Introduction
Non-small cell lung cancer (NSCLC) accounts for
approximately 80% of lung cancer and is the leading cause of
cancer-related death worldwide (1).
Despite great advances in therapeutic methods, the 5-year overall
survival of NSCLC is still <15% (2). Therefore, it is urgent to explore novel
biomarkers for disease prevention and clinical treatment of
NSCLC.
MicroRNAs (miRNAs) are a class of endogenous,
non-coding small RNAs with 19–25 nucleotides in length (3,4). miRNAs
binding to the 3′-UTR of target mRNA to incise the target mRNAs or
inhibit the translation of proteins at post-transcriptional level
(3). Accumulating evidence has
identified that miRNAs participate in multiple processes of cancer,
including cell growth, metastasis and apoptosis (5). miR-650 functions as an oncogene and is
overexpressed in multiple diseases, including colorectal cancer,
chronic lymphocytic leukemia, osteosarcoma and glioma (6–9). Mraz
et al indicated that the expression of miR-650 was high and
affected the biology of chronic lymphocytic leukemia (10). Moreover, miR-650 affected the release
of allograft rejection-associated cytokines from HRGECs and
regulated chemotaxis of macrophages (11). However, miR-650 inhibited the
proliferation and metastasis through targeting to Gfi1 in oral
cancer and leukemia (12,13). Therefore, we investigated miR-650
expression and the roles of miR-650 in NSCLC.
Inhibitor of growth 4 (ING4) is a member of IMG
family, which is involved in nucleoprotein modification by
acetylation of histone (14,15). ING4 containing a highly conserved
C-terminal plant homeodomain finger motif, may regulate several
biological activities, including DNA repair, cell cycle and
apoptosis (16). Moreover, ING4 has
been reported to be a tumor suppressor gene in various cancers,
including head and neck squamous cell carcinomas, pancreatic
cancer, breast cancer and clear cell renal carcinoma (17–21).
Downregulation of ING4 improved angiogenesis of transformed gastric
epithelial cells (22). ING4
inhibited cell proliferation, cell cycle progress, migration and
invasion in melanoma (23).
Moreover, Chen et al demonstrated that ING4 suppressed tumor
angiogenesis and acted as a prognostic marker in human colorectal
cancer (24). In lung cancer, ING4
suppressed the proliferation and increased apoptosis, and ING4
overexpression enhanced radiosensitivity (25). In this study, miR-650 was validated
to be upregulated in NSCLC and upregulation of miR-650 improved the
overall survival of NSCLC, while ING4 demonstrated the opposite
results. miR-650 promoted cell proliferation and invasion and ING4
could partially reverse the function of miR-650. ING4 was confirmed
as a direct and functional target of miR-650, and miR-650 enhanced
Wnt-1/β-catenin pathway in A549 cells.
Patients and methods
Tissue samples
Forty-nine NSCLC patients were collected at Jining
No. 1 People's Hospital (Jining, China) from January 2015 to June
2018 and 49 pairs of cancer tissues and adjacent normal tissues
were obtained. The tissue specimens were immediately frozen in
liquid nitrogen and stored at −80°C. None of the patients accepted
any treatment prior to surgery and the patient details are shown in
Table I.
 | Table I.miR-650 expression and
clinicopathological features in 49 non-small cell lung cancer. |
Table I.
miR-650 expression and
clinicopathological features in 49 non-small cell lung cancer.
|
|
| miR-650
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | Cases (n=49) | 26 High (%) | 23 Low (%) |
P-valuea |
|---|
| Sex |
|
|
| 0.469 |
|
Male | 25 | 12 (48.0) | 13 (52.0) |
|
|
Female | 24 | 14 (58.3) | 10 (41.7) |
|
| Age (years) |
|
|
| 0.648 |
|
≤60 | 23 | 13 (56.5) | 10 (43.5) |
|
|
>60 | 26 | 13 (50.0) | 13 (50.0) |
|
| Tumor size
(mm) |
|
|
| 0.124 |
|
≤5.0 | 27 | 17 (63.0) | 10 (37.0) |
|
|
>5.0 | 22 | 9
(40.9) | 13 (59.1) |
|
| TNM stage |
|
|
| 0.016a |
|
I–II | 26 | 18 (69.2) | 8
(30.8) |
|
|
III–IV | 23 | 8
(34.8) | 15 (65.2) |
|
| Local invasion |
|
|
| 0.066 |
|
T1-T2 | 26 | 17 (65.4) | 9
(34.6) |
|
|
T3-T4 | 23 | 9
(39.1) | 14 (60.9) |
|
| Lymph node
metastasis |
|
|
| 0.029a |
|
0–2 | 23 | 16 (69.6) | 7
(30.4) |
|
|
>2 | 26 | 10 (38.5) | 16 (61.5) |
|
| Histology |
|
|
| 0.765 |
|
Adenocarcinoma | 33 | 18 (54.5) | 15 (45.5) |
|
|
Squamous | 16 | 8
(50.0) | 8
(50.0) |
|
The Ethics Committee of Jining No. 1 People's
Hospital approved this study. Patients who participated in this
research had complete clinical data. The signed informed consents
were obtained from the patients or the guardians.
Cell lines and culture condition
Two human lung cell lines, A549 (cat. no. CCL-185) a
lung adenocarcinoma and NCI-H460 (cat. no. HTB-177) a large cell
carcinoma and one normal bronchial epithelial cell line MRC-5 (cat.
no. CCL-171) were purchased from American Type Culture Collection.
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
was utilized to culture all the cells at 37°C in a humidified
atmosphere of 5% CO2.
Cell transfection
The miR-650 mimic, the miR-650 inhibitor and
negative control (NC) were designed and synthesized by GenePharma,
while pcDNA3.1-ING4 and pcDNA3.1-NC vectors were purchased from
Guangzhou RiboBio Co., Ltd. A549 cells at 70% confluence in 6-well
plates were transfected with the vectors by Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). After incubated for 6
h at 37°C in a humidified atmosphere of 5% CO2, the
cells were replaced with fresh normal RPMI-1640 medium in each
well.
Total RNA extraction and RT-qPCR
The TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was applied to extract total RNA from tissue
samples and cell lines. For the quantification of ING4 mRNA, the
PrimeScript RT reagent kit (Takara Bio) was utilized to perform
reverse transcription. The temperature conditions for reverse
transcription were as follows: 37°C for 15 min and 85°C for 5 sec.
SYBR Premix Ex Taq (Takara Bio, Inc.) was applied to carry out qPCR
on Applied Biosystems 7500 Real-time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The expression of
miR-650 was determined by using TaqMan microRNA assay (Applied
Biosystems; Thermo Fisher Scientific, Inc.). GAPDH and U6 served as
internal control for ING4 and miR-650 respectively. PCR conditions
were as follows: 1 cycle of 95°C for 30 sec, followed by 40 cycles
of a two-step cycling program (95°C for 5 sec; 60°C for 30 sec).
PCR primer sequences were as follows: miR-650 forward
5′-AGAGGAGGCAGCGCTCT-3′ and reverse 5′-CAGTGCGTGTCGTGGAGT-3′; U6
forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′, reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; ING4 forward
5′-TTTCAGAGGGAGGGTCCTTT-3′ and reverse 5′-GCCAGAGCCTAGATGACCTG-3′;
GAPDH forward, 5′-CTGGGCTACACTGAGCACC-3′, reverse,
5′-AAGTGGTCGTTGAGGGCAATG-3′. The 2−∆∆Cq method was
applied to assess the relative expression of the mRNA levels of
miR-650 and ING4 (26).
Western blot analysis
RIPA containing protease inhibitors (Beyotime
Institute of Biotechnology) was adopted to extract the total
proteins on ice. Total protein concentration was quantified using
BCA assay kit (Beyotime Biotechnology), equal amounts of proteins
(50 μg) were separated by 10% SDS-PAGE, and transferred onto
PVDF membranes (EMD Millipore). Then the membranes were blocked in
5% non-fat milk to block the non-specific antigen, and then the
primary antibodies were incubated at 4°C at room temperature for
1.5 h overnight with anti-ING4 antibody (cat. no. ab113425; dil
1:1,000), anti-Wnt1 (cat. no. ab15251; dil 1:1,000) anti-β-catenin
(cat. no. ab16051; dil 1:1,000) and anti-GADPH antibody (cat. no.
ab125247; dil 1:3,000) all from Abcam. After the membranes were
washed with TBST, the corresponding secondary antibody was
incubated with HRP-conjugated (dil 1:4,000; Abcam) at room
temperature for 2 h. Enhanced chemiluminescence solution (Pierce;
Thermo Fisher Scientific, Inc.) was adopted to visualize the
protein bands and then were analyzed with AlphaEase FC 4.0.1
software (ProteinSimple).
CCK8 assay
CCK8 (Dojindo) was employed to calculate the
capacity of cell proliferation. A549 cells at density 3,000
cells/well, were seeded into 96-well plates and cultured for 24,
48, 72 or 96 h at 37°C. Subsequently, each well was added with 10
µl CCK8 reagent and incubated 2 h. Finally, microplate reader
(Bio-Rad Laboratories) was employed to determine cell proliferation
with the absorbance at 450 nm.
Transwell assays
Transwell chambers (8 µM pore size, Corning Inc.)
covered with Matrigel (BD Biosciences) were applied to measure the
capacity of cell invasion. A549 cells were suspended in normal
medium without FBS, and 200 µl were seeded into the upper chamber,
while the lower chambers were filled with 500 µl RPMI-1640 medium
supplemented with 20% FBS functioned as the attractant. After
incubation for 48 h, the cells remaining on the upper surface of
the membranes were removed by cotton swabs, whereas the invaded
cells were fixed and stained by paraformaldehyde and 10% crystal
violet (Sigma-Aldrich; Merck Millipore) respectively. Microscopy
(Olympus Corporation; magnification, ×200) was employed to count
the invaded cell numbers in five fields.
Luciferase reporter assay
To verify the putative targets of miR-650, we
adopted bioinformatics tool TargetScan (http://targetscan.org/) to predict the candidate
genes. The binding site at mRNA 3′-UTR was mutated from UGCCUCC to
ACGGAGG, and then inserted into pmirGlo vectors, which were
designated as pmirGlo-ING4-WT and pmirGlo-ING4-MUT. Both the
wild-type and the mutant sequences and miR-650 mimic were
co-transfected in A549 cells. Dual Luciferase Assay System (Promega
Corporation) was employed to calculate the firefly luciferase
activity with renilla luciferase activity as the normalization.
Statistical analysis
SPSS 19.0 was used to analyze all the data and
presented as mean ± standard deviation (SD). The one way analysis
of variance followed by Tukey's post-hoc test and student's t-test
(for comparisons between two groups) were employed to perform the
statistical analysis. The relationship between expression of
miR-650 and clinical and the pathological variables was analyzed
using Pearson's χ2 test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Overexpression of miR-650 is
associated with poor prognosis
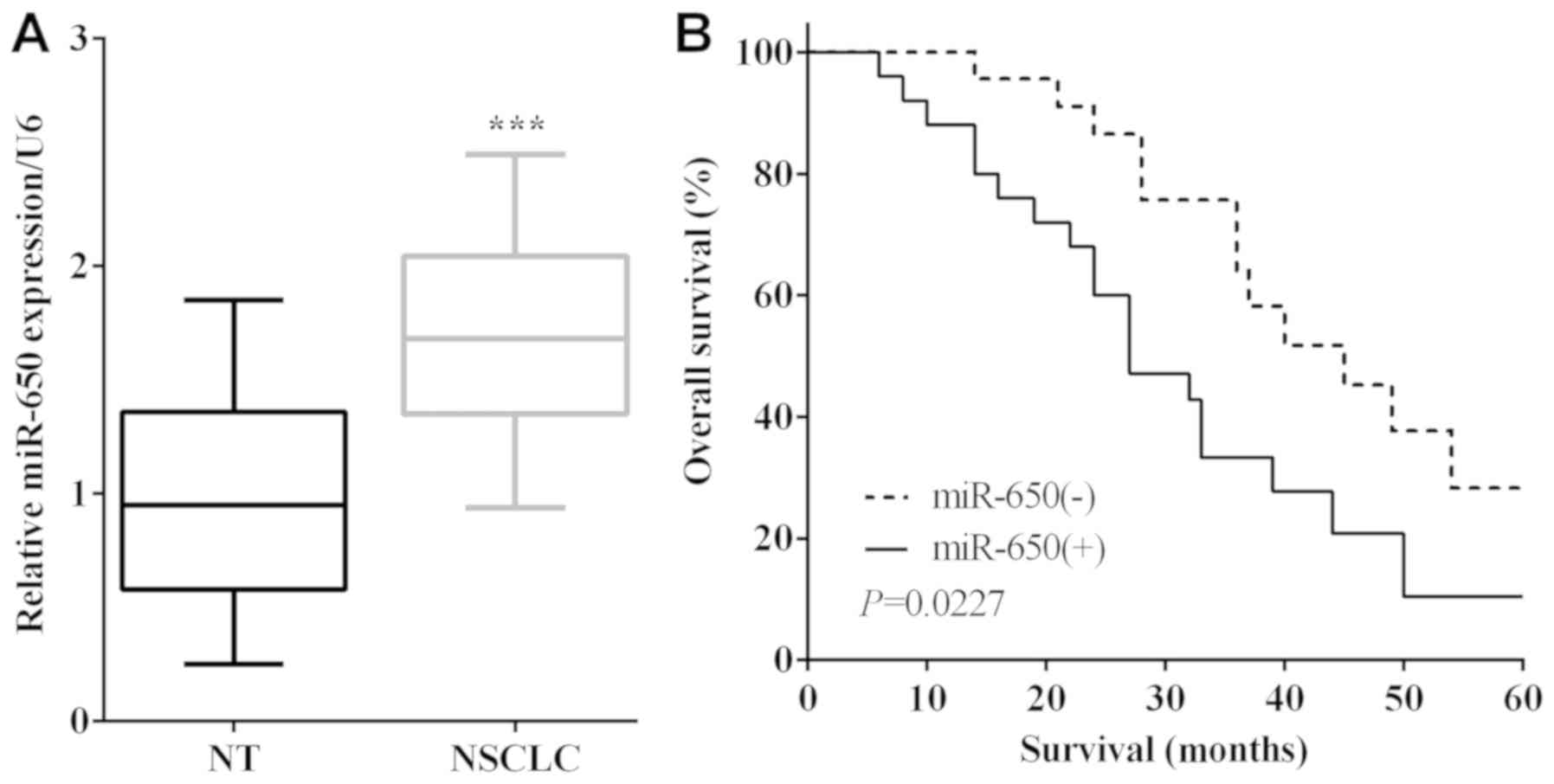
miR-650 expression in NSCLC tissues and their
matched adjacent normal tissues was calculated by RT-qPCR. The mRNA
levels of miR-650 were lower in normal lung tissues than that in
NSCLC tissues (P<0.0001) (Fig.
1A). To verify the connection between the expression of miR-650
and overall survival of NSCLC patients, the survival curves were
plotted. As shown in Fig. 1B,
patients with higher expression of miR-650 had a shorter OS than
those with lower expression (P=0.0227). To investigate the
relationship between miR-650 and NSCLC clinicopathological
characteristics, 49 patients were divided into two groups according
to miR-650 expression, sex, age, tumor size, TNM stage, local
invasion, lymph node metastasis and histology. Statistical
differences were detected by chi-square test and it was found that
miR-650 was associated with tumor size (P=0.016) and lymph node
metastasis (P=0.029) (Table I).
miR-650 enhances cell proliferation
and invasion in NSCLC
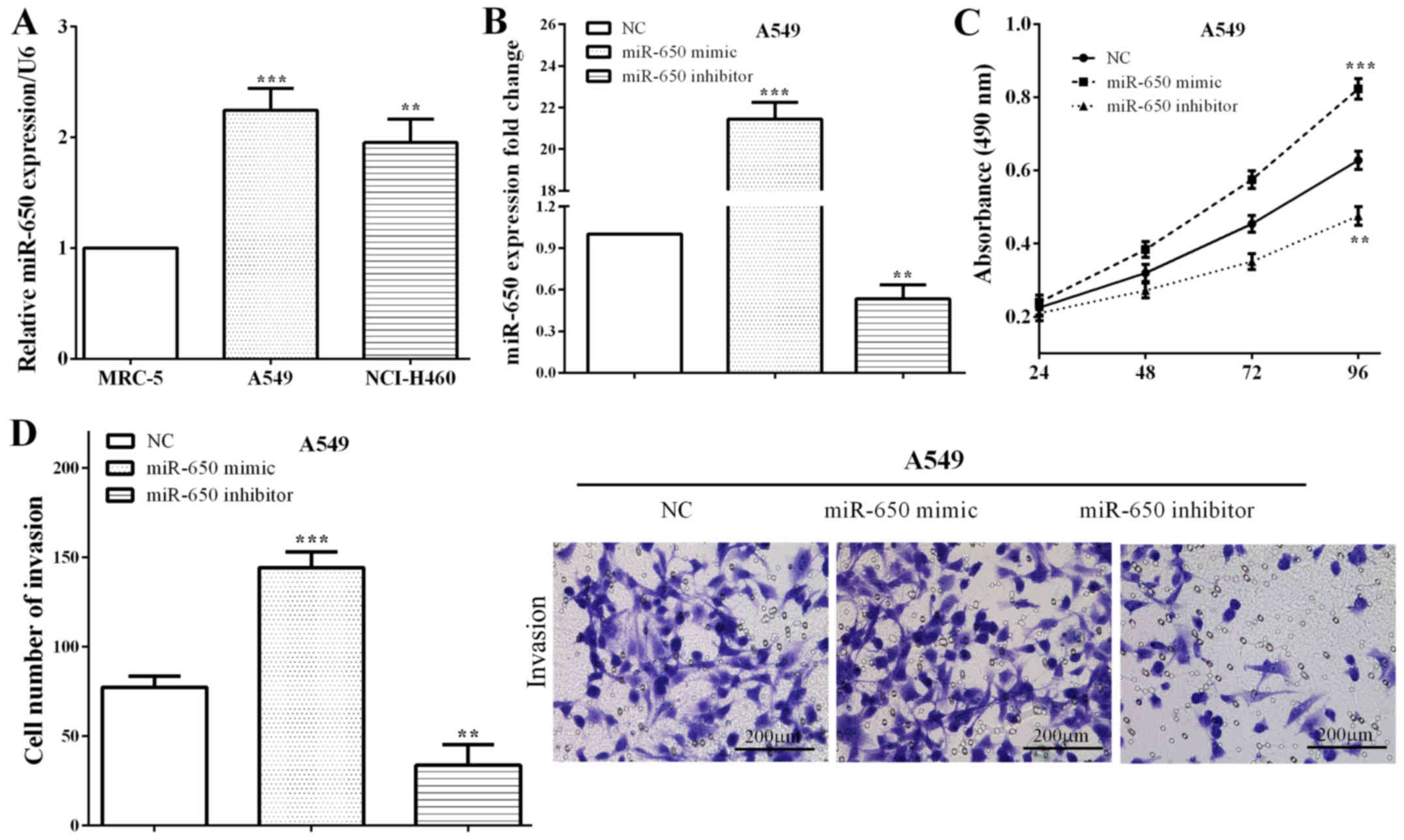
Expression of miR-650 was calculated in NSCLC cell
lines (A549 and NCI-H460) and a bronchial epithelial cell line
(MRC-5). Similar to results in tissues, miR-650 was overexpressed
in NSCLC A549 cells (P=0.0004) and NCI-H460 (P=0.0014) versus that
in normal MRC-5 cells (Fig. 2A),
which suggested that miR-650 may play important roles in growth and
metastasis of NSCLC. The miR-650 mimic or miR-650 inhibitor as well
as the negative control (NC) was transfected with A549 cells. We
measured the expression of miR-650 by RT-qPCR, and it demonstrated
that miR-650 was upregulated in A549 cells transfected miR-650
mimic (P<0.0001), while downregulated after transfected with
miR-650 inhibitor (P=0.0013) (Fig.
2B). Subsequently, CCK8 and Transwell assays were performed to
calculate the proliferative and invasive abilities of miR-650. As
expected, the cell proliferation (P=0.0008) and invasion (P=0.0004)
were enhanced by miR-650 mimic, whereas, miR-650 reduced the
proliferative (P=0.0019) and invasive (P=0.0044) capacities in A549
cells (Fig. 2C and D), which
illustrated that miR-650 acted as an oncogene in NSCLC.
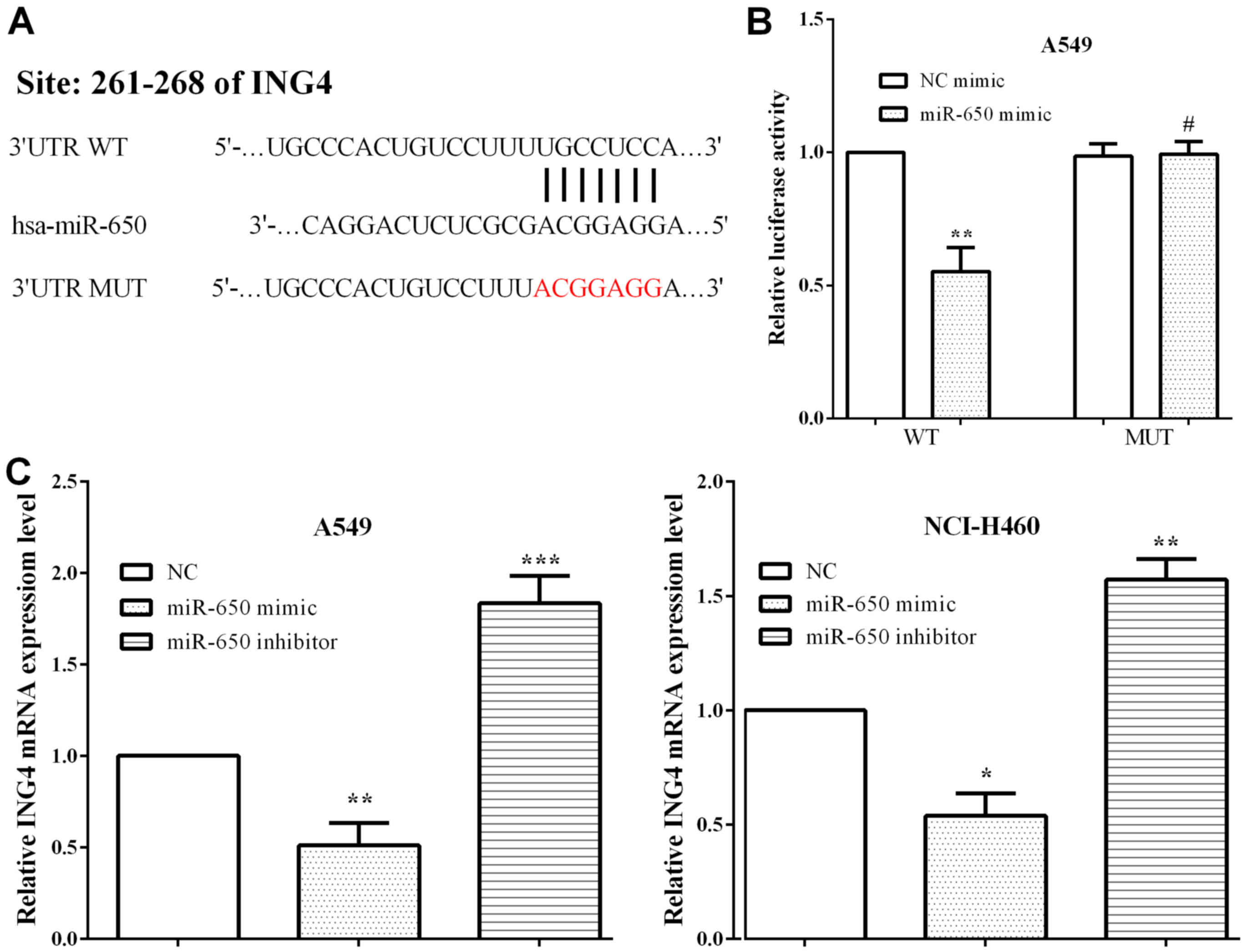
ING4 is a target gene of miR-650
TargetScan was adopted to explore the potential
targets of miR-650, and ING4 was identified (Fig. 3A). To evaluate whether miR-650
directly targeted the 3′-UTR of ING4 mRNA, luciferase reporter
assay was performed. A549 cells were co-transfected with the
miR-650 mimic and pmirGlo-ING4-WT or pmirGlo-ING4-MUT. As expected,
we discovered that the miR-650 mimic reduced the luciferase
activity of pmirGlo-ING4-WT (P=0.0010), whereas the activity of
pmirGlo-ING4-MUT was not altered (P=0.8645) (Fig. 3B). To verify that the expression of
ING4 was mediated by miR-650, RT-qPCR was applied to assess ING4
expression in A549 and NCI-H460 cells when exogenously altered by
miR-650. It was shown that miR-650 mimic decreased ING4 expression
(P=0.0024 and 0.0364) while miR-650 inhibitor enhanced the
expression of ING4 in A549 and NCI-H460 cells (P=0.0007 and 0.0044)
(Fig. 3C).
miR-650 regulates NSCLC progress
through ING4/Wnt1/β- catenin signaling pathway
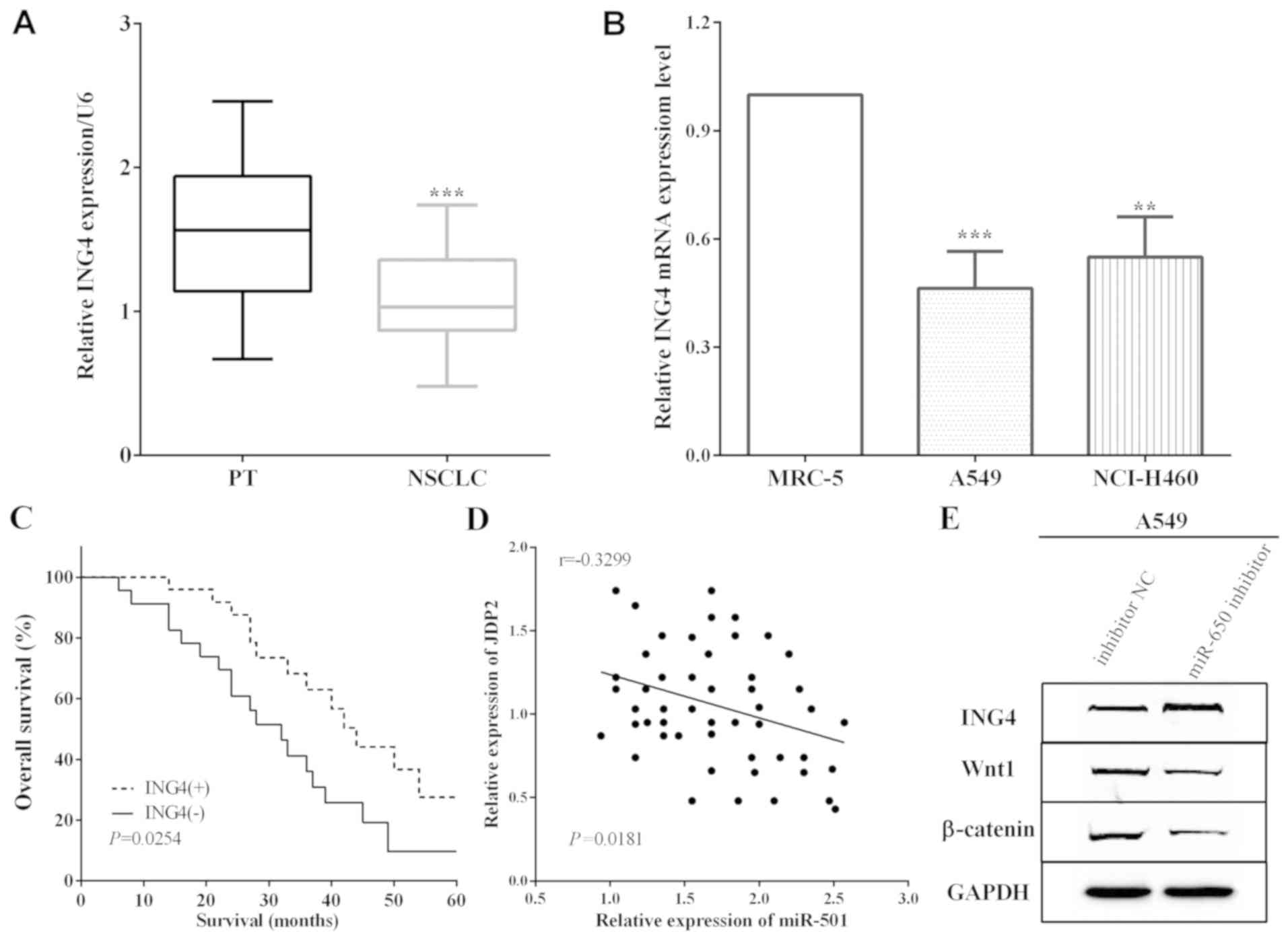
ING4 expression was measured by RT-qPCR in cancer
and adjacent normal lung tissues, and it was demonstrated that ING4
expession was low in NSCLC tissues versus adjacent normal lung
tissues (P<0.0001) (Fig. 4A).
Thus, the association between the expression of ING4 and the 5-year
overall survival was detected and it was established that
downregulation of ING4 predicted poor prognosis (P=0.0254)
(Fig. 4B). Moreover, ING4 expression
in cells was calculated and the RT-qPCR results indicated that ING4
was downregulated in A549 and NCI-H460 cells versus MRC-5 (P=0.0008
and 0.0022) (Fig. 4C). The
correlation between miR-650 and ING4 levels and the expression of
miR-650 had a negative association with ING4 expression in NSCLC
tissues (P=0.0181) (Fig. 4D). The
expression of Wnt1 and β-catenin was restrained by miR-650
inhibitor in A549 cells (Fig.
4E).
ING4 reverses partial roles of miR-650
on cell proliferative and invasive abilities
To verify the functions of ING4 in miR-650
overexpressed cells, pcDNA3.1-ING4 plasmid was transfected into
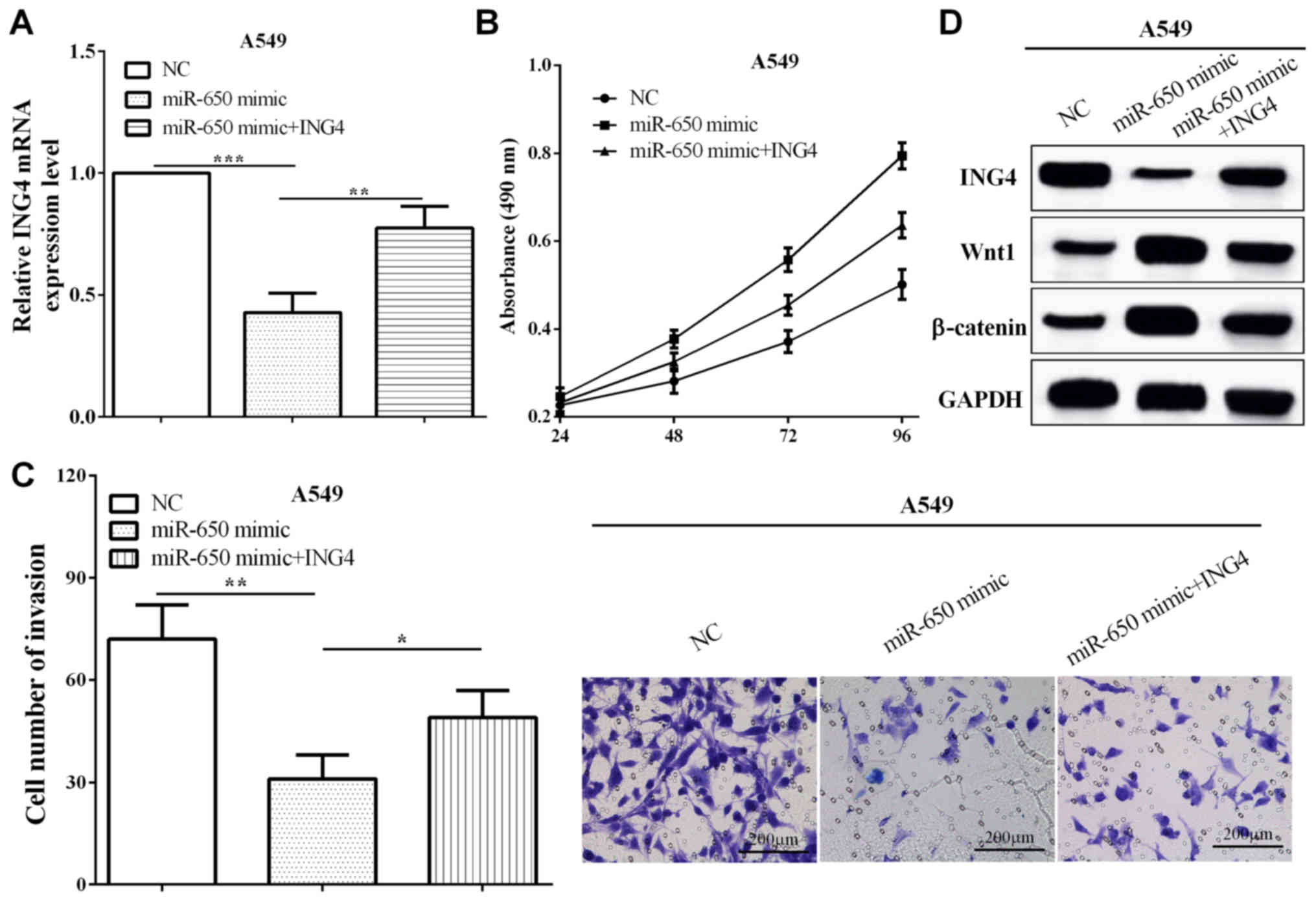
miR-650 overexpressed A549 cells (P=0.0075), as shown in Fig. 5A. Subsequently, CCK8 and Transwell
assays demonstrated that ING4 suppressed the proliferation and
invasion compared with cells only transfected with miR-650 mimic
(P=0.0030 and 0.0427) (Fig. 5B and
C). These data demonstrated that ING4 reversed partial roles of
miR-650 on NSCLC cell proliferation and invasion. In addition,
miR-650 mimic inhibited the expression of ING4, while the
expression of Wnt1 and β-catenin was increased. Re-expression of
ING4 reversed the reduction of Wnt1 and β-catenin in A549 (Fig. 5D). The results indicated that miR-650
promoted NSCLC cell proliferation and invasion by targeting ING4
through Wnt-1/β-catenin pathway.
Discussion
Non-small cell lung cancer, including lung
adenocarcinoma, squamous cell carcinoma and large cell carcinoma,
has a high morbidity and mortality (2,27).
Therefore, it is urgent to explore new biomarkers for disease
prevention and clinical treatment of NSCLC.
miRNAs bind to the 3′-UTR of the target mRNA to
incise the target mRNAs or to inhibit the translation of proteins
at post-transcriptional level (3).
miR-650 has been reported to act as a prognostic factor and
contributed to the docetaxel chemoresistance in lung adenocarcinoma
(28). Our results were consistent
with these findings. miR-650 was overexpressed in NSCLC and
upregulation of miR-650 predicted poor 5-year overall survival.
Moreover, You et al (29)
indicated that miR-650 enhanced cell proliferation, migration and
EMT through binding to ING4 in colorectal cancer and Zeng et
al (30) in hepatocellular
carcinoma. Consistent with all the above findings, we discovered
that miR-650 promoted cell proliferation and invasion by targeting
ING4 in NSCLC. However, miR-650 suppressed disease progress of
rheumatoid arthritis synovial fibroblasts and high-risk
non-metastatic colorectal cancer (31,32).
Therefore, we considered that the expression of miR-650 had tissue
specificity.
ING4 acted as a tumor suppressor, and was a
potential target for tumor therapy (33,34).
ING4 suppressed cell proliferation and induced cell apoptosis in
melanoma (35). Similarly, ING4
inhibited cell growth, invasion of EMT and suppressed tumor
angiogenesis (36). Moreover, ING4
suppressed cell proliferation, invasion and enhanced apoptosis in
osteosarcoma (37). Our results are
consistent with all these findings. ING4 expression was low in
NSCLC tissues and cell lines, and downregulation of ING4 predicted
poor prognosis. ING4 has been reported to be a target of several
miRNAs, including miR-330, miR-423 and miR-761 (38–40). We
discovered that ING4 was a direct target of miR-650 and miR-650
mediated the expression of ING4 through directly binding to the
3′-UTR of mRNA. Moreover, miR-650 suppressed Wnt-1/β-catenin
pathway by targeting ING4 in A549 cells. In addition, ING4 reversed
the effects of miR-650 in proliferation and invasion in A549
cells.
In brief, miR-650 was upregulated in NSCLC and
upregulation of miR-650 indicated a shorter overall survival of
NSCLC, while ING4 demonstrated the opposite results. miR-650
promoted cell proliferation and invasion through Wnt-1/β-catenin
pathway by binding to ING4. ING4 was able to partially reverse the
function of miR-650 in cell proliferation and invasion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XT wrote the manuscript. YD performed RT-qPCR and
western blot analysis. XiaW and XiuW were responsible for CCK8,
transwell and luciferase reporter assays. LZ analyzed and
interpreted the patients' data. HB helped with statistical
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Jining No. 1 People's
Hospital (Jining, China) approved this study. Patients who
participated in this research had complete clinical data. The
signed informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heist RS and Engelman JA: SnapShot:
non-small cell lung cancer. Cancer Cell. 21:448.e22012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng L, Xie Y, Zhang H and Wu Y:
Down-regulation of NDRG2 gene expression in human colorectal cancer
involves promoter methylation and microRNA-650. Biochem Biophys Res
Commun. 406:534–538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang YQ, Tian T, Zhu HY, Liang JH, Wu W,
Wu JZ, Xia Y, Wang L, Fan L, Li JY, et al: NDRG2 mRNA levels and
miR-28-5p and miR-650 activity in chronic lymphocytic leukemia. BMC
Cancer. 18:10092018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yun JH, Moon S, Lee HS, Hwang MY, Kim YJ,
Yu HY, Kim Y, Han BG, Kim BJ and Kim JM: MicroRNA-650 in a copy
number-variable region regulates the production of interleukin 6 in
human osteosarcoma cells. Oncol Lett. 10:2603–2609. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun B, Pu B, Chu D, Chu X, Li W and Wei D:
MicroRNA-650 expression in glioma is associated with prognosis of
patients. J Neurooncol. 115:375–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mraz M, Dolezalova D, Plevova K, Stano
Kozubik K, Mayerova V, Cerna K, Musilova K, Tichy B, Pavlova S,
Borsky M, et al: MicroRNA-650 expression is influenced by
immunoglobulin gene rearrangement and affects the biology of
chronic lymphocytic leukemia. Blood. 119:2110–2113. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin P, Chen H, Xie J, Zhou C and Zhu X:
Essential role of microRNA-650 in the regulation of B-cell
CLL/lymphoma 11B gene expression following transplantation: A novel
mechanism behind the acute rejection of renal allografts. Int J Mol
Med. 40:1840–1850. 2017.PubMed/NCBI
|
|
12
|
Ningning S, Libo S, Chuanbin W, Haijiang S
and Qing Z: MiR-650 regulates the proliferation, migration and
invasion of human oral cancer by targeting growth factor
independent 1 (Gfi1). Biochimie. 156:69–78. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan C, Xu L, Du P and Pang J: miRNA-650
exerts anti-leukemia activity by inhibiting cell proliferation
through Gfi1 targeting. Tumori. 104:369–374. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guérillon C, Bigot N and Pedeux R: The ING
tumor suppressor genes: Status in human tumors. Cancer Lett.
345:1–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Doyon Y, Cayrou C, Ullah M, Landry AJ,
Côté V, Selleck W, Lane WS, Tan S, Yang XJ and Côté J: ING tumor
suppressor proteins are critical regulators of chromatin
acetylation required for genome expression and perpetuation. Mol
Cell. 21:51–64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagashima M, Shiseki M, Pedeux RM, Okamura
S, Kitahama-Shiseki M, Miura K, Yokota J and Harris CC: A novel
PHD-finger motif protein, p47ING3, modulates p53-mediated
transcription, cell cycle control, and apoptosis. Oncogene.
22:343–350. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui S, Gao Y, Zhang K, Chen J, Wang R and
Chen L: The emerging role of inhibitor of growth 4 as a tumor
suppressor in multiple human cancers. Cell Physiol Biochem.
36:409–422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gunduz M, Nagatsuka H, Demircan K, Gunduz
E, Cengiz B, Ouchida M, Tsujigiwa H, Yamachika E, Fukushima K,
Beder L, et al: Frequent deletion and down-regulation of ING4, a
candidate tumor suppressor gene at 12p13, in head and neck squamous
cell carcinomas. Gene. 356:109–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Y, Mou X, Wang S, Liu XE and Sun X:
ING4 expressing oncolytic vaccinia virus promotes anti-tumor
efficiency and synergizes with gemcitabine in pancreatic cancer.
Oncotarget. 8:82728–82739. 2017.PubMed/NCBI
|
|
20
|
Keenen MM and Kim S: Tumor suppressor ING4
inhibits estrogen receptor activity in breast cancer cells. Breast
Cancer (Dove Med Press). 8:211–221. 2016.PubMed/NCBI
|
|
21
|
Ren Y, Zhao S, Chen H, Fu YM and Zhao B:
Association between the expression of inhibitor of growth family
member 4 and the progression of clear cell renal carcinoma. Oncol
Lett. 14:2453–2457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Fu R, Xu M, Huang Y, Sun G and Xu
L: N-methyl-N-nitro-N-nitrosoguanidine-mediated ING4 downregulation
contributed to the angiogenesis of transformed human gastric
epithelial cells. Life Sci. 199:179–187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai L, Li H, Chen C, Cheng X, Wang Y, Liu
J, Wang Y and Hao L: Role of inhibitor of growth 4 in the
suppression of human melanoma cells through the Fas/FasL-mediated
apoptosis pathway. Int J Mol Med. 41:1055–1061. 2018.PubMed/NCBI
|
|
24
|
Chen Y, Huang Y, Hou P, Zhang Z, Zhang Y,
Wang W, Sun G, Xu L, Zhou J, Bai J, et al: ING4 suppresses tumor
angiogenesis and functions as a prognostic marker in human
colorectal cancer. Oncotarget. 7:79017–79031. 2016.PubMed/NCBI
|
|
25
|
Pan X, Wang R, Bian H, De W, Zhang P, Wei
C and Wang Z: Overexpression of inhibitor of growth 4 enhances
radiosensitivity in non-small cell lung cancer cell line SPC-A1.
Technol Cancer Res Treat. 16:533–545. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ettinger DS, Akerley W, Borghaei H, Chang
AC, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Govindan R,
Grannis FW Jr, et al National comprehensive cancer network, :
Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw.
11:645–653, quiz 653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang JY, Cui SY, Chen YT, Song HZ, Huang
GC, Feng B, Sun M, De W, Wang R and Chen LB: MicroRNA-650 was a
prognostic factor in human lung adenocarcinoma and confers the
docetaxel chemoresistance of lung adenocarcinoma cells via
regulating Bcl-2/Bax expression. PLoS One. 8:e726152013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
You Q, Li H, Liu Y, Xu Y, Miao S, Yao G,
Xue Y, Geng J, Jin X and Meng H: MicroRNA-650 targets inhibitor of
growth 4 to promote colorectal cancer progression via mitogen
activated protein kinase signaling. Oncol Lett. 16:2326–2334.
2018.PubMed/NCBI
|
|
30
|
Zeng ZL, Li FJ, Gao F, Sun DS and Yao L:
Upregulation of miR-650 is correlated with down-regulation of ING4
and progression of hepatocellular carcinoma. J Surg Oncol.
107:105–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu X, Chen H, Zhang Q, Xu J, Shi Q and
Wang M: MiR-650 inhibits proliferation, migration and invasion of
rheumatoid arthritis synovial fibroblasts by targeting AKT2. Biomed
Pharmacother. 88:535–541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou C, Cui F, Li J, Wang D, Wei Y, Wu Y,
Wang J, Zhu H and Wang S: MiR-650 represses high-risk
non-metastatic colorectal cancer progression via inhibition of
AKT2/GSK3β/E-cadherin pathway. Oncotarget. 8:49534–49547.
2017.PubMed/NCBI
|
|
33
|
Li S, Fan T, Liu H, Chen J, Qin C and Ren
X: Tumor suppressor ING4 overexpression contributes to
proliferation and invasion inhibition in gastric carcinoma by
suppressing the NF-κB signaling pathway. Mol Biol Rep.
40:5723–5732. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yuan S, Jin J, Shi J and Hou Y: Inhibitor
of growth-4 is a potential target for cancer therapy. Tumour Biol.
37:4275–4279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma Y, Cheng X, Wang F, Pan J, Liu J, Chen
H, Wang Y and Cai L: ING4 inhibits proliferation and induces
apoptosis in human melanoma A375 cells via the Fas/caspase-8
apoptosis pathway. Dermatology. 232:265–272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qu H, Yin H, Yan S, Tao M, Xie Y and Chen
W: Inhibitor of growth 4 suppresses colorectal cancer growth and
invasion by inducing G1 arrest, inhibiting tumor angiogenesis and
reversing epithelial-mesenchymal transition. Oncol Rep.
35:2927–2935. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li M, Zhu Y, Zhang H, Li L, He P, Xia H,
Zhang Y and Mao C: Delivery of inhibitor of growth 4 (ING4) gene
significantly inhibits proliferation and invasion and promotes
apoptosis of human osteosarcoma cells. Sci Rep. 4:73802014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu X, Feng Y, Sun L, Qu L and Sun C: Roles
of microRNA-330 and its target gene ING4 in the development of
aggressive phenotype in hepatocellular carcinoma cells. Dig Dis
Sci. 62:715–722. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li S, Zeng A, Hu Q, Yan W, Liu Y and You
Y: miR-423-5p contributes to a malignant phenotype and temozolomide
chemoresistance in glioblastomas. Neuro Oncol. 19:55–65. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yan A, Yang C, Chen Z, Li C and Cai L:
MiR-761 promotes progression and metastasis of non-small cell lung
cancer by targeting ING4 and TIMP2. Cell Physiol Biochem. 37:55–66.
2015. View Article : Google Scholar : PubMed/NCBI
|