Introduction
Small cell lung cancer (SCLC) represents 13–15% of
all lung cancer diagnoses in the United States between 2006 and
2017 (1,2). Similar to non-small cell lung cancer,
the primary risk factor for SCLC remains smoking tobacco (3). Early diagnosis of SCLC is challenging
due to the lack of specific symptoms and its extremely aggressive
nature which is characterized by rapid tumor growth, quick doubling
time and early metastasis. A statistically significant improvement
in 2- and 5-year survival in limited- and extensive-stage SCLC
cohorts from the Surveillance, Epidemiology, and End Results (SEER)
Registry database, analyzed by Joinpoint regression, has been
reported (1). However, the prognosis
of patients with SCLC is poor with a 5-year survival rate of <5%
and an average overall survival time of only 2–4 months for
patients who do not receive effective treatment (4). At present, platinum and etoposide
remain the preferred first-line chemotherapy regimen for the
treatment of extensive-stage SCLC, and surgery is recommended for
patients with limited-stage SCLC (5). Although SCLC is sensitive to
chemotherapy and radiotherapy, the majority of patients relapse or
progress after first-line therapy. Surgery in the form of lobectomy
is a potential option for TNM stage I (T1-2N0M0) without
mediastinal or supraclavicular involvement (6). However, the role of surgery in SCLC
treatment remains controversial. Therefore, the present study
performed a period propensity score matching analysis using the
SEER database to examine the effects of surgery on survival in
patients with SCLC.
Materials and methods
Patients and methods
This was a retrospective, population-based study
using cases registered in the SEER database made publicly available
through online access. Data were retrieved using the Surveillance
Research Program, National Cancer Institute SEER*Stat software
(seer.cancer.gov/seerstat) version 8.3.5.
Informed consent from the study population was waived, as the
authors had no access to the identities of the patients, and no
identifiable patient information was included.
Data collection
The following database was used for selection of
cases: SEER Program (www.seer.cancer.gov) SEER*Stat Database:
Incidence-SEER 18 Regs Research Data + Hurricane Katrina Impacted
Louisiana Cases, Nov 2017 Sub (2000–2015) <Katrina/Rita
Population Adjustment> - Linked To County Attributes-Total U.S.,
1969–2016 Counties, National Cancer Institute, DCCPS, Surveillance
Research Program, released April 2018, based on the November 2017
submission. Only patients with SCLC [based on International
Classification of Diseases for Oncology, 3rd edition (ICD-O-3)
(7) codes: 8041/3-8045/3] between
January 2010 and December 2015 were included in the present study.
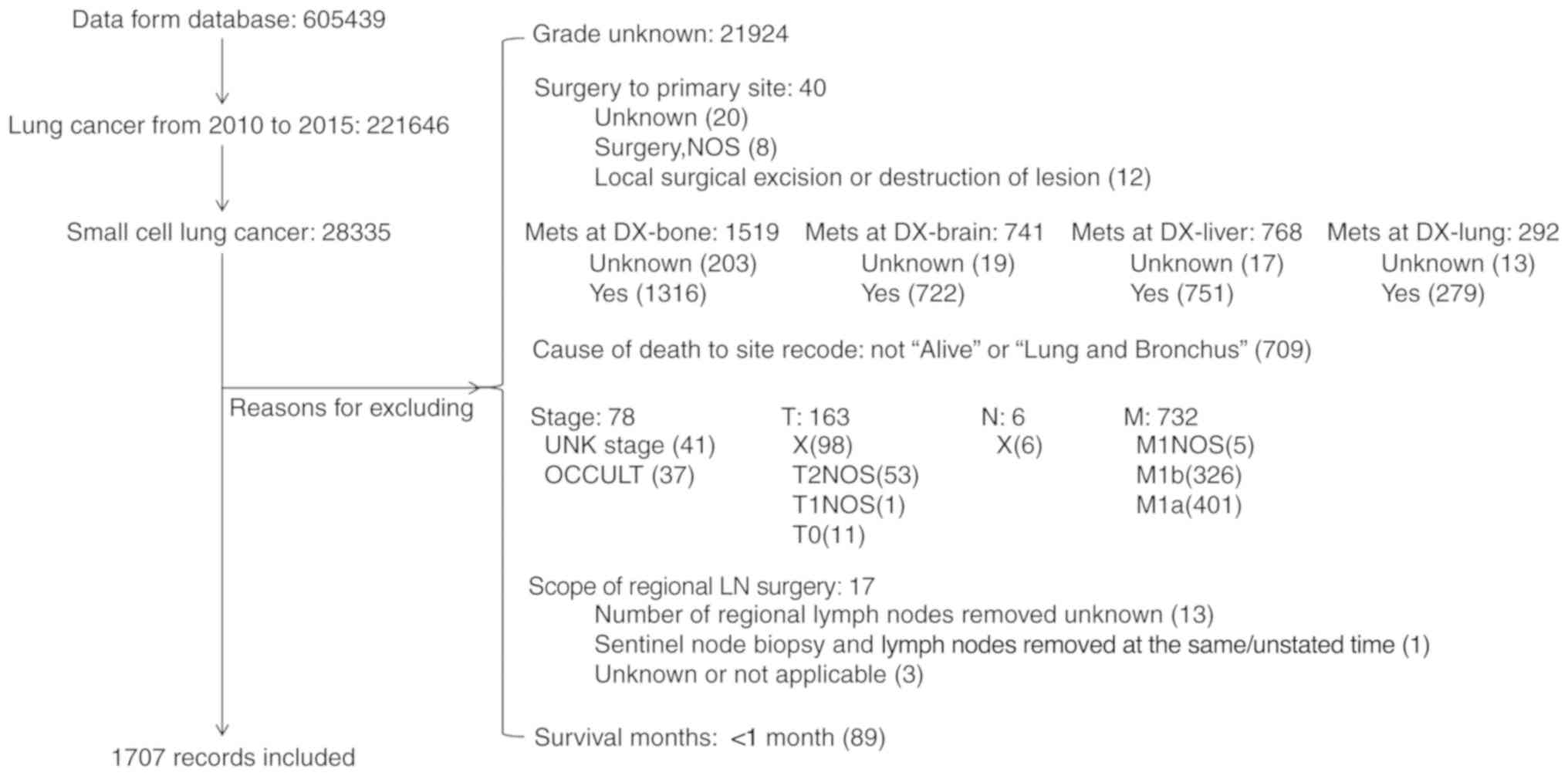
The exclusion criteria were: i) An ambiguous or unknown
classification of observed clinical characteristics, ii) cause of
death to site (COD) recode not as ‘Alive’ or ‘Lung and Bronchus’,
iii) distant metastasis at the brain, liver and lung, iv) M1, v) T0
and finally vi) a survival time of <1 month (Fig. 1). Individual data for each case were
retrieved from the database including sex, age at diagnosis, race,
histology, grade, surgery to primary site (SPS),
Tumor-Node-Metastasis (TNM) stage (8), COD and survival time.
Subgroup definitions
In the SEER database, grades were recorded as
follows: i) Grade I, well differentiated; ii) grade II, moderately
differentiated; iii) grade III, poorly differentiated and iv)
undifferentiated and anaplastic. SPS was divided into: i)
Non-surgery, no surgery of primary site; ii) Sublobectomy,
Partial/Wedge/Segmental Resection, Lingulectomy, Partial Lobectomy,
Sleeve Resection iii) lobe/s, lobectomy or bilobectomy; iv)
Pneumonectomy. The T, N, M and Stage were recorded in the database
accordingly to the AJCC cancer staging manual, 7th ed. (8).
Statistical analyses
Statistical analysis were performed using Stata 15.0
(Stata Corp. LLC). The propensity score matching was performed
using the ‘psmatch2’ module in the software. Student's t-test was
used to analyze the differences of means between two samples.
Differences of cause-specific survival (CSS) between subgroups and
the role of surgery in each subgroup was estimated using the
Kaplan-Meier product method and compared by a log-rank test. Cox
regression analysis was used to evaluate the effects of multiple
variables on survival. The difference of incidence of COD was
examined using χ2 test. Quantitative data were converted
into categorical data, with the exception of survival time. All
statistical tests were two-sided and P<0.05 was considered to
indicate a statistically significant difference.
Results
Patients selection and demography of
included patients
Based on the patient selection criteria described
previously (Fig. 1), 221,646 records
of lung cancer between January 2010 and December 2015 were
identified from the SEER database. Among them, 28,335 (12.78%) were
SCLC, and 1,707 met the inclusion criteria of the present study and
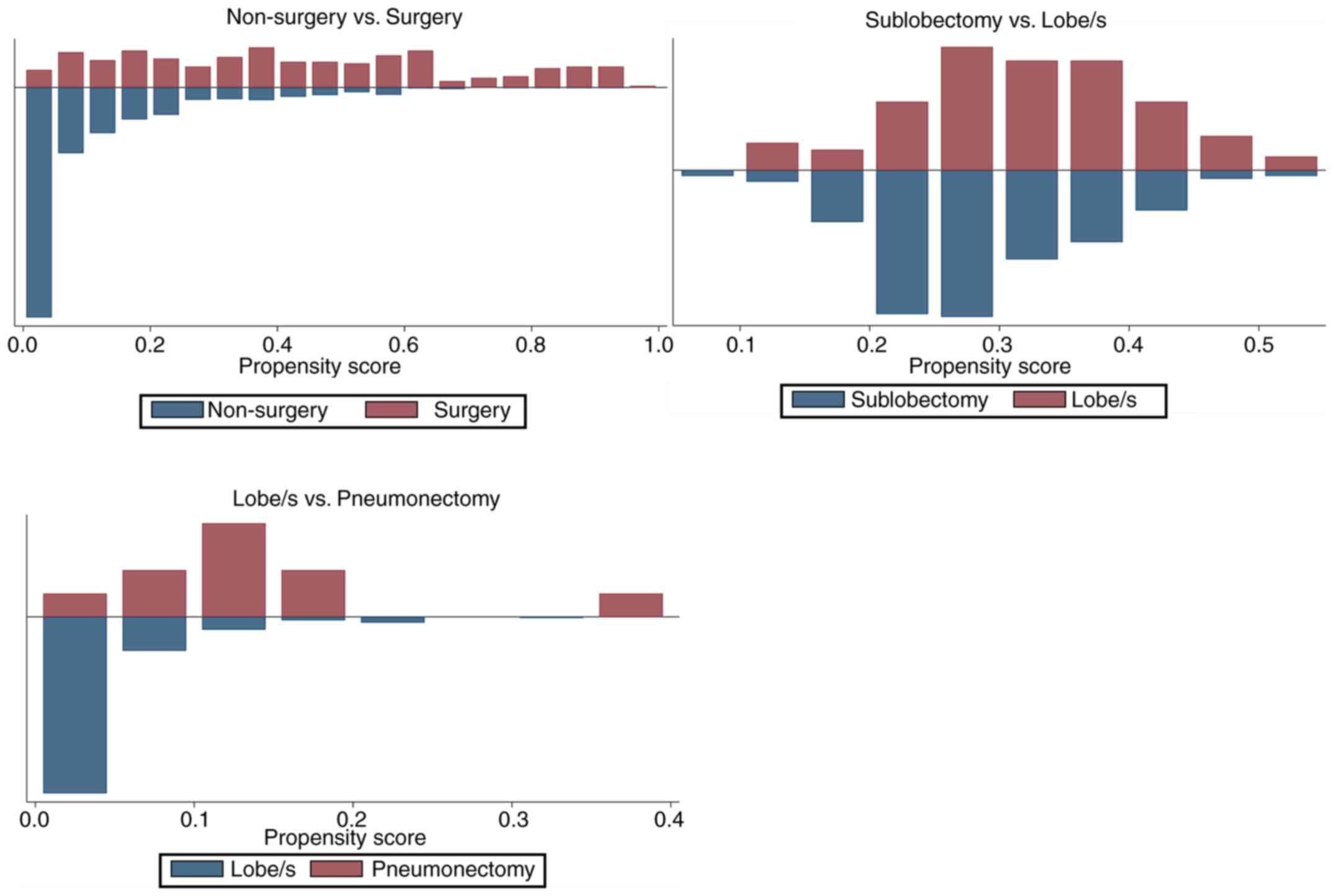
were subsequently extracted for analysis. After propensity score
matching, 294 pairs were selected for comparison between
non-surgery and surgery, 84 pairs were selected for comparison
between sublobectomy and lobe/s and 10 pairs were selected for
comparison between lobe/s and pneumonectomy. The results of the
propensity score matching are presented in Tables I and II, and Fig.
2. Following matching, the clinicopathological features of
grade, histology, stage, T and N were balanced in the non-surgery
vs. surgery group, as well as T in the sub-lobectomy vs. lobe/s
group and T and stage in the lobe/s vs. pneumonectomy group
(P>0.05 in the matched groups; P<0.05 in the unmatched
groups) (Table I). The mean and
median biases in the matched groups were lower compared with those
in the unmatched groups, and the overall differences in the
clinicopathological features between the three different surgical
groups were statistically insignificant (P=0.13, 0.96 and 0.28,
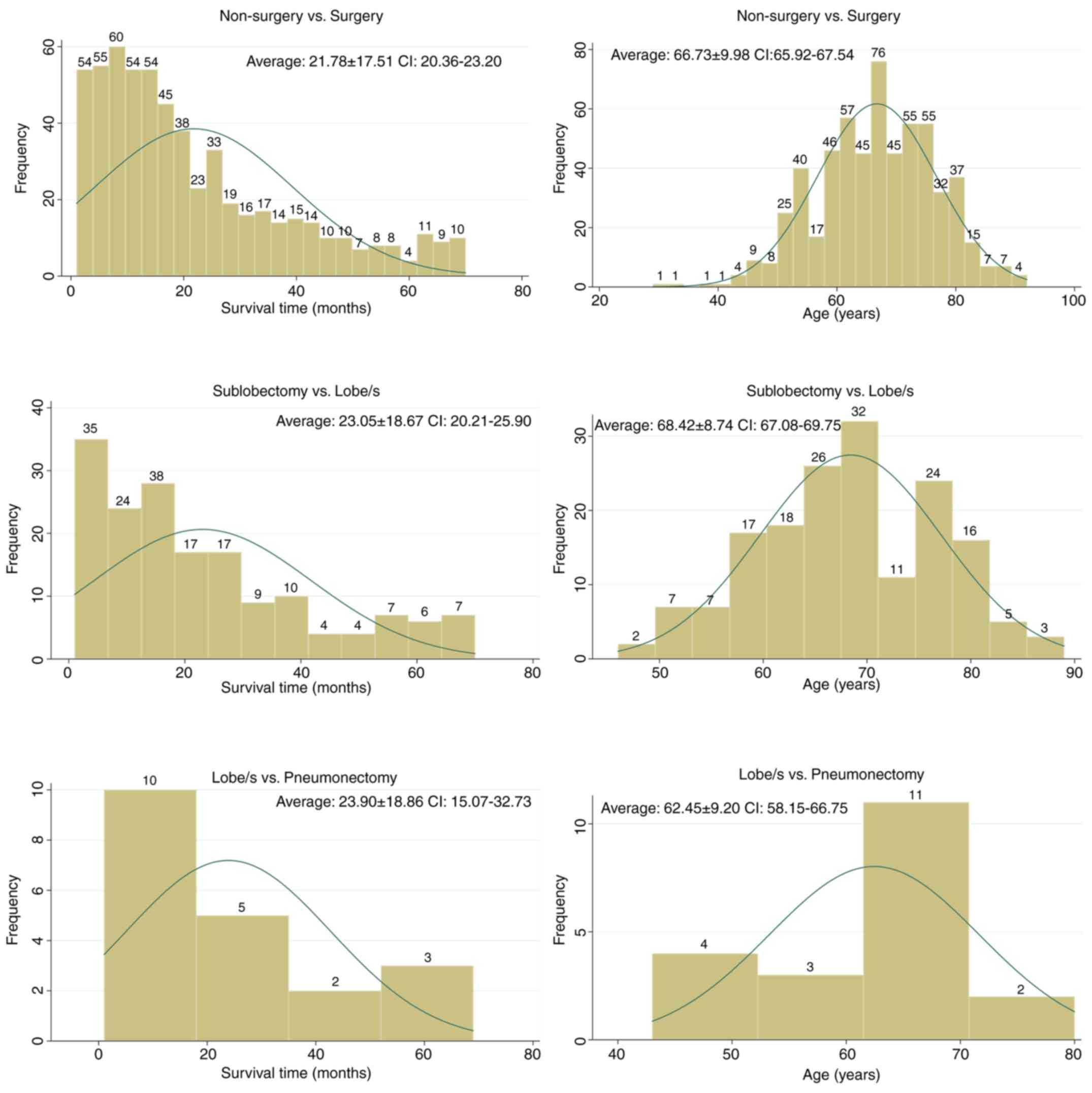
respectively) (Table II; Fig. 2). Fig.
3 shows the distribution of survival time and age for each
surgical group.
 | Table I.Propensity score matching test between
the surgery groups. |
Table I.
Propensity score matching test between
the surgery groups.
|
|
|
| Mean |
|
| t-test |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Surgery | Features | Un/Matched | Treated | Control | %bias | %reduct |bias| | t | P-value | V(T)/V(C) |
|---|
| Non-surgery vs.
surgery | Sex | U | 0.43197 | 0.43100 | 0.2 |
| 0.03 | 0.976 | . |
|
|
| M | 0.43197 | 0.39116 | 8.2 | −4,086.7 | 1.00 | 0.315 | . |
|
| Age | U | 4.19730 | 4.19670 | 0.1 |
| 0.01 | 0.994 | 0.79a |
|
|
| M | 4.19730 | 4.37070 | −17.3 | −32,360.8 | −2.15 | 0.032 | 0.87 |
|
| Grade (8) | U | 3.35710 | 3.56480 | −33.3 |
| −5.66 | <0.001 | 1.63a |
|
|
| M | 3.35710 | 3.30270 | 8.7 | 73.8 | 0.92 | 0.356 | 0.90 |
|
| Histology | U | 2.07480 | 1.16140 | 67.6 |
| 14.41 | <0.001 | 5.82a |
|
|
| M | 2.07480 | 1.91160 | 12.1 | 82.1 | 1.16 | 0.248 | 1.14 |
|
| Stage (8) | U | 2.75170 | 4.77570 | −131.9 |
| −21.61 | <0.001 | 1.33a |
|
|
| M | 2.75170 | 2.57480 | 11.5 | 91.3 | 1.29 | 0.197 | 0.96 |
|
| T (8) | U | 2.54080 | 4.29650 | −113.3 |
| −17.05 | <0.001 | 0.80 |
|
|
| M | 2.54080 | 2.43200 | 7.0 | 93.8 | 0.90 | 0.366 | 1.00 |
|
| N (8) | U | 0.60884 | 1.61220 | −110.9 |
| −16.30 | <0.001 | 0.68a |
|
|
| M | 0.60884 | 0.54082 | 7.5 | 93.2 | 1.01 | 0.313 | 0.98 |
|
| Race | U | 4.75170 | 4.68440 | 8.1 |
| 1.23 | 0.217 | 0.84 |
|
|
| M | 4.75170 | 4.73810 | 1.6 | 79.8 | 0.21 | 0.836 | 0.98 |
| Sub-lobectomy vs.
lobe/s | Sex | U | 0.40476 | 0.45000 | −9.1 |
| −0.70 | 0.485 | . |
|
|
| M | 0.40476 | 0.38095 | 4.8 | 47.4 | 0.31 | 0.754 | . |
|
| Age | U | 4.29760 | 4.17000 | 13.3 |
| 1.06 | 0.290 | 1.36 |
|
|
| M | 4.29760 | 4.20240 | 10.0 | 25.4 | 0.63 | 0.529 | 1.22 |
|
| Grade (8) | U | 3.40480 | 3.35000 | 7.9 |
| 0.61 | 0.541 | 1.03 |
|
|
| M | 3.40480 | 3.36900 | 5.2 | 34.8 | 0.33 | 0.742 | 0.97 |
|
| Histology | U | 1.85710 | 2.12500 | −15.6 |
| −1.18 | 0.240 | 0.85 |
|
|
| M | 1.85710 | 1.61900 | 13.8 | 11.1 | 0.99 | 0.323 | 1.29 |
|
| Stage (8) | U | 2.64290 | 2.74500 | −6.0 |
| −0.48 | 0.631 | 1.37 |
|
|
| M | 2.64290 | 2.55950 | 4.9 | 18.4 | 0.32 | 0.751 | 1.34 |
|
| T (8) | U | 2.22620 | 2.60500 | −25.8 |
| −2.04 | 0.042 | 1.29 |
|
|
| M | 2.22620 | 2.20240 | 1.6 | 93.7 | 0.11 | 0.916 | 1.29 |
|
| N (8) | U | 0.59524 | 0.60500 | −1.2 |
| −0.09 | 0.927 | 1.43 |
|
|
| M | 0.59524 | 0.58333 | 1.4 | −22.0 | 0.09 | 0.929 | 1.34 |
|
| Race | U | 4.83330 | 4.73500 | 13.3 |
| 0.98 | 0.326 | 0.65 |
|
|
| M | 4.83330 | 4.88100 | −6.5 | 51.6 | −0.50 | 0.616 | 1.33 |
| Lobe/s vs.
pneumonectomy | Sex | U | 0.30000 | 0.45000 | −30.6 |
| −0.90 | 0.350 | . |
|
|
| M | 0.30000 | 0.60000 | −61.1 | −100.0 | −1.34 | 0.196 | . |
|
| Age | U | 3.90000 | 4.17000 | −23.4 |
| −0.90 | 0.360 | 2.42 |
|
|
| M | 3.90000 | 4.20000 | −26.0 | −11.1 | −0.60 | 0.556 | 3.02 |
|
| Grade (8) | U | 3.10000 | 3.35000 | −31.8 |
| −1.10 | 0.270 | 1.63 |
|
|
| M | 3.10000 | 3.20000 | −12.7 | 60.0 | −0.25 | 0.806 | 0.91 |
|
| Histology | U | 2.90000 | 2.12500 | 40.6 |
| 1.30 | 0.190 | 1.28 |
|
|
| M | 2.90000 | 3.40000 | −26.2 | 35.5 | −0.55 | 0.591 | 0.96 |
|
| Stage (8) | U | 3.80000 | 2.74500 | 66.5 |
| 2.10 | 0.040 | 1.09 |
|
|
| M | 3.80000 | 3.80000 | 0.0 | 100.0 | 0.00 | 1.000 | 1.2 |
|
| T (8) | U | 3.90000 | 2.60500 | 87.1 |
| 2.90 | <0.001 | 1.35 |
|
|
| M | 3.90000 | 4.00000 | −6.7 | 92.3 | −0.14 | 0.891 | 0.95 |
|
| N (8) | U | 0.80000 | 0.60500 | 25.0 |
| 0.80 | 0.440 | 1.05 |
|
|
| M | 0.80000 | 0.80000 | 0.0 | 100.0 | 0.00 | 1.000 | 1.56 |
|
| Race | U | 4.40000 | 4.73500 | −31.5 |
| −1.20 | 0.220 | 2.43 |
|
|
| M | 4.40000 | 4.10000 | 28.2 | 10.4 | 0.53 | 0.605 | 0.97 |
 | Table II.The efficacy of the propensity score
matching. |
Table II.
The efficacy of the propensity score
matching.
| Group | Sample (n) | Ps R2 | LR
χ2 |
P>χ2 | MeanBias | MedBias | B | R | %Var |
|---|
| Non-surgery vs.
surgery | Unmatched
(1,413) | 0.309 | 484.81 | <0.01 | 58.20 | 50.50 | 157.8a | 1.15 | 71.00 |
|
| Matched (294) | 0.015 | 12.59 | 0.13 | 9.30 | 8.50 | 29.4a | 1.22 | 0.00 |
| Sublobectomy vs.
lobe/s | Unmatched
(200) | 0.030 | 10.19 | 0.25 | 11.50 | 11.20 | 41.5a | 1.09 | 0.00 |
|
| Matched (84) | 0.011 | 2.53 | 0.96 | 6.00 | 5.10 | 24.50 | 1.40 | 0.00 |
| Lobe/s vs.
pneumonectomy | Unmatched
(200) | 0.161 | 12.98 | 0.11 | 42.10 | 31.70 | 111.8a | 1.78 | 0.00 |
|
| Matched (10) | 0.353 | 9.78 | 0.28 | 20.10 | 19.40 | 109.9a | 7.50* | 0.00 |
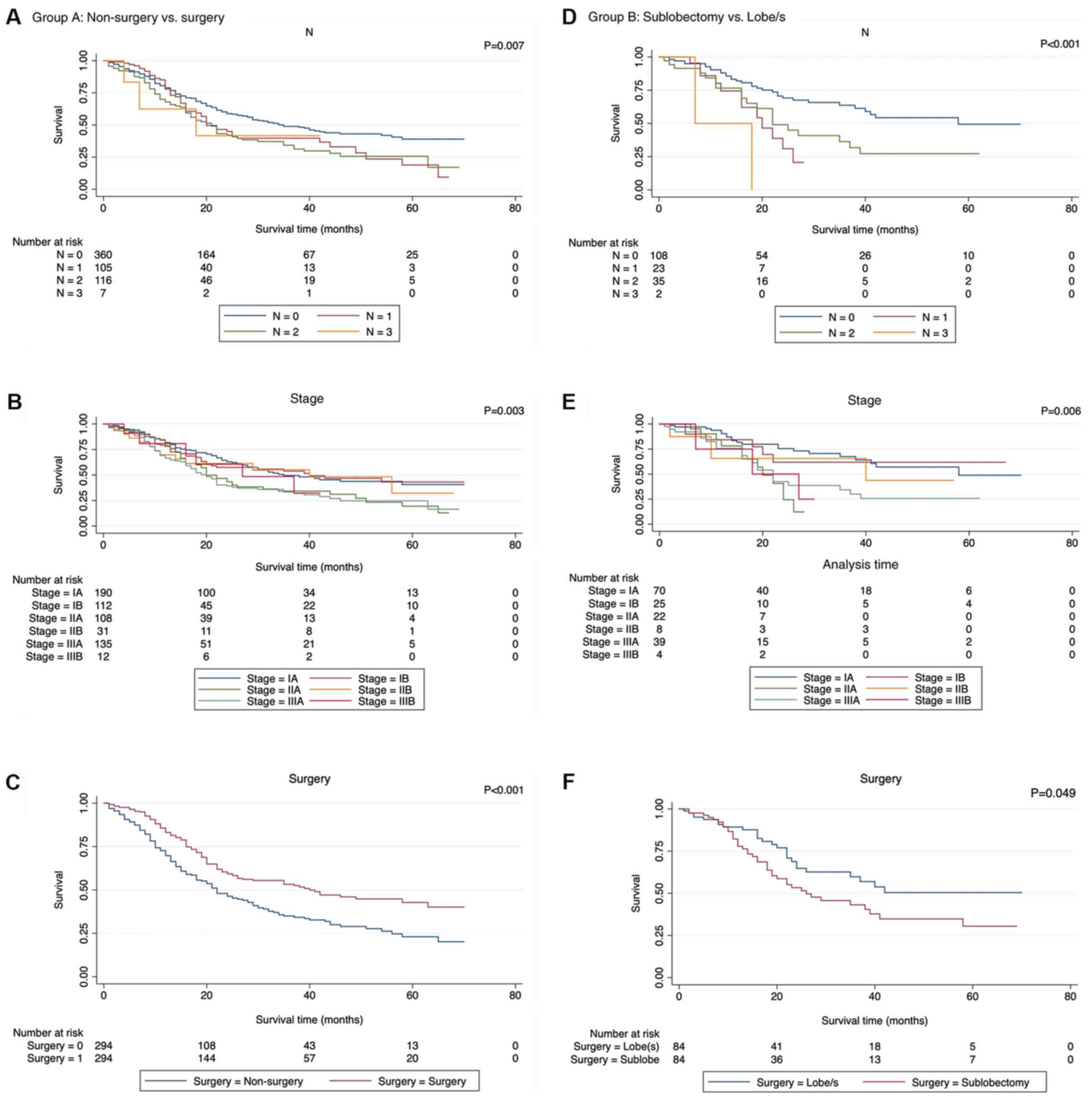
Role of surgery in SCLC
The potential prognostic factors were analyzed by
univariate analysis using the Kaplan-Meier method and were compared
with the log-rank test (Table
III), which revealed that there were significant differences in
CSS in N (P=0.01), Stage (P<0.001), and Surgery (P<0.001)
when comparing non-surgery with surgery, and in N (P<0.001),
Stage (P=0.006), and Surgery (P=0.049) when comparing sublobectomy
with lobe/s (Fig. 4). However, the
difference was not significant when comparing lobe/s with
pneumonectomy. Cox regression analysis, (Table IV), which included all
characteristics for clinical purposes revealed that the differences
were significant for age [P<0.001; hazard ratio (HR)=1.21, 95%
confidence interval (CI) 1.07–1.36] and surgery (P<0.001, HR
0.59, 95% CI, 0.47–0.76) when comparing non-surgery with surgery.
However, no significant differences were detected when comparing
sublobectomy with lobe/s, and lobe/s with pneumonectomy. The
results of the survival functions of the clinicopathological
features stratified by surgery are presented in Table V and Fig.
5. No statistical analysis was performed for certain
subclinical features since the testing was not possible when no
failures were observed and/or no observation was present in the
database. There were significant differences among subgroups of
50–60 years, 70–80 years in age; male, female in sex; Caucasian in
race; small cell cancer, not otherwise specified (NOS) in
histology; grade III in grade; T1a in T; N0 in N; IA, IB in stage
when comparing non-surgery with surgery. Significant differences
were detected between 80–90 years in age and N0, N1 in N; IIA in
stage when comparing sublobectomy with lobe/s (Table V). Although statistically
insignificant, more patients in the following clinicopathological
subgroups had survival benefits from surgery compared with
non-surgery (Fig. 6): 60–70 and
80–90 years in age; Hispanic and African descent in Race; oat cell
carcinoma, small cell carcinoma with intermediate cell, and
combined small cell carcinoma in histology; Grade I, II, IV; T1b
and T2a. Similar survival benefits in patients who received lobe/s
compared with sublobectomy were observed in the following
clinicopathological subgroups (Fig.
7): 60–70 years, 70–80 years in age; male and female in sex;
African descent and Caucasian in race; small cell carcinoma with
NOS and combined small cell carcinoma in histology; Grade III and
IV; T1a and T1b. Generally, patients who did not receive surgery
(P<0.001) or received sublobectomy (P=0.03) were at an increased
risk of mortality when compared with patients who received surgery
or lobe/s respectively (Table VI).
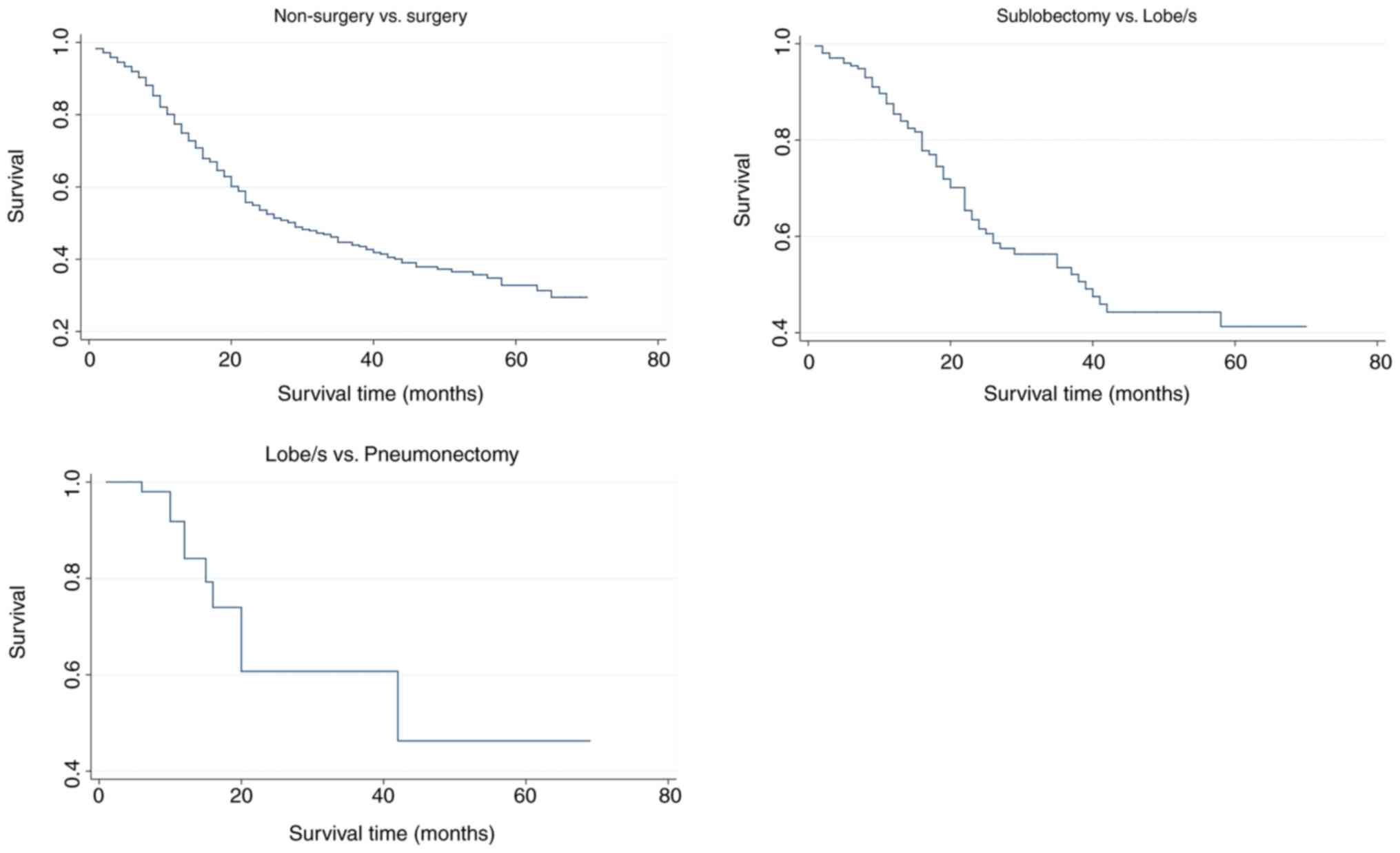
Fig. 8 shows the cumulative survival
curves of each group.
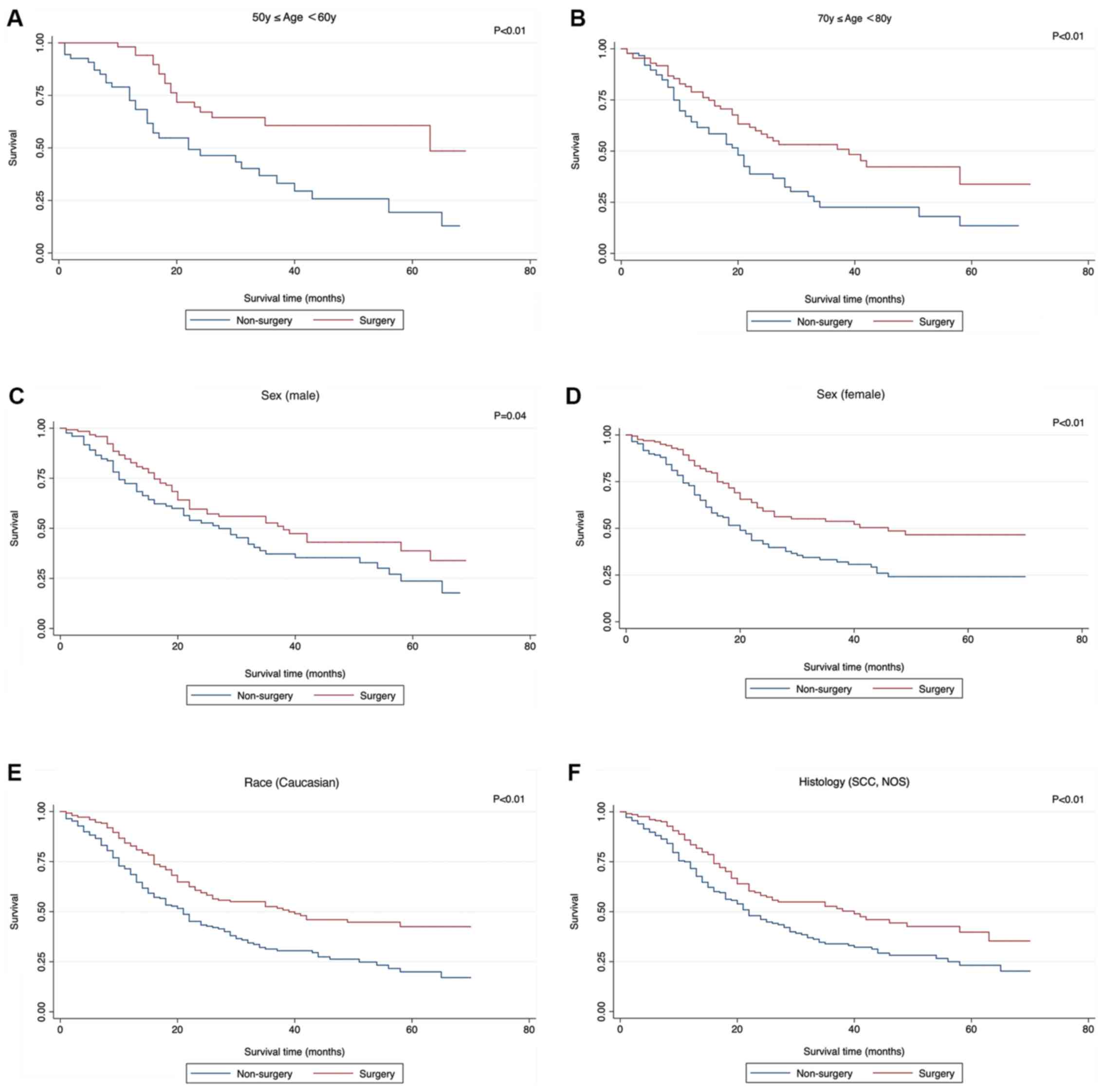 | Figure 5.Significant survival functions of
surgery stratified by clinicopathological features when comparing
Non-surgery with surgery. (A) Age, ≥60 and <70 years; (B) age,
≥70 and <80 years; (C) sex, male; (D), sex, female; (E) race,
Caucasian; (F) histology, SCC, NOS; SCC, small cell cancer; NOS,
not otherwise specified. (G) grade III; (H) T1a; (I) N0; (J) Stage
IA; (K) Stage IB. SCC, small cell cancer; NOS, not otherwise
specified. |
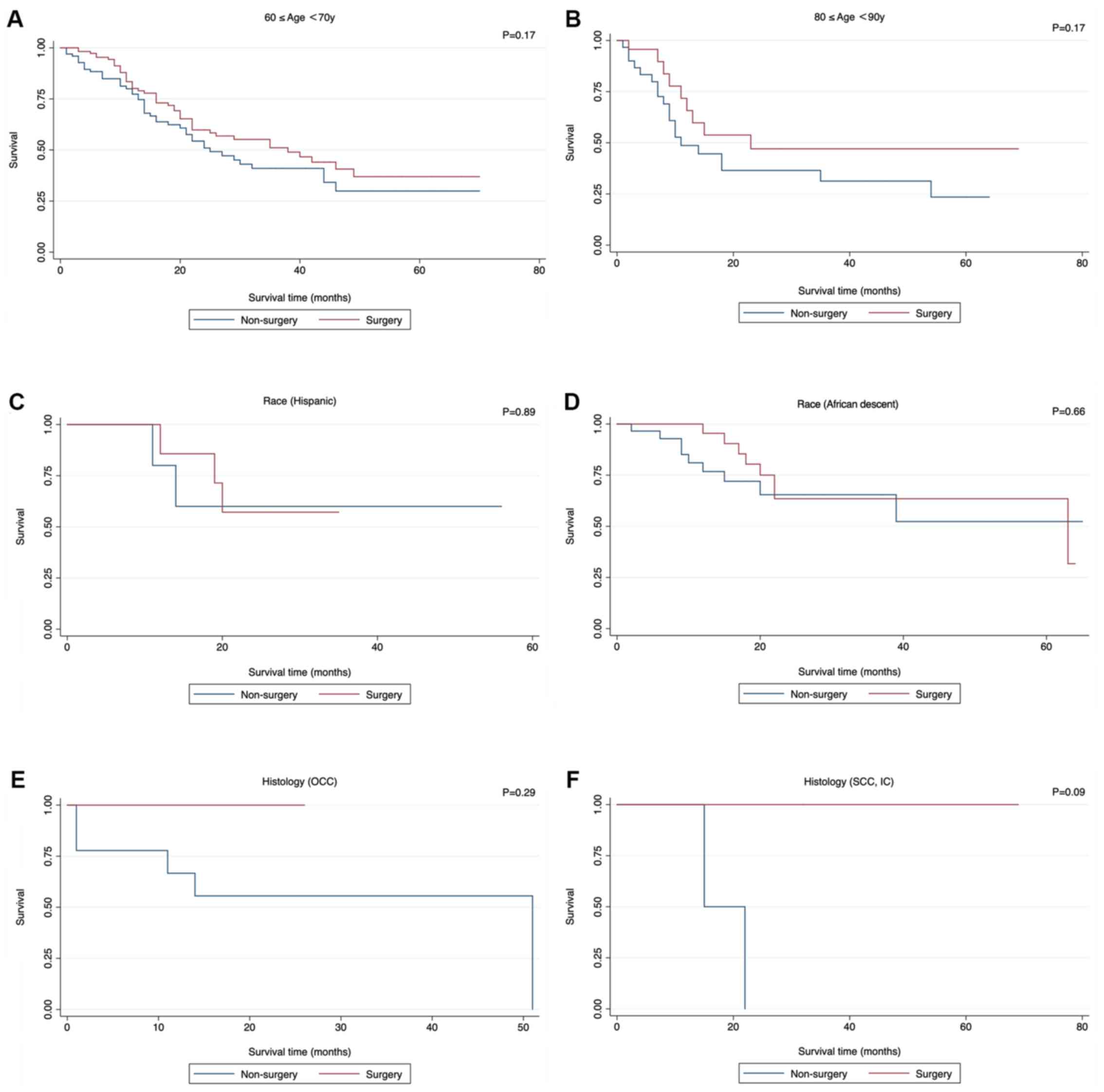 | Figure 6.Insignificant survival functions of
surgery stratified by clinicopathological features when comparing
Non-surgery with surgery. (A) Age, ≥60 and <70 years; (B) age,
≥80 and <90 years; (C) race, Hispanic; (D); race, African
descent; (E); histology, OCC; (F) histology, SCC, IC. OCC, Oat cell
carcinoma; SCC, IC, Small cell carcinoma, Intermediate cell; CSCC,
Combined small cell carcinoma. (G) Histology (CSCC); (H) Grade I;
(I) Grade II; (J) Grade IV; (K) T1b; (L) T2a. CSCC, Combined small
cell carcinoma. |
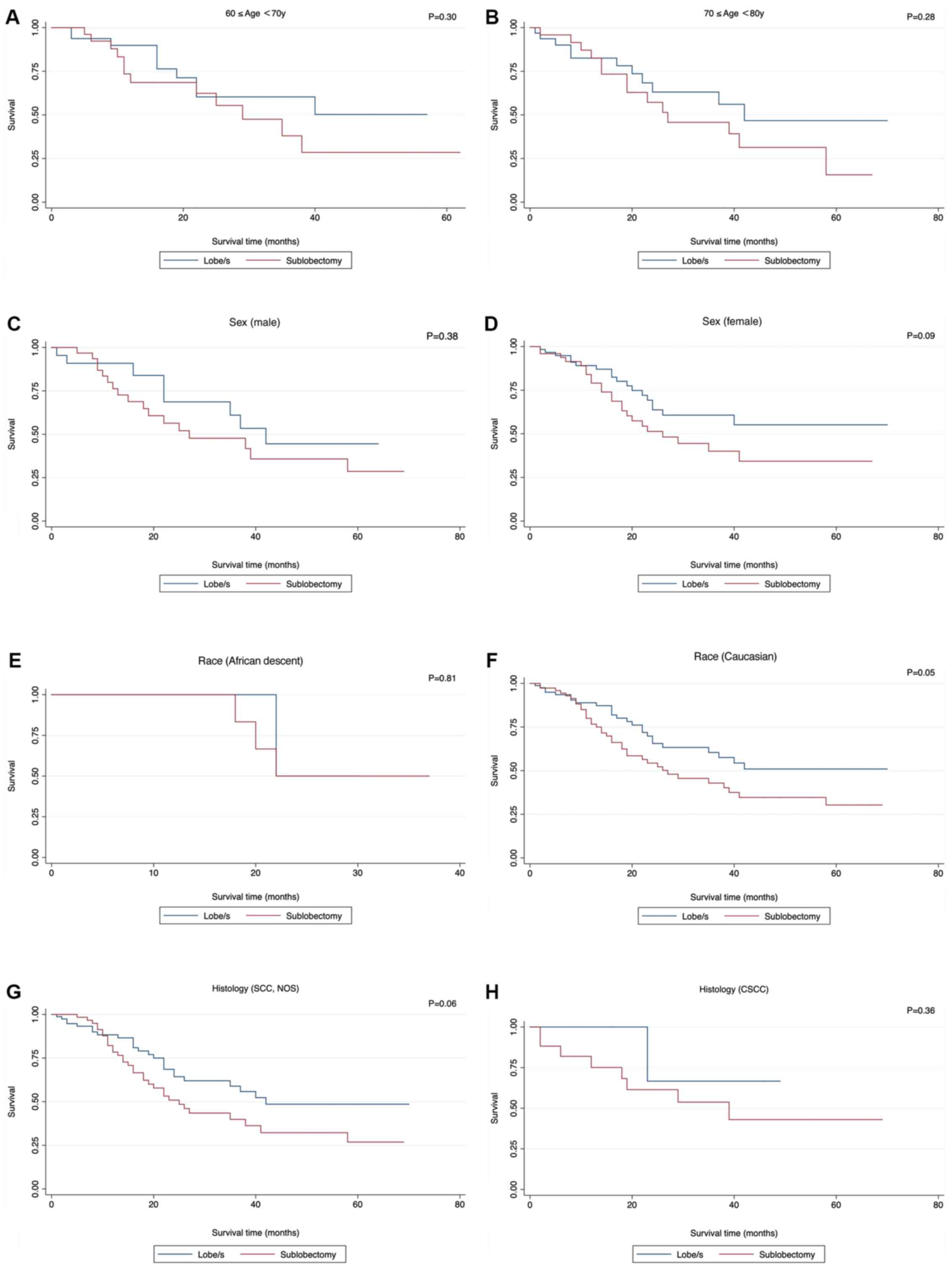 | Figure 7.Insignificant survival functions of
surgery stratified by clinicopathological features when comparing
Sublobectomy with Lobe/s. (A) Age, ≥60 and <70 years; (B) age,
≥70 and <80 years; (C) sex, male; (D), sex, female; (E) race,
African descent; (F) race, Caucasian; (G) histology, SCC, NOS; (H)
histology, CSCC; SCC, small cell cancer; NOS, not otherwise
specified; CSCC, combined small cell carcinoma. (I) grade III; (J)
grade IV; (K) T1a; (L) T1b. SCC, small cell cancer; NOS, not
otherwise specified; CSCC, combined small cell carcinoma. |
 | Table III.Demographics of the included patients
and results of univariate analysis. |
Table III.
Demographics of the included patients
and results of univariate analysis.
| A, Non-surgery vs.
surgery group |
|---|
|
|---|
| Features | NP | NE | NEE | P-value |
|---|
| Age, years |
|
|
| 0.341 |
|
<40 | 3 | 2 | 2.02 |
|
| ≥40,
<50 | 22 | 10 | 12.62 |
|
| ≥50,
<60 | 114 | 51 | 60.07 |
|
| ≥60,
<70 | 214 | 91 | 98.62 |
|
| ≥70,
<80 | 178 | 92 | 79.70 |
|
| ≥80,
<90 | 53 | 28 | 21.19 |
|
| ≥90,
<100 | 4 | 2 | 1.78 |
|
| Sex |
|
|
| 0.714 |
|
Male | 252 | 118 | 120.99 |
|
|
Female | 336 | 158 | 155.01 |
|
| Race |
|
|
| 0.279 |
|
Hispanic | 15 | 5 | 7.69 |
|
|
AI/AN | 2 | 0 | 0.09 |
|
|
API | 12 | 7 | 5.15 |
|
| African
descent | 52 | 17 | 27.18 |
|
|
Caucasian | 506 | 247 | 235.81 |
|
|
Unknown | 1 | 0 | 0.09 |
|
| Histology |
|
|
| 0.678 |
| SCC,
NOS | 462 | 222 | 213.83 |
|
|
OCC | 11 | 5 | 5.69 |
|
| SCC,
IC | 4 | 2 | 2.89 |
|
|
CSCC | 111 | 47 | 53.59 |
|
| Grade (8) |
|
|
| 0.149 |
| I | 10 | 2 | 3.93 |
|
| II | 23 | 7 | 14.32 |
|
|
III | 281 | 127 | 125.92 |
|
| IV | 274 | 140 | 131.83 |
|
| T (8) |
|
|
| 0.135 |
|
T1a | 189 | 86 | 92.83 |
|
|
T1b | 128 | 60 | 63.02 |
|
|
T2a | 173 | 77 | 79.92 |
|
|
T2b | 29 | 19 | 10.54 |
|
| T3 | 53 | 25 | 21.82 |
|
| T4 | 16 | 9 | 7.86 |
|
| N (8) |
|
|
| 0.007 |
| 0 | 360 | 150 | 176.48 |
|
| 1 | 105 | 54 | 45.92 |
|
| 2 | 116 | 69 | 51.28 |
|
| 3 | 7 | 3 | 2.33 |
|
| Stage (8) |
|
|
| 0.003 |
| IA | 190 | 77 | 99.00 |
|
| IB | 112 | 44 | 53.56 |
|
|
IIA | 108 | 56 | 45.88 |
|
|
IIB | 31 | 13 | 13.80 |
|
|
IIIA | 135 | 80 | 57.93 |
|
|
IIIB | 12 | 6 | 5.84 |
|
| Surgery |
|
|
| <0.001 |
| No | 294 | 161 | 125.40 |
|
|
Yes | 294 | 115 | 150.60 |
|
| B, Sublobectomy
vs. lobe/s group |
|
|
Features | NP | NE | NEE | P-value |
|
| Age, years |
|
|
| 0.384 |
|
<40 | N/A | N/A | N/A |
|
| ≥40,
<50 | 2 | 2 | 0.79 |
|
| ≥50,
<60 | 28 | 9 | 13.77 |
|
| ≥60,
<70 | 61 | 22 | 21.40 |
|
| ≥70,
<80 | 57 | 25 | 23.78 |
|
| ≥80,
<90 | 20 | 8 | 6.26 |
|
| ≥90,
<100 | N/A | N/A | N/A |
|
| Sex |
|
|
| 0.586 |
|
Male | 56 | 25 | 22.92 |
|
|
Female | 112 | 41 | 43.08 |
|
| Race |
|
|
| 0.979 |
|
Hispanic | 2 | 1 | 0.70 |
|
|
AI/AN | N/A | N/A | N/A |
|
|
API | N/A | N/A | N/A |
|
| African
descent | 8 | 4 | 4.17 |
|
|
Caucasian | 157 | 61 | 61.07 |
|
|
Unknown | 1 | 0 | 0.06 |
|
| Histology |
|
|
| 0.743 |
| SCC,
NOS | 144 | 57 | 55.32 |
|
|
OCC | 1 | 0 | 0.36 |
|
| SCC,
IC | N/A | N/A | N/A |
|
|
CSCC | 23 | 9 | 10.31 |
|
| Grade (8) |
|
|
| 0.355 |
| I | 4 | 0 | 1.38 |
|
| II | 5 | 1 | 2.54 |
|
|
III | 72 | 27 | 29.03 |
|
| IV | 87 | 38 | 33.05 |
|
| T (8) |
|
|
| 0.258 |
|
T1a | 85 | 29 | 37.15 |
|
|
T1b | 27 | 11 | 11.03 |
|
|
T2a | 36 | 15 | 11.00 |
|
|
T2b | 2 | 2 | 0.85 |
|
| T3 | 12 | 6 | 3.82 |
|
| T4 | 6 | 3 | 2.15 |
|
| N (8) |
|
|
| <0.001 |
| 0 | 108 | 33 | 46.06 |
|
| 1 | 23 | 12 | 6.44 |
|
| 2 | 35 | 19 | 13.11 |
|
| 3 | 2 | 2 | 0.39 |
|
| Stage (8) |
|
|
| 0.006 |
| IA | 70 | 21 | 32.86 |
|
| IB | 25 | 6 | 8.97 |
|
|
IIA | 22 | 12 | 6.30 |
|
|
IIB | 8 | 3 | 2.76 |
|
|
IIIA | 39 | 21 | 13.51 |
|
|
IIIB | 4 | 3 | 1.60 |
|
| Surgery |
|
|
| 0.049 |
| No | 84 | 26 | 33.90 |
|
|
Yes | 84 | 40 | 32.10 |
|
|
| C, Lobe/s vs.
pneumonectomy group |
|
| Feature | NP | NE | NEE | P-value |
|
| Age, years |
|
|
| 0.210 |
|
<40 | – | – | – |
|
| ≥40,
<50 | 2 | 1 | 1.27 |
|
| ≥50,
<60 | 4 | 1 | 0.85 |
|
| ≥60,
<70 | 10 | 8 | 4.75 |
|
| ≥70,
<80 | 3 | 0 | 2.44 |
|
| ≥80,
<90 | 1 | 0 | 0.68 |
|
| ≥90,
<100 | N/A | N/A | N/A |
|
| Sex |
|
|
| 0.544 |
|
Male | 6 | 3 | 3.91 |
|
|
Female | 14 | 7 | 6.09 |
|
| Race |
|
|
| 0.632 |
|
Hispanic | 1 | 0 | 0.68 |
|
|
AI/AN | 1 | 0 | <0.001 |
|
|
API | 1 | 1 | 0.31 |
|
| African
descent | 4 | 2 | 1.68 |
|
|
Caucasian | 13 | 7 | 7.33 |
|
|
Unknown | N/A | N/A | N/A |
|
| Histology |
|
|
| 0.235 |
| SCC,
NOS | 7 | 2 | 3.62 |
|
|
OCC | N/A | N/A | N/A |
|
| SCC,
IC | 1 | 0 | 0.88 |
|
|
CSCC | 12 | 8 | 5.50 |
|
| Grade (8) |
|
|
| 0.438 |
| I | 1 | 1 | 0.31 |
|
| II | 4 | 2 | 2.83 |
|
|
III | 8 | 3 | 3.97 |
|
| IV | 7 | 4 | 2.90 |
|
| T (8) |
|
|
| 0.113 |
|
T1a | 1 | 0 | 0.88 |
|
|
T1b | N/A | N/A | N/A |
|
|
T2a | 5 | 2 | 3.14 |
|
|
T2b | 2 | 1 | 0.17 |
|
| T3 | 8 | 4 | 4.28 |
|
| T4 | 4 | 3 | 1.52 |
|
| N (8) |
|
|
| 0.710 |
| 0 | 6 | 2 | 3.09 |
|
| 1 | 9 | 5 | 4.59 |
|
| 2 | 5 | 3 | 2.32 |
|
| 3 | N/A | N/A | N/A |
|
| Stage (8) |
|
|
| 0.650 |
| IA | 1 | 0 | 0.88 |
|
| IB | 2 | 1 | 1.27 |
|
|
IIA | 3 | 1 | 1.19 |
|
|
IIB | 1 | 0 | 0.88 |
|
|
IIIA | 12 | 7 | 5.30 |
|
|
IIIB | 1 | 1 | 0.48 |
|
| Surgery |
|
|
| 0.185 |
| No | 10 | 6 | 4.02 |
|
|
Yes | 10 | 4 | 5.98 |
|
 | Table IV.Multivariate analysis of different
surgery types. |
Table IV.
Multivariate analysis of different
surgery types.
| A, Non-surgery vs.
surgery group |
|---|
|
|---|
|
| Multivariate
analysis |
|---|
|
|
|
|---|
| Characteristic | Hazard ratio | 95% Confidence
interval | P-value |
|---|
| Age | 1.21 | 1.07–1.36 | <0.01 |
| Sex | 0.97 | 0.76–1.23 | 0.78 |
| Race | 1.11 | 0.92–1.35 | 0.27 |
| Histology | 0.98 | 0.90–1.06 | 0.60 |
| Grade | 1.11 | 0.91–1.36 | 0.29 |
| T | 1.13 | 0.98–1.30 | 0.09 |
| N | 1.27 | 0.85–1.88 | 0.24 |
| Stage | 1.01 | 0.81–1.27 | 0.92 |
| Surgery | 0.59 | 0.47–0.76 | <0.01 |
|
| B, Sublobectomy
vs. lobe/s group |
|
|
| Multivariate
analysis |
|
|
|
|
Characteristic | Hazard
ratio | 95% Confidence
interval | P-value |
|
| Age | 1.23 | 0.94–1.61 | 0.92 |
| Sex | 1.03 | 0.61–1.71 | 0.92 |
| Race | 0.84 | 0.51–1.40 | 0.51 |
| Histology | 0.99 | 0.81–1.20 | 0.91 |
| Grade | 1.64 | 1.03–2.60 | 0.29 |
| T | 1.27 | 1.00–1.63 | 0.05 |
| N | 2.04 | 0.90–4.64 | 0.09 |
| Stage | 0.83 | 0.52–1.32 | 0.92 |
| Surgery | 1.67 | 0.96–2.90 | 0.07 |
|
| C, Lobe/s vs.
Pneumonectomy group |
|
|
| Multivariate
analysis |
|
|
|
|
Characteristic | Hazard
ratio | 95% Confidence
interval | P-value |
|
| Age | 0.28 | 0.05–1.47 | 0.13 |
| Sex | 0.22 | 0.03–1.58 | 0.13 |
| Race | 0.20 | 0.03–1.59 | 0.51 |
| Histology | 2.47 | 0.93–6.52 | 0.07 |
| Grade | 0.77 | 0.18–3.37 | 0.73 |
| T | 2.80 | 0.20–39.85 | 0.45 |
| N | 0.86 | 0.13–5.87 | 0.88 |
| Stage | 2.06 | 0.12–34.16 | 0.61 |
| Surgery | 2.39 | 0.18–32.14 | 0.51 |
 | Table V.Survival functions of
clinicopathological features stratified by surgery. |
Table V.
Survival functions of
clinicopathological features stratified by surgery.
| A. Non-surgery vs.
surgery group |
|---|
|
|---|
|
| Non-surgery | Surgery |
|
|---|
|
|
|
|
|
|---|
| Feature | NE | NEE | NE | NEE | P-value |
|---|
| Age, years |
|
|
|
| <0.01 |
|
<40 | 2 | 2.0 | NT | NT | NS |
| ≥40,
<50 | 6 | 6.2 | 4 | 3.8 | 0.91 |
| ≥50,
<60 | 33 | 20.8 | 18 | 30.2 | <0.01 |
| ≥60,
<70 | 46 | 39.6 | 45 | 51.4 | 0.17 |
| ≥70,
<80 | 53 | 39.8 | 39 | 52.2 | <0.01 |
| ≥80,
<90 | 19 | 15.5 | 9 | 12.5 | 0.17 |
| ≥90,
<100 | 2 | 2.0 | NT | NT | NS |
| Sex |
|
|
|
| <0.01 |
|
Male | 65 | 54.0 | 53 | 64.0 | 0.04 |
|
Female | 96 | 71.3 | 62 | 86.7 | <0.01 |
| Race |
|
|
|
| <0.01 |
|
Hispanic | 2 | 1.9 | 3 | 3.1 | 0.89 |
|
AI/AN | NT | NT | NT | NT | NS |
|
API | 3 | 3.1 | 4 | 3.9 | 0.91 |
| African
descent | 9 | 8.1 | 8 | 8.9 | 0.66 |
|
Caucasian | 147 | 111.3 | 100 | 135.7 | <0.01 |
| Histology |
|
|
|
| <0.01 |
| SCC,
NOS | 139 | 113.5 | 83 | 108.5 | <0.01 |
|
OCC | 5 | 4.2 | 0 | 0.8 | 0.29 |
| SCC,
IC | 2 | 0.8 | 0 | 1.2 | 0.09 |
|
CSCC | 15 | 9.7 | 32 | 37.3 | 0.05 |
| Grade (8) |
|
|
|
| <0.01 |
| I | 1 | 0.5 | 1 | 1.5 | 0.37 |
| II | 3 | 2.0 | 4 | 5.0 | 0.42 |
|
III | 77 | 55.6 | 50 | 71.4 | <0.01 |
| IV | 80 | 69.5 | 60 | 70.5 | 0.07 |
| T (8) |
|
|
|
| <0.01 |
|
T1a | 56 | 34.5 | 30 | 51.5 | <0.01 |
|
T1b | 42 | 36.8 | 18 | 23.2 | 0.16 |
|
T2a | 38 | 29.7 | 39 | 47.4 | 0.05 |
|
T2b | 15 | 15.1 | 4 | 3.9 | 0.96 |
| T3 | 7 | 9.1 | 18 | 15.9 | 0.36 |
| T4 | 3 | 2.5 | 6 | 6.5 | 0.68 |
| N (8) |
|
|
|
| <0.01 |
| 0 | 100 | 68.6 | 50 | 81.4 | <0.01 |
| 1 | 22 | 24.3 | 32 | 29.7 | 0.49 |
| 2 | 38 | 32.2 | 31 | 36.8 | 0.15 |
| 3 | 1 | 1.8 | 2 | 1.2 | 0.31 |
| Stage (8) |
|
|
|
| <0.01 |
| IA | 50 | 31.1 | 27 | 45.9 | <0.01 |
| IB | 31 | 20.8 | 13 | 23.2 | <0.01 |
|
IIA | 32 | 32.0 | 24 | 24.0 | 1.00 |
|
IIB | 7 | 7.2 | 6 | 5.9 | 0.93 |
|
IIIA | 39 | 33.5 | 41 | 46.5 | 0.20 |
|
IIIB | 2 | 2.7 | 4 | 3.3 | 0.58 |
|
| B, Sublobectomy
vs. lobe/s group |
|
|
|
Sub-lobectomy | lobe/s |
|
|
|
|
|
|
| Feature | NE | NEE | NE | NEE | P-value |
|
| Age, years |
|
|
|
| 0.05 |
|
<40 | N/A | N/A | N/A | N/A | NS |
| ≥40,
<50 | 2 | 2.0 | NT | NT | NS |
| ≥50,
<60 | 5 | 5.5 | 4 | 3.5 | 0.75 |
| ≥60,
<70 | 12 | 9.6 | 10 | 12.4 | 0.30 |
| ≥70,
<80 | 14 | 11.3 | 11 | 13.7 | 0.28 |
| ≥80,
<90 | 7 | 4.0 | 1 | 4.1 | 0.03 |
| ≥90,
<100 | N/A | N/A | N/A | N/A | NS |
| Sex |
|
|
|
| 0.06 |
|
Male | 17 | 14.9 | 8 | 10.1 | 0.38 |
|
Female | 23 | 17.7 | 18 | 23.3 | 0.09 |
| Race |
|
|
|
| 0.05 |
|
Hispanic | 1 | 1.0 | NT | NT | NS |
|
AI/AN | N/A | N/A | N/A | N/A | N/A |
|
API | N/A | N/A | N/A | N/A | NS |
| African
descent | 3 | 2.8 | 1 | 1.2 | 0.81 |
|
Caucasian | 36 | 28.4 | 25 | 32.6 | 0.05 |
| Histology |
|
|
|
| 0.04 |
| SCC,
NOS | 32 | 25.1 | 25 | 31.9 | 0.06 |
|
OCC | NT | NT | NT | NT | NS |
| SCC,
IC | N/A | N/A | N/A | N/A | NS |
|
CSCC | 8 | 6.8 | 1 | 2.2 | 0.36 |
| Grade (8) |
|
|
|
| 0.06 |
| I | NT | NT | NT | NT | NS |
| II | 1 | 1.0 | 0 | 0.0 | 1.00 |
|
III | 18 | 14.5 | 9 | 12.5 | 0.17 |
| IV | 21 | 17.0 | 17 | 21.0 | 0.19 |
| T (8) |
|
|
|
| 0.07 |
|
T1a | 14 | 10.7 | 7 | 10.4 | 0.52 |
|
T1b | 5 | 3.5 | 1 | 2.5 | 0.48 |
|
T2a | 5 | 1.0 | 7 | 11.0 | 0.22 |
|
T2b | 1 | 0.6 | 2 | 2.4 | 0.32 |
| T3 | 12 | 12.6 | 9 | 8.4 | 0.24 |
| T4 | 3 | 3.0 | NT | NT | NS |
| N (8) |
|
|
|
| 0.02 |
| 0 | 22 | 16.2 | 11 | 16.8 | 0.04 |
| 1 | 5 | 1.2 | 7 | 10.8 | <0.001 |
| 2 | 11 | 12.2 | 8 | 6.8 | 0.56 |
| 3 | 2 | 2.0 | NT | NT | NS |
| Stage (8) |
|
|
|
| 0.02 |
| IA | 14 | 10.7 | 7 | 10.4 | 0.14 |
| IB | 5 | 3.5 | 1 | 2.5 | 0.20 |
|
IIA | 5 | 1.0 | 7 | 11.0 | <0.01 |
|
IIB | 1 | 0.6 | 2 | 2.4 | 0.56 |
|
IIIA | 12 | 12.6 | 9 | 8.4 | 0.77 |
|
IIIB | 3 | 3.0 | NT | NT | NS |
|
| C, Lobe/s vs.
Pneumonectomy group |
|
|
| lobe/s |
Pneumonec-tomy |
|
|
|
|
|
|
| Feature | NE | NEE | NE | NEE | P-value |
|
| Age, years |
|
|
|
| 0.42 |
|
<40 | N/A | N/A | N/A | N/A | NS |
| ≥40,
<50 | NT | NT | 1 | 1.0 | NS |
| ≥50,
<60 | 0 | 0.0 | 1 | 1.0 | 1.00 |
| ≥60,
<70 | 6 | 6.7 | 2 | 1.3 | 0.42 |
| ≥70,
<80 | NT | NT | NT | NT | NS |
| ≥80,
<90 | NT | NT | NT | NT | NS |
| ≥90,
<100 | N/A | N/A | N/A | N/A | NS |
| Sex |
|
|
|
| 0.18 |
|
Male | 2 | 1.2 | 1 | 1.9 | 0.3 |
|
Female | 4 | 2.8 | 3 | 4.2 | 0.35 |
| Race |
|
|
|
| 0.24 |
|
Hispanic | NT | NT | NT | NT | NS |
|
AI/AN | NT | NT | NT | NT | NS |
|
API | 1 | 1.0 | N/A | N/A | NS |
| African
descent | 1 | 0.5 | 1 | 1.5 | 0.32 |
|
Caucasian | 4 | 2.9 | 3 | 4.1 | 0.39 |
| Histology |
|
|
|
| 0.70 |
| SCC,
NOS | 1 | 0.2 | 1 | 1.8 | 0.03 |
|
OCC | N/A | N/A | N/A | N/A | NS |
| SCC,
IC | NT | NT | NT | NT | NS |
|
CSCC | 5 | 5.3 | 3 | 2.7 | 0.80 |
| Grade (8) |
|
|
|
| 0.20 |
| I | 1 | 1.0 | NT | NT | NS |
| II | 1 | 0.8 | 1 | 1.3 | 0.71 |
|
III | 2 | 1.7 | 1 | 1.3 | 0.70 |
| IV | 2 | 0.9 | 2 | 3.1 | 0.14 |
| T (8) |
|
|
|
| 0.31 |
|
T1a | NT | NT | NT | NT | NS |
|
T1b | N/A | N/A | N/A | N/A | NS |
|
T2a | 1 | 0.2 | 1 | 1.8 | 0.05 |
|
T2b | 0 | 0.0 | 1 | 1.0 | 1.00 |
| T3 | 3 | 2.9 | 1 | 1.1 | 0.91 |
| T4 | 2 | 1.7 | 1 | 1.3 | 0.69 |
| N (8) |
|
|
|
| 0.24 |
| 0 | 0 | 0.5 | 2 | 1.6 | 0.45 |
| 1 | 4 | 2.1 | 1 | 2.9 | 0.06 |
| 2 | 2 | 1.8 | 1 | 1.2 | 0.78 |
| 3 | N/A | N/A | N/A | N/A | NS |
| Stage (8) |
|
|
|
| 0.48 |
| IA | NT | NT | NT | NT | NS |
| IB | 1 | 1.0 | N/A | N/A | NS |
|
IIA | 1 | 0.5 | 0 | 0.5 | 0.32 |
|
IIB | NT | NT | NT | NT | NS |
|
IIIA | 4 | 3.6 | 3 | 3.4 | 0.74 |
|
IIIB | 1 | 1.0 | NT | NT | NS |
 | Table VI.The incidence of cause of death to
site. |
Table VI.
The incidence of cause of death to
site.
| Surgery | Alive | Death | Total | P |
|---|
| Non-surgery | 133 | 161 | 294 |
<0.01a |
| Surgery | 179 | 115 | 294 |
|
| Sublobectomy | 44 | 40 | 84 | 0.03a |
| Lobectomy or
bilobectomy | 58 | 26 | 84 |
|
| Lobectomy or
bilobectomy | 4 | 6 | 10 | 0.37 |
| Pneumonectomy | 6 | 4 | 10 |
|
Discussion
As SCLC responds to chemotherapy and radiotherapy,
surgical treatment is considered to be an option for stage I–IIA
(T1-2, N0, M0) SCLC (9,10). The most recent National Comprehensive
Cancer Network (NCCN) guidelines recommend that patients with SCLC
at clinical stage I–IIA (T1-2, N0, M0) after a standard staging
evaluation may be eligible for surgical resection (9). After analyzing the SEER database,
Schreiber et al (11)
concluded that the use of surgery, and particularly lobectomy, in
selected patients with limited-stage SCLC was associated with
improved survival outcomes. However, there was inherent selection
bias in their study (11).
Therefore, the present study performed the period propensity score
matching analysis using the SEER database to further examine the
role of surgery on survival in patients with SCLC.
Following propensity score matching analysis, the
present study identified that the clinicopathological features of N
stage and surgery were important factors for postoperative CSS in
patients with SCLC who received surgery, including sublobectomy,
lobe/s and pneumonectomy. This finding was corroborated by the
results of previous studies (5,11,12). The
IASLC proposals (12) demonstrated
that there was a significant difference in the survival of patients
who underwent surgery between N0 patients and those with
node-positive disease for both clinical and pathological staging,
independent of T category. Takenaka et al (5) reported that the 5-year survival rates
of the patients with SCLC with or without surgical resection,
according to the clinical stage were as follows: 62 and 25% in
stage I (P<0.01), 33 and 24% in stage II (P=0.95) and 18 and 18%
in stage III (P=0.35). The study of Schreiber et al
(11) also demonstrated that the
overall survival for patients with SCLC with N0, N1 and N2 who
received surgery were significantly improved, when compared with
those who did not receive surgery (P<0.01). In addition, the
significance of surgery was corroborated following Cox regression
analysis (P<0.001) (Table IV).
These results suggested that the role of surgery for patients with
SCLC was significant.
To further identify who would benefit from surgery,
the survival functions of surgery stratified by the
clinicopathological features were analyzed using log-rank tests
(Table V). The results revealed that
patients who did not receive surgery in any of the subgroups,
including sex, histology and grade had an increased risk of COD
compared with patients who received surgery. These results suggest
that surgery should be performed irrespective of sex, histology,
grade and clinicopathological features. Previous studies have
reported that increasing age was an independent adverse prognostic
factor in SCLC (13–15). However, the results of the present
study demonstrated that patients between 50 and 90 years of age
benefited from surgery, although analysis was not tested in the
subgroups of age <40 years or between 90 and 100 years.
Furthermore, age was an independent prognostic factor (P<0.001;
Table IV), which suggested that
surgery should also be performed even in elderly patients with
SCLC. This result was similar with the treatment of thoracic
irradiation for limited-stage SCLC, in which it was reported that
in the dose range examined, age did not appear to have an effect on
the delivery, tolerance or efficacy of TI in the combined modality
management of SCLC (16). Concerning
T, there was significant difference in the T1a subgroup
(P<0.001) and better survival trends in the T1b and T2a
subgroups. Furthermore, patients with SCLC with N0 (P<0.001) and
stage Ia (P<0.001) and Ib (P<0.001) would have an increased
benefit from surgery. These results, which were in accordance with
those reported by the IASLC (12),
clarified why the most recent NCCN guidelines recommend that
patients with SCLC with clinical stage I–IIA (T1-2, N0, M0) after a
standard staging evaluation may be considered for surgical
resection (9).
As with the comparison of non-surgery with surgery,
following propensity score matching analysis, the present study
identified that the clinicopathological features of N, stage and
surgery were important factors in postoperative CSS in patients
with SCLC who received sublobectomy compared with those who
received lobe/s. However, no independent prognostic factor was
identified in the Cox regression model.
When the survival functions of surgery stratified by
clinicopathological features were analyzed using log-rank tests
(Table V), more patients who
received sublobectomy in all subgroups of sex, histology were at
risk of COD compared with those who received lobe/s. On the other
hand, the results also demonstrated that there were more patients
between 60 and 90 years of age, who benefited from lobe/s, although
this analysis was not performed in the subgroups ages <50 years,
as no failure events were observed, and in the subgroups ages
<40 years and between 90 and 100 years, due to the absence of
observations. Another study comparing treatment strategies for
stage I SCLC using the National Cancer Database (17) demonstrated that lobectomy was
associated with an improved survival compared with limited
resection (HR 0.64; 95% CI, 0.53–0.78; P<0.001). Schreiber et
al (11) revealed that the
median survival time for lobectomy and sublobectomy was 40 and 23
months, respectively (P<001). These results confirmed that,
similar with the NCCN recommendation (9), bi-/lobecotomy was the preferred
operation for SCLC compared with sublobectomy, even in elder
patients irrespective of sex and histology. When analyzing T,
although there was no significant difference, it was the patients
with SCLC with T1a to T2b, who had received lobe/s, who exhibited
better survival trends. Simultaneously, the present study also
demonstrated that more patients with N0-1, stage Ia-IIb who
received sublobectomy, rather than lobe/s, were at risk of COD.
Despite the recommendation in the most recent NCCN guidelines that
patients with SCLC with clinical stage I–IIA (T1-2, N0, M0) after a
standard staging evaluation may be considered for surgical
resection (9) and multiple medical
societies concluding that the survival advantage of surgical
resection is only observed in patients with stage I disease
(5,10,18,19), the
results of the present study suggest that patients with SCLC with
up to stage IIB (N1) may benefit from lobe/s. Combs et al
(20) also stated that patients with
stages I, II and III SCLC that underwent surgical resection as part
of the initial treatment with chemotherapy may exhibit an improved
overall survival rate.
Due to the inclusion criterion, only 10 patients who
received pneumonectomy were included in the present study, and
therefore results were too limited to be extensively discussed.
Despite the large sample size, a limitation of the SEER database,
and consequently of the present study, was the lack of information
regarding performance and smoking status, which may have an
important impact on postoperative survival, and the use of
perioperative effective treatments, including systemic therapy,
mediastinal radiation therapy and prophylactic cranial irradiation.
In addition, the different surgical types of lobectomy and
bilobectomy were recorded as a single category ‘lobectomy or
bilobectomy’, and the present study was unable to analyze the
difference between them.
In conclusion, surgery should be taken into
consideration when initial treatment strategy is made in patients
with SCLC with a clinical stage I–IIA (T1-2, N0, M0), and should
not be overlooked in patients >50 years, irrespective of sex,
histology and the grade of the clinicopathological features. There
is also evidence to suggest that certain patients with SCLC with
stage IIB (N1) may also benefit from lobectomy or bilobectomy,
although further investigation is required. In addition, lobe/s is
preferred compared with sublobectomy when surgery is performed.
However, the present study was unable to conclusively state the
role of pneumonectomy for SCLC.
Acknowledgements
The authors would like to thank the Surveillance,
Epidemiology, and End Results (SEER) Program for providing free
access to the database.
Funding
This study was funded by the Wu Jieping Medical
Foundation (grant no. 320.6750.18470) and the Qi Lu Cancer Research
Foundation of Chinese Society of Clinical Oncology (grant no.
Y-Q201801-006).
Availability of data and materials
The datasets generated during and/or analyzed during
the current study are available in the Surveillance, Epidemiology,
and End Results (SEER; www.seer.cancer.gov) Program SEER*Stat Database.
Authors' contributions
XD, DT, JX, WL, SY, HZ and JZ participated in the
design of the study and performed statistical analysis. LL, ZT and
XC contributed to the acquisition and interpretation of data and
critically revised the article for intellectual content. All
authors were involved in the writing of the manuscript and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there is no competing
interests.
References
|
1
|
Govindan R, Page N, Morgensztern D, Read
W, Tierney R, Vlahiotis A, Spitznagel EL and Piccirillo J: Changing
epidemiology of small-cell lung cancer in the United States over
the last 30 years: Analysis of the surveillance, epidemiologic, and
end results database. J Clin Oncol. 24:4539–4544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kalemkerian GP and Schneider BJ: Advances
in small cell lung cancer. Hematol Oncol Clin North Am. 31:143–156.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pleasance ED, Stephens PJ, O'Meara S,
McBride DJ, Meynert A, Jones D, Lin ML, Beare D, Lau KW, Greenman
C, et al: A small-cell lung cancer genome with complex signatures
of tobacco exposure. Nature. 463:184–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stinchcombe TE and Gore EM: Limited-stage
small cell lung cancer: Current chemoradiotherapy treatment
paradigms. Oncologist. 15:187–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takenaka T, Takenoyama M, Inamasu E,
Yoshida T, Toyokawa G, Nosaki K, Hirai F, Yamaguchi M, Shimokawa M,
Seto T and Ichinose Y: Role of surgical resection for patients with
limited disease-small cell lung cancer. Lung Cancer. 88:52–56.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schneider BJ, Saxena A and Downey RJ:
Surgery for early-stage small cell lung cancer. J Natl Compr Canc
Netw. 9:1132–1139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Allen PW: ICDO-international
classification of diseases for oncology. Pathology. 23:2801991.
View Article : Google Scholar
|
|
8
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th. Springer;
New York, NY: 2010
|
|
9
|
National Comprehensive Cancer Network.
NCCN Clinical Practice Quidelines in Oncology (NCCN guidelines), .
Small Cell Lung Cancer, 2018. https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdfOctober
22–2018
|
|
10
|
Jett JR, Schild SE, Kesler KA and
Kalemkerian GP: Treatment of small cell lung cancer: Diagnosis and
management of lung cancer, III ed: American College of chest
physicians evidence-based clinical practice guidelines. Chest. 143
(Suppl 5):e400S–e419S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schreiber D, Rineer J, Weedon J, Vongtama
D, Wortham A, Kim A, Han P, Choi K and Rotman M: Survival outcomes
with the use of surgery in limited-stage small cell lung cancer:
Should its role be re-evaluated? Cancer. 116:1350–1357. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nicholson AG, Chansky K, Crowley J,
Beyruti R, Kubota K, Turrisi A, Eberhardt WE, van Meerbeeck J and
Rami-Porta R; Staging and Prognostic Factors Committee, Advisory
Boards, and Participating Institutions; Staging and Prognostic
Factors Committee Advisory Boards and Participating Institutions, :
The international association for the study of lung cancer lung
cancer staging project: Proposals for the revision of the clinical
and pathologic staging of small cell lung cancer in the forthcoming
eighth edition of the TNM classification for lung cancer. J Thorac
Oncol. 11:300–311. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tas F, Ciftci R, Kilic L and Karabulut S:
Age is a prognostic factor affecting survival in lung cancer
patients. Oncol Lett. 6:1507–1513. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Allan SG, Stewart ME, Love S, Cornbleet
MA, Smyth JF and Leonard RC: Prognosis at presentation of small
cell carcinoma of the lung. Eur J Cancer. 26:703–705. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lara JD, Brunson A, Riess JW, Kelly K,
Lara PN Jr and Gandara DR: Clinical predictors of survival in young
patients with small cell lung cancer: Results from the California
cancer registry. Lung Cancer. 112:165–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Quon H, Shepherd FA, Payne DG, Coy P,
Murray N, Feld R, Pater J, Sadura A and Zee B: The influence of age
on the delivery, tolerance, and efficacy of thoracic irradiation in
the combined modality treatment of limited stage small cell lung
cancer. Int J Radiat Oncol Biol Phys. 43:39–45. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paximadis P, Beebe-Dimmer JL, George J,
Schwartz AG, Wozniak A and Gadgeel S: Comparing treatment
strategies for stage I small-cell lung cancer. Clin Lung Cancer.
19:e559–e565. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rudin CM, Ismaila N, Hann CL, Malhotra N,
Movsas B, Norris K, Pietanza MC, Ramalingam SS, Turrisi AT III and
Giaccone G: Treatment of small-cell lung cancer: American society
of clinical oncology endorsement of the American college of chest
physicians guideline. J Clin Oncol. 33:4106–4111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inoue M, Sawabata N and Okumura M:
Surgical intervention for small-cell lung cancer: What is the
surgical role? Gen Thorac Cardiovasc Surg. 60:401–405. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Combs SE, Hancock JG, Boffa DJ, Decker RH,
Detterbeck FC and Kim AW: Bolstering the case for lobectomy in
stages I, II and IIIA small-cell lung cancer using the national
cancer data base. J Thorac Oncol. 10:316–323. 2015. View Article : Google Scholar : PubMed/NCBI
|