Introduction
In 2012, 300,400 newly diagnosed cases of oral
cancer were reported, and 145,400 patients succumbed to oral cancer
worldwide (1). Oral squamous cell
carcinomas (OSCCs) are the most common type of oral cancer
(2). Interleukin (IL)-8 is a key
cytokine that promotes tumor progression (3). Additionally, a previous study
demonstrated that OSCC cells may secrete IL-8 (4). IL-8 released by tumor cells recruits
neutrophils from the circulating blood to the local tumor
microenvironment (3). Neutrophils
entering the tumor microenvironment exert various biological
functions, including promotion of tumor angiogenesis and tumor cell
proliferation (5,6).
The forkhead/winglike spiral transcription factor
forkhead box P3 (FOXP3) is a member of the forkhead
transcription factor family (7). As
a key transcription factor, FOXP3 serves an important role
in the formation of regulatory T cells (Tregs) and their
immunosuppressive function (7).
Several studies on FOXP3 have focused on Tregs (8–10), and
previous studies demonstrated that FOXP3 is expressed not
only in Tregs, but also in various types of cancer (11–13),
including pancreatic cancer (11). A
study by Hinz et al (11)
indicated that pancreatic cancer cells expressing FOXP3
attenuate activated T-cell proliferation. By specifically reducing
FOXP3 expression in pancreatic cancer cells, its inhibitory
effect on T-cell proliferation can be partially reduced (11).
Neutrophil infiltration of a tumor microenvironment
is a common manifestation of tumor pathology (5,6,14,15).
Tumor-associated neutrophils (TANs) are classified as the N1 type,
with an antitumor effect, and the N2 type, with a tumor-promoting
effect (16,17). The phenotype of TANs is associated
with factors in the tumor microenvironment, including transforming
growth factor β and interferon β (16,17). A
study by Nozawa et al (18)
revealed that TANs promote tumor cell proliferation in pancreatic
cancer. Additionally, neutrophils are not only common immune killer
cells, but also a potential immunoregulatory cell type (19). Whether FOXP3 is expressed in
neutrophils and whether its expression serves a role in tumor
progression, to the best of our knowledge, has not been reported on
so far.
In the present study, quantitative PCR (qPCR) was
performed to detect the effect of cytokine IL-8 on the expression
levels of FOXP3 in neutrophils, and a Cell Counting Kit 8
(CCK-8) proliferation assay was used to evaluate the effect of
FOXP3 expression on the proliferation of OSCC cells in
vitro. Furthermore, immunofluorescence staining was conducted
to detect the expression levels of FOXP3 in neutrophils in
OSCC tissue samples in vivo. The present study broadens the
range of known mechanisms via which neutrophils promote tumor cell
proliferation and tumor progression.
Materials and methods
Human samples
Cancer tissue samples and paracarcinoma tissues (1.5
cm away from the cancer) were collected from patients with OSCC at
the College of Stomatology, Guangxi Medical University (Nanning,
China) between July 2017 and December 2017. Patients with OSCC
included in the present study had not been previously treated, and
were candidates for surgical resection of primary tumors and
selective or radical neck dissection. The exclusion criteria were:
i) Patients with other severe systemic disorders or distant
metastasis; and ii) patients with samples that were inadequate for
immunofluorescence staining.
Samples from 23 patients with OSCC were included in
the present study. The median age of the patients was 52 years
(range, 24–82 years); 14 patients were male, and 9 patients were
female. According to the Union for International Cancer Control 7th
Edition staging system (20), the
patients were divided into stage II (n=6), III (n=7) and IV (n=10)
groups. Each sample was stained using hematoxylin and eosin as
described previously (21).
Histopathological diagnoses were confirmed by at least two
independent pathologists. Notable intercellular bridging and
keratinized beads were observed in the cancer tissues, histological
features which are consistent with squamous cell carcinoma. Tissue
samples were soaked in 10% buffered formalin at room temperature
for 48 h, followed by paraffin embedding. Blood samples were
obtained from 9 healthy donors (5 men and 4 women; median age, 45;
age range, 28–56) and placed into heparinized tubes at room
temperature and processed within 2 h of blood collection. The
Ethics Committee of Guangxi Medical University approved the study
protocol, and all patients and healthy donors provided written
informed consent.
Cells and cell culture
SCC-9 cells were obtained from the Fuheng Cell
Center (Shanghai, China). The cell line was authenticated by
Shanghai Biowing Applied Biotechnology Co., Ltd., using short
tandem repeat (STR) profiling (22),
according to the American National Standards Institute Standard
(ASN-0002) set forth by the American Type Culture Collection
Standards Development Organization (22). The STR results revealed that this
cell line had no multiple alleles and no cross contamination of
human cells. The DNA of the cell line was found to match perfectly
with the type of cells in a cell line retrieval, and the DSMZ
database (dsmz.de/services/human-and-animal-cell-lines/online-str-analysis)
demonstrated that the cells, called SCC-9, corresponded to the cell
number CRL-1629. SCC-9 cells were maintained in DMEM/F12 medium
(cat. no. 319-085-CL; Wisent Biotechnology) supplemented with 10%
FBS (cat. no. 086-110; Wisent Biotechnology) and 1%
penicillin/streptomycin solution (cat. no. 450-201-EL; Wisent
Biotechnology). Neutrophils were isolated from healthy donor blood
samples by means of Polymorphprep™ (cat. no. 1114683; Axis-Shield
Diagnostics, Ltd.) according to the manufacturer's protocol, and
neutrophils were resuspended in RPMI-1640 medium (cat. no.
350-006-CL; Wisent Biotechnology) supplemented with 10% FBS and 1%
penicillin/streptomycin solution. All cells were cultured at 37°C
in a humidified atmosphere containing 5% CO2, and all
subsequent culturing were performed under the same conditions.
Isolation of neutrophils and
co-culture with SCC-9 cells
To isolate neutrophils, heparinized blood was
layered on Polymorphprep according to the manufacturer's protocol.
Briefly, 5 ml heparinized blood was carefully layered over 5 ml
Polymorphprep in a 15 ml centrifuge tube. The samples were
centrifuged at 500 × g for 30 min at 20°C. Subsequently, the lower
phase containing neutrophils was collected, diluted with PBS (cat.
no. P1010; Beijing Solarbio Science & Technology Co., Ltd.) and
centrifuged at 400 × g for 10 min at 20°C. The neutrophils were
resuspended in RPMI-1640 medium supplemented with 10% FBS.
The co-culture experiments were conducted in 96-well
plates, as described previously (23). The SCC-9 cells were plated at a
density of 5×105 cells/ml in DMEM/F12 medium containing
10% FBS. After 24 h, the medium was discarded, and the cells were
incubated in 100 µl RPMI-1640 medium supplemented with 10% FBS.
Neutrophils were directly added to the tumor cells at a final
density of 5×105 cells/ml. To investigate the effect of
FOXP3 on the proliferation of SCC-9 cells in co-culture, the
cells were treated with IL-8 (100 ng/ml; cat. no. 200-08;
PeproTech, Inc.) or peptide P60 (P60; 100 µM; cat. no. 350582;
Abbiotec, Inc.), a specific peptide inhibitor of FOXP3
(24). The plates were incubated for
24 h at 37°C in a humidified atmosphere containing 5%
CO2.
Cell proliferation assay
Cell proliferation was assessed using the CCK-8
assay (cat. no. 70-CCK805; Hangzhou MultiSciences (Lianke) Biotech
Co., Ltd.) according to the manufacturer's protocol. For co-culture
experiments, SCC-9 cells were plated at a density of
5×105 cells/ml in DMEM/F12 medium containing 10% FBS.
After 24 h, the medium was discarded, and the cells were incubated
in 100 µl of RPMI-1640 medium supplemented with 10% FBS.
Neutrophils were directly added to the tumor cells at a final
density of 5×105 cells/ml. To investigate the effect of
FOXP3 on the proliferation of SCC-9 cells in co-culture, the
cells were treated with human recombinant IL-8 (100 ng/ml; cat. no.
200-08; PeproTech, Inc.) or P60. Subsequently, 100 µl RPMI-1640
medium containing 10% FBS and 10 µl CCK-8 reagent was added.
Optical density was measured at 450 nm on a microplate reader after
2 h. The experiment was independently repeated three times.
Reverse-transcription (RT)-qPCR
To examine the effects of IL-8 on FOXP3
expression in neutrophils, neutrophils (1×106
cells/well) were cultured in 24-well plates in RPMI-1640 medium
supplemented with 10% FBS and treated with recombinant human IL-8
(100 ng/ml, diluted in distilled water) or treated with the same
volume of PBS (control group) for 12 h.
For mRNA analysis, RNA (200 ng per sample) was
extracted from neutrophils using RNAiso Plus reagent (cat. no.
9108; Takara Bio, Inc.) according to the manufacturer's protocol.
Complementary DNA (cDNA) was synthesized with the PrimeScript™ RT
Reagent kit with gDNA Eraser (Perfect Real Time) (cat. no. RR047A;
Takara Bio, Inc.) according to the manufacturer's protocol. The
reverse transcription temperature protocol used was: 37°C for 15
min followed by 85°C for 5 sec. The primers for GAPDH and
FOXP3 were purchased from Takara (Takara Bio, Inc.). The
primer sequences were as follows: FOXP3 forward,
5′-GAAACAGCACATTCCCAGAGTTC-3′ and reverse, 5′-ATGGCCCAGCGGATGAG-3′
(25); and GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse, 5′-TGGTGAAGACGCCAGTGGA-3′.
GAPDH was used as an internal control. qPCR was performed on
an ABI 7500 real-time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using TB Premix Ex Taq™ II (cat. no. RR820A;
Takara Bio, Inc.) according to the manufacturer's protocol. The
thermocycling conditions were: Denaturation at 95°C for 30 sec;
followed by 40 cycles at 95°C for 5 sec and 60°C for 34 sec, and a
final extension step of 95°C for 15 sec, 60°C for 1 min, 95°C for
15 sec and 60°C for 15 sec. The experiment was independently
repeated three times. The 2−ΔΔCq method was used to
calculate the relative fold in gene expression determined from
quantitative PCR experiments (26,27). The
fold change in cDNA of the target gene relative to the GAPDH
endogenous control was determined by the following equation: Fold
change=2−ΔΔCq, where
ΔΔCq=[(CqFOXP3-CqGAPDH) (experimental
group)-(CqFOXP3-CqGAPDH) (control group)].
The Ct value is the number of amplification cycles at which the
fluorescence signal reaches a set threshold.
Immunofluorescence staining
Tissue samples were cut into 4-µm-thick sections.
The sections were deparaffinized with xylene and rehydrated in a
descending ethanol series of 70, 80, 90, 95 and 100%, followed by
antigen retrieval with citrate buffer (cat. no. mvs-0066; Fuzhou
Maixin Biotech Co., Ltd.) with pH 6.0, microwaved on high power to
boiling point for 3 min and subsequently blocked with 5% BSA
(Beijing Solarbio Science & Technology Co., Ltd.; cat. no.
SW3015) for 30 min. The sections were incubated at 4°C overnight
with the following primary antibodies at 1:100 dilution: Rabbit
polyclonal anti-human FOXP3 antibody (cat. no. ab10901;
Abcam), mouse monoclonal anti-human myeloperoxidase (MPO) antibody
(cat. no. ab25989; Abcam) and mouse monoclonal anti-human IL-8
antibody (cat. no. sc-8427; Santa Cruz Biotechnology, Inc.). The
sections were washed with PBS, followed by incubation with the
following secondary antibodies at 1:1,000 dilution for 1 h at 37°C:
Goat anti-rabbit immunoglobulin G (IgG) antibody (cat. no.
ab150077; Abcam) and goat anti-mouse IgG antibody (cat. no.
ab150114; Abcam). Sections were washed with PBS followed by
incubation with DAPI (5 µg/ml; cat. no. C0060; Beijing Solarbio
Science & Technology Co., Ltd.) at 37°C for 10 min. The tissue
sections were washed with PBS before mounting on the slides with
Solarbio Fluorescence Mounting medium (cat. no. S2100; Beijing
Solarbio Science & Technology Co., Ltd.). SCC-9 cells were
fixed with 4% paraformaldehyde at room temperature, and
permeabilized with 0.3% Triton X-100 (Beijing Solarbio Science
& Technology Co., Ltd.; cat. no. T8200) for 20 min. After
blocking with 5% BSA at 37°C for 30 min, the samples were stained
as described above. Images were captured under a fluorescence
microscope (magnification, ×400; Olympus Corporation), and the
integrated optical density (IOD) of FOXP3 protein expression
in three randomly selected fields was measured using Image Pro-Plus
6.0 software (version 6.0; Media Cybernetics, Inc.). The IOD mean
value of each section was determined after analyzing three random
images.
Statistical analysis
Each experiment was repeated three times and the
data are expressed as the mean ± standard deviation. IBM SPSS
Statistics software (version 20.0; IBM Corp.) was used to perform
statistical analyses. A paired Student's t-test was used to analyze
the difference between FOXP3 mRNA expression in neutrophils.
An unpaired Student's t-test was used to analyze the
immunofluorescence results of FOXP3 protein expression in
neutrophils. Cell proliferation assays were analyzed using a
one-way ANOVA with a post-hoc Tukey's. P<0.05 was considered to
indicate a statistically significant difference.
Results
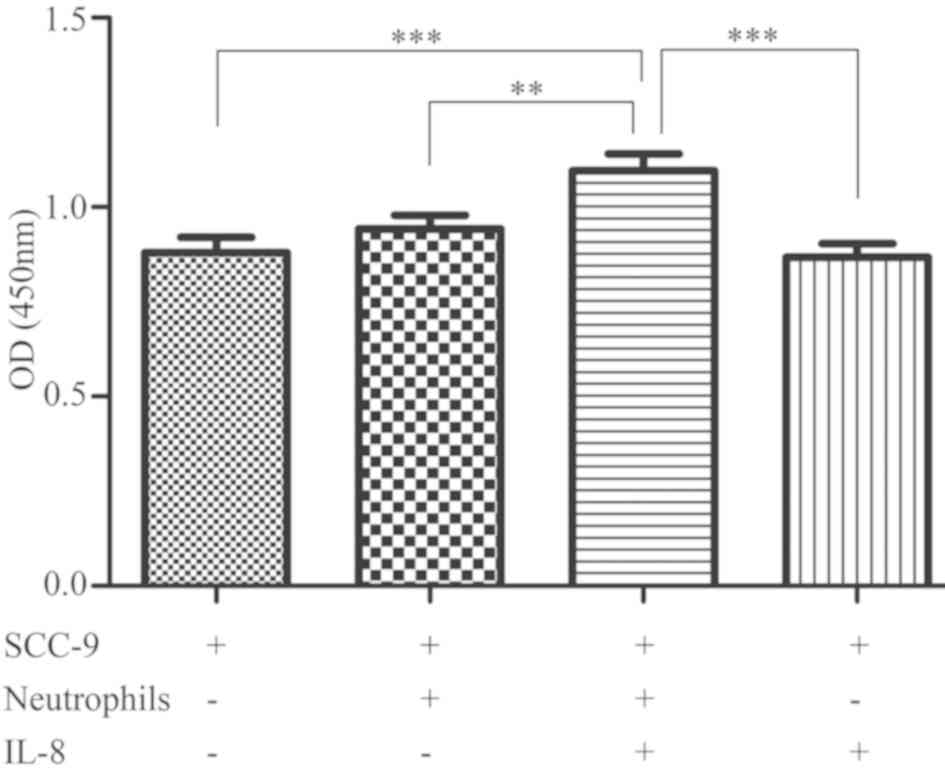
Neutrophils and IL-8 promote SCC-9
cell proliferation
OSCC cells secrete IL-8, and high concentrations of
IL-8 recruit neutrophils to the cancer microenvironment; thus,
there are large quantities of IL-8 and neutrophils in the tumor
microenvironment (3,4). To investigate the effects of IL-8 and
neutrophils on the proliferation of OSCC cells, neutrophils and
OSCC cells were co-cultured and treated with IL-8 for 24 h, and
SCC-9 cell proliferation was assessed using a CCK-8 assay. The
results revealed that IL-8 and neutrophils exerted a synergistic
effect on SCC-9 cells and together promoted their proliferation
(P<0.001; Fig. 1).
It has previously been shown that neutrophils adhere
to the surface of epithelial cells through adhesion molecules
(28), and IL-8 promotes the
adhesion of neutrophils by upregulating the expression of these
adhesion molecules (29). To confirm
that the increased proliferation in the SCC-9/neutrophil/IL-8 group
was not due to increased adhesion promoted by neutrophils, after
washing, neutrophils were detected by immunofluorescence staining
using an MPO antibody, which has been used as a neutrophil-specific
antibody in previous studies (30,31). The
results confirmed that there were no adherent neutrophils on the
surface of SCC-9 cells in 96-well plates after washing (Fig. S1).
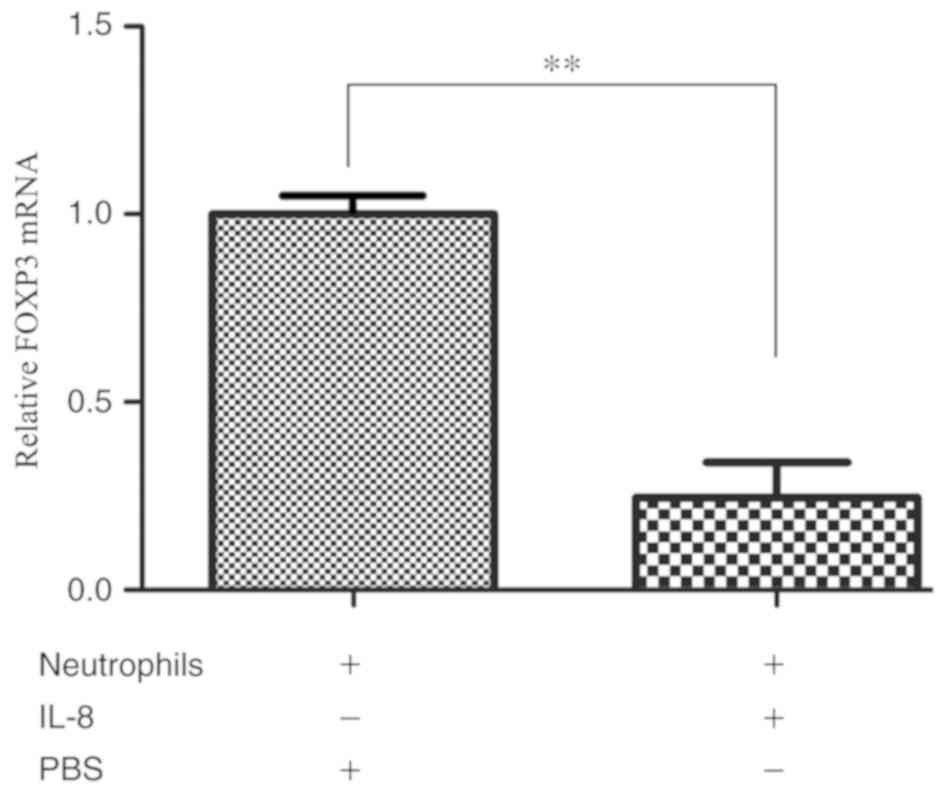
IL-8 downregulates FOXP3 mRNA
expression in neutrophils
Various studies have demonstrated that FOXP3
is expressed in a variety of tumor cells and is involved in tumor
cell proliferation and apoptosis (11,12,32). To
test if IL-8 induces a change in FOXP3 expression in
neutrophils, human peripheral blood neutrophils were stimulated
with recombinant human IL-8 and the mRNA expression levels of
FOXP3 in neutrophils were evaluated. The results indicated
that IL-8 downregulated FOXP3 mRNA expression in neutrophils
(P=0.005; Fig. 2).
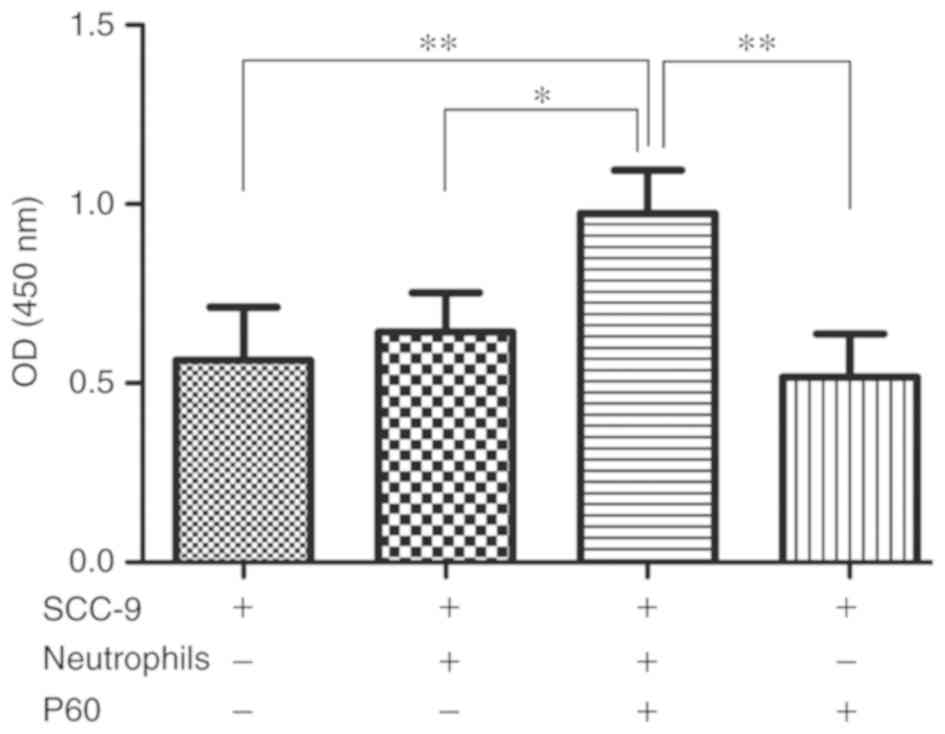
Neutrophils and an inhibitor of FOXP3
promote proliferation of SCC-9 cells
To investigate the effect of FOXP3 in
neutrophils on the proliferation of SCC-9 cells, neutrophils and
OSCC cells were co-cultured and treated with P60, a specific
peptide inhibitor of FOXP3, for 24 h. Subsequently, SCC-9
cell proliferation was assessed using a CCK-8 assay. The results
revealed that P60 treatment of co-cultured neutrophils and SCC-9
cells increased the proliferation of SCC-9 cells in compared with
SCC-9 cells alone (P=0.004; Fig. 3),
suggesting that a combination of neutrophils and an inhibitor of
FOXP3 together promote the proliferation of SCC-9 cells.
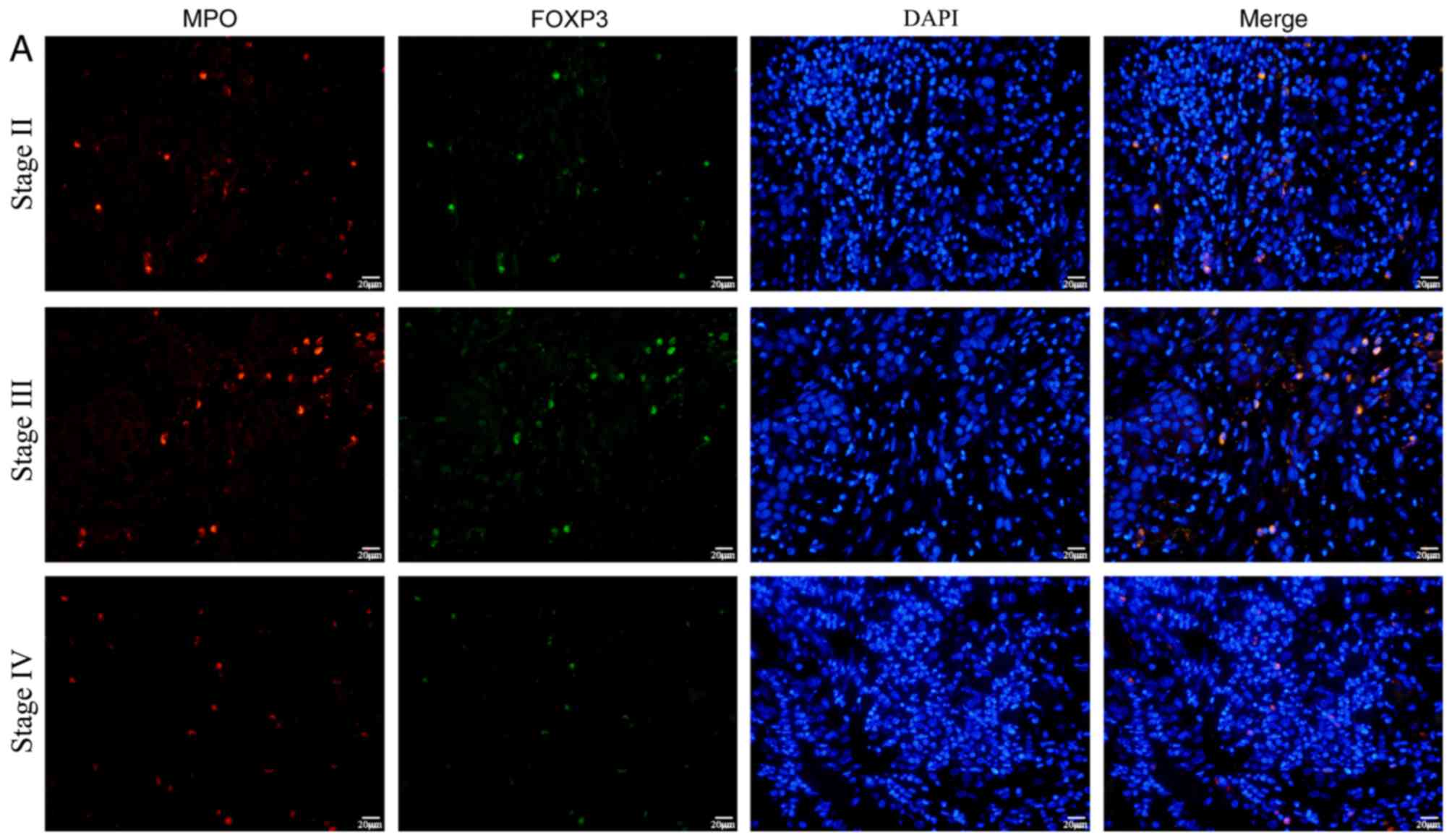
Protein expression levels of FOXP3 in
neutrophils in the OSCC tumor microenvironment
To investigate the association of FOXP3 in
neutrophils and OSCC in vivo, cancer tissue samples from 23
patients were stained by immunofluorescence to detect FOXP3
expression in neutrophils infiltrating the tumor microenvironment.
MPO was also stained as marker of neutrophils (30,31). The
results revealed that FOXP3 protein expression in
neutrophils was significantly lower in patients with stage IV
cancer compared with those with stage II (P=0.001) and III
(P=0.008) (Fig. 4).
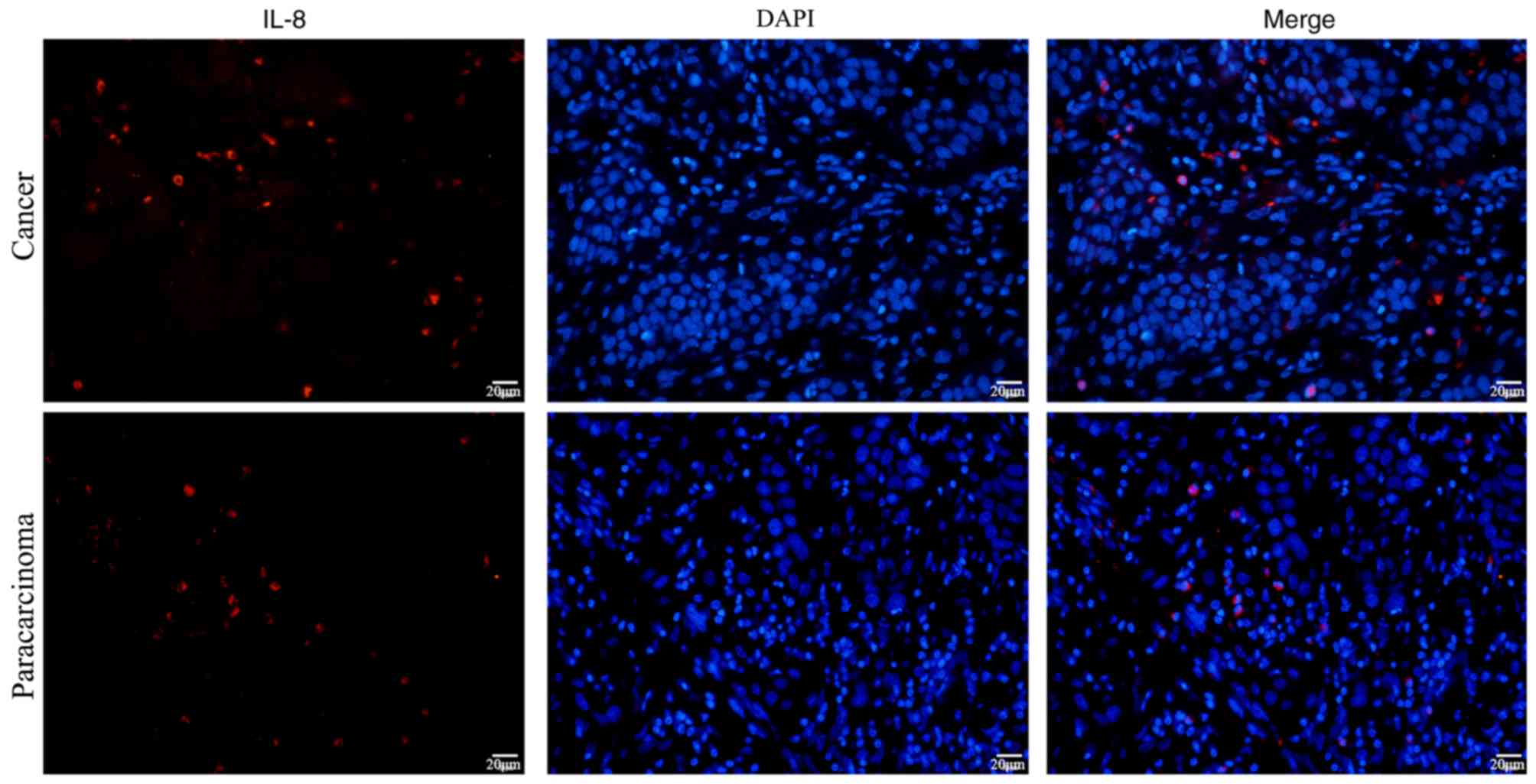
Protein expression levels of IL-8 in
the OSCC tumor microenvironment
Fujita et al (33) reported that high expression of IL-8
in oral cancer is associated with poor prognosis, suggesting that
IL-8 may promote proliferation and metastasis of oral cancer cells.
The aforementioned in vitro co-culture experimental results
revealed that IL-8 downregulated FOXP3 mRNA expression in
neutrophils, and neutrophils treated with inhibitor of FOXP3
enhanced proliferation of SCC-9 cells. To investigate if IL-8
protein expression is also altered in vivo,
immunofluorescence was conducted to detect IL-8 protein expression
in cancer and paracarcinoma tissue samples from patients with OSCC.
The results demonstrated that IL-8 protein was expressed in both
OSCC tissues and paracarcinoma tissues (Fig. 5).
Discussion
Recent studies (34–36)
suggest that FOXP3 is expressed in a variety of tumor cells
and its protumor or antitumor roles are a controversial topic. Hinz
et al (11) demonstrated that
T-cell proliferation is observed after specific silencing of
FOXP3 expression with small interfering RNAs in pancreatic
cancer cells. This result indicates that pancreatic cancer cells
expressing FOXP3 inhibit T-cell proliferation, and thereby
promote tumor progression. However, FOXP3 also performs a
tumor suppressor function in breast cancer cells (37,38).
Zhang et al (37) reported
that FOXP3 is expressed in breast cancer cells and is
negatively associated with breast cancer metastasis. Furthermore, a
previous study demonstrated that FOXP3 inhibits adhesion and
invasiveness of breast cancer cells by downregulating CD44
(37). To date, however,
FOXP3 expression and its role in neutrophils, to the best of
our knowledge, have not been reported on. The present study
demonstrated that IL-8 downregulated the expression of FOXP3
in neutrophils, and following P60 inhibition of FOXP3,
neutrophils promoted the proliferation of SCC-9 cells. A study by
Casares et al (24) reported
that P60 enters the cells, downregulates FOXP3 nuclear
translocation and inhibits the function of FOXP3 protein
in vitro. It has been identified that P60 alone does not
alter effector T cell or Treg proliferation in response to
stimulation in vitro (24).
In the present study, SCC-9 cells were treated with P60 for 24 h,
and there was no statistically significant difference identified in
the proliferation of SCC-9 cells compared with the control group
(P=0.656). Therefore, P60 alone may not affect the proliferation of
SCC-9 cells. The results revealed that IL-8 downregulated
FOXP3 expression in neutrophils, but its specific signaling
pathway was not examined in the present study. According to other
studies, IL-6 and IL-27 inhibit FOXP3 expression by
activating the STAT3 signaling pathway (39,40).
Furthermore, a study by Qu et al (41) revealed that IL-8 increased STAT3
phosphorylation, whereas knockout of IL-8 reduced phosphorylation
of STAT3, suggesting that IL-8 may also suppress FOXP3
expression in neutrophils by activating the STAT3 signaling
pathway.
TANs serve an important role in the progression of
tumor development. For example, neutrophil-released neutrophil
elastase (NE) causes a release of growth factors, thereby promoting
tumor cell proliferation (6). NE
also promotes tumor cell proliferation by degrading insulin
receptor substrate 1 (42). In
addition, in the tumor microenvironment, neutrophils promote the
proliferation of tumor cells by releasing neutrophil extracellular
traps (NETs) into the microenvironment (43,44). The
present study revealed that IL-8 downregulated FOXP3
expression in neutrophils, and IL-8 treatment combined with
co-culturing with neutrophils promoted the proliferation of SCC-9
cells. In addition, the present study demonstrated that the
expression of FOXP3 in neutrophils in samples from patients
with stage IV tumors was lower compared with that in stage III and
II patients. This suggested that downregulation of FOXP3 in
neutrophils in the cancer microenvironment may be associated with
progression of OSCC. Chung et al (45) demonstrated that in
Foxp3-deficient mice, the microglia produced increased
quantities of reactive oxygen species (ROS) when treated with
lipopolysaccharide compared with the wild-type mice. FOXP3
negatively regulated the production of ROS by activating NF-κB
(45). A previous study on NETs
suggested that increased levels of ROS in neutrophils can promote
the formation and release of NETs (46). Considering the results of these
previous studies, it was hypothesized that IL-8 recruited
neutrophils from the blood vessel to the local tumor
microenvironment and downregulated FOXP3 expression in
neutrophils. The downregulation of FOXP3 expression in
neutrophils may subsequently lead to increased production of ROS in
neutrophils, and potentially promote the production and release of
NETs, stimulating the proliferation of OSCC cells.
In summary, IL-8 in the tumor microenvironment may
recruit neutrophils, and downregulation of FOXP3 in
neutrophils by IL-8 may promote the progression of OSCC. These
finding expands the range of known mechanisms through which
neutrophils promote proliferation of tumor cells and thus, tumor
progression. However, additional studies are required to fully
elucidate the underlying mechanisms.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank the pathologists,
Professor Haiyun Qing and Professor Yiping Yang (College of
Stomatology, Guangxi Medical University) for their assistance in
pathological diagnosis.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81360403)
and the Medical and Health Appropriate Technology Development and
Promotion Project of Guangxi (grant no. S2018067).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FL and HK conceived the study and participated in
its design. CZ and FL drafted the manuscript. CZ, XC and WF
performed the cell experiments. QT, ZZ and TY performed sample
collection and immunofluorescence staining. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Guangxi Medical University. Written informed consent
was provided by all patients and healthy volunteers.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nasry WHS, Rodriguez-Lecompte JC and
Martin CK: Role of COX-2/PGE2 mediated inflammation in oral
squamous cell carcinoma. Cancers (Basel). 10:E3482018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haqqani AS, Sandhu JK and Birnboim HC:
Expression of interleukin-8 promotes neutrophil infiltration and
genetic instability in mutatect tumors. Neoplasia. 2:561–568. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lalla RV, Spiro JD, Tanzer ML and Kreutzer
DL: Association of fibrin and interleukin 8 in human oral squamous
cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
95:452–457. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bekes EM, Schweighofer B, Kupriyanova TA,
Zajac E, Ardi VC, Quigley JP and Deryugina EI: Tumor-recruited
neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately
the levels of tumor angiogenesis and efficiency of malignant cell
intravasation. Am J Pathol. 179:1455–1470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wada Y, Yoshida K, Tsutani Y, Shigematsu
H, Oeda M, Sanada Y, Suzuki T, Mizuiri H, Hamai Y, Tanabe K, et al:
Neutrophil elastase induces cell proliferation and migration by the
release of TGF-alpha, PDGF and VEGF in esophageal cell lines. Oncol
Rep. 17:161–167. 2007.PubMed/NCBI
|
|
7
|
Bennett CL, Christie J, Ramsdell F,
Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT,
Chance PF and Ochs HD: The immune dysregulation,
polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused
by mutations of FOXP3. Nat Genet. 27:20–21. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia H, Qi H, Gong Z, Yang S, Ren J, Liu Y,
Li MY and Chen GG: The expression of FOXP3 and its role in human
cancers. Biochim Biophys Acta Rev Cancer. 1871:170–178. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wolf D, Wolf AM, Rumpold H, Fiegl H,
Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E and
Marth C: The expression of the regulatory T cell-specific forkhead
box transcription factor FoxP3 is associated with poor prognosis in
ovarian cancer. Clin Cancer Res. 11:8326–8331. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hinz S, Pagerols-Raluy L, Oberg HH,
Ammerpohl O, Grüssel S, Sipos B, Grützmann R, Pilarsky C,
Ungefroren H, Saeger HD, et al: Foxp3 expression in pancreatic
carcinoma cells as a novel mechanism of immune evasion in cancer.
Cancer Res. 67:8344–8350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu R, Liu C, Chen D, Yang WH, Liu X, Liu
CG, Dugas CM, Tang F, Zheng P, Liu Y and Wang L: FOXP3 controls an
miR-146/NF-κB negative feedback loop that inhibits apoptosis in
breast cancer cells. Cancer Res. 75:1703–1713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang HY and Sun H: Up-regulation of Foxp3
inhibits cell proliferation, migration and invasion in epithelial
ovarian cancer. Cancer Lett. 287:91–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trellakis S, Bruderek K, Dumitru CA,
Gholaman H, Gu X, Bankfalvi A, Scherag A, Hütte J, Dominas N,
Lehnerdt GF, et al: Polymorphonuclear granulocytes in human head
and neck cancer: Enhanced inflammatory activity, modulation by
cancer cells and expansion in advanced disease. Int J Cancer.
129:2183–2193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang N, Feng Y, Wang Q, Liu S, Xiang L,
Sun M, Zhang X, Liu G, Qu X and Wei F: Neutrophils infiltration in
the tongue squamous cell carcinoma and its correlation with CEACAM1
expression on tumor cells. PloS One. 9:e899912014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fridlender ZG, Sun J, Kim S, Kapoor V,
Cheng G, Ling L, Worthen GS and Albelda SM: Polarization of
tumor-associated neutrophil phenotype by TGF-beta: ‘N1’ versus ‘N2’
TAN. Cancer Cell. 16:183–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Piccard H, Muschel RJ and Opdenakker G: On
the dual roles and polarized phenotypes of neutrophils in tumor
development and progression. Crit Rev Oncol Hematol. 82:296–309.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nozawa H, Chiu C and Hanahan D:
Infiltrating neutrophils mediate the initial angiogenic switch in a
mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA.
103:12493–12498. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tateda K, Moore TA, Newstead MW, Tsai WC,
Zeng X, Deng JC, Chen G, Reddy R, Yamaguchi K and Standiford TJ:
Chemokine-dependent neutrophil recruitment in a murine model of
legionella pneumonia: Potential role of neutrophils as
immunoregulatory cells. Infect Immun. 69:2017–2024. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of malignant tumours (UICC International
Union Against Cancer)7th. Wiley-Blackwell; Oxford: 2009
|
|
21
|
Bancroft J and Stevens A: Theories and
practice of histological techniquesChurchill Livingstone; New York,
USA: pp. 124–150. 1996
|
|
22
|
Capes-Davis A, Reid YA, Kline MC, Storts
DR, Strauss E, Dirks WG, Drexler HG, MacLeod RA, Sykes G, Kohara A,
et al: Match criteria for human cell line authentication: Where do
we draw the line? Int J Cancer. 132:2510–2519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grandel U, Heygster D, Sibelius U, Fink L,
Sigel S, Seeger W, Grimminger F and Hattar K: Amplification of
lipopolysaccharide-induced cytokine synthesis in non-small cell
lung cancer/neutrophil cocultures. Mol Cancer Res. 7:1729–1735.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Casares N, Rudilla F, Arribillaga L,
Llopiz D, Riezu-Boj JI, Lozano T, Lopez-Sagaseta J, Guembe L,
Sarobe P, Prieto J, et al: A peptide inhibitor of FOXP3 impairs
regulatory T cell activity and improves vaccine efficacy in mice. J
Immunol. 185:5150–5159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fecci PE, Mitchell DA, Whitesides JF, Xie
W, Friedman AH, Archer GE, Herndon JE II, Bigner DD, Dranoff G and
Sampson JH: Increased regulatory T-cell fraction amidst a
diminished CD4 compartment explains cellular immune defects in
patients with malignant glioma. Cancer Res. 66:3294–3302. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Winer J, Jung CK, Shackel I and Williams
PM: Development and validation of real-time quantitative reverse
transcriptase-polymerase chain reaction for monitoring gene
expression in cardiac myocytes in vitro. Anal Biochem. 270:41–49.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmittgen TD, Zakrajsek BA, Mills AG,
Gorn V, Singer MJ and Reed MW: Quantitative reverse
transcription-polymerase chain reaction to study mRNA decay:
Comparison of endpoint and real-time methods. Anal Biochem.
285:194–204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McDonald RJ, St George JA, Pan LC and Hyde
DM: Neutrophil adherence to airway epithelium is reduced by
antibodies to the leukocyte CD11/CD18 complex. Inflammation.
17:145–151. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Crowe SE, Alvarez L, Dytoc M, Hunt RH,
Muller M, Sherman P, Patel J, Jin Y and Ernst PB: Expression of
interleukin 8 and CD54 by human gastric epithelium after
Helicobacter pylori infection in vitro. Gastroenterology.
108:65–74. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mole DJ, Webster SP, Uings I, Zheng X,
Binnie M, Wilson K, Hutchinson JP, Mirguet O, Walker A, Beaufils B,
et al: Kynurenine-3-monooxygenase inhibition prevents multiple
organ failure in rodent models of acute pancreatitis. Nat Med.
22:202–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen CB, Liu LS, Zhou J, Wang XP, Han M,
Jiao XY, He XS and Yuan XP: Up-regulation of HMGB1 exacerbates
renal ischemia-reperfusion injury by stimulating inflammatory and
immune responses through the TLR4 signaling pathway in mice. Cell
Physiol Biochem. 41:2447–2460. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zuo T, Wang L, Morrison C, Chang X, Zhang
H, Li W, Liu Y, Wang Y, Liu X, Chan MW, et al: FOXP3 is an X-linked
breast cancer suppressor gene and an important repressor of the
HER-2/ErbB2 oncogene. Cell. 129:1275–1286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fujita Y, Okamoto M, Goda H, Tano T,
Nakashiro K, Sugita A, Fujita T, Koido S, Homma S, Kawakami Y and
Hamakawa H: Prognostic significance of interleukin-8 and
CD163-positive cell-infiltration in tumor tissues in patients with
oral squamous cell carcinoma. PLoS One. 9:e1103782014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun X, Feng Z, Wang Y, Qu Y and Gai Y:
Expression of Foxp3 and its prognostic significance in colorectal
cancer. Int J Immunopathol Pharmacol. 30:201–206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi JY, Ma LJ, Zhang JW, Duan M, Ding ZB,
Yang LX, Cao Y, Zhou J, Fan J, Zhang X, et al: FOXP3 Is a HCC
suppressor gene and Acts through regulating the TGF-β/Smad2/3
signaling pathway. BMC Cancer. 17:6482017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang S, Liu Y, Li MY, Ng CSH, Yang SL,
Wang S, Zou C, Dong Y, Du J, Long X, et al: FOXP3 promotes tumor
growth and metastasis by activating Wnt/β-catenin signaling pathway
and EMT in non-small cell lung cancer. Mol Cancer. 16:1242017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang C, Xu Y, Hao Q, Wang S, Li H, Li J,
Gao Y, Li M, Li W, Xue X, et al: FOXP3 suppresses breast cancer
metastasis through downregulation of CD44. Int J Cancer.
137:1279–1290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu R, Liu C, Chen D, Yang WH, Liu X, Liu
CG, Dugas CM, Tang F, Zheng P, Liu Y and Wang L: FOXP3 Controls an
miR-146/NF-κB negative feedback loop that inhibits apoptosis in
breast cancer cells. Cancer Res. 75:1703–1713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yao Z, Kanno Y, Kerenyi M, Stephens G,
Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl
R, et al: Nonredundant roles for Stat5a/b in directly regulating
Foxp3. Blood. 109:4368–4375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huber M, Steinwald V, Guralnik A, Brüstle
A, Kleemann P, Rosenplänter C, Decker T and Lohoff M: IL-27
inhibits the development of regulatory T cells via STAT3. Int
Immunol. 20:223–234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qu JQ, Yi HM, Ye X, Li LN, Zhu JF, Xiao T,
Yuan L, Li JY, Wang YY, Feng J, et al: MiR-23a sensitizes
nasopharyngeal carcinoma to irradiation by targeting IL-8/Stat3
pathway. Oncotarget. 6:28341–28356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Houghton AM, Rzymkiewicz DM, Ji H, Gregory
AD, Egea EE, Metz HE, Stolz DB, Land SR, Marconcini LA, Kliment CR,
et al: Neutrophil elastase-mediated degradation of IRS-1
accelerates lung tumor growth. Nat Med. 16:219–223. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cools-Lartigue J, Spicer J, Najmeh S and
Ferri L: Neutrophil extracellular traps in cancer progression. Cell
Mol Life Sci. 71:4179–4194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tohme S, Yazdani HO, Al-Khafaji AB, Chidi
AP, Loughran P, Mowen K, Wang Y, Simmons RL, Huang H and Tsung A:
Neutrophil extracellular traps promote the development and
progression of liver metastases after surgical stress. Cancer Res.
76:1367–1380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chung HS, Lee JH, Kim H, Lee HJ, Kim SH,
Kwon HK, Im SH and Bae H: Foxp3 is a novel repressor of microglia
activation. Glia. 58:1247–1256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lood C, Blanco LP, Purmalek MM,
Carmona-Rivera C, De Ravin SS, Smith CK, Malech HL, Ledbetter JA,
Elkon KB and Kaplan MJ: Neutrophil extracellular traps enriched in
oxidized mitochondrial DNA are interferogenic and contribute to
lupus-like disease. Nat Med. 22:146–153. 2016. View Article : Google Scholar : PubMed/NCBI
|