| 0.665b |
|
(−) | 32 | 11 |
| 28 | 15 |
| 32 | 11 |
| 34 | 9 |
|
|
(+) | 9 | 1 |
| 8 | 2 |
| 8 | 2 |
| 9 | 1 |
|
Discussion
Dysregulation of miRNA biogenesis components has
been reported to be associated with carcinogenesis (18), cancer chemosensitivity (19), angiogenesis (13), metastasis (20), proliferation and migration (21), and the prognosis of patients with
cancer (20,22) in various cancers, including ccRCC
(12,13). Although the dysregulation and
pathophysiological role of DICER1 in ccRCC has been
investigated (12,13), studies of other miRNA biogenesis
components, such as DROSHA, DGCR8, and AGO2 are
needed. In the present study, we investigated the mRNA levels of
miRNA biogenesis components and analyzed their clinicopathological
relevance in the TCGA KIRC cohort and a Korean ccRCC cohort.
First, our hierarchical clustering results revealed
considerable dysregulation of miRNA biogenesis-related genes in
ccRCC tissues compared with ST from TCGA KIRC cohort. Among the
dysregulated genes, DICER 1 was significantly downregulated
in ccRCC tissues. Similarly, in our Korean ccRCC cohort, DICER
1 was significantly downregulated. These results are consistent
with those of previous studies of ccRCC in which downregulated
DICER1 mRNA levels were detected (12). Particularly, one study revealed that
DICER1 was more downregulated in VHL-deficient (VHL mutation
and methylation) ccRCCs compared with ST (13). Because nearly all samples (71 of 72
patients with ccRCC) were VHL-deficient (data not shown) in the
dataset of 72 patients with ccRCC of TCGA KIRC cohort, we did not
evaluate whether DICER1 mRNA levels were downregulated in
VHL-deficient ccRCCs compared with wild-type VHL ccRCCs. Notably,
we found for the first time that DROSHA and DGCR8
were significantly downregulated in the ccRCC tissues of both
cohorts. On the other hand, AGO2 was only significantly
downregulated in the Korean ccRCC cohort. Although we did not
identify the exact cause underlying the downregulation of AGO2 in
only the Korean ccRCC cohort, it is possible that racial
differences between the two cohorts affected the AGO2 mRNA levels.
These results demonstrate that the downregulated mRNA levels of
DICER1, DROSHA, and DGCR8 are common in ccRCC samples
and that downregulation of the three components is important in the
pathophysiology of ccRCC.
Accumulating evidence indicates significant
correlations between the inter-individual levels of miRNA
biogenesis components in paired samples of cancer and ST:
DGCR8 and AGO2 in colorectal cancer (23); DICER1 and DROSHA in
triple negative breast cancer (24);
and DICER1 and DROSHA, as well as DROSHA and
DGCR8, in TCGA papillary thyroid carcinoma (25). Therefore, we investigated the
correlation between the mRNA levels of the components in both
cohorts. Our data revealed significant correlations between
DICER1 and DROSHA as well as between DROSHA
and DGCR8 in both cohorts.
Recent studies showed that aberrant regulation of
miRNA biogenesis components is associated with specific
clinicopathological parameters (10,26–29). For
example, DICER1 mRNA levels were significantly correlated
with overall TNM staging (12).
Subsequent studies evaluated the clinicopathological implications
of dysregulated miRNA biogenesis components in ccRCC. In contrast
to a recent study (12), our results
revealed that there was no significant correlation between the mRNA
levels of any components and stages of T and M in TCGA KIRC cohort.
Furthermore, DICER1 and DGCR8 mRNA levels were
significantly correlated with histologic grade of ccRCC. However,
we found no significant correlation between dysregulation of the
miRNA biogenesis components and any clinicopathological parameters
in the Korean ccRCC cohort, including sex, age, T stage, Fuhrman
grade/ISUP grade (histologic grade), lymphovascular invasion, and
peri-renal fat invasion. These differences between cohorts may be
attributed to the small numbers of enrolled patients and racial
differences. Because few studies have examined the
clinicopathological implications of dysregulated the miRNA
biogenesis components in ccRCC, further studies focusing on these
issues in ccRCC are required.
Because RT-qPCR and RNA-Seq are different
experimental assays utilizing different methodologies and show
varying sensitivities, dynamic ranges of the results, and
experimental principles, the results of the present study should be
interpreted with caution. Nevertheless, we detected downregulation
of DICER1, DROSHA, DGCR8, and AGO2 and significant
correlations between DICER1 and DROSHA as well as
between DROSHA and DGCR8 in ccRCC. Collectively,
DICER1, DROSHA, DGCR8, and AGO2 were significantly
downregulated and correlated with each other in ccRCC, suggesting
that they serve important roles in the pathophysiology of
ccRCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Research Foundation of Korea (grant funded by the Korea Government
Ministry of Science, ICT and Future Planning; grant nos.
2017R1C1B5016670 and 2014R1A5A2010008).
Availability of data and materials
The datasets analyzed during the present study are
available from The Cancer Genome Atlas (tcga-data.nci.nih.gov/tcga/tcgaDownload.jsp) and the
UCSC Xena (xena.ucsc.edu). The datasets generated
in the present study are available from the corresponding author
upon reasonable request.
Authors' contributions
SSL and SK contributed to the conception and design
of the study, analysis of the data, interpretation of the results,
and the writing of the manuscript. HM and SK contributed to the
acquisition of data. SSL, HM and SK performed the experiments. JYH,
BHK and MSC contributed to the conception and design of the study,
and assisted with the determination of the clinical implications of
the study. SK reviewed and edited the manuscript. All authors read
and approved the manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy and integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
The biospecimens for this retrospective study were
provided by the Keimyung Human Bio-Resource Bank, a member of the
National Biobank of Korea, which is supported by the Ministry of
Health and Welfare. The experimental study was approved by the
Institutional Review Board of Keimyung University and Dongsan
Medical Center (approval no. 2015-10-021-004) and was performed in
accordance with the principles of the Declaration of Helsinki. The
requirement for written informed consent was waived due to the
retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ccRCC
|
clear cell renal cell carcinoma
|
|
miRNA
|
microRNA
|
|
TCGA KIRC
|
The Cancer Genome Atlas Kidney Clear
Cell Carcinoma
|
|
qPCR
|
quantitative PCR
|
|
DGCR8
|
DiGeroge syndrome critical region gene
8
|
|
AGO2
|
Argonaute 2
|
|
ST
|
adjacent non-neoplastic surround
tissues
|
References
|
1
|
Bhatt JR and Finelli A: Landmarks in the
diagnosis and treatment of renal cell carcinoma. Nat Rev Urol.
11:517–525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kovacs G, Akhtar M, Beckwith BJ, Bugert P,
Cooper CS, Delahunt B, Eble JN, Fleming S, Ljungberg B, Medeiros
LJ, et al: The Heidelberg classification of renal cell tumours. J
Pathol. 183:131–133. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Campbell D, Walker R, Mathew T and Craig
J: Commentary on ‘Influence of industry on renal guideline
development’. Clin J Am Soc Nephrol. 2:2112007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Papachristou DJ, Korpetinou A,
Giannopoulou E, Antonacopoulou AG, Papadaki H, Grivas P, Scopa CD
and Kalofonos HP: Expression of the ribonucleases Drosha, Dicer,
and Ago2 in colorectal carcinomas. Virchows Arch. 459:431–440.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mockenhaupt S, Schurmann N and Grimm D:
When cellular networks run out of control: Global dysregulation of
the RNAi machinery in human pathology and therapy. Prog Mol Biol
Transl Sci. 102:165–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim S, Song ML, Min H, Hwang I, Baek SK,
Kwon TK and Park JW: miRNA biogenesis-associated RNase III
nucleases Drosha and Dicer are upregulated in colorectal
adenocarcinoma. Oncol Lett. 14:4379–4383. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hata A and Kashima R: Dysregulation of
microRNA biogenesis machinery in cancer. Crit Rev Biochem Mol Biol.
51:121–134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tchernitsa O, Kasajima A, Schäfer R, Kuban
RJ, Ungethüm U, Györffy B, Neumann U, Simon E, Weichert W, Ebert MP
and Röcken C: Systematic evaluation of the miRNA-ome and its
downstream effects on mRNA expression identifies gastric cancer
progression. J Pathol. 222:310–319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shaikhibrahim Z, Lindstrot A, Ochsenfahrt
J, Fuchs K and Wernert N: Epigenetics-related genes in prostate
cancer: Expression profile in prostate cancer tissues,
androgen-sensitive and -insensitive cell lines. Int J Mol Med.
31:21–25. 2013.PubMed/NCBI
|
|
12
|
Ma X, Fan Y, Gao Y, Zhang Y, Huang Q, Ai
Q, Ni D, Chen W, Zhang P, Song E, et al: Dicer is down-regulated in
clear cell renal cell carcinoma and in vitro Dicer knockdown
enhances malignant phenotype transformation. Urol Oncol.
32:46.e9–e17. 2014. View Article : Google Scholar
|
|
13
|
Fan Y, Li H, Ma X, Gao Y, Bao X, Du Q, Ma
M, Liu K, Yao Y, Huang Q, et al: Dicer suppresses the malignant
phenotype in VHL-deficient clear cell renal cell carcinoma by
inhibiting HIF-2α. Oncotarget. 7:18280–18294. 2016.PubMed/NCBI
|
|
14
|
Lin J, Horikawa Y, Tamboli P, Clague J,
Wood CG and Wu X: Genetic variations in microRNA-related genes are
associated with survival and recurrence in patients with renal cell
carcinoma. Carcinogenesis. 31:1805–1812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of clear cell renal cell
carcinoma. Nature. 499:43–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene F and Trotti A: AJCC cancer staging handbook: From the AJCC
cancer staging manual. Springer; New York: 2010
|
|
17
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gurtner A, Falcone E, Garibaldi F and
Piaggio G: Dysregulation of microRNA biogenesis in cancer: The
impact of mutant p53 on Drosha complex activity. J Exp Clin Cancer
Res. 35:452016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X, Li WF, Wu X, Zhang HC, Chen L,
Zhang PY, Liu LY, Ma D, Chen T, Zhou L, et al: Dicer regulates
non-homologous end joining and is associated with chemosensitivity
in colon cancer patients. Carcinogenesis. 38:873–882. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
To SKY, Mak ASC, Eva Fung YM, Che CM, Li
SS, Deng W, Ru B, Zhang J and Wong AST: β-catenin downregulates
Dicer to promote ovarian cancer metastasis. Oncogene. 36:5927–5938.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu Z, Wang L, Wang C, Ju X, Wang M, Chen
K, Loro E, Li Z, Zhang Y, Wu K, et al: Cyclin D1 induction of Dicer
governs microRNA processing and expression in breast cancer. Nat
Commun. 4:28122013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Diaz-Garcia CV, Agudo-López A, Pérez C,
López-Martín JA, Rodríguez-Peralto JL, de Castro J, Cortijo A,
Martínez-Villanueva M, Iglesias L, García-Carbonero R, et al:
DICER1, DROSHA and miRNAs in patients with non-small cell lung
cancer: Implications for outcomes and histologic classification.
Carcinogenesis. 34:1031–1038. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim B, Lee JH, Park JW, Kwon TK, Baek SK,
Hwang I and Kim S: An essential microRNA maturing microprocessor
complex component DGCR8 is up-regulated in colorectal carcinomas.
Clin Exp Med. 14:331–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Passon N, Gerometta A, Puppin C, Lavarone
E, Puglisi F, Tell G, Di Loreto C and Damante G: Expression of
Dicer and Drosha in triple-negative breast cancer. J Clin Pathol.
65:320–326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim J, Park WJ, Jeong KJ, Kang SH, Kwon
SY, Kim S and Park JW: Racial differences in expression levels of
miRNA machinery-related genes, Dicer, Drosha, DGCR8, and AGO2, in
Asian Korean papillary thyroid carcinoma and comparative validation
using the cancer genome atlas. Int J Genomics. 2017:57897692017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vaksman O, Hetland TE, Trope' CG, Reich R
and Davidson B: Argonaute, Dicer, and Drosha are up-regulated along
tumor progression in serous ovarian carcinoma. Hum Pathol.
43:2062–2069. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin RJ, Lin YC, Chen J, Kuo HH, Chen YY,
Diccianni MB, London WB, Chang CH and Yu AL: microRNA signature and
expression of Dicer and Drosha can predict prognosis and delineate
risk groups in neuroblastoma. Cancer Res. 70:7841–7850. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stratmann J, Wang CJ, Gnosa S, Wallin A,
Hinselwood D, Sun XF and Zhang H: Dicer and miRNA in relation to
clinicopathological variables in colorectal cancer patients. BMC
Cancer. 11:3452011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shu GS, Yang ZL and Liu DC:
Immunohistochemical study of Dicer and Drosha expression in the
benign and malignant lesions of gallbladder and their
clinicopathological significances. Pathol Res Pract. 208:392–397.
2012. View Article : Google Scholar : PubMed/NCBI
|