Introduction
In the majority of patients with non-small cell lung
cancer (NSCLC), stage progression occurs before the cancer is
diagnosed, thereby delaying the patient receiving radical surgery
(1). In such cases, non-invasive
treatment methods are required (2).
Epidermal growth factor receptor (EGFR) mutations in patients with
positive NSCLC are the preferred therapeutic targets (3); however, in those not harboring the EGFR
mutation, NSCLC treatment methods are limited; thus, chemotherapy
is preferred in these patients (4,5).
Platinum-based chemotherapy is extensively used, and the efficacy
of cisplatin therapy has been demonstrated (6). However, resistance to platinum-based
drugs in patients with NSCLC cannot be disregarded; thus, it is
important to understand the molecular mechanism underlying
platinum-based pharmacotherapeutic resistance among patients with
NSCLC (7).
MicroRNAs are small non-coding RNAs that regulate
various cellular processes via binding to the 3′-untranslated
region (3′-UTR) of target mRNAs, thus degrading mRNAs or inhibiting
their translation (8). The role of
post-transcriptional regulation by microRNAs has attracted
increasing attention. Numerous studies have reported that microRNA
expression is associated with tumorigenesis and progression, as
well as chemotherapeutic resistance (9,10), and
that microRNA-152, specifically, is downregulated in patients with
NSCLC (11,12). However, to the best of our knowledge,
few studies have investigated the association between microRNA-152
expression and chemotherapeutic resistance to platinum-based drugs
in NSCLC. Therefore, the present study aimed to investigate the
changes in microRNA-152 expression in cisplatin-resistant A549
cells and its effects on cisplatin sensitivity.
Materials and methods
Cells and cell culture
The A549 cell line was obtained from the Type
Culture Collection of the Chinese Academy of Sciences. The
cisplatin-resistant A549 (A549/cis) cell line was obtained from the
Cell Culture Center. Both cell lines were cultured in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.). All cells were
maintained in a humidified incubator at 37°C and 5% CO2.
After three passages, cisplatin, at a final concentration of 2
µg/ml, was added to the A549/cis cell culture. Cells were
maintained in a humidified incubator at 37°C for 24 h.
Chemotherapeutic resistance in the A549/cis cells was determined
using the Cell Counting Kit-8 (CCK-8) (Abmole Bioscience, Inc.)
method.
Transient transfection
Once the A549/cis cells reached 80% confluence, they
were transfected with microRNA-152 mimics or unrelated mimics
(negative control), using Lipofectamine® 2000
(GenePharma Co., Ltd.), in accordance with the manufacturer's
protocol. The following microRNA-152 mimics sequences were used:
Forward, 5′-UCAGUGCAUGACAGAACUUGG-3′; reverse,
5′-AAGUUCUGUCAUGCACUGAUU-3′. The following negative control
sequences were used: Forward, 5′-UUCUCCGAACGUGUCACGUAA-3′; reverse,
5′-ACGUGACACGUUCGGAGAAUG-3′. The working concentration of
microRNA-152 mimics was 50 nM, the concentration of unrelated
microRNA-152 mimics was the same as that of microRNA-152 mimics. To
avoid the influence of cis to A549/cis cells, cells
(1×105 cells/ml) were cultured in a drug-free medium for
at least 2 weeks, and then transfected. Subsequent experiments were
conducted 48 h later.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR) analysis
Total RNA of A549 cells and A549/cis cells was
isolated using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
MicroRNA was isolated using the mirVana miRNA Isolation kit
(Ambion; Thermo Fisher Scientific, Inc.). MicroRNA-152 expression
was detected using TaqMan MicroRNA Assay primers (Applied
Biosystems; Thermo Fisher Scientific, Inc.; http://www.targetscan.org/). The thermocycling
conditions are as follows: Initial activation of Taq polymerase at
95°C for 10 min, 40 cycles of PCR amplification at 95°C for 15 sec,
annealing/elongation at 60°C for 1 min. The following primers were
used: Bcl-2: Forward, 5′-TTCTTTGAGTTCGGTGGGGTC-3′; reverse,
5′-TGCATATTTGTTTGGGGCAGG-3′; NF-κB: Forward,
5′-CTGCATTTCCACAGTTTCCAGAACC-3′; reverse,
5′-ACGCTGCTCTTCTATAGGAACTTGG-3′. GAPDH: Forward,
5′-ACCACAGTCCATGCCATCAC-3′, reverse, 5′-TCCACCACCCTGTTGCTGTA-3′.
Bcl-2 and NF-κB expression levels were normalized to those of
GAPDH, and microRNA-152 expression levels were normalized to those
of U6. Relative expression levels were quantified using the
2−ΔΔCq method (13).
Western blot analysis
Mouse anti Bcl-2 (cat. no., B9804; dilution,
1:1,000), NF-κB (cat. no., N8523; dilution, 1:1,000), GAPDH (cat.
no., G9295; dilution, 1:35,000) were purchased from Sigma-Aldrich
(Merck KGaA), the secondary antibody Goat Anti-Mouse IgG (H+L)
(cat. no., SA00001-1; dilution, 1:8,000) was purchased from
ProteinTech Group, Inc. Cultured A549 cells, A549/cis cells,
A549/cis cells transfected with microRNA-152 mimics, and A549/cis
cells transfected with unrelated microRNA-152 mimics were lysed in
RIPA buffer with 1% phenylmethylsulfonyl fluoride; the latter two
were cultured for 48 h after transfection. Total protein was
determined using BCA method. Extracted proteins were loaded 50 µg
and separated via 10% SDS-PAGE and electro-transferred onto a PVDF
membrane. The blocking reagent was 5% skim milk, then the PVDF
membrane was incubated overnight at 4°C in freshly prepared TBST.
The blots were probed with primary antibodies at 4°C overnight and
subsequently incubated with horseradish peroxidase-conjugated
secondary antibodies at room temperature for 1 h. Signals were
visualized using ECL substrates (Pierce; Thermo Fisher Scientific,
Inc.). GAPDH was considered an endogenous control.
Cell proliferation assay
A549/cis cells were divided into 6 groups: Cells
without cisplatin treatment and transfection (untreated group);
cells without cisplatin treatment transfected with unrelated
microRNA mimics (miR control group); cells without cisplatin
treatment transfected with microRNA mimics (miR mimics group);
untransfected cells with cisplatin treatment (cis group); cells
with cisplatin treatment transfected with unrelated microRNA mimics
(cis+miR control group); cells with cisplatin treatment transfected
with microRNA mimics (cis+miR mimics group). Cells were treated
with 10 µl CCK-8 reagent (Abmole Bioscience, Inc.) 24 h after
transfection and incubated at 37°C for 1.5 h. The absorbance was
measured at 450 nM, using a microtiter plate reader. Meanwhile,
cell viability was measured. Cell viability (%) was calculated as
follows: (OD sample-OD blank)/(OD control-OD blank) ×100. The half
maximal inhibitory concentration (IC50) was also
calculated. Morphological changes and apoptosis in cells were
assessed using a terminal deoxynucleotidyl transferase
dUTP-mediated nick-end labeling (TUNEL) assay. Briefly, the cells
were seeded at a concentration of 1×105 cells/ml in
24-well plates and incubated for 24 h. Cells were then washed with
ice-cold PBS three times for 5 min and fixed with 4%
paraformaldehyde at room temperature for 1 h, followed with acetic
acid/ethanol (1:3) for post-fixation at −20°C for 5 min. The TUNEL
assay was performed using the In Situ Cell Death Detection
kit (Roche Applied Science), according to the manufacturer's
protocol. Cell nuclei were stained with DAPI (Beyotime Institute of
Biotechnology) at 25°C for 10 min. Glycerinum (Beyotime Institute
of Biotechnology) was used to mount the slides. TUNEL-positive
nuclei were defined as those with dark green fluorescent staining
and were identified via fluorescence microscopy. To quantify
TUNEL-positive cells, the number of green fluorescence-positive
cells was counted in 4–6 random fields at ×200 magnification. Cell
nuclei were counterstained with 4,6-diamidino-2-phenylindole at
25°C for 10 min (Beyotime Institute of Biotechnology). The
experiments were repeated 3 times.
Flow cytometry
Apoptosis was assessed via flow cytometry. Briefly,
six groups as aforementioned, cells were cultured in 24-well plates
at a density of 1×105 cells/well, and then trypsinized,
harvested, washed and stained with Annexin V-fluorescein
isothiocyanate and propidium iodide (PI) for 15 min at 4°C using
the apoptosis detection kit (BD Biosciences), according to the
manufacturer's protocol. The stained cells were analyzed using a
flow cytometer (FACScalibur; BD Biosciences). The proportion of
cells at each stage of the cell cycles was analyzed in each cell
group by Cell Quest software version 5.1 (Becton, Dickinson and
Company). After 24 h of treatment, 500 µl of PI was added in each
group for 15 min at room temperature to stain the nuclei, and cell
cycle analysis was performed using a FACstar Plus cytometer
(Becton, Dickinson and Company).
Statistical analysis
All experiments were performed in triplicate. Data
are presented as the mean ± standard deviation. Paired Student's
t-test was used for comparison between two groups. One-way analysis
of variance was used for comparisons between multiple groups,
followed by the Dunnett's method as a post hoc test, using SPSS
software (version 21.0; IBM Corp.) P<0.05 was considered to
indicate a statistically significant result.
Results
Expression of microRNA-152, Bcl-2, and
NF-κB in A549/cis cells
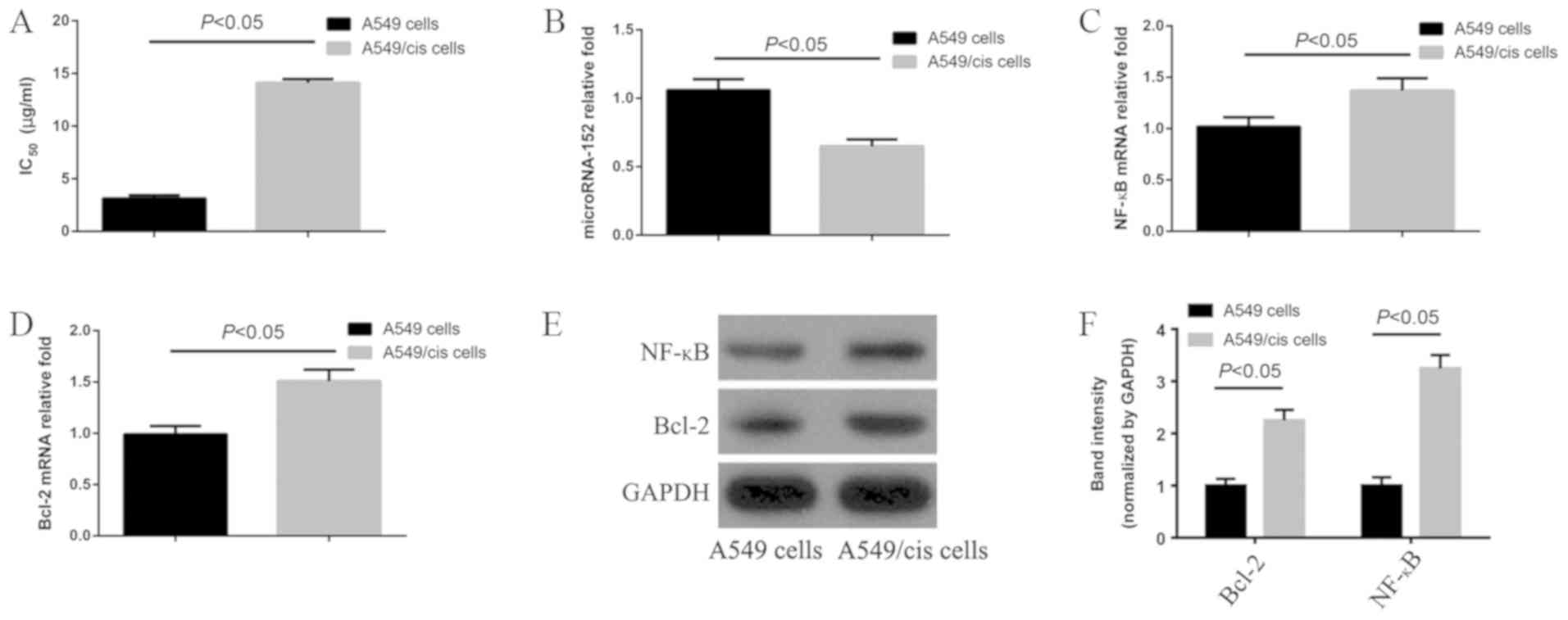
After 48 h of incubation with cisplatin, the
IC50 of A549 cells and A549/cis cells was 3.128±0.12
µg/ml and 14.107±0.35 µg/ml, respectively, which was significantly
different (P<0.05). The resistance index was approximately 4.51
(Fig. 1A). MicroRNA-152 was
significantly downregulated (P<0.05) in A549/cis cells compared
with that in A549 cells (Fig. 1B).
RT-qPCR and western blotting revealed that Bcl-2 and NF-κB were
significantly upregulated in A549/cis cells compared with that in
A549 cells (all P<0.05; Fig.
1C-F). Further analysis revealed that these improvements were
1.53±0.21-fold (Bcl-2) (Fig. 1C) and
1.37±0.13-fold (NF-κB) (Fig.
1D).
MicroRNA-152 increases cisplatin
sensitivity in A549/cis cells
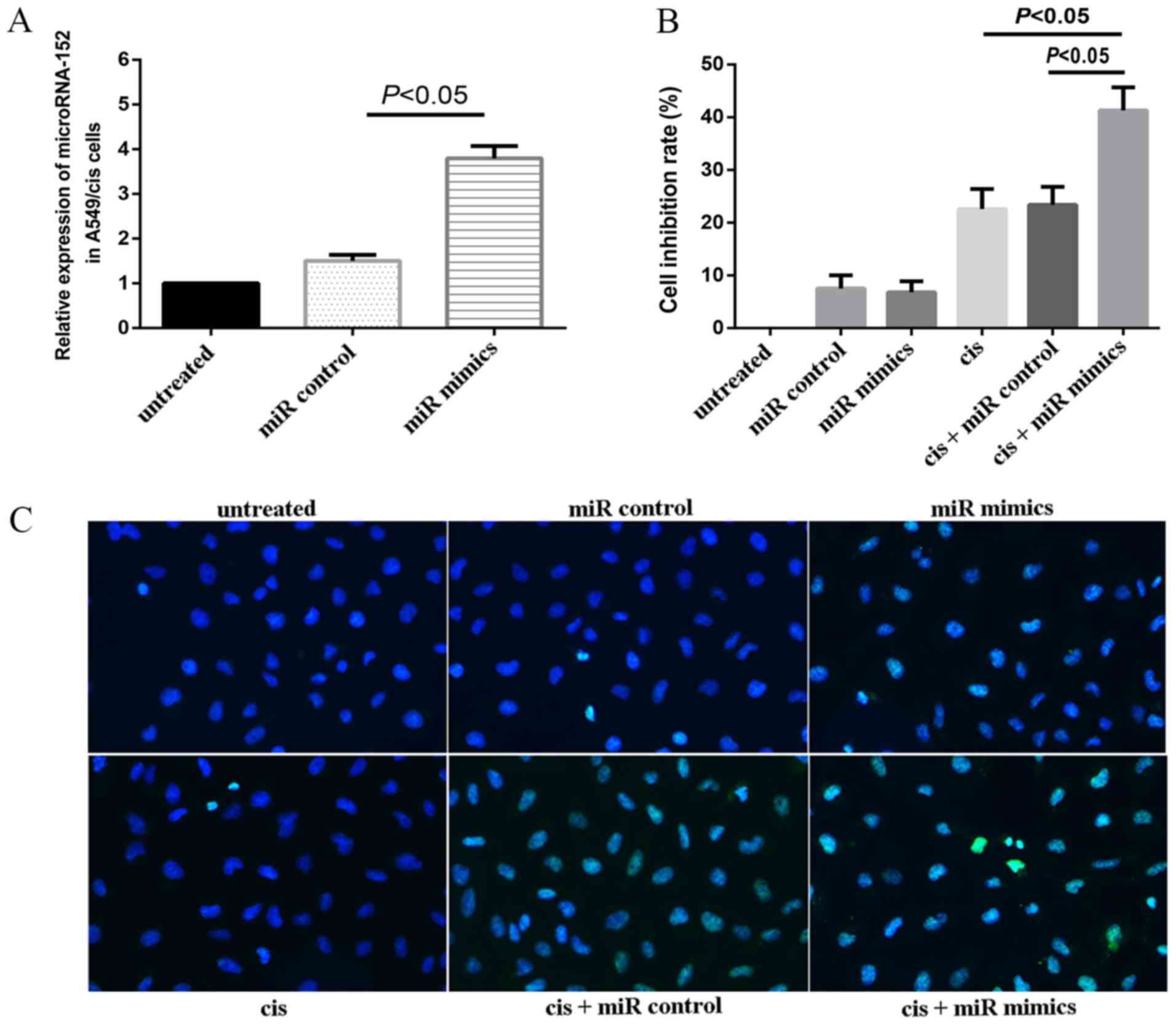
In order to verify the transfection efficiency,
unrelated microRNA-152 mimics (negative control) and microRNA-152
mimics were transfected into the A549/cis cells. Cells transfected
with the microRNA-152 mimics exhibited significantly increased
levels of microRNA-152 expression compared with untreated cells and
cells transfected with the miR control (P<0.05; Fig. 2A). In order to further determine the
role of microRNA-152 in chemotherapeutic resistance in NSCLC,
A549/cis cells were transfected with microRNA-152, and
proliferation was assessed using a CCK-8 assay in the present
study. Cell inhibition rates of miR control, miR mimics, cis,
cis+miR control, and cis+miR mimics were 7.5±2.5, 6.8±2.1,
22.6±3.8, 23.4±3.4 and 41.3±4.4%, respectively (Fig. 2B). The inhibition rate of the cis+miR
mimics group was significantly greater than that of cis and cis+miR
control groups (both P<0.05). As presented in the figure
(Fig. 2C), the nuclei of normal
cells were uniformly diffused with light blue fluorescence
following staining, under the ultraviolet laser at 450 nm upon
fluorescence microscopy (untreated group). Following treatment, the
morphology of apoptotic cells changed: Cells started to form
granules, and diffuse fluorescence was observed in the nucleus and
cytoplasm of cells, leading to the formation of apoptotic bodies
(Fig. 2C).
MicroRNA-152 increases
cisplatin-induced apoptosis in A549/cis cells
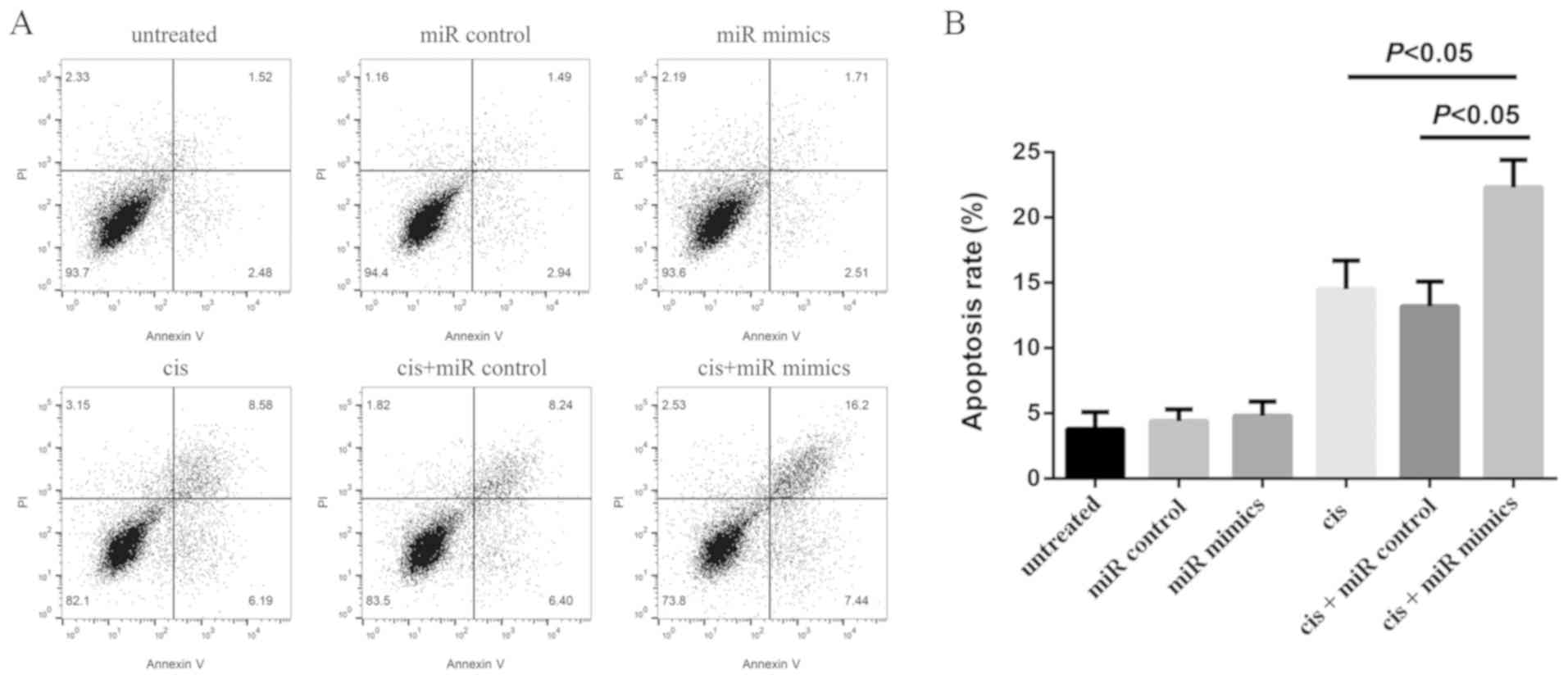
Flow cytometry analysis was performed in order to
assess the rate of apoptosis in A549/cis cells in the present
study. It was revealed that microRNA-152 overexpression induced
apoptosis in A549/cis cells (Fig.
3A). Furthermore, it revealed that the apoptotic rates of
untreated, miR control, miR mimics, cis, cis+miR control and
cis+miR mimics groups were 3.8±1.3, 4.4±0.9, 4.8±1.1, 14.5±2.2,
13.2±1.9 and 22.3±2.1%, respectively (Fig. 3B).
Effect of microRNA-152 upregulation on
the cell cycle of A549/cis cells
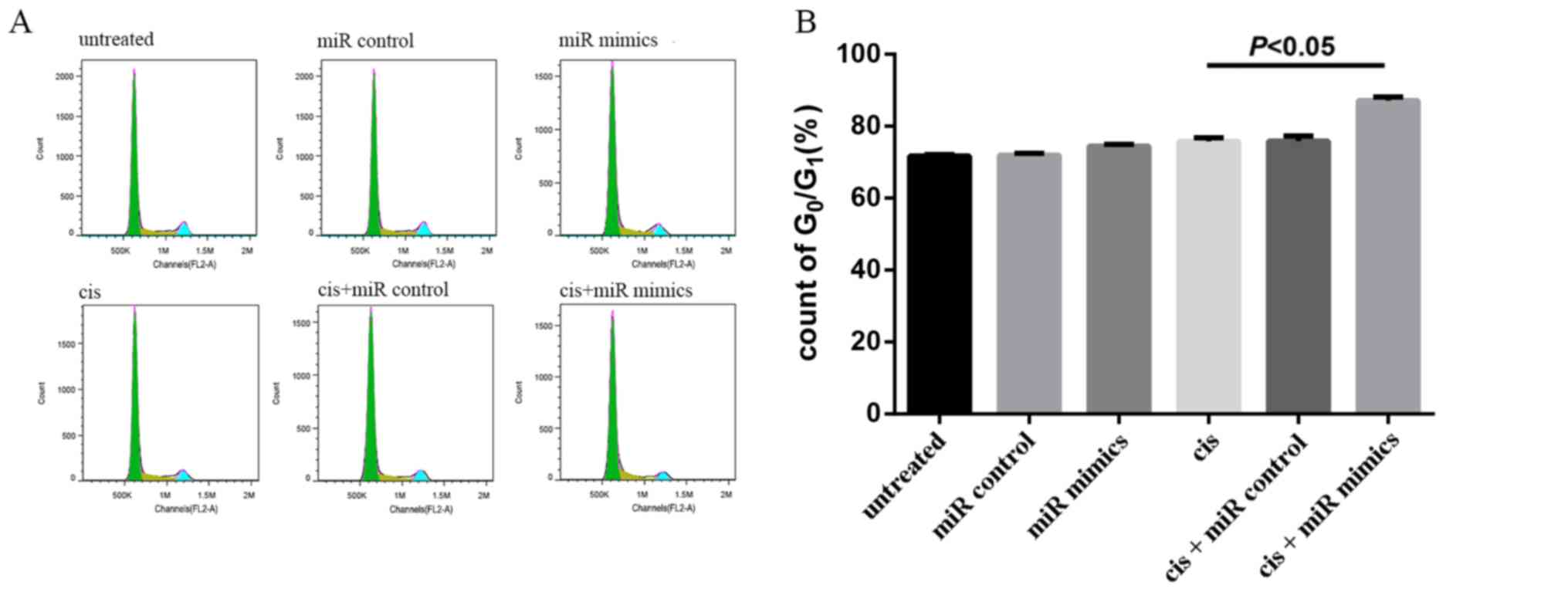
MicroRNA-152 was transfected into A549/cis cells
treated with cisplatin (2 µg/ml) for 48 h to examine its effects on
cell cycle progression. The G0/G1 phase
accounted for 71.69±0.45, 71.95±0.52, 74.43±0.54, 75.81±0.97,
75.90±1.42, and 87.1±1% in untreated, miR control, miR mimic, cis,
cis+miR control and cis+miR mimic groups, respectively, in A549/cis
cells. The cis+miR mimic and cis groups were significantly
different (P<0.05; Fig. 4).
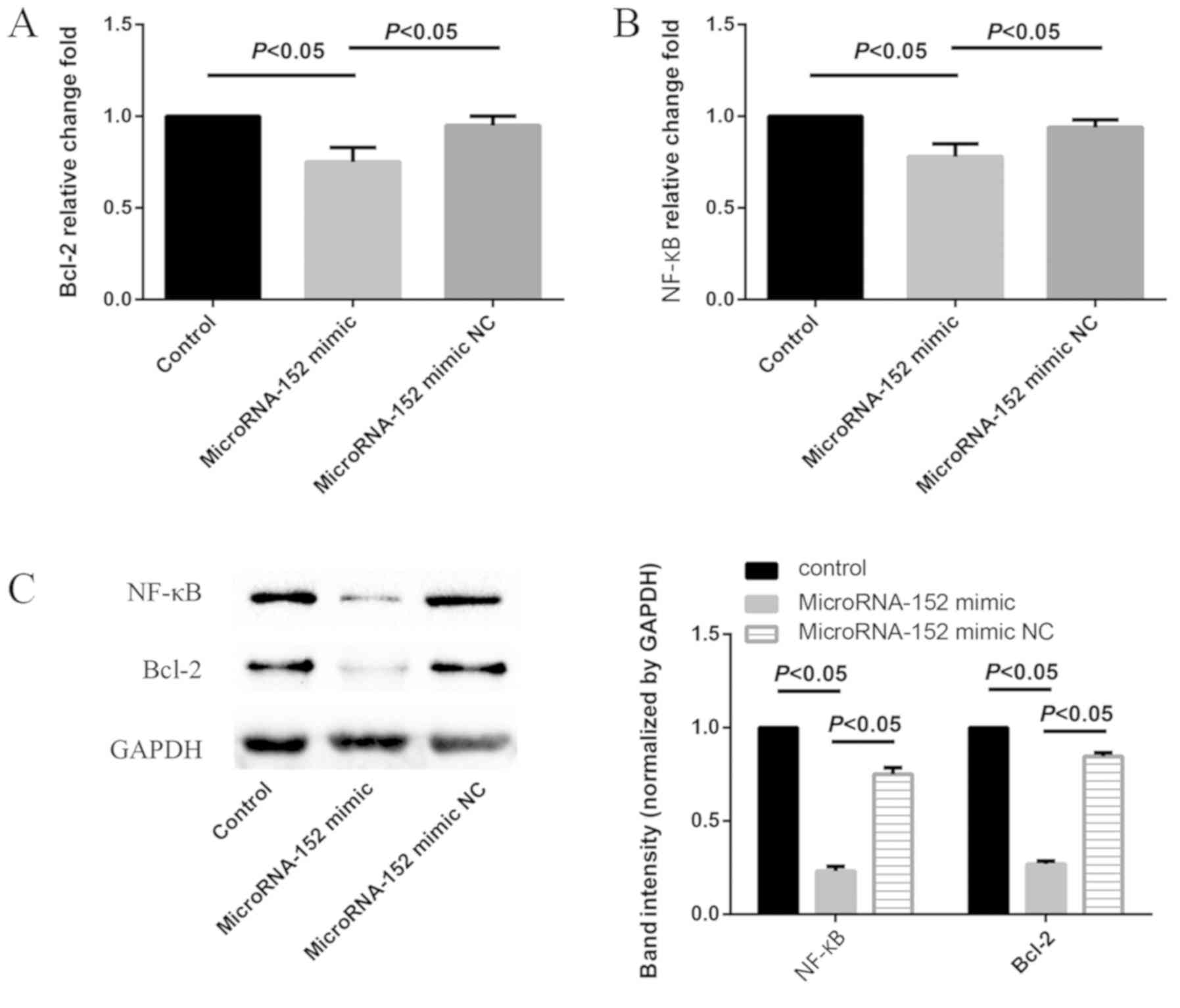
MicroRNA-152 downregulates Bcl-2 and
NF-κB in A549/cis cells
In order to determine the effect of microRNA-152 on
Bcl-2 and NF-κB expression in A549/cis cells, microRNA-152 mimics
were transfected into A549/cis cells. Consequently, Bcl-2 and NF-κB
were significantly downregulated, as demonstrated by the results of
the RT-qPCR and western blot analyses (all P<0.05; Fig. 5).
Discussion
Systemic chemotherapy helps to comprehensively treat
lung cancer; however, chemotherapeutic resistance among tumors
often results in chemotherapeutic failure, and is the primary
reason underlying tumor recurrence and metastasis. Cisplatin is a
first-line chemotherapeutic drug for NSCLC. However, cisplatin
resistance is one of the primary obstacles to its curative effect
(14–16); thus, it is important to overcome
issues including chemotherapeutic and intervention resistance in
tumor cells.
The molecular mechanism underlying cellular
cisplatin resistance is complex, being primarily involved in drug
transport, drug detoxification, apoptosis and a number of other
aspects, with the following common effects: First, molecular pumps
on the cell membrane are aberrantly expressed and cause the efflux
of the majority of drugs, thereby decreasing the intracellular drug
concentration. Resistance can be induced by overexpression of P-gp
on the cell membrane, for example, [encoded by multi-drug
resistance (MDR)1, a member of the multidrug resistance gene MDR
family], an energy-dependent molecular pump that actively
transports cisplatin or other chemotherapeutic drugs outside the
cell, thus decreasing the intracellular drug concentration or its
redistribution in tumor cells (17).
Secondly, the effect of drug detoxification in cells is enhanced;
accordingly, drug metabolism and excretion are increased,
potentially accompanied by a decrease in drug toxicity, e.g., drug
resistance caused by aberrant expression of glutathione
S-transferase PI family members, which can increase drug polarity,
eliminate drug metabolites, prevent DNA binding of drugs in tumor
cells, and catalyze the interaction of glutathione and
electrophiles, thereby eliminating reactive oxygen species-mediated
injury resulting from chemotherapeutic drugs, accompanied by
prevention of lipid oxidation on the cell membrane (18). Thirdly, abnormalities in the DNA
repair capacity, e.g., mutation in topoisomerase TOPOII, can result
in cisplatin resistance (19).
Finally, aberrant activity of apoptosis regulators may cause
resistance (20). Cisplatin may
enhance apoptosis via certain apoptotic regulators, thereby
potentially suppressing the efficacy of cisplatin treatment upon
aberrant expression (21).
Recently, numerous studies on different types of
tumor have focused on the association between microRNA and
tumorigenesis, tumor progression and chemotherapeutic resistance
(22–24). MicroRNAs are a class of small
non-coding RNAs of ~21-25 nucleotides in length, which suppress
target mRNAs at the post-transcriptional level, thereby regulating
cell proliferation and apoptosis (25–27).
Accumulating evidence has indicated a close association between
microRNA and tumors, which may affect tumor progression,
differentiation, metastasis and chemotherapeutic resistance
(28). Furthermore, chemotherapeutic
resistance is a bottleneck issue warranting urgent resolution in
clinical oncology, and potential microRNA targets to reverse
chemotherapeutic resistance need to be identified. Upregulation of
microRNA-200c reportedly increased the chemosensitivity of breast
cancer cells to epirubicin (29),
and overexpression of microRNA-1915 reversed multidrug resistance
in colon cancer cells (30). By
contrast, downregulation of microRNA-93 enhanced apoptosis in
cisplatin-resistant ovarian cancer cells (31), and inhibition of microRNA-328 also
induced cellular apoptosis and decreased cell proliferation in
A549/cis cells treated with cisplatin (32). There have been a number of studies
that focus microRNA-152 and cancer in recent years (33,34),
which have demonstrated that microRNA-148a and microRNA-152
decrease tamoxifen resistance in estrogen receptor-positive breast
cancer via downregulating activated leukocyte cell adhesion
molecule. MicroRNA-152 has been demonstrated to function as a tumor
suppressor in cervical cancer (35).
In the present study, microRNA-152 was downregulated
in cisplatin-resistant A549/cis cells compared with in
non-resistant A549 cells; hence, it may be concluded that
microRNA-152 downregulation suppressed apoptosis in tumor cells,
which is not conducive to the treatment of the tumor. In addition,
these data are concurrent with previous reports, for instance,
microRNA-152 reportedly inhibited the growth and invasiveness of
NSCLC, while microRNA-152 downregulation increased tumor cell
proliferation (12).
Thus, the present study preliminarily reports an
association between cisplatin resistance and microRNA expression in
NSCLC. Although numerous studies have reported that microRNA-152 is
downregulated in tumors and serves as a tumor suppressor (36,37), the
association between microRNA-152 and chemotherapeutic resistance
has been unclear. In the present study, microRNA-152 levels were
lower in A549 cells than in A549/cis cells; thus, A549/cis cells
exhibited adequate cisplatin resistance. However, following
transfection of microRNA-152 mimics into A549/cis cells, the
susceptibility of these cells to cisplatin improved. Therefore, it
can be speculated that the upregulation of intracellular
microRNA-152 resulted from transfection of microRNA-152 mimics,
which triggered corresponding signaling pathways, thereby enhancing
cisplatin resistance in cells. This was further confirmed via TUNEL
staining. Following transfection of mimics and cisplatin treatment,
dense granular fluorescence was observed in the nucleus or
cytoplasm of A549/cis cells. Furthermore, Annexin V-FITC/PI double
staining flow cytometric analysis was performed in order to
determine the apoptotic rate of cultured cells in vitro.
Flow cytometric analysis revealed that the apoptotic rate of group
cis+miR mimics significantly increased, suggesting that
transfection of microRNAs into A549/cis cells increased their
sensitivity to cisplatin.
However, it is currently unclear which signaling
pathway is involved in the underlying molecular mechanisms. Hence,
the present study analyzed the levels of Bcl-2 and NF-κB in
different cells. Bcl-2 and NF-κB were significantly upregulated in
A549/cis cells. However, following transfection of the microRNA-152
mimic, Bcl-2 and NF-κB were downregulated. These results suggest
that microRNA-152 may play a regulatory role in chemotherapeutic
sensitivity by regulating Bcl-2 and NF-κB.
Bcl-2 and the NF-κB are well-known anti-apoptotic
proteins. Numerous studies have reported that Bcl-2 and NF-κB are
upregulated in various different types of tumor, both exerting
significant anti-apoptotic effects in the proliferation of
malignant tumor cells and are closely associated with tumor cell
invasiveness and metastasis, including NSCLC, gastric cancer,
ovarian cancer, and colon cancer (38,39).
Hall et al (40) reported
that Bcl-2 serves as a potential chemotherapeutic target in tumors
of the reproductive system, with Bcl-2 upregulation suggesting a
poor prognosis. Sun et al (41) reported that NF-κB was upregulated in
lung adenocarcinoma A549 cells, and it suppressed the
chemotherapeutic sensitivity of A549 cells. In the present study,
microRNA-152 upregulation suppressed Bcl-2 and NF-κB and weakened
their anti-apoptotic effects, thereby providing a potential
explanation behind the improvement in the chemotherapeutic
sensitivity of A549 cells to cisplatin. However, further studies
are required in order to determine whether Bcl-2 and NF-κB are
direct targets of microRNA-152 or are simply components of the
microRNA-152 regulatory machinery.
To further understand apoptosis in A549/cis cells
transfected with microRNA-152, PI staining was performed in order
to analyze the effect of microRNA-152 on the cell cycle
distribution. Consequently, cells were arrested in the
G0/G1 phase of the cell cycle. However,
whether Bcl-2 and NF-κB are direct target genes of microRNA-152 or
downstream of microRNA-152 regulatory pathways needs to be further
confirmed.
The present study revealed that microRNA-152 is
downregulated in A549/cis cells, with concomitant upregulation of
Bcl-2 and NF-κB. Overexpression of microRNA-152 strengthened the
inhibitory effects on cell proliferation and increased the
apoptotic rate, accompanied by significant downregulation of Bcl-2
and NF-κB in A549/cis cells. The present results indicate that
microRNA-152 upregulation may decrease cisplatin resistance in
NSCLC, its effects potentially mediated via regulation of Bcl-2 and
NF-κB signal transduction.
Acknowledgements
The authors would like to thank Dr Bing Bai
(Department of Endocrine, The Fifth Affiliated Hospital of
Zhengzhou University) and Dr Youcai Tang (Laboratory of
Rehabilitation Medicine, The Fifth Affiliated Hospital of Zhengzhou
University) for coordinating the project and technical assistance
throughout the course of this work.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ and HL conceived and designed the study. WZ, SY
and DG acquired the data. WZ, JC and SM analyzed the data. WZ, WL,
SY, DG, JC, SM, YX and ML contributed to the interpretation of the
data. WZ and HL wrote and revised the paper. YX and ML provided
administrative, technical, or material support. WZ and HL
supervised the study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu F, Li J, Jang C, Wang J and Xiong J:
The role of Axl in drug resistance and epithelial-to-mesenchymal
transition of non-small cell lung carcinoma. Int J Clin Exp Pathol.
7:6653–6661. 2014.PubMed/NCBI
|
|
2
|
Kocher F, Pircher A, Mohn-Staudner A,
Romeder F, Duller W, Steinmaurer M, Eckmayr J, Schmid T, Hilbe W,
Fiegl M and Greil R: Multicenter phase II study evaluating
docetaxel and cisplatin as neoadjuvant induction regimen prior to
surgery or radiochemotherapy with docetaxel, followed by adjuvant
docetaxel therapy in chemonaive patients with NSCLC stage II, IIIA
and IIIB (TAX-AT 1.203 Trial). Lung Cancer. 85:395–400. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Juhász E, Kim JH, Klingelschmitt G and
Walzer S: Effects of erlotinib first-line maintenance therapy
versus placebo on the health-related quality of life of patients
with metastatic non-small-cell lung cancer. Eur J Cancer.
49:1205–1215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pircher A, Manzl C, Fiegl M, Popper H,
Pirker R and Hilbe W: Overcoming resistance to first generation
EGFR TKIs with cetuximab in combination with chemotherapy in an
EGFR mutated advanced stage NSCLC patient. Lung Cancer. 83:408–410.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rolfo C, Giovannetti E, Hong DS, Bivona T,
Raez LE, Bronte G, Buffoni L, Reguart N, Santos ES, Germonpre P, et
al: Novel therapeutic strategies for patients with NSCLC that do
not respond to treatment with EGFR inhibitors. Cancer Treat Rev.
40:990–1004. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanna N, Shepherd FA, Fossella FV, Pereira
JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M,
Muller T, et al: Randomized phase III trial of pemetrexed versus
docetaxel in patients with non-small-cell lung cancer previously
treated with chemotherapy. J Clin Oncol. 22:1589–1597. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu C, Wang Y, Xia Y, He S, Wang Z, Chen Y,
Wu C, Shu Y and Jiang J: Wilms' tumor 1 enhances
Cisplatin-resistance of advanced NSCLC. FEBS Lett. 588:4566–4572.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Daniels MG, Bowman RV, Yang IA, Govindan R
and Fong KM: An emerging place for lung cancer genomics in 2013. J
Thorac Dis. 5 (Suppl 5):S491–S497. 2013.PubMed/NCBI
|
|
9
|
Ke Y, Zhao W, Xiong J and Cao R:
Downregulation of miR-16 promotes growth and motility by targeting
HDGF in non-small cell lung cancer cells. FEBS Lett. 587:3153–3157.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xin C, Zhang H and Liu Z: miR-154
suppresses colorectal cancer cell growth and motility by targeting
TLR2. Mol Cell Biochem. 387:271–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng Z, Ma R, Tan W and Zhang L: MiR-152
suppresses the proliferation and invasion of NSCLC cells by
inhibiting FGF2. Exp Mol Med. 46:e1122014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Su Y, Wang Y, Zhou H, Lei L and Xu L:
MicroRNA-152 targets ADAM17 to suppress NSCLC progression. FEBS
Lett. 588:1983–1988. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amable L: Cisplatin resistance and
opportunities for precision medicine. Pharmacol Res. 106:27–36.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fennell DA, Summers Y, Cadranel J, Benepal
T, Christoph DC, Lal R, Das M, Maxwell F, Visseren-Grul C and Ferry
D: Cisplatin in the modern era: The backbone of first-line
chemotherapy for non-small cell lung cancer. Cancer Treat Rev.
44:42–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim ES: Chemotherapy resistance in lung
cancerLung Cancer and Personalized Medicine. Springer International
Publishing; pp. 189–209. 2016, View Article : Google Scholar
|
|
17
|
Li S, Lei Y, Jia Y, Li N, Wink M and Ma Y:
Piperine, a piperidine alkaloid from Piper nigrum re-sensitizes
P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells.
Phytomedicine. 19:83–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang W, Liu G and Zheng J: Human renal
UOK130 tumor cells: A drug resistant cell line with highly
selective over-expression of glutathione S-transferase-pi isozyme.
Eur J Pharmacol. 568:61–67. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mansoori B, Mohammadi A, Davudian S,
Shirjang S and Baradaran B: The different mechanisms of cancer drug
resistance: A brief review. Adv Pharm Bull. 7:339–348. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rodriguez-Barrueco R, Nekritz EA, Bertucci
F, Yu J, Sanchez-Garcia F, Zeleke TZ, Gorbatenko A, Birnbaum D,
Ezhkova E, Cordon-Cardo C, et al: miR-424(322)/503 is a breast
cancer tumor suppressor whose loss promotes resistance to
chemotherapy. Genes Dev. 31:553–566. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heavey S, Barr M, Edwards C, O'Byrne K and
Gately K: 7 NFkB-IκBα interaction: A mechanism of resistance to
cisplatin in NSCLC. Lung Cancer. 75 (Suppl 1):S32012. View Article : Google Scholar
|
|
22
|
Li Y, Liang Y, Sang Y, Song X, Zhang H,
Liu Y, Jiang L and Yang Q: MiR-770 suppresses the chemo-resistance
and metastasis of triple negative breast cancer via direct
targeting of STMN1. Cell Death Dis. 9:142018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Bao W, Liu Y, Wang S, Xu S, Li X,
Li Y and Wu S: miR-98-5p contributes to cisplatin resistance in
epithelial ovarian cancer by suppressing miR-152 biogenesis via
targeting Dicer1. Cell Death Dis. 9:4472018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan W, Liao Y, Qiu Y, Liu H, Tan D, Wu T,
Tang M, Zhang S and Wang H: miRNA 146a promotes chemotherapy
resistance in lung cancer cells by targeting DNA damage inducible
transcript 3 (CHOP). Cancer Lett. 428:55–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang JS, Li BJ, Lu HW, Chen Y, Lu C, Zhu
RX, Liu SH, Yi QT, Li J and Song CH: Serum miR-152, miR-148a,
miR-148b, and miR-21 as novel biomarkers in non-small cell lung
cancer screening. Tumour Biol. 36:3035–3042. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dou H, Wang Y, Su G and Zhao S: Decreased
plasma let-7c and miR-152 as noninvasive biomarker for
non-small-cell lung cancer. Int J Clin Exp Med. 8:9291–9298.
2015.PubMed/NCBI
|
|
27
|
Zeng K, Chen X, Xu M, Liu X, Hu X, Xu T,
Sun H, Pan Y, He B and Wang S: CircHIPK3 promotes colorectal cancer
growth and metastasis by sponging miR-7. Cell Death Dis. 9:4172018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rottiers V, Najafi-Shoushtari SH, Kristo
F, Gurumurthy S, Zhong L, Li Y, Cohen DE, Gerszten RE, Bardeesy N,
Mostoslavsky R and Näär AM: MicroRNAs in metabolism and metabolic
diseases. Cold Spring Harb Symp Quant Biol. 76:2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen J, Tian W, Cai H, He H and Deng Y:
Down-regulation of microRNA-200c is associated with drug resistance
in human breast cancer. Med Oncol. 29:2527–2534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu K, Liang X, Cui D, Wu Y, Shi W and Liu
J: miR-1915 inhibits Bcl-2 to modulate multidrug resistance by
increasing drug-sensitivity in human colorectal carcinoma cells.
Mol Carcinog. 52:70–78. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fu X, Tian J, Zhang L, Chen Y and Hao Q:
Involvement of micro RNA-93, a new regulator of PTEN/Akt signaling
pathway, in regulation of chemotherapeutic drug cisplatin
chemosensitivity in ovarian cancer cells. FEBS Lett. 586:1279–1286.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang C, Wang S, Ma F and Zhang W:
miRNA-328 overexpression confers cisplatin resistance in non-small
cell lung cancer via targeting of PTEN. Mol Med Rep. 18:4563–4570.
2018.PubMed/NCBI
|
|
33
|
Chen L, Wang Y, He J, Zhang C, Chen J and
Shi D: Long noncoding RNA H19 promotes proliferation and invasion
in human glioma cells by downregulating miR-152. Oncol Res. 2018.
View Article : Google Scholar
|
|
34
|
Chen MJ, Cheng YM, Chen CC, Chen YC and
Shen CJ: MiR-148a and miR-152 reduce tamoxifen resistance in ER+
breast cancer via downregulating ALCAM. Biochem Biophys Res Commun.
483:840–846. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Marques JHM, Mota AL, Oliveira JG, Lacerda
JZ, Stefani JP, Ferreira LC, Castro TB, Aristizábal-Pachón AF and
Zuccari DAPC: Melatonin restrains angiogenic factors in
triple-negative breast cancer by targeting miR-152-3p: In vivo and
in vitro studies. Life Sci. 208:131–138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma J, Yao Y, Wang P, Liu Y, Zhao L, Li Z,
Li Z and Xue Y: MiR-152 functions as a tumor suppressor in
glioblastoma stem cells by targeting Krüppel-like factor 4. Cancer
Lett. 355:85–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu Y, Huang A, Li T, Su X, Ding H, Li H,
Qin X, Hou L, Zhao Q, Ge X, et al: MiR-152 reduces human umbilical
vein endothelial cell proliferation and migration by targeting
ADAM17. FEBS Lett. 588:2063–2069. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Batsi C, Markopoulou S, Kontargiris E,
Charalambous C, Thomas C, Christoforidis S, Kanavaros P,
Constantinou AI, Marcu KB and Kolettas E: Bcl-2 blocks
2-methoxyestradiol induced leukemia cell apoptosis by a
p27(Kip1)-dependent G1/S cell cycle arrest in conjunction with
NF-kappaB activation. Biochem Pharmacol. 78:33–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Luna-López A, González-Puertos VY,
Romero-Ontiveros J, Ventura-Gallegos JL, Zentella A, Gomez-Quiroz
LE and Königsberg M: A noncanonical NF-κB pathway through the p50
subunit regulates Bcl-2 overexpression during an
oxidative-conditioning hormesis response. Free Radic Biol Med.
63:41–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hall C, Troutman SM, Price DK, Figg WD and
Kang MH: Bcl-2 family of proteins as therapeutic targets in
genitourinary neoplasms. Clin Genitourin Cancer. 11:10–19. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun C, Chan F, Briassouli P and
Linardopoulos S: Aurora kinase inhibition downregulates NF-kappaB
and sensitises tumour cells to chemotherapeutic agents. Biochem
Biophys Res Commun. 352:220–225. 2007. View Article : Google Scholar : PubMed/NCBI
|