Introduction
Renal cell carcinoma (RCC) is a cancer that
originates from the proximal tubular epithelium, and 70–80% of RCC
cases are clear cell RCC (1). Each
year, approximately 200,000 patients are newly diagnosed with RCC
and approximately 100,000 succumb to this malignancy, while the
incidence of the disease has been steadily increasing in recent
years (2,3). It was estimated that almost 25% of
patients present with metastases at diagnosis (4). Surgical resection is a potentially
curative therapeutic method for early and localized RCC; however,
>30% of patients with RCC develop metastases after radical
nephrectomy (5). In addition, the
10-year relative overall survival rates are poor (6). Therefore, exploring biomarkers
associated with tumorigenesis and progression may improve the
potential therapeutic strategies and the prognosis of RCC.
Expression of the human gene cell cycle-related and
expression-elevated protein in tumor (CREPT), also named regulation
of nuclear pre-mRNA domain-containing protein 1B gene, is
upregulated in various types of cancer, and has been indicated to
increase cyclin D1 transcription and enhance cell proliferation by
directly interacting with RNA polymerase (RNAP) II (7–12).
Recently, CREPT was demonstrated to recognize and interact with
RNAP II through the N-terminal RPR domain and C-terminal domain
(13). CREPT enhances cell growth
and promotes tumorigenesis by promoting the G1- to S-phase
transition (7,14). Zhang et al (15) demonstrated that CREPT promotes the
expression of cyclin D1 and c-myc through the regulation of Wnt
signaling as an underlying mechanism of its oncogenic role to
enhance the proliferative and migratory ability of cells. In
addition, the expression of CREPT was associated with the survival
time of patients with stomach cancer (7). However, to date, the role of CREPT in
the development of RCC has remained elusive.
In the present study, the expression of CREPT in RCC
tissues was determined by western blot and immunohistochemical
(IHC) analyses, and an association between CREPT expression and
survival rate was revealed. Knockdown of CREPT inhibited the
proliferation, colony formation and invasion of RCC cells in
vitro. Furthermore, silencing of CREPT was indicated to block
the G1- to S-phase cell cycle transition by regulating the
expression of c-myc and cyclin D1 in RCC.
Materials and methods
Patients and tissues
A total of 90 patients with histologically confirmed
RCC were analyzed. All of the patients underwent curative radical
or partial nephrectomy at the Department of Urology at Peking
University People's Hospital (Beijing, China) between July 2010 and
January 2014. The freshly obtained tissues were immediately
snap-frozen in liquid nitrogen and stored at −80°C for analysis.
None of the patients received any adjuvant therapy prior to
surgical resection. The clinicopathological characteristics of the
patients with RCC are presented in Table
I. In the present study, the Tumor-Nodes-Metastasis (TNM)
staging system from 2009 was utilized to classify RCC patients
(16). Tumor grade was assessed with
the Fuhrman four-grade scale (17).
 | Table I.Relationships between CREPT and
features of patients with RCC. |
Table I.
Relationships between CREPT and
features of patients with RCC.
|
|
| CREPT expression |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Number of
cases | Low | High | χ2 | P-value |
|---|
| Type |
|
|
| 4.582 | 0.032 |
|
Normal | 90 | 62 | 28 |
|
|
|
RCC | 90 | 48 | 42 |
|
|
| Fuhrman grade |
|
|
| 15.453 | 0.000 |
|
I+II | 65 | 43 | 22 |
|
|
|
III | 25 | 5 | 20 |
|
|
| TNM stage |
|
|
| 11.967 | 0.001 |
|
I+II | 65 | 42 | 23 |
|
|
|
III+IV | 25 | 6 | 19 |
|
|
| Sex |
|
|
| 2.087 | 0.149 |
|
Male | 55 | 26 | 29 |
|
|
|
Female | 35 | 22 | 13 |
|
|
| Age (years) |
|
|
| 0.268 | 0.605 |
|
≥60 | 36 | 18 | 18 |
|
|
|
<60 | 54 | 30 | 24 |
|
|
| Size (cm) |
|
|
| 0.179 | 0.673 |
| ≥4 | 45 | 25 | 20 |
|
|
|
<4 | 45 | 23 | 22 |
|
|
| BMI
(kg/m2) |
|
|
| 0.658 | 0.417 |
|
≥23 | 50 | 27 | 23 |
|
|
|
<23 | 40 | 25 | 15 |
|
|
| Histological
type |
|
|
| 0.097 | 0.756 |
|
ccRCC | 76 | 40 | 36 |
|
|
|
Others | 14 | 8 | 6 |
|
|
All the patients provided informed consent prior to
enrolment in the present study and the protocol was reviewed and
approved by the Ethics Committee of Peking University People's
Hospital (Beijing, China).
Follow up
After curative surgery, all of the patients were
followed up every 6 months until death or recurrence. The median
follow up was 23.5 months (range, 8–53 months). The follow up of
the 85 patients included in the present study was completed in July
2014.
Histological examination
Samples obtained from the kidney were fixed in 10%
formalin for 24 h at room temperature and routinely processed for
paraffin embedding. Histological sections (4 µm) were stained with
hematoxylin for 1 min and eosin for 5 min at room temperature, and
examined by a senior pathologist.
Immunohistochemical analysis
IHC analysis was performed using an IHC polymer
double detection kit (OriGene Technologies, Inc., Rockville, MD,
USA). In brief, after being deparaffinized and rehydrated, the
tissue sections were incubated with 0.3% hydrogen peroxide for 10
min to eliminate endogenous peroxidase activity at room
temperature. Subsequently, antigen retrieval was performed by
heating the sections in 10 mM citrate buffer for 2.5 min. After
washing three times with PBS, the sections were incubated with
mouse anti-CREPT monoclonal antibody (15) (dilution, 1:100; kindly provided by Dr
Zhijie Chang, Tsinghua University, Beijing, China) overnight at 4°C
in a humidified chamber. After washing three times with PBS, the
specimens were incubated with mouse secondary antibody (dilution,
1:200; OriGene Technologies, Inc.) for 30 min at room temperature.
The sections were stained with diaminobenzidine for 50 sec at room
temperature and the nuclei were counterstained with Meyer's
hematoxylin for 1 min at room temperature.
IHC evaluation
Nuclear immunoreactivity for CREPT was evaluated
using a semi-quantitative method by experienced pathologists who
were blinded to the clinicopathological data of the patients. The
percentage of positive tumor cells (0%, 0; 1–10%, 1; 11–50%, 2;
51–80%, 3; 81–100%, 4) and the staining intensity (negative, 0;
weak, 1; moderate, 2; strong, 3) were evaluated. The numeric values
of the two parameters were multiplied to obtain an immunoreactivity
score (IRS) ranging from 0 to 12 (18). For statistical analysis, patients
were divided into two groups with low or high CREPT expression
based on the IRS (IRS=0–4 or 6–12, respectively. The IRS value
could not be 5).
Cell culture
The 786-O and 769P human renal cell carcinoma cell
lines and the HK-2 normal renal tubular cell line were obtained
from the American Type Culture Collection (Manassas, VA, USA) and
cultured in RPMI-1640 medium containing 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a 5%
CO2 atmosphere.
CREPT RNA interference
To knock down the expression of CREPT, short hairpin
RNA (shRNA) targeting CREPT was used. 786-O and 769P cells were
transfected with lentiviral vector containing plasmid
pLL3.7-CREPT-short hairpin RNA (pLL3.7-CREPT-sh) or
pLL3.7-CREPT-empty vector (pLL3.7-CREPT-EV), respectively. Cells
were transfected with lentiviral vector at a MOI of 30 for 48 h
using Lipofectamine® 3000 according to the
manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc.). The plasmids pLL3.7-CREPT-sh and pLL3.7-CREPT-EV were kindly
provided by Dr Zhijie Chang (Tsinghua University, Beijing, China).
The sequences of shRNA used were as follows: Forward,
5′-TGGACCTGAATTCACTAGAGATTCAAGAGATCTCTAGTGAATTCAGGTCCTTTTTTC-3′ and
reverse,
5′-GAAAAAAGGACCTGAATTCACTAGAGATCTCTGGAATCTCTAGTGAATTCAGGTCCA-3′.
The underlined sequences represent the ‘short inverted repeat
sequences’ and the sequences between the underlined sequences were
the loop of the shRNA. Stably transfected 786-O and 769P cell lines
were acquired by screening the lentiviral vector-infected cells
with puromycin at a concentration of 2 µg/ml for 2 weeks. The
expression of CREPT in the stably transfected cell lines was
determined by western blot analysis.
Western blot analysis
Proteins extracted from tissues and cells were
extracted using RIPA buffer (Solarbio Life Sciences). Protein
quantification were determined using BCA protein assays (Solarbio
Life Sciences). A total of 20 µg of protein per lane were separated
by SDS-PAGE (10% gel) and transferred onto polyvinylidene fluoride
membranes (EMD Millipore, Bedford, MA, USA). Membranes were blocked
with 10% skimmed milk in Tris-buffered saline containing Tween-20
(TBST) for 1 h at room temperature. Subsequently, the membranes
were incubated with primary antibodies overnight at 4°C. The
following primary antibodies were used: Mouse anti-CREPT monoclonal
antibody (1:100 dilution; kindly provided by Dr Zhijie Chang)
(15); rabbit anti-c-myc monoclonal
antibody (1:1,000; cat. no. 5605); rabbit anti-cyclin D1 monoclonal
antibody (1:1,000 dilution; cat. no. 2978); and rabbit anti-β-actin
monoclonal antibody (1:2,000 dilution; cat. no. 4970) (all from CST
Biological Reagents Co., Ltd.). After three washes with TBST, the
membranes were incubated with secondary antibody (goat anti-rabbit
IgG H&L horseradish peroxidase-conjugated; 1:2,000; cat. no.
ab205718 or goat anti-rabbit IgG H&L horseradish
peroxidase-conjugated; 1:2,000; ab205719; Abcam) at 37°C for 1 h
and then washed with TBST five times prior to exposure.
Quantitative analysis of protein expression was assessed via
measurement of the gray value of each protein band using ImageJ
v1.8.0, (National Institutes of Health, Bethesda, MD, USA). β-actin
was used as the loading control.
Cell Counting Kit (CCK)-8 assay
Cell proliferation was examined using a CCK-8 assay.
A total of 1.5×103 786-O or 769P cells stably
transfected with CREPT-EV or CREPT-sh in 100 µl complete medium
were individually seeded in 96-well plates with three repeats per
condition. At different time-points, 10 µl CCK-8 stain was added
into each well, followed by incubation for 1.5 h prior to
measurement of the absorbance at 450 nm.
Colony formation assay
The 786-O and 769P cells stably transfected with
CREPT-EV or CREPT-sh were individually seeded into 6-well plates at
a density of 1,000 cells/well. After culturing at 37°C for two
weeks, the cells were stained with 0.1% crystal violet for 10 min
at room temperature. Subsequently, the cells were washed three
times with PBS and the number of clones were counted.
Wound-healing assay
The in vitro wound-healing assay was
performed as described previously (19). The cells were seeded in 6-well plates
to generate a confluent monolayer. The monolayer was scratched with
a sterile 200-µl pipette tip and the floating cells were carefully
removed by rinsing with PBS. The cells were cultured in RPMI-1640
medium without FBS at 37°C in an atmosphere containing 5%
CO2. Images of the wounds were captured at 0 and 48 h
after scraping. The sizes of the wound closure areas were analyzed
using Image-Pro plus 6.0 (Media Cybernetics, Inc., Rockville, MA,
USA).
Cell invasion assay
The cell invasion assays were performed using
Matrigel Invasion Chambers with a pore size of 8 µm (Corning, Inc.,
Corning, NY, USA). A total of 3×104 786-O and 769P cells
in 300 µl serum-free medium were seeded into the upper chamber, and
600 µl supplemented medium was added to the bottom of the chamber.
After culturing for 24 h, the cells that passed through the
membrane were fixed with 4% paraformaldehyde for 20 min at room
temperature and subsequently stained with crystal violet for 20 min
at room temperature. The cells on the upper surface of the chamber
were wiped off and after washing with PBS, the invaded cells were
counted in five random fields under a microscope. All the assays
were performed three times.
Flow cytometry and cell cycle
analysis
Cells were collected and washed with ice-cold PBS.
The cell cycle was determined by flow cytometry (Beckman Coulter,
Brea, CA, USA) after staining with propidium iodide (Liankenbio,
Hangzhou, China) according to the manufacturer's protocols. The
data was analyzed using CytExpert software (version 2.0; Beckman
Coulter, Inc.).
Statistical analysis
All experiments were performed at least three times
and the values are expressed as the mean ± standard deviation. The
statistical significance between the migration and invasion of
cells in different groups was analyzed with a Student's t-test.
One-way analysis of variance and a Dunnett's Multiple Comparison
post-hoc test were used to analyze the expression of CREPT in the
cell lines. Differences in the cell cycle distribution and
differences of protein expression in different groups were analyzed
using two-way analysis of variance, and Bonferroni post-hoc test
was used to determine these differences. The statistical analyses
for clinical samples were performed using the SPSS software 19.0
(IBM Corp., Armonk, NY, USA). The χ2 test and Fisher's
exact test were used to analyze the association between CREPT
expression measured by immunohistochemistry and the
clinicopathological characteristics of RCC. Survival analyses were
performed via drawing Kaplan-Meier curves, and the differences
between subgroups were analyzed using the log-rank test. P<0.05
was considered to indicate a statistically significant
difference.
Results
CREPT is overexpressed in RCC tissues
and cell lines
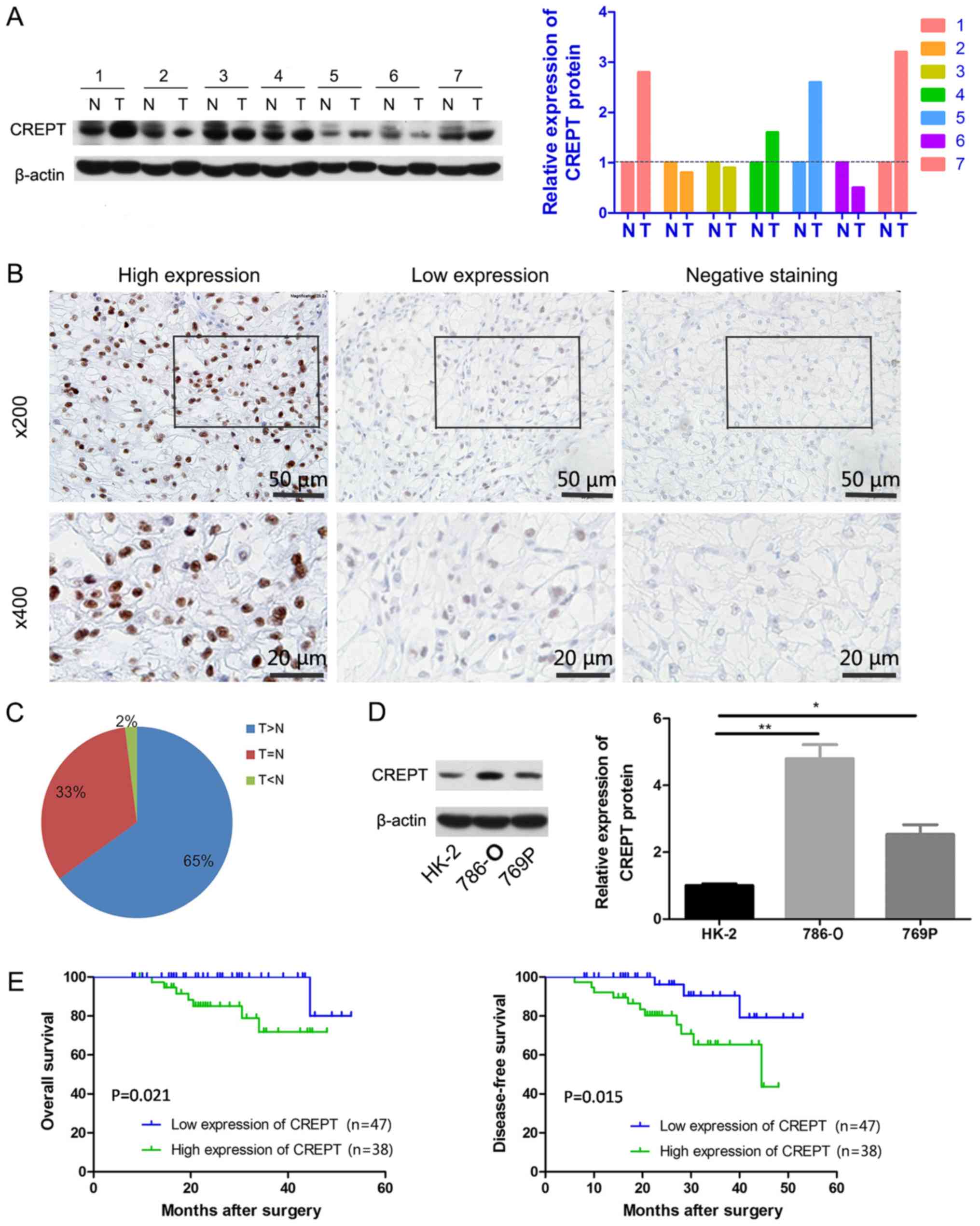
To investigate CREPT expression in RCC, the protein
expression was first assessed in seven pairs of RCC and matched
adjacent tissues through western blot analysis. CREPT was
overexpressed in four of the seven pairs of tissue samples
(Fig. 1A). Furthermore, IHC staining
for CREPT was performed on 90 RCC samples. CREPT staining was
detected in the nuclei of tumor cells, but not in the cytoplasm
(Fig. 1B). Quantification of the
staining indicated that the expression of CREPT in tumor tissues
was increased in 65% (58/90), unchanged in 33% (30/90) and
decreased in 2% (2/90) of the samples compared with that in
adjacent normal renal tissues (Fig. 1B
and C). CREPT expression was then examined via western blot
analysis in RCC cell lines, 786-O and 769P, and a normal renal
tubular cell line, HK-2. As presented in Fig. 1D, CREPT expression was increased in
786-O and 769P cells, and considerably lower in HK-2. Taken
together, CREPT was overexpressed in RCC.
Association between CREPT expression
and clinicopathological characteristics of patients with RCC
As presented in Table
I, the expression of CREPT in RCC tissues was increased
compared with normal tissues (P=0.032), and was significantly
associated with the TNM stage (P=0.001) and Fuhrman grade
(P<0.001). However, there were no significant associations
identified between CREPT expression and sex (P=0.149), age
(P=0.605), tumor size (P=0.673), body mass index (P=0.417) and
histological type (P=0.756). Furthermore, survival analysis was
performed for 85 of the patients. The 85 patients were stratified
into two groups according to the expression levels of CREPT (high
or low). The high CREPT expression group comprised 38 patients, of
whom 11 had recurrence or metastasis, and 7 cases died of
recurrence. The remaining 47 patients had relatively lower levels
of CREPT expression, among whom 3 cases had recurrence or
metastasis and only one patient died of recurrence. The
Kaplan-Meier analysis revealed that patients with increased CREPT
expression had significantly worse overall survival rates (P=0.021;
Fig. 1E) and disease-free survival
rates (P=0.015; Fig. 1E) compared
with patients with low CREPT expression levels. Together, these
data indicate that CREPT is a powerful prognostic factor for
overall survival and disease-free survival of patients with
RCC.
Knockdown of CREPT inhibits the
proliferation and colony formation of RCC cells
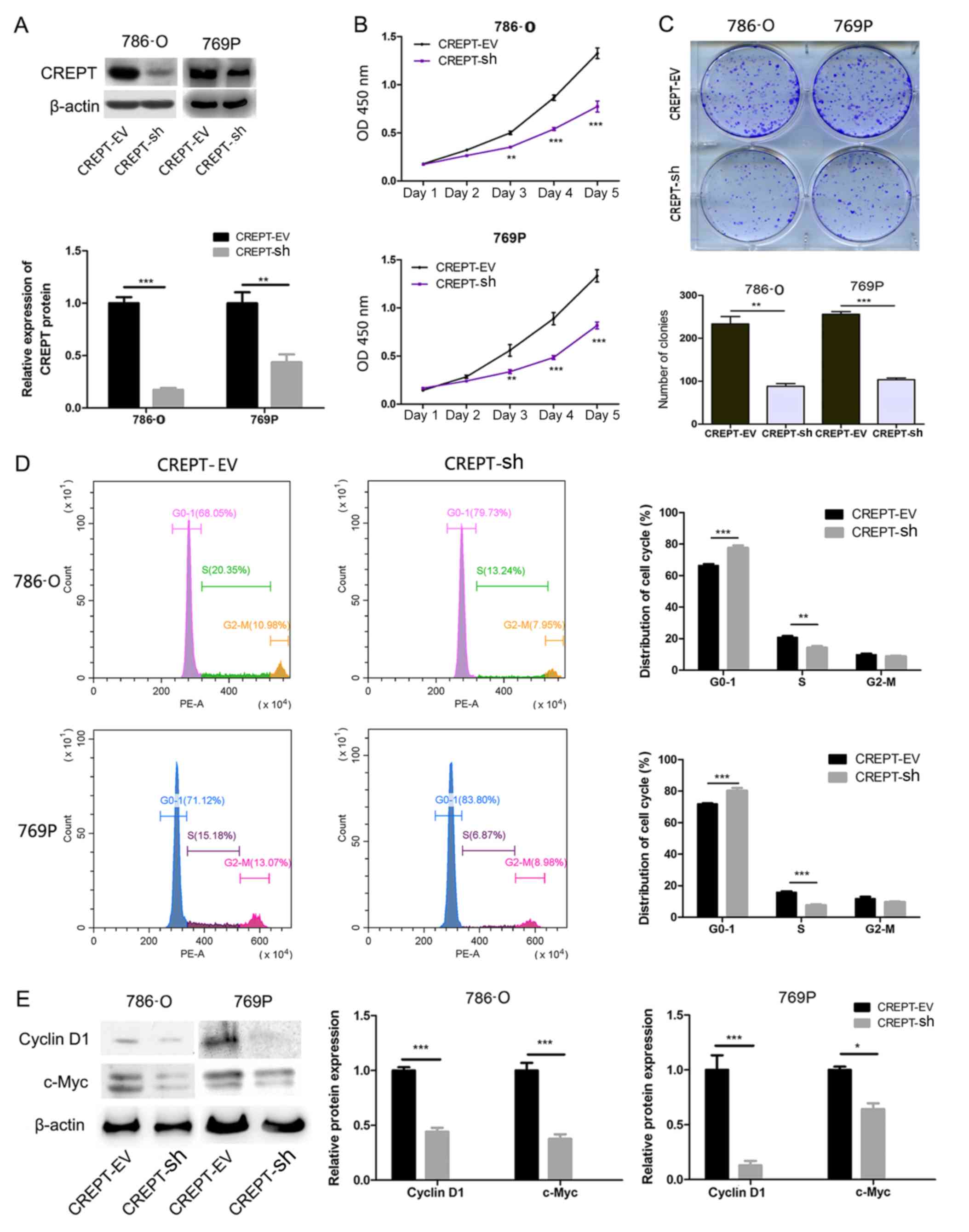
siRNA-mediated knockdown was performed to evaluate
the effect of CREPT on the biological behavior of RCC cells,. By
using western blot analysis, it was confirmed that the expression
levels of CREPT in the RCC cells transfected with CREPT-sh were
significantly lower compared with those in cells transfected with
CREPT-EV (Fig. 2A). The effect of
CREPT knockdown on the proliferation of RCC cells was then assessed
using CCK-8 assays. As presented in Fig.
2B, the proliferation of 786-O and 769P cells stably
transfected with CREPT-sh was significantly decreased from day 3
onwards compared with cells transfected with CREPT-EV. Furthermore,
the effect of CREPT on the colony formation ability was evaluated
using clonogenic assays. After knocking down CREPT expression in
786-O and 769P cells, the number and size of the colonies was
significantly decreased in both 786-O cells (P<0.01) and for
769P cells (P<0.001; Fig. 2C).
Taken together, these results suggest that CREPT is involved in
regulating the proliferation and colony formation of RCC cells.
Knockdown of CREPT induces cell cycle
arrest at the G1 phase
Effect of CREPT on the cell cycle was examined by
flow cytometric analysis. The results suggested that silencing of
CREPT in 786-O or 769P cells resulted in G1 arrest (Fig. 2D).
Furthermore, knockdown of CREPT led to a decrease in
the expression of cyclin D1. Considering that c-myc and cyclin D1
are key regulators of the cell cycle, the protein expression levels
of c-myc and cyclin D1 in stably transfected 786-O or 769P cells
were assessed via western blot analysis. The results indicated that
the expression levels of cyclin D1 and c-myc were markedly
decreased in CREPT-sh cells compared with those in CREPT-EV cells
(Fig. 2E).
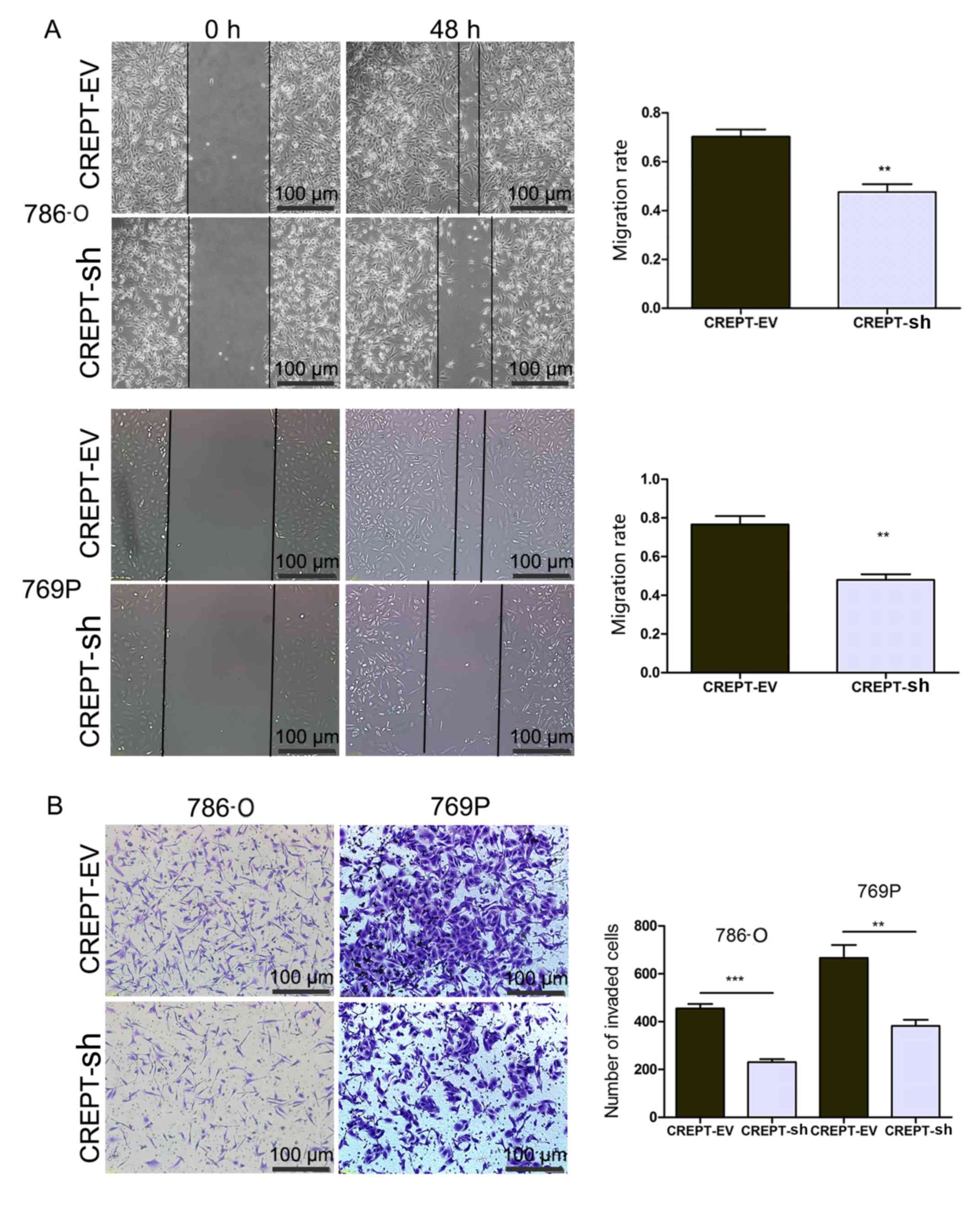
Silencing of CREPT inhibits the
migration and invasion of RCC cells
To evaluate the effect of CREPT on the metastatic
ability of RCC cells, the two stably transfected cell lines were
subjected to wound healing and invasion assays. Of note,
downregulation of CREPT significantly reduced the migration rate
and the number of invaded 7860 or 769p cells (Fig. 3A and B).
Discussion
Although clinical treatment strategies and
preliminary health checks have improved in recent years, the
prognosis for patients with RCC remains unsatisfactory, which is
partly due to the high rate of recurrence and distant metastasis.
At present, the established TNM staging and Fuhrman grading systems
are used as prognostic indicators for RCC (20–22). Our
understanding of the pathogenesis of RCC has increased in recent
years, and various prognostic indicators of RCC progression have
been identified, including the expression levels of leucine
zipper-EF-hand containing transmembrane protein 1, lactate
dehydrogenase and microRNAs (miRs) (23–26).
However, there is still a lack of reliable biomarkers for
predicting RCC progression and monitoring the response to drug
treatment. In particular, patients with the same TNM stage and/or
Fuhrman grade of RCC have a high variability in disease recurrence
and metastasis. Therefore, novel biomarkers with the ability to
effectively predict differential prognoses for patients with the
same TNM stage and/or Fuhrman grade are required. It is desirable
to identify novel molecular markers associated with the progression
of RCC, which may also be of great significance for the improvement
of therapeutic strategies and patient prognosis.
Previous findings have shown the upregulation of
CREPT in various tumors and studied the role it serves in
tumorigenesis and disease progression. In colorectal cancer, CREPT
upregulation is involved in cell proliferation and is directly
controlled by miR-383 through targeting the 3′-untranslated region
of the CREPT gene (27).
Additionally, upregulation of CREPT was associated with
histological grade of colorectal cancer and was involved in
conferring sensitivity of colorectal cancer cells to 5-fluorouracil
treatment (28). Consistent with the
expression of CREPT in colorectal cancer, the results of the
present study confirmed that CREPT protein was localized to the
nuclear region of RCC cells, and that the expression levels of
CREPT were significantly higher in RCC tissues compared with those
in normal adjacent tissues. Furthermore, CREPT expression was
associated with the TNM stage and Fuhrman grade of RCC, but not
with sex, age, tumor size and histological type of patients with
RCC, which indicated that overexpression of CREPT is a major
promoter of the development and progression of RCC. In addition, it
was indicated that patients with increased CREPT expression had a
significantly lower overall and disease-free survival rate than
those with low CREPT expression. Therefore, CREPT may be a negative
prognosticator for RCC.
In the present in vitro study, the effects of
CREPT on RCC cells were assessed through silencing of CREPT in RCC
cell lines (786-O and 769P). The results indicated that the
proliferation, colony formation, migration and invasion of 786-O
and 769P cells were significantly suppressed after knockdown of
CREPT. Therefore, CREPT may be used as a novel marker to identify
malignant progression in patients with RCC.
The dysregulation of the cell cycle is a common
phenomenon in almost all types of human cancer (29–31). In
the present study, it was indicated that silencing of CREPT
resulted in cell cycle arrest at G1 phase, which may explain the
inhibitory effect of CREPT on the proliferation of RCC cells.
Cyclin D1, a cell cycle regulator, was confirmed to have a critical
role in the initiation and progression of RCC (32,33).
Dysfunction or abnormal expression of numerous regulatory genes may
cause the development of cancer through upregulating the expression
of cyclin D1 (33). In gastric
cancer, silencing of CREPT suppressed cell proliferation through
the induction of G0/G1 phase cell cycle arrest via reducing the
expression of cyclin D1 and Cyclin D-dependent kinase 4, and
inducing the expression of p53 and p21 (34). Consistent with the findings in
gastric cancer, the present results indicated that cyclin D1 may be
regulated by CREPT in RCC. C-myc is also a key regulator of the
cell cycle and may be regulated by CREPT through the Wnt signaling
pathway (15). In the present study,
CREPT was demonstrated to decrease the expression of c-myc and
promote the progression of RCC. However, no evidence for CREPT
regulating the expression of cyclin D1 and c-myc directly was
provided. Further investigation of the underlying mechanisms of
CREPT-mediated regulation of cyclin D1 and c-Myc is required.
Furthermore, previous studies have revealed that CREPT may be
regulated by miR-383 in colorectal cancer, and influences the
sensitivity of colorectal cancer to fluorouracil, as well as
gastric cancer cell apoptosis (27,28,34),
indicating a pleiotropic effect of CREPT on various cancer types
and complex mechanisms by which CREPT promotes tumor progression.
Therefore, further studies are required in order to elucidate the
potential molecular mechanisms underlying CREPT in promoting tumor
progression..
In conclusion, the present study is the first to
report that CREPT is significantly overexpressed in RCC and is
associated with the TNM stage, Fuhrman grade and prognosis of
patients with RCC, to the best of the our knowledge. Knockdown of
CREPT suppressed RCC cell growth, colony formation and invasion,
and caused cell cycle arrest through restraining the expression of
cyclin D1 and c-myc. Thus, CREPT may be a promising novel
prognostic marker and a potential target for the treatment of
RCC.
Acknowledgements
Not applicable.
Funding
The present study was funded by The Fund for
Fostering Young Scholars of Peking University Health Science Center
(grant no. BMU2018PY012) and The Fund for Beijing Municipal Health
Commission (grant no. 2127000132).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HQY and QFC performed the experiments and drafted
the manuscript. HYZ participated in the interpretation of data and
revision of manuscript. SHW and WNC were involved in the
acquisition and analysis of data. XWZ contributed to collecting
specimen used in this study. ZJC conceived of the study. TX and XJY
participated in the design of the study.
Ethics approval and consent to
participate
The protocol utilized in the present study was
reviewed and approved by The Ethics Committee of Peking University
People's Hospital (Beijing, China). All patients provided informed
consent prior to enrolment in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors have no conflict of interests to
declare.
References
|
1
|
Matsuura K, Nakada C, Mashio M, Narimatsu
T, Yoshimoto T, Tanigawa M, Tsukamoto Y, Hijiya N, Takeuchi I,
Nomura T, et al: Downregulation of SAV1 plays a role in
pathogenesis of high-grade clear cell renal cell carcinoma. BMC
Cancer. 11:5232011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miyamoto H, Miller JS, Fajardo DA, Lee TK,
Netto GJ and Epstein JI: Non-invasive papillary urothelial
neoplasms: The 2004 WHO/ISUP classification system. Pathol Int.
60:1–8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Montironi R, Santinelli A, Pomante R,
Mazzucchelli R, Colanzi P, Filho AL and Scarpelli M: Morphometric
index of adult renal cell carcinoma. Comparison with the Fuhrman
grading system. Virchows Arch. 437:82–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cindolo L, Patard JJ, Chiodini P, Schips
L, Ficarra V, Tostain J, de La Taille A, Altieri V, Lobel B,
Zigeuner RE, et al: Comparison of predictive accuracy of four
prognostic models for nonmetastatic renal cell carcinoma after
nephrectomy: A multicenter European study. Cancer. 104:1362–1371.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang Z, Chu PG, Woda BA, Liu Q, Balaji
KC, Rock KL and Wu CL: Combination of quantitative IMP3 and tumor
stage: A new system to predict metastasis for patients with
localized renal cell carcinomas. Clin Cancer Res. 14:5579–5584.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim SP, Alt AL, Weight CJ, Costello BA,
Cheville JC, Lohse C, Allmer C and Leibovich BC: Independent
validation of the 2010 American Joint Committee On Cancer TNM
classification for renal cell carcinoma: Results from a large,
single institution cohort. J Urol. 185:2035–2039. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu D, Wu Y, Wang Y, Ren F, Wang D, Su F,
Zhang Y, Yang X, Jin G, Hao X, et al: CREPT accelerates
tumorigenesis by regulating the transcription of cell-cycle-related
genes. Cancer Cell. 21:92–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma J, Ren Y, Zhang L, Kong X, Wang T, Shi
Y and Bu R: Knocking-down of CREPT prohibits the progression of
oral squamous cell carcinoma and suppresses cyclin D1 and c-Myc
expression. PLos One. 12:e1743092017.
|
|
9
|
Liang Z, Feng Q, Xu L, Li S and Zhou L:
CREPT regulated by miR-138 promotes breast cancer progression.
Biochem Biophys Res Commun. 493:263–269. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng G, Li W, Zuo B, Guo Z, Xi W, Wei M,
Chen P, Wen W and Yang AG: High expression of CREPT promotes tumor
growth and is correlated with poor prognosis in colorectal cancer.
Biochem Biophys Res Commun. 480:436–442. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu T, Li WM, Wang WP, Sun Y, Ni YF, Xing
H, Xia JH, Wang XJ, Zhang ZP and Li XF: Inhibiting CREPT reduces
the proliferation and migration of non-small cell lung cancer cells
by down-regulating cell cycle related protein. Am J Transl Res.
8:2097–2113. 2016.PubMed/NCBI
|
|
12
|
Ren F, Wang R, Zhang Y, Liu C, Wang Y, Hu
J, Zhang L and Chang Z: Characterization of a monoclonal antibody
against CREPT, a novel protein highly expressed in tumors. Monoclon
Antib Immunodiagn Immunother. 33:401–408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mei K, Jin Z, Ren F, Wang Y, Chang Z and
Wang X: Structural basis for the recognition of RNA polymerase II
C-terminal domain by CREPT and p15RS. Sci China Life Sci.
57:97–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Qiu H, Hu W, Li S and Yu J: RPRD1B
promotes tumor growth by accelerating the cell cycle in endometrial
cancer. Oncol Rep. 31:1389–1395. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Liu C, Duan X, Ren F, Li S, Jin
Z, Wang Y, Feng Y, Liu Z and Chang Z: CREPT/RPRD1B, a recently
identified novel protein highly expressed in tumors, enhances the
β-catenin. TCF4 transcriptional activity in response to Wnt
signaling. J Biol Chem. 289:22589–22599. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fisseler-Eckhoff A: New TNM classification
of malignant lung tumors 2009 from a pathology perspective.
Pathologe. 30 (Suppl 2):S193–S199. 2009.(In German). View Article : Google Scholar
|
|
17
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sinn BV, von Minckwitz G, Denkert C,
Eidtmann H, Darb-Esfahani S, Tesch H, Kronenwett R, Hoffmann G,
Belau A, Thommsen C, et al: Evaluation of Mucin-1 protein and mRNA
expression as prognostic and predictive markers after neoadjuvant
chemotherapy for breast cancer. Ann Oncol. 24:2316–2324. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Delahunt B, Sika-Paotonu D, Bethwaite PB,
William Jordan T, Magi-Galluzzi C, Zhou M, Samaratunga H and
Srigley JR: Grading of clear cell renal cell carcinoma should be
based on nucleolar prominence. Am J Surg Pathol. 35:1134–1139.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Erdoğan F, Demirel A and Polat O:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Int J Clin Pract. 58:333–336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu J, Huang B, Li S, Zhang X, Xie T and Xu
Y: Knockdown of LETM1 inhibits proliferation and metastasis of
human renal cell carcinoma cells. Oncol Lett. 16:6377–6382.
2018.PubMed/NCBI
|
|
24
|
Wang Y, Li G, Wan F, Dai B and Ye D:
Prognostic value of D-lactate dehydrogenase in patients with clear
cell renal cell carcinoma. Oncol Lett. 16:866–874. 2018.PubMed/NCBI
|
|
25
|
Chen P, Zhao L, Pan X, Jin L, Lin C, Xu W,
Xu J, Guan X, Wu X, Wang Y, et al: Tumor suppressor microRNA-136-5p
regulates the cellular function of renal cell carcinoma. Oncol
Lett. 15:5995–6002. 2018.PubMed/NCBI
|
|
26
|
Zhang XL, Xu G, Zhou Y and Yan JJ:
MicroRNA-183 promotes the proliferation and metastasis of renal
cell carcinoma through targeting Dickkopf-related protein 3. Oncol
Lett. 15:6003–6008. 2018.PubMed/NCBI
|
|
27
|
Li J, Smith AR, Marquez RT, Li J, Li K,
Lan L, Wu X, Zhao L, Ren F, Wang Y, et al: MicroRNA-383 acts as a
tumor suppressor in colorectal cancer by modulating CREPT/RPRD1B
expression. Mol Carcinog. 57:1408–1420. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuang YS, Wang Y, Ding LD, Yang L, Wang Y,
Liu SH, Zhu BT, Wang XN, Liu HY, Li J, et al: Overexpression of
CREPT confers colorectal cancer sensitivity to fluorouracil. World
J Gastroenterol. 24:475–483. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim JK and Diehl JA: Nuclear cyclin D1: An
oncogenic driver in human cancer. J Cell Physiol. 220:292–296.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nojima H: G1 and S-phase checkpoints,
chromosome instability, and cancer. Methods Mol Biol. 280:3–49.
2004.PubMed/NCBI
|
|
31
|
Massague J: G1 cell-cycle control and
cancer. Nature. 432:298–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Z, Fu Q, Lv J, Wang F and Ding K:
Prognostic implication of p27Kip1, Skp2 and Cks1 expression in
renal cell carcinoma: A tissue microarray study. J Exp Clin Cancer
Res. 27:512008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lima MS, Pereira RA, Costa RS, Tucci S,
Dantas M, Muglia VF, Ravinal RC and Barros-Silva GE: The prognostic
value of cyclin D1 in renal cell carcinoma. Int Urol Nephrol.
46:905–913. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun M, Si G, Sun HS and Si FC: Inhibition
of CREPT restrains gastric cancer growth by regulation of cycle
arrest, migration and apoptosis via ROS-regulated p53 pathway.
Biochem Biophys Res Commun. 496:1183–1190. 2018. View Article : Google Scholar : PubMed/NCBI
|