Introduction
Three-dimensional (3D) cell culture has emerged in
the field of cancer research as a tool for capturing critical
biological properties of cancer, especially for solid tumors.
Multicellular spheroids (MCSs) developed in 3D culture are believed
to more closely mimic solid tumors with respect to cell-cell
interactions, hypoxia, drug penetration, and nutrition gradients,
which are irreproducible in conventional two-dimensional (2D) cell
culture (1,2). MCSs generated from cancer cell lines
alter their molecular phenotypes in various types of cancer,
including ovarian, colon, prostate, head and neck, endometrial, and
lung (3–11). Along with the molecular phenotypic
changes, functional properties in 3D culture, e.g. cell
proliferation and sensitivity to drugs, also change compared to
their parental cells grown in 2D adherent culture (3–7,12). Evidence for MCSs derived from bladder
cancer cell lines is limited. Our group found that MCSs generated
from bladder cancer cell lines changed protein levels of delta N
p63 alpha, E-cadherin, and N-cadherin, implicating
epithelial-mesenchymal transition between MCSs in suspension and
parental cells grown in 2D adherent culture (13). Intriguingly, CHIR99021, a GSK3
inhibitor and Wnt pathway activator, promoted cell proliferation
only in 3D culture but not in 2D culture (14). These data further corroborate the
importance of 3D cell culture to discover new biological aspects of
cancer cells.
Methodologies preparing MCSs from cell lines vary
among researches, including the U-bottom ultra-low adherence plates
method (6,7,9), the
hanging drop method (8), the
microgravity system using a rotating bioreactor (10), culture within extracellular matrix
(4,5), and nano-imprinted plates based on
scaffold-based technologies (12).
The difference among these methodologies can cause biological
differences among MCSs prepared by each method. Particularly, the
presence of extracellular matrix impacts structural, molecular, and
functional characteristics of MCSs (13,15,16). We
have adopted a modified aggregation-based method using plates
coated in-house with poly-2-hydroxyethyl methacrylate (poly-HEMA),
which prevents cells from attaching to plates, enhances cell
aggregation, and consequently yields MCSs in suspension (3,14). This
method enables rapid, easy, and cost-effective preparation of MCSs
from conventional cell lines.
In this study, we described the process of
spontaneous formation of MCSs in suspension from human bladder
cancer cell lines by the aggregation-based method. Histological
evaluation for these MCSs was carried out using
immunohistochemistry with antibodies against luminal and basal
markers for urothelium. We also compared molecular characteristics
of bladder cancer cells between MCSs in suspension and parental
cells grown in conventional 2D culture. Cellular proliferation and
chemosensitivity to cisplatin (CDDP) and gemcitabine were also
assessed in 3D and 2D cultures for comparison.
Materials and methods
Cell culture
RT4 and 5637 were selected to examine as
representative luminal and basal human bladder cancer cell lines,
respectively (17). RT4 and 5637
were purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA) in November 2015, and cultured as previously
described (14). Briefly, cells were
cultured at 37°C under 5% CO2 in RPMI 1640 containing
10% fetal bovine serum. MCSs from cell lines were prepared by the
aggregation-based method. 2×105 or 1,000 cells
(respectively) were seeded in 6- or 96-well U-bottom plates coated
with poly-HEMA (Sigma, St. Louis, MO, USA). MCSs were formed 24 h
after seeding and used in further experiments. Images of cultured
cells were taken by EVOS Cell Imaging Systems (Thermo Fischer
Scientific, Waltham, MA, USA).
Histological evaluation
Histological evaluation was performed as previously
described (15). MCSs were embedded
in iPGell (Diagnocine, Hackensack, NJ, USA) and fixed in 10%
buffered formalin. Sections of 4 µm were stained with
hematoxylin-eosin for histological evaluation. Immunohistochemistry
was performed using primary antibodies; Ki 67 (ab16667, Abcam,
Cambridge, MA, USA), cleaved caspase 3 (9661, Cell Signaling
Technology, Danvers, MA, USA), cytokeratin 14 (CK14) (ab181595,
Abcam), cytokeratin 20 (CK20) (ab76126, Abcam), cytokeratin 5 (CK5)
(PRB-160P, Covance, Princeton, NJ, USA), peroxisome
proliferator-activated receptor gamma (PPARγ) (2435, Cell Signaling
Technology), forkhead box A1 (FOXA1) (ab170933, Abcam), and p63
(CM163A, Biocare Medical, Pacheco, CA, USA).
Western blotting
Western blotting was performed as previously
described (14). Primary antibodies
used included; β-actin (A2228, Sigma), FOXA1 (ab170933, Abcam),
CK20 (ab76126, Abcam), CK5 (PRB-160P, Covance), CK14 (ab181595,
Abcam), and PPARγ (2435, Cell Signaling Technology).
Immunocytochemistry
Immunocytochemistry was performed as previously
described (15). Briefly, Cells were
seeded on Lab-Tek II Chamber Slide (Thermo Fischer Scientific) 24 h
before fixation. Cells were fixed with 4% paraformaldehyde/PBS for
15 min. After permeabilization and blocking with Dako Protein Block
Seum-Free (Dako) containing 1% Triton X-100 for 15 min, cells were
incubated at 4°C with anti-CK14 (Abcam) or CK20 antibody (Abcam)
overnight followed by incubation at room temperature with Alexa-488
conjugated secondary antibody (Thermo Fischer Sicentific) for 1 h.
After counterstaining with Hoechst 33342 (Thermo Fischer
Sicentific), images of fluorescent cells were obtained using EVOS
Cell Imaging Systems (Thermo Fischer Sicentific).
Quantitative real-time polymerase
chain reaction (qRT-PCR)
qRT-PCR analysis was performed as previously
described (14). Briefly, total RNA
was extracted from cells using the RNeasy system (Qiagen, Hilden,
Germany) after RT4 and 5637 cells were grown in 2D adherent culture
and in 3D suspension culture. qRT-PCR was performed using the
StepOnePlus system (Applied Biosystems, Foster City, CA, USA).
TaqMan gene expression assays for KRT20 (CK20), KRT18 (CK18), KRT14
(CK14), KRT5 (CK5), and glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) were used. All primers were purchased from Thermo Fischer
Scientific. GAPDH was used for normalization.
Viability assay and growth assay
Viability assay and growth assay were performed as
previously described (14). Briefly,
for viability assay in 2D culture, 3,000 and 1,500 cells of RT4 and
5637 were seeded in a 96-well tissue-treated plate in triplicate,
respectively. For viability assay in 3D culture, MCSs were prepared
in 96-well U-bottom plates as described above. Twenty-four hours
after seeding, cells were treated with PBS or CDDP (Sigma) or
gemcitabine (Sigma) for 72 h. To measure cell viability,
CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison,
WI, USA) and FLUOstar OPTIMA (BMG Labtech, Ortenberg, Germany) were
used according to manufacture's protocols. Relative cell
proliferation was calculated by dividing the viability of the
indicated day by that of day 1. Following this, dose-response
curves of CDDP and gemcitabine were depicted and IC50
was calculated using GraphPad Prism 7 (GraphPad Software, San
Diego, CA, USA) with non-linear (curve fit) regression algorithms.
For growth assay in 3D cultures, Images of MCSs were collected at
days 1 and 6 by the EVOS Cell Imaging Systems, and the areas
occupied by the MCSs were measured using Image J software (National
Institutes of Health, Bethesda, MD, USA). Relative growth rates
were calculated by dividing the area of MCSs at day 6 by that at
day 1.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 7 (GraphPad Software). Data are presented as mean ± standard
deviation (SD). Two-group analysis was performed by the unpaired
t-test for the results of RT-qPCR, and results were considered
statistically significant at P≤0.05.
Results
Bladder cancer cell lines RT4 and 5637
spontaneously form MCSs in suspension by the aggregation-based
method
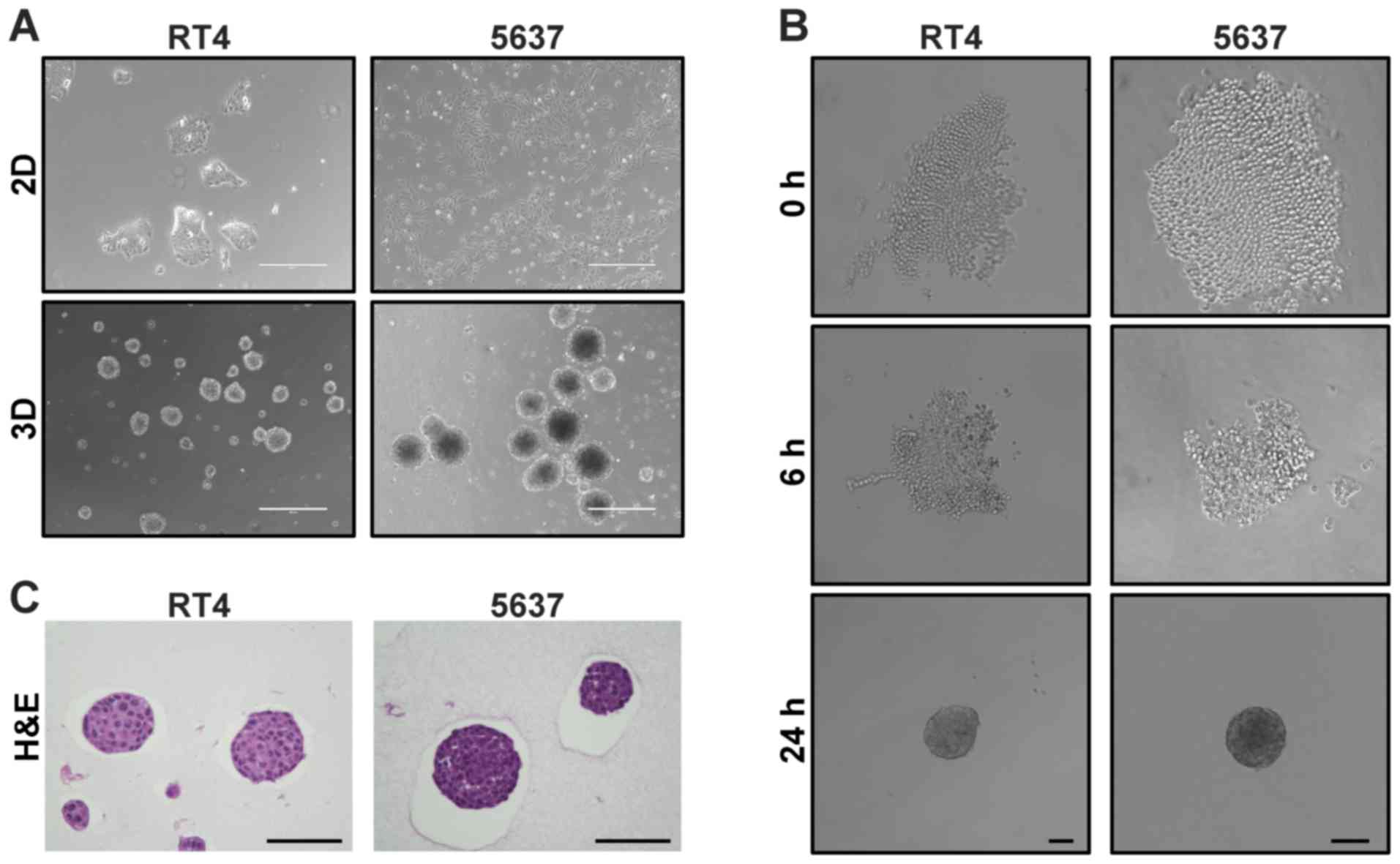
Twenty-four hours after RT4 and 5637 cells were
seeded in a 6-well plate coated with poly-HEMA, multiple MSCs were
found floating (Fig. 1A).
5637-derived MCSs were apparently darker than RT4-derived MCSs.
Time-lapse images showed that both cells aggregated with each other
over time, eventually forming a round-shaped MCS in suspension
(Fig. 1B). Hematoxylin-eosin
staining of MCSs demonstrated that MCSs derived from RT4 and 5637
were packed with cells (Fig. 1C).
Cells in RT4-derived MCSs had larger cytoplasm and lower
nuclear/cytoplasmic ratio than cells in 5637-derived MCSs (Fig 1C). These data demonstrated rapid
generation of MCSs in suspension from bladder cancer cell lines RT4
and 5637 by the aggregation-based method.
RT4- and 5637-derived MCSs consist of
cells expressing different levels of luminal/basal differentiation
markers
Molecular subtype membership has been established in
bladder cancer, exhibiting a broad spectrum of bladder cancer
biology. Subtypes are clustered into luminal and basal at the
highest level (18). We sought to
characterize RT4- and 5637-derived MCSs with the expression levels
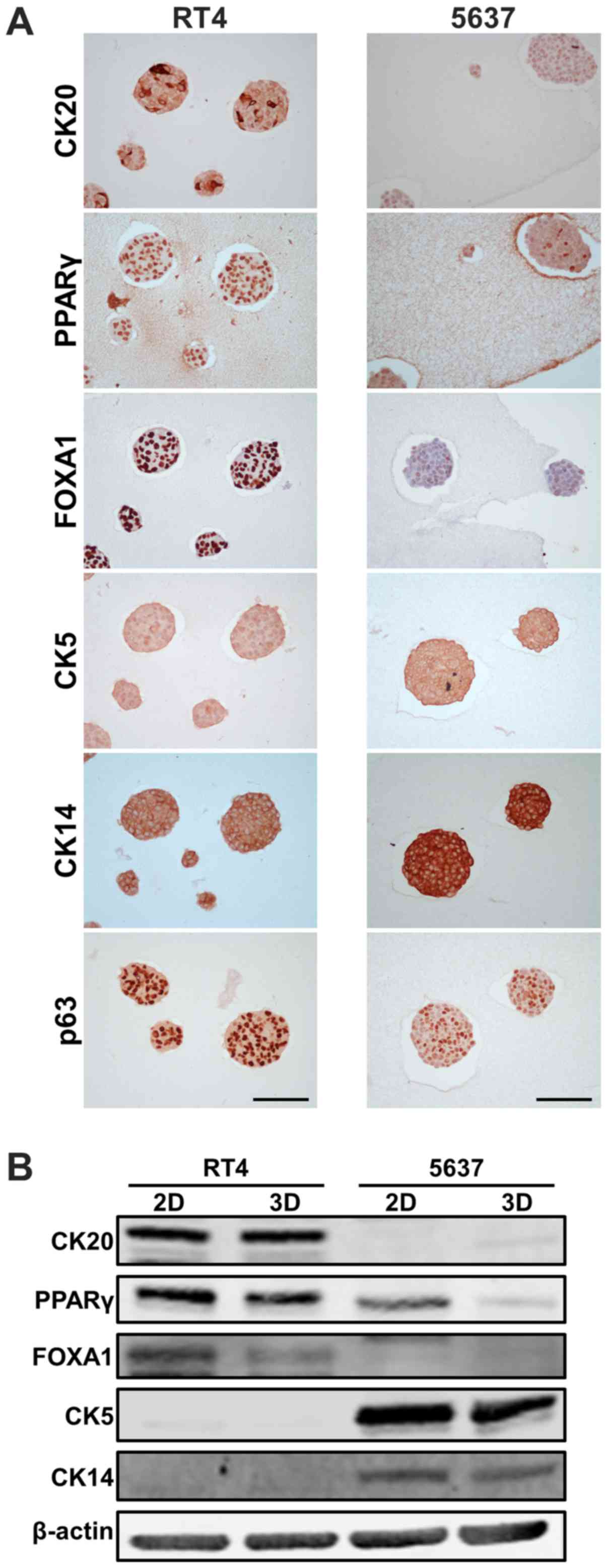
of luminal/basal markers. Immunohistochemistry showed that luminal
markers, including CK20, PPARγ, and FOXA1, were expressed more in
RT4-derived MCSs than in 5637-derived MCSs, while basal markers of
CK5 and CK14 were expressed more in 5637-derived MCSs than in
RT4-derived MCSs (Fig. 2A). These
data were consistent with the findings that RT4 and 5637 were
luminal and basal subtypes according to transcriptome analysis in
conventional 2D adherent culture (17). Heterogeneous expression of CK20 in
RT4-derived MCSs, and that of PPARγ and p63 in 5637-derived MCSs
were observed within a single MCS (Fig.
2A), demonstrating that one MCS consisted of cells
heterogeneously expressing luminal/basal markers. Heterogeneous
expression of CK20 was also observed among RT4 cells grown in 2D
adherent culture (Fig. S1).
Next, expression levels of luminal/basal markers
were compared between MCSs in 3D suspension culture and parental
cells grown in 2D adherent culture. Western blotting showed that
luminal markers, including CK20, PPARγ, and FOXA1, were expressed
more in RT4-derived MCSs than in 5637-derived MCSs, while basal
markers of CK5 and CK14 were expressed more in 5637-derived MCSs
than in RT4-derived MCSs (Fig. 2B),
which was in line with the immunohistochemical findings. PPARγ and
FOXA1 were expressed less in MCSs than in parental cells grown in
2D culture, while other markers examined were equally expressed in
cells in both culture conditions (Fig.
2B). Quantitative real-time polymerase chain reaction analysis
demonstrated significant difference in mRNA expression levels of
KRT20, KRT18, and KRT14 between MCSs in 3D suspension
culture and parental cells grown in 2D adherent culture (Fig. S2).
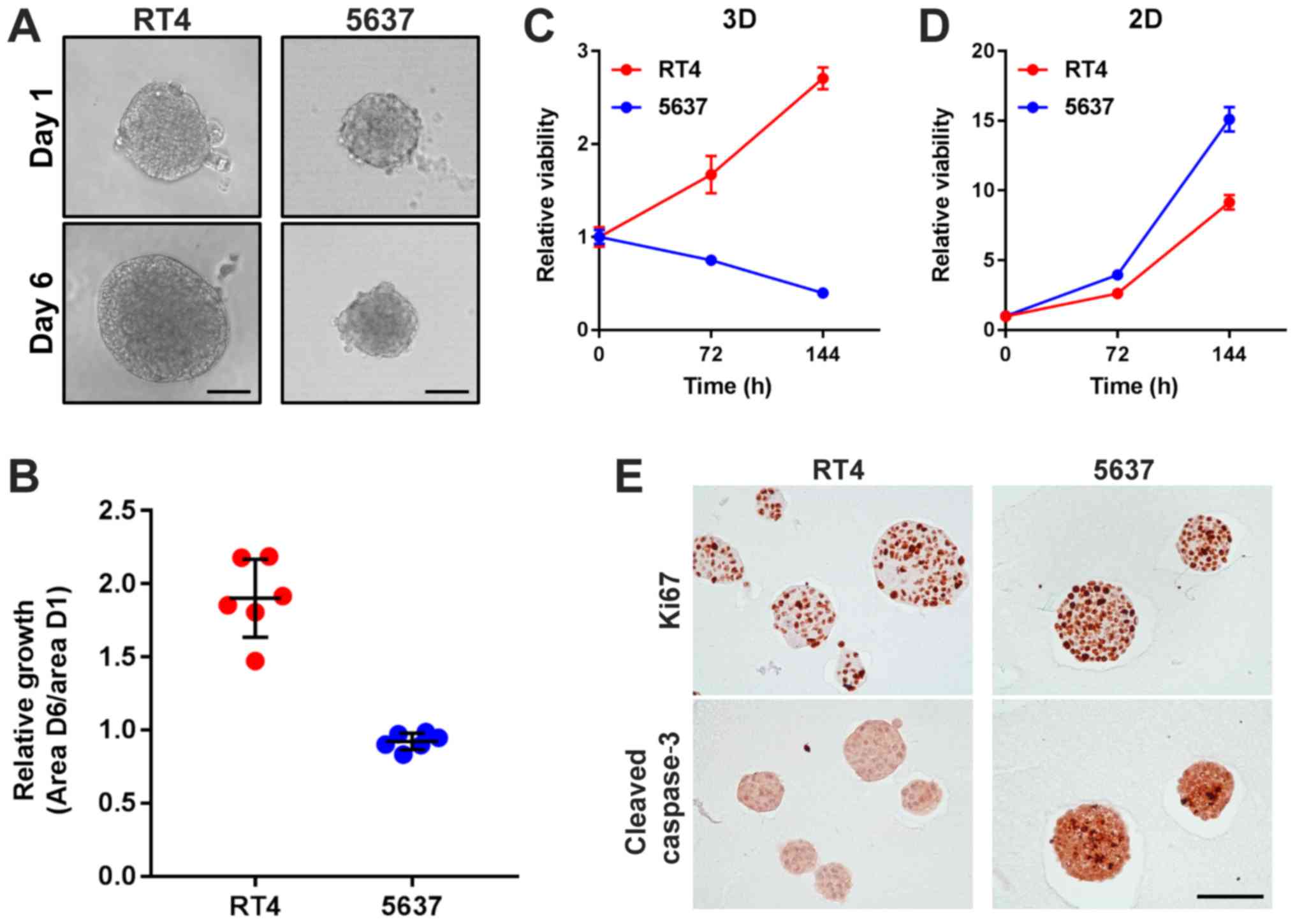
RT4-derived MCSs grow more rapidly than 5637-derived
MCSs, but RT4 proliferates less than 5637 in 2D adherent culture.
Next, cellular ability of proliferation was examined both in 3D
suspension culture and in 2D adherent culture. RT4-derived MCSs
apparently grew over time under microscopic evaluation, while
5637-derived MCSs slightly shrunk over 120 h of culture (Fig. 3A and B). Consistent with the
microscopic findings, cellular viability of RT4-derived MCSs
increased and that of 5637-derived MCSs decreased with time
(Fig 3C). In 2D adherent culture,
cellular viability of both RT4 and 5637 increased with time
(Fig. 3D). Of interest, cell
viability of 5637 decreased with time when cultured as MCSs in
suspension but increased more rapidly than that of RT4 in 2D
culture (Fig. 3C and D).
Immunohistochemistry revealed that Ki67 staining was expressed in
almost all of the cells in both RT4- and 5637-derived MCSs, while
cleaved caspase 3 was profoundly found in 5637-derived MCSs but not
in RT4-derived MCSs (Fig. 3E). These
data suggested that 5637 cells were more susceptible to apoptosis
than RT4 cells in MCSs in suspension, resulting in more efficient
growth of RT4-derived MCSs than 5637-derived MCSs.
Cells in RT4- and 5637-derived MCSs
are more resistant to CDDP and gemcitabine than parental cells
grown in 2D adherent culture
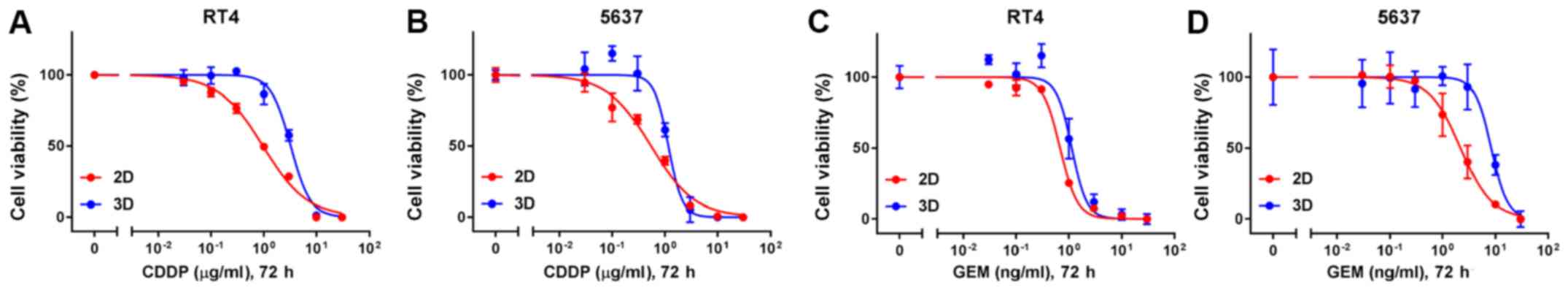
Lastly, chemosensitivity was compared between MCSs
in 3D suspension culture and cells grown in 2D adherent culture.
CDDP and gemcitabine, which are the main chemotherapeutic agents of
the current standard regimen to treat advanced urothelial cancer,
were selected to test. IC50 of CDDP of both RT4- and
5637-derived MCSs were higher than those of parental cells grown in
2D adherent culture (Fig. 4A and B
and Table I). Similarly,
IC50 of gemcitabine of both RT4- and 5637-derived MCSs
were higher than those of parental cells in 2D adherent culture
(Fig. 4C and D and Table I). RT4 cells were more resistant to
CDDP than 5637 cells both in 2D and in 3D cultures (Fig. 4A and B and Table I). Likewise, RT4 cells were more
sensitive to gemcitabine than 5637 cells both in 2D and in 3D
cultures (Fig. 4C and D and Table I). The difference in sensitivity to
CDDP and gemcitabine between RT4 and 5637 cells were more
conspicuous in 3D culture than 2D culture (Table I).
 | Table I.IC50 of CDDP and
gemcitabine on proliferation of RT4 and 5637 cells grown as MCSs in
3D suspension culture and in 2D adherent culture. |
Table I.
IC50 of CDDP and
gemcitabine on proliferation of RT4 and 5637 cells grown as MCSs in
3D suspension culture and in 2D adherent culture.
|
|
| CDDP, µg/ml | Gemcitabine,
ng/ml |
|---|
|
|
|
|
|
|---|
| Cell line | Culture type | IC50 | 95% CI | IC50 | 95% CI |
|---|
| RT4 | 2D | 0.9601 | 0.8388–1.098 | 0.6785 | 0.6159–0.7458 |
|
| 3D | 3.245 | 2.984–3.521 | 1.134 | 0.9862–1.362 |
| 5637 | 2D | 0.5424 | 0.4442–0.6599 | 2.2 | 1.852–2.615 |
|
| 3D | 1.158 | NA-1.317 | 8.309 | 6.662–9.9 |
Discussion
In the present study, we demonstrated that human
bladder cancer cell lines RT4 and 5637 spontaneously aggregated
with each other over time and formed round MCSs by being prevented
from attaching to culture plates and kept in suspension. MCSs were
composed of cells differentially expressing luminal/basal markers.
PPARγ and FOXA1 of luminal markers were less expressed in MCSs than
in parental cells grown in 2D adherent culture. Cells in MCSs in
suspension proliferated less efficiently, and were more resistant
to CDDP and gemcitabine than parental cells grown in 2D
culture.
Morphology of MCSs varies among cell lines, from a
round sphere with a smooth surface to a loose aggregate with
irregular structure (3–7,9,12). Formation of the round contour of MCSs
was apparently associated with the presence of E-cadherin, which is
an adhesion molecule tightening cell-cell contact, in cell lines of
ovarian, colon, and head and neck cancer (3,6,9). Cell lines lacking E-cadherin expression
tended to form loose aggregates in 3D culture (3,6,9). As for bladder cancer, RT4 and 5637 have
E-cadherin expression (13,17), and were able to form round MCSs
(Fig. 1). Functional blocking of
E-cadherin disrupted round MCSs into loose aggregates of 5637 cells
in 3D suspension culture (data not shown). Moreover, J82, which is
one of the mesenchymal cell lines of bladder cancer and lacks a
detectable level of E-cadherin (17), also formed loose aggregates in 3D
suspension culture (13). These data
suggest that adhesion molecules like E-cadherin would be
indispensable to form a round sphere with a smooth surface in 3D
suspension culture.
Cells cultured as MCSs are generally less
proliferative than parental cells grown in 2D adherent culture
irrespectively of methodologies to produce MCSs (3,5,6,9), which
was shown to be true of RT4 and 5637 cells (Fig. 3C and D). Less proliferative ability
of MCSs in suspension could be attributable to the absence of
cellular contact with the extracellular matrix, as the cellular
adhesion to the extracellular matrix like basement membrane
activates intracellular signaling of proliferation (19). Another reason for the less
proliferation of MCSs could be explained by anoikis, a particular
form of apoptosis in epithelial cells induced by the lack of
cellular contact with the extracellular matrix (20). Interestingly, 5637 cells could
proliferate more rapidly than RT4 cells in 2D adherent culture but
were less proliferative as MCSs in 3D suspension culture (Fig. 3C and D). Cleaved caspase 3, an
apoptotic marker, were found only in 5637-derived MCSs but not in
RT4-derived MCSs (Fig. 3E).
Therefore, 5637 cells might be more vulnerable to anoikis than RT4
cells, leading to the dynamic difference of cellular proliferation
between culture conditions.
IC50 of CDDP and gemcitabine of RT4 and
5637 as MCSs were consistently higher than those of parental cells
in 2D adherent culture (Fig. 4 and
Table 1). These results were
expected from the abovementioned less proliferative characteristics
of MCSs because cytotoxic agents typically target rapidly dividing
cells. Intriguingly, in vitro screening experiments
identified cytotoxic drugs especially to slow-proliferating cancer
cells, which are supposedly responsible for resistance to typical
chemotherapeutic agents and late relapse (21). These drugs targeting
slow-proliferating cells might be more effective to RT4 and 5637
cells in 3D culture than in 2D culture, which would indicate the
impact of culture conditions on drug sensitivity of cancer
cells.
We showed that PPARγ and FOXA1 of luminal markers
were expressed less in RT4- and 5637-derived MCSs than in parental
cells grown in 2D culture, while delta N p63 alpha, a
transcriptional factor positively controlling basal cell gene
signature in bladder cancer cells (22), was previously found reversibly
upregulated in MCSs in suspension (14). These findings lead to the hypothesis
that cellular plasticity of bladder cancer cells within the
luminal/basal spectrum could occur depending on culture conditions
(23). Of note, divergent
luminal/basal subtypes were found within the same tumor of human
bladder cancer despite sharing identical genomic alterations,
implicating subtype-specific plasticity of bladder cancer cells
(24). We are currently planning to
perform transcriptome analysis based on our hypothesis to
comprehensively characterize their phenotypic alterations between
culture conditions.
In summary, we described the process of developing
MCSs from two bladder cancer cell lines, RT4 and 5637, using the
aggregation-based method. Molecular and functional characteristics
of RT4 and 5637 cells were found remarkably different between MCSs
in 3D suspension culture and parental cells grown in conventional
2D adherent culture. Culturing cell lines as MCSs in suspension is
a notable platform to decipher alternative aspects of biology of
bladder cancer cells, which could not be unraveled by the
conventional 2D adherent culture.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the grant from the
Greenberg Bladder Cancer Institute.
Availability of data and materials
The datasets used analyzed in the present study are
available from the corresponding author on reasonable request.
Authors' contributions
TY and TJB conceived and designed the study. TY and
XL performed the experiments. TY performed the statistical
analysis. NAS, MK, GJ and DJM contributed to the analysis and
interpretation of the data. TY and TJB wrote the paper. NAS, MK, GJ
and DJM provided discussion and critically revised the paper for
important intellectual content and gave final approval of the paper
to be published. DJM and TJB supervised the study. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sharma SV, Haber DA and Settleman J: Cell
line-based platforms to evaluate the therapeutic efficacy of
candidate anticancer agents. Nat Rev Cancer. 10:241–253. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamada KM and Cukierman E: Modeling tissue
morphogenesis and cancer in 3D. Cell. 130:601–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JM, Mhawech-Fauceglia P, Lee N,
Parsanian LC, Lin YG, Gayther SA and Lawrenson K: A
three-dimensional microenvironment alters protein expression and
chemosensitivity of epithelial ovarian cancer cells in vitro. Lab
Invest. 93:528–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Härmä V, Virtanen J, Mäkelä R, Happonen A,
Mpindi JP, Knuuttila M, Kohonen P, Lötjönen J, Kallioniemi O and
Nees M: A comprehensive panel of three-dimensional models for
studies of prostate cancer growth, invasion and drug responses.
PLoS One. 5:e104312010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luca AC, Mersch S, Deenen R, Schmidt S,
Messner I, Schäfer KL, Baldus SE, Huckenbeck W, Piekorz RP, Knoefel
WT, et al: Impact of the 3D microenvironment on phenotype, gene
expression, and EGFR inhibition of colorectal cancer cell lines.
PLoS One. 8:e596892013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Riedl A, Schlederer M, Pudelko K, Stadler
M, Walter S, Unterleuthner D, Unger C, Kramer N, Hengstschläger M,
Kenner L, et al: Comparison of cancer cells in 2D vs 3D culture
reveals differences in AKT-mTOR-S6K signaling and drug responses. J
Cell Sci. 130:203–218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ekert JE, Johnson K, Strake B, Pardinas J,
Jarantow S, Perkinson R and Colter DC: Three-dimensional lung tumor
microenvironment modulates therapeutic compound responsiveness in
vitro-implication for drug development. PLoS One. 9:e922482014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paullin T, Powell C, Menzie C, Hill R,
Cheng F, Martyniuk CJ and Westerheide SD: Spheroid growth in
ovarian cancer alters transcriptome responses for stress pathways
and epigenetic responses. PLoS One. 12:e01829302017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmidt M, Scholz CJ, Polednik C and
Roller J: Spheroid-based 3-dimensional culture models: Gene
expression and functionality in head and neck cancer. Oncol Rep.
35:2431–2440. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grun B, Benjamin E, Sinclair J, Timms JF,
Jacobs IJ, Gayther SA and Dafou D: Three-dimensional in vitro cell
biology models of ovarian and endometrial cancer. Cell Prolif.
42:219–228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kadletz L, Heiduschka G, Domayer J, Schmid
R, Enzenhofer E and Thurnher D: Evaluation of spheroid head and
neck squamous cell carcinoma cell models in comparison to monolayer
cultures. Oncol Lett. 10:1281–1286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Imamura Y, Mukohara T, Shimono Y,
Funakoshi Y, Chayahara N, Toyoda M, Kiyota N, Takao S, Kono S,
Nakatsura T and Minami H: Comparison of 2D- and 3D-culture models
as drug-testing platforms in breast cancer. Oncol Rep.
33:1837–1843. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshida T, Okuyama H, Nakayama M, Endo H,
Tomita Y, Nonomura N, Nishimura K and Inoue M: Dynamic change in
p63 protein expression during implantation of urothelial cancer
clusters. Neoplasia. 17:574–585. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshida T, Sopko NA, Kates M, Liu X, Joice
G, McConkey DJ and Bivalacqua TJ: Three-dimensional organoid
culture reveals involvement of Wnt/β-catenin pathway in
proliferation of bladder cancer cells. Oncotarget. 9:11060–11070.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshida T, Kates M, Sopko NA, Liu X, Singh
AK, Bishai WR, Joice G, McConkey DJ and Bivalacqua TJ: Ex vivo
culture of tumor cells from N-methyl-N-nitrosourea-induced bladder
cancer in rats: Development of organoids and an immortalized cell
line. Urol Oncol. 36:160.e23–160.e32. 2018. View Article : Google Scholar
|
|
16
|
Okuyama H, Kondo J, Sato Y, Endo H,
Nakajima A, Piulats JM, Tomita Y, Fujiwara T, Itoh Y, Mizoguchi A,
et al: Dynamic change of polarity in primary cultured spheroids of
human colorectal adenocarcinoma and its role in metastasis. Am J
Pathol. 186:899–911. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Warrick JI, Walter V, Yamashita H, Chung
E, Shuman L, Amponsa VO, Zheng Z, Chan W, Whitcomb TL, Yue F, et
al: Cooperate to drive luminal subtype in bladder cancer: A
molecular analysis of established human cell lines. Sci Rep.
6:385312016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Robertson AG, Kim J, Al-Ahmadie H,
Bellmunt J, Guo G, Cherniack AD, Hinoue T, Laird PW, Hoadley KA,
Akbani R, et al: Comprehensive molecular characterization of
muscle-invasive bladder cancer. Cell. 174:10332018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pickup MW, Mouw JK and Weaver VM: The
extracellular matrix modulates the hallmarks of cancer. EMBO Rep.
15:1243–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frisch SM and Francis H: Disruption of
epithelial cell-matrix interactions induces apoptosis. J Cell Biol.
124:619–626. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kondoh E, Mori S, Yamaguchi K, Baba T,
Matsumura N, Cory Barnett J, Whitaker RS, Konishi I, Fujii S,
Berchuck A and Murphy SK: Targeting slow-proliferating ovarian
cancer cells. Int J Cancer. 126:2448–2456. 2010.PubMed/NCBI
|
|
22
|
Choi W, Porten S, Kim S, Willis D, Plimack
ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee IL, et al:
Identification of distinct basal and luminal subtypes of
muscle-invasive bladder cancer with different sensitivities to
frontline chemotherapy. Cancer Cell. 25:152–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SH, Hu W, Matulay JT, Silva MV,
Owczarek TB, Kim K, Chua CW, Barlow LJ, Kandoth C, Williams AB, et
al: Tumor evolution and drug response in patient-derived organoid
models of bladder cancer. Cell. 173:515–528. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hovelson DH, Udager AM, McDaniel AS,
Grivas P, Palmbos P, Tamura S, Lazo de la Vega L, Palapattu G,
Veeneman B, El-Sawy L, et al: Targeted DNA and RNA sequencing of
paired urothelial and squamous bladder cancers reveals discordant
genomic and transcriptomic events and unique therapeutic
implications. Eur Urol. 74:741–753. 2018. View Article : Google Scholar : PubMed/NCBI
|