Introduction
Non-coding RNAs (ncRNAs), which account for 98% of
human RNAs (1), do not encode
proteins but play an important role in the regulation of gene
expression, including transcription, translation and RNA splicing
(2,3). NcRNAs include microRNAs (miRNAs/miRs),
small nuclear RNAs, PIWI-interacting RNAs, long non-coding RNAs
(lncRNA) and circular RNAs (circRNAs) (4,5).
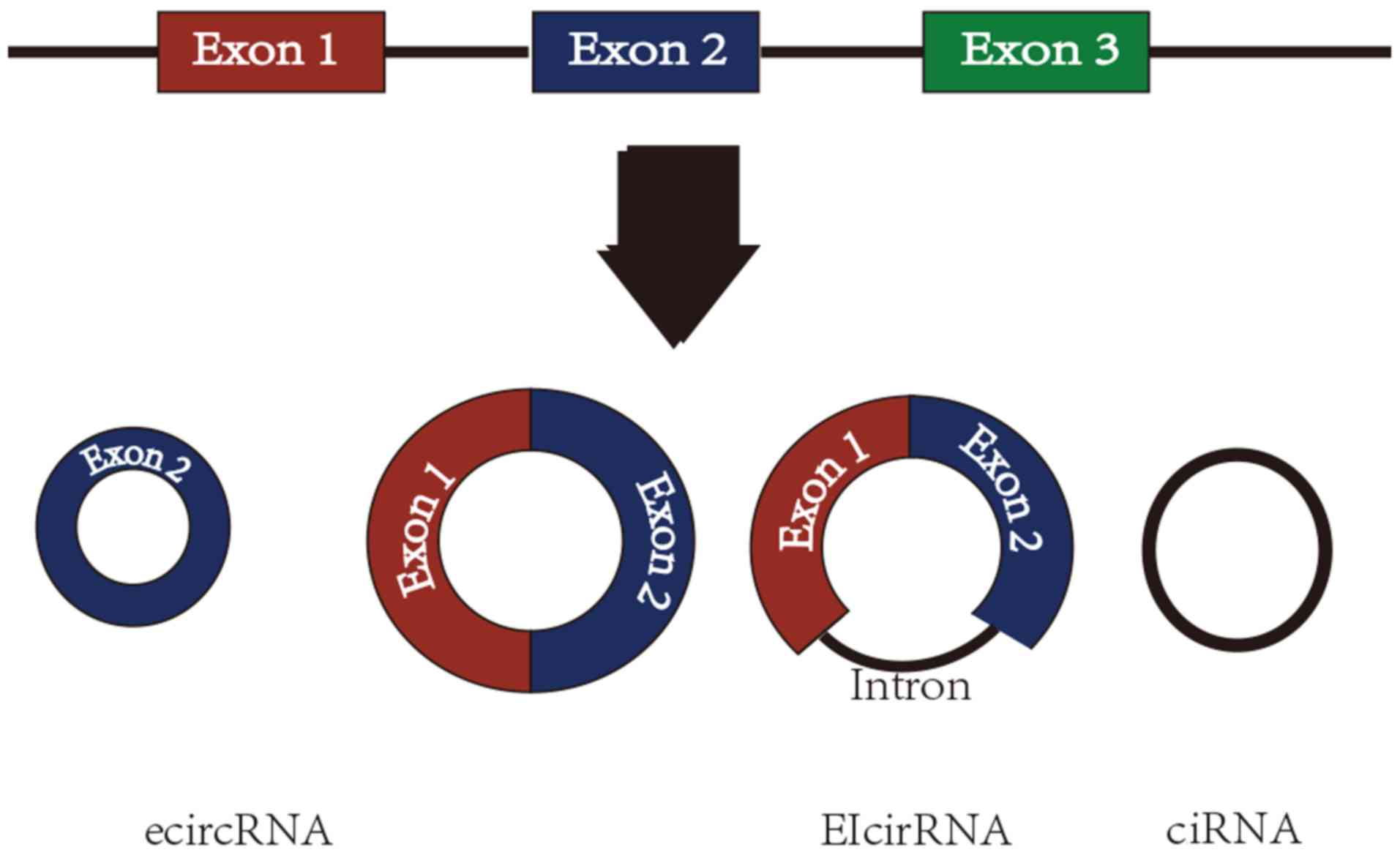
circRNAs are formed by back-splicing events where the 5′ and 3′
ends join to form covalently closed continuous loops without
polyadenylated tails (6). There are
three types of circRNAs: i) Exonic circRNAs (ecircRNAs; with one or
more exons) (7,8); ii) exon-intron circRNA (EIcirRNA)
(9); and iii) circularized intron
RNA (ciRNA) (10). However, 85% of
circRNAs originate from exons (2)
(Fig. 1). circRNAs were initially
considered as byproducts of transcription (11) after being first discovered in plant
viruses in 1976 (12). However, the
growing number of publications on circRNAs has illustrated the
biological importance of circRNAs.
circRNAs can function as miRNA sponges. Certain
circRNAs can act as competing endogenous RNAs that negatively
influence miRNAs, thus regulating transcription and translation.
Cerebellar degeneration-related protein 1 antisense RNA was
reported to bind to miR-7, indicating that circRNAs have
miRNA-binding capacity (3). miRNAs
have antagonizing activities in the human and mouse brain (3), and can inhibit miR-7, thus increasing
the expression levels of miR-7 targets, including epidermal growth
factor receptor and insulin receptor substrate 2 (13). Besides, the testis-specific circRNA,
sex-determining region Y was also reported to serve as a miR-138
sponge (13). The circRNA-miRNA-mRNA
axis has critical functions in tumorigenesis (14). The function of certain circRNAs
depends largely on their target pathways. For example, circ-itchy
E3 ubiquitin protein ligase (ITCH) could increase the level of
ITCH, which inhibits the Wnt/β-catenin pathway (15,16).
circRNAs can act as platforms for protein interaction. For example,
mannose binding lectin (Mbl) specifically binds to the circ-Mbl,
which is generated from its own RNA (17), and circ-forkhead box O3 can inhibit
cell-cycle progression by interacting with Cdk (18). circRNAs were reported to regulate
translation and are naturally resistant to exonucleases (19). The presence of internal ribosome
entry sites (IRESs) and appropriate open reading frames (ORFs)
induce cap-independent translation (2). The ORF can be translated in an
Escherichia coli cell-free translation system (20). A recent study demonstrated that
efficient circRNA translation occurred in HeLa cells, and circRNA
can be translated without such particular sequences (21). Furthermore, ribosomes can be
recruited to an internal initiation codon by IRES (21). Circ-ZNF609 was reported to be
translated by this mechanism and its high degree of methylation
facilitates its translational activity (22).
circRNAs are conserved, stable and specific to the
developmental stages and tissues (23,24). In
comparison with linear RNAs, circRNAs were reported to be highly
conserved at the nucleotide level in several eukaryotes from yeast
to human (23). Due to their natural
resistance to exonucleases, circRNAs appear to be extremely stable
transcripts with half-lives of >24-48 h (25). Multiple previous results of RNA
sequencing (RNA-seq) from brain tissues and differentiated cell
lines indicated that circRNAs are tissue-specific (24). The various expressions at different
developmental stages of mouse brain suggest that characteristics of
circRNAs may depend on the developmental stage (24,26).
The development of high-throughput RNA-seq
technology (27) accelerated the
discovery of various circRNAs. RNA libraries were established using
Ribo-Zero (ribosome RNA depletion) and RNase R (28). Later, computational algorithms
emerged for detecting circRNAs, which were most commonly found at
back-splicing junctions (29).
CircBase (http://www.circbase.org/) (30) contains data from Homo sapiens, Mus
musculus, Caenorhabditis elegans, Drosophila melanogaster and
Latimeria chalumnae species. Specifically, the CircBase
database contains sequences, gene descriptions and genomic
locations. CIRCpedia v2 (http://www.picb.ac.cn/rnomics/circpedia) (31) provides circRNA annotation for six
species (human, mouse, rat, zebrafish, fly and worm) and contains
an analysis tool for investigating the differential expression of
circRNAs. CircInteractome (http://circinteractome.nia.nih.gov) (32) contains 109 datasets of RNA-binding
proteins and functions by searching RNA-binding sites. CircNet
database (http://circnet.mbc.nctu.edu.tw/) (33) assembled by transcriptome sequencing
datasets, containing circRNA, miRNA and gene networks, and
tissue-specific circRNA expression profiles. CirclncRNAnet
(http://app.cgu.edu.tw/circlnc) (34) provides networks of lncRNAs or
circRNAs of interest, based on next-generation sequencing (NGS)
data. CircRNADb (http://reprod.njmu.edu.cn/circrnadb) (35) focuses on protein-coding annotations
and provides information on exon splicing, genome sequence, IRES,
ORF and references. CircR2Disease (http://bioinfo.snnu.edu.cn/CircR2Disease/) (36) provides experimental evidence for the
associations between circRNAs and diseases and helps find the
appropriate algorithms or models for circRNA-disease associations.
Tissue-specific circRNA database (http://gb.whu.edu.cn/TSCD) (37) provides tissue-specific circRNAs in
the main tissues of humans and mice and provides evidence to
identify new markers for organogenesis and development of diseases.
Similarly, cancer-specific circRNA database (http://gb.whu.edu.cn/CSCD) (38) aims to identify cancer-specific
circRNAs.
Exosomes are 50–140-nm nanovesicles that transport
abundant bioactive substances (39),
which enhance cell-to-cell communication (40). Li et al (41) reported that circRNAs are stable in
exosomes and demonstrated that circ-isoleucyl-tRNA synthetase 1 in
tumor cell-derived exosomes function in the metastasis of
pancreatic cancer (42). Zhang et
al (43) demonstrated that
exosome circRNAs circ-DB promoted the tumor growth of
hepatocellular carcinoma, through the absorption of miR-34a and
activation of the USP7/Cyclin A2 pathway. Since circRNAs are
abundant in exosomes, they may cause disease-specific differential
gene expression (44) and serve as
biomarkers.
The presence of circRNAs in human peripheral blood,
including in serum (45), plasma
(42) and peripheral blood
mononuclear cells (46), and saliva
(47) and bone marrow (48) indicates their potential as disease
biomarkers. Furthermore, circRNAs play a critical role in the
pathogenesis and diagnosis of cancers, including gastric cancer
(49), breast cancer (50) and esophageal cancer (51). However, the roles and mechanisms of
circRNAs in hematological malignancies have not been fully
clarified. Hematological malignancies are diseases of unusual stem
and progenitor cells, which originate from genetic and epigenetic
changes resulting in the dysregulation of self-renewal,
proliferation and differentiation of cells (52). The present review provided an
overview of the recent advancements of circRNAs associated with
hematological malignancies, including acute myeloid leukemia (AML),
chronic myeloid neoplasms, B- and T-/natural killer (NK)-cell
lymphoma and multiple myeloma (MM), and discussed their relevance
to clinical practice.
Acute myeloid leukemia
AML is a class of highly heterogeneous diseases
derived from bone marrow hematopoietic cells and is characterized
by the rapid growth of abnormal white blood cells, reduction in the
normal production of blood cells, and infiltration and disruption
of other organs in the body (53–55).
Over the past few years, NGS has revealed an accumulation of
mutations in genes regulating the splicing process in ~10% of
patients with AML (56),
highlighting the important role of the alterations in gene
regulation in the molecular pathogenesis of AML.
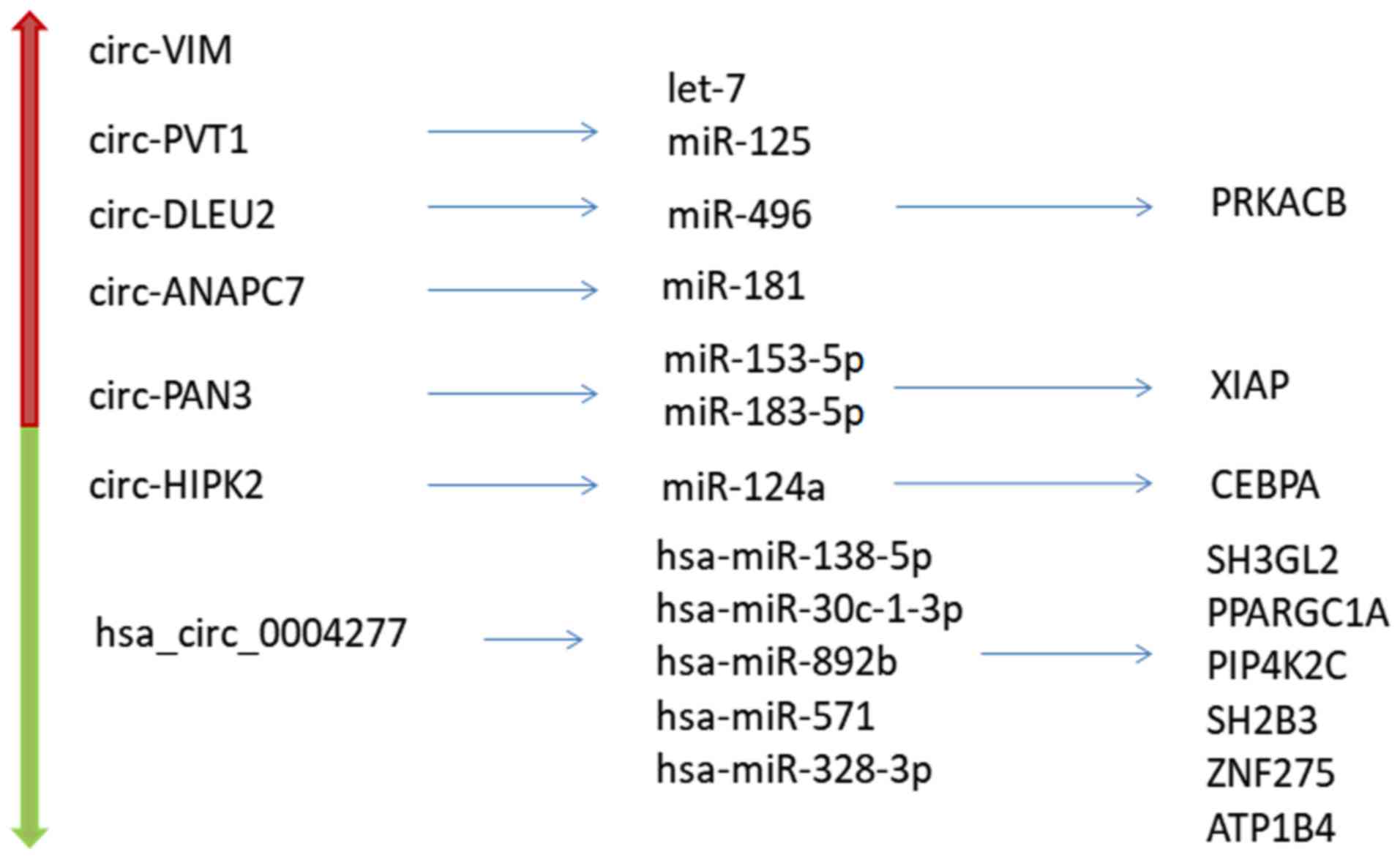
circ-Vimentin (VIM)
VIM, a component of type III intermediate filament
protein, is involved in the regulation of lymphocyte adhesion and
transcellular migration, and is associated with poor clinical
outcome in older patients with AML (57).
Yi et al (58)
observed that circ-VIM expression level in de novo patients
with AML (particularly patients with non-acute promyelocytic
leukemia and cytogenetically normal patients with AML) was
significantly upregulated compared with that in healthy controls. A
receiver operating characteristic (ROC) curve analysis indicated
that high expression level of circ-VIM may serve as a promising
diagnostic biomarker and treatment target (58).
circ-PVT1 oncogene (PVT1)
In AML, 8q24 amplifications were reported to be
associated with two fusion genes, PVT1-zinc finger, MIZ-type
containing 7 (NSMCE2) and BF104016-NSMCE2 (59,60).
circ-PVT1, generated from exon 2 of PVT1, is highly expressed in
gastric cancer (49), non-small cell
lung carcinoma (61) and
osteosarcoma (62) compared with
levels in matched para-carcinoma tissue; circ-PVT1 is highly
expressed in head and neck squamous cell carcinoma and its
expression is significantly associated with mutant p53 (63).
circ-PVT1 is highly expressed in AML compared with
its level in normal bone marrow cells (48) and circ-PVT1 was found to act as a
sponge for let-7 (64) and miR-125
families (65). Therefore, circ-PVT1
may be a potential novel therapeutic target.
hsa_circ_0004277
Li et al (66)
reported that hsa_circ_0004277 expression was significantly lower
in the AML group than in healthy controls and patients who entered
complete remission (CR) post-treatment. These previous results
indicated that hsa_circ_0,004277 variation is associated with AML
progression.
Furthermore, hsa_circ_0004277 might be a diagnostic
biomarker as well as a therapeutic target in AML (66). Additionally, hsa_circ_0004277 has
several downstream targets including hsa-miR-138-5p,
hsa-miR-30c-1-3p, hsa-miR-892b, hsa-miR-571 and hsa-miR-328-3p,
revealed by bioinformatics analysis (66). Further downstream gene targets were
predicted by bioinformatics analysis, with the most probable being
SH3 domain containing GRB2 like 2, endophilin A1, PPARG coactivator
1α, phosphatidylinositol-5-phosphate 4-kinase type 2γ, SH2B adaptor
protein 3, zinc finger protein 275 and ATPase
Na+/K+ transporting family member β4
(66).
hsa_circ_0075001
Hirsch et al (67) reported a correlation between total
nucleophosmin expression and hsa_circ_0075001. High
hsa_circ_0075001 expression was strongly associated with a
significantly lower expression of genes involved in the Toll-like
receptor (TLR) signaling pathway (67). TLR1 expression was associated with
leukemic stem cell survival in AML, as well as enhanced TLR1/TLR2
activation in leukemic stem cell differentiation (68,69).
Thus, hsa_circ_0075001 is a potential biomarker for classification
and risk stratification.
circ-DLEU2
Wu et al (70)
found that circ-DLEU2 was highly expressed in tissue samples from
patients with AML and AML cell lines. Increased circ-DLEU2 resulted
in promoted proliferation and inhibited apoptosis in AML cell
lines. Furthermore, circ-DLEU2 suppressed miR-496 and further
increased PRKACB expression (70).
circ-ANAPC7
Chen et al (71) compared the circRNA expression profile
among five AML samples and five iron-deficiency anemia samples
collected from donor bone marrow, which revealed 282 significantly
upregulated and 416 downregulated circRNAs in patients with AML.
Reverse transcription-quantitative PCR results indicated
significant upregulation of circ-ANAPC7 in AML and suggested that
circ-ANAPC7 was a potential biomarker for AML diagnosis and a
potential novel therapeutic target. Further bioinformatics analysis
indicated that circ-ANAPC7 may serve as a sponge for the miR-181
family (71).
circ-PAN3
Shang et al (72) compared the expression profiles of
circRNAs between drug-resistant THP-1/ADM cell lines and naïve
THP-1 cell lines. In total, 49 circRNAs showed significant
differential expression. Meanwhile, overexpression of circ-PAN3 was
identified in the bone marrow samples from patients with
refractory/recurrent AML. circ-PAN3 had binding sites on miR-153-5p
and miR-183-5p. Further experiments indicated that circ-PAN3 was
possibly responsible for ADM resistance in AML and that circ-PAN3
regulated THP-1/ADM cells via the
circ-PAN3-miR-153-5p/miR-183-5p-XIAP axis (72).
circ-HIPK2
Acute promyelocytic leukemia (APL) is a type of AML
specialized for the promyelocytic leukemia-retinoic acid receptor α
(PML/RARα) fusion protein, which induces oncogenic transcription by
blocking cell differentiation at the promyelocytic stage, resulting
in malignant transformation (73).
Ribo-minus RNA-seq analysis revealed 4,313
APL-expressed circRNAs in NB4 cell lines (74). Moreover, 508 circRNAs were
dynamically expressed during all-trans retinoic acid treatment.
circ-HIPK2 was downregulated in samples of patients with APL
compared with that in healthy control samples and other subtypes of
AML cases. The expression level of circ-HIPK2 was elevated
significantly when patients with APL achieved CR (74). Ina previous study, circ-HIPK2 was
found to function as a competing endogenous RNA, sponging miR-124,
regulating astrocyte activation (75). Furthermore, miR-124a inhibited the
downstream protein CEBPA (76).
Therefore, circ-HIPK2 may affect APL differentiation through the
miR-124-3p-CEBPA axis (74). The
association of circRNAs expression in AML is shown in Fig. 2.
Chronic myeloid leukemia
Chronic myeloid leukemia (CML) is a stem cell
disorder of uncontrolled myeloid proliferation characterized by the
Philadelphia chromosome (77). The
reciprocal translocation t(9;22) (q34; q11.2), results in the
BCR-ABL1 fusion. The progression of the disease involves 2–3
phases: Indolent chronic phase (CP), accelerated phase and blast
phase (77,78). The introduction of imatinib and other
tyrosine kinase inhibitors (TKIs) can change the disease course;
however, resistance develops in ~13% of patients (78).
circ-BA9.3
Fusion genes, encoded by abnormal chromosomal
translocations were associated with hematological malignancies
(79). Guarnerio et al
(80) reported that tumors harbor
circRNAs derived from chromosomal translocations and genomic
fusion, which form aberrant fusion-circRNAs (f-circRNA).
Furthermore, such f-circRNAs can be functionally relevant and
tumor-promoting, with potential diagnostic and therapeutic
implications (80). F-circRNAs such
as PML-RARα and MLL-AF9 were found in hematological malignancies
(80). The oncogene BCR-ABL1, which
encodes a hyperactive tyrosine kinase, was found to be the most
important factor that led to CML pathogenesis (81,82).
TKIs induce long-lasting remission in patients with CML; however,
8–13% of chronic CML cases were resistant to imatinib, and ~18% of
patients in the CP of CML lost their sensitivity to imatinib and
suffered from relapse and progression (83).
Pan et al (84) reported that circ-BA9.3, a f-circRNA
with 1,137 nucleotides, was the circular RNA of BCR-ABL1, and was
found in 30 patients with CML, including 23 with imatinib-resistant
and seven with imatinib-sensitive CML. circ-BA9.3 can improve the
translation efficiency of BCR-ABL1 or prevent the oncoprotein from
degrading (84). Additionally, the
authors reported that circ-BA9.3 may cause severe carcinogenicity
and resistance to TKI, such as imatinib, dasatinib and nilotinib by
increasing the protein level of the BCR-ABL1 oncogene (84). circ-BA9.3 is involved in TKI
resistance and may be a target for the diagnosis and treatment of
patients with CML (84).
hsa_circ_0080145
Using high-throughput sequencing technology, Liu
et al (85) identified 361
circRNAs that were aberrantly expressed in CML. Among these,
hsa_circ_0080145 was significantly upregulated in CML.
ceRNA regulatory network analysis revealed that
hsa_circ_0024002, hsa-circ_0080145 and hsa_circ_0037781 had the
most target miRNAs, including leukemia-associated miRNAs, such as
miR-16, miR-181a and miR-29b, among the differentially expressed
circRNAs. miR-16 is upregulated in the peripheral lymphoid
(86) and miR-181a inhibits vascular
inflammation in human macrophages, suggesting that these circRNAs
may be involved in the pathogenesis of CML (87). Furthermore, the miR-29 family is
associated with malignant hematopoiesis and downregulated the
expression of BCR-ABL1 (88).
Notably, hsa_circ_0080145 was upregulated in samples
of patients with CML, as well as in CML cell lines. The dual
luciferase reporter assay showed a sponge function of
hsa_circ_0080145 for miR-29b. Therefore, circ_0080145 may be a
potential prognostic and therapeutic biomarker for CML (85).
Chronic lymphocytic leukemia
Chronic lymphocytic leukemia (CLL) is the most
frequently occurring leukemia in adults, characterized by
significant expansion of dysfunctional B cells with co-expression
of CD5, CD19, CD20 and CD23 in the peripheral blood, lymphoid
organs and bone marrow (89). The
diagnosis of CLL is mainly based on the precise immunophenotype of
peripheral blood or bone marrow lymphocytes (90).
Xia et al (91) reported that circ-CBFB
(hsa_circ_0000707), derived from the CBFB transcript (NM_001755),
was significantly overexpressed in CLL compared to that in healthy
controls. In addition, circ-CBFB regulates proliferation and
inhibits apoptosis of CLL cells (91).
High circ-CBFB expression is an independent
prognosis factor in patients with CLL. Mechanistically, circ-CBFB
acts as a sponge for miR-607 and causes increased expression of the
downstream target FZD3, thus regulating the activation of the
Wnt/β-catenin pathway in CLL (91).
The hyperactivation of FZD3 is known to be associated with CLL
hematopoiesis (92). In summary, the
circ-CBFB-miR-607-FZD3-Wnt/β-catenin axis is a potential target for
CLL therapy. The expressions and functions of circRNAs in leukemia
are shown in Table I.
 | Table I.circRNAs and their utility in
haematological malignancies. |
Table I.
circRNAs and their utility in
haematological malignancies.
| Type of cancer | circRNA |
Upregulated/downregulated in cancer | Function | (Refs.) |
|---|
| AML | circ-VIM | Upregulated | Diagnostic
biomarker, treatment target | (58) |
|
| circ-PVT1 | Upregulated | Therapeutic
target | (48) |
|
|
hsa_circ_0004277 | Downregulated | Diagnostic
biomarker, therapeutic target | (66) |
|
|
hsa_circ_0075001 | Upregulated | Classification | (67) |
|
| circ-DLEU2 | Upregulated | Diagnostic
biomarker | (70) |
|
| circ-ANAPC7 | Upregulated | Diagnosis or
therapeutic target | (71) |
|
| circ-PAN3 | Upregulated | Potential target
for drug resistance | (72) |
|
| circ-HIPK2 | Downregulated | Therapeutic
target | (74) |
| CML | circ-BA9.3 | Upregulated in
resistant CML | Diagnostic
biomarker, therapeutic target | (84) |
|
|
hsa_circ_0080145 | Upregulated | Prognosis
biomarker, therapeutic target | (85) |
| CLL | circ-CBFB | Upregulated | Therapeutic
target | (91) |
B-cell lymphoma and multiple myeloma
Dahl et al (93) performed RNA-seq profiling of various
lymphomas and multiple myeloma cell lines including four different
mantle cell lymphoma (MCL) cell lines, REC-1, Granta-519, UPN2 and
Z138, and the MM cell line, NCI-H929. Additionally, a novel circRNA
derived from IKZF3 was detected, which is highly expressed in
NCI-H929 (93). circRNAs from genes
involved in lymphomagenesis and the development of MM were also
detected, including FOXP1, SETD3, EZH2, ATM, XPO1, IKZF3, CD11A and
WHSC1.
The differential expression levels of various
circRNAs in different B-cell malignancies may potentially assist in
distinguishing between different B-cell malignancies in the near
future.
Conclusions
With the development of NGS and bioinformatics
techniques, the importance of ncRNAs in tumor studies has been
demonstrated in recent years (4).
circRNAs have become topic of interest due to their biological
functions, including the modulation of miRNA activity, protein
interaction and regulation of translation (6,17,19).
circRNAs have promising potential to serve as diagnostic,
prognostic biomarkers and therapeutic targets for diseases
(48,58,66,70,71,74). The
present review summarizes the function of differentially expressed
circRNAs that contribute to the pathogenesis, diagnosis, treatment
and prognosis of various hematological malignancies.
The detailed mechanisms and the function of the
specific circRNAs mentioned in the present review remain to be
clarified. In the future, circRNAs that have been thoroughly
explored in preclinical studies should be further studied in
clinical settings in order to be potentially used as biomarkers for
timely diagnosis and as therapeutic targets for the treatment of
various diseases.
Acknowledgements
Not applicable.
Funding
Funding was received from The Natural Science
Foundation, China (grant no. 81570203).
Availability of data and materials
Not applicable.
Authors' contributions
MM, YW, ZL and MZ contributed to the concept and
collated the references, and MM draft the manuscript, ZL and MZ
reviewed and edited the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
circRNA
|
circular RNA
|
|
miRNA
|
microRNA
|
|
ncRNA
|
non-coding RNA
|
|
ecircRNAs
|
exonic circRNAs
|
|
EIcirRNA
|
exon-intron circRNA
|
|
ciRNA
|
circularized intron RNA
|
|
IRES
|
internal ribosome entry site
|
|
ORF
|
open reading frame
|
|
NGS
|
next generation sequencing
|
|
AML
|
acute myeloid leukemia
|
|
APL
|
acute promyelocytic leukemia
|
|
CML
|
chronic myeloid leukemia
|
|
MPN
|
myeloproliferative neoplasm
|
|
CLL
|
chronic lymphocytic leukemia
|
|
MCL
|
mantle cell lymphoma
|
|
MM
|
multiple myeloma
|
References
|
1
|
ENCODE Project Consortium: An integrated
encyclopedia of DNA elements in the human genome. Nature.
489:57–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pamudurti NR, Bartok O, Jens M,
Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E,
Perez-Hernandez D, Ramberger E, et al: Translation of CircRNAs. Mol
Cell. 66:9–21.e27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mattick JS and Makunin IV: Non-coding RNA.
Hum Mol Genet. 15:R17–R29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu Z, Yan Y, Zeng S, Dai S, Chen X, Wei J
and Gong Z: Circular RNAs: Clinical relevance in cancer.
Oncotarget. 9:1444–1460. 2017.PubMed/NCBI
|
|
6
|
Lasda E and Parker R: Circular RNAs:
Diversity of form and function. RNA. 20:1829–1842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL
and Yang L: Complementary sequence-mediated exon circularization.
Cell. 159:134–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kelly S, Greenman C, Cook PR and
Papantonis A: Exon skipping is correlated with exon
circularization. J Mol Biol. 427:2414–2417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Z, Huang C, Bao C, Chen L, Lin M, Wang
X, Zhong G, Yu B, Hu W, Dai L, et al: Exon-intron circular RNAs
regulate transcription in the nucleus. Nat Struct Mol Biol.
22:256–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin
QF, Xing YH, Zhu S, Yang L and Chen LL: Circular intronic long
noncoding RNAs. Mol Cell. 51:792–806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cocquerelle C, Mascrez B, Hétuin D and
Bailleul B: Mis-splicing yields circular RNA molecules. FASEB J.
7:155–160. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kolakofsky D: Isolation and
characterization of sendai virus DI-RNAs. Cell. 8:547–555. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Xie Q, He D, Ling Y, Li Y, Li J
and Zhang H: Circular RNA: New star, new hope in cancer. BMC
Cancer. 18:8342018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li F, Zhang L, Li W, Deng J, Zheng J, An
M, Lu J and Zhou Y: Circular RNA ITCH has inhibitory effect on ESCC
by suppressing the Wnt/β-catenin pathway. Oncotarget. 6:6001–6013.
2015.PubMed/NCBI
|
|
16
|
Huang G, Zhu H, Shi Y, Wu W, Cai H and
Chen X: cir-ITCH plays an inhibitory role in colorectal cancer by
regulating the Wnt/β-catenin pathway. PLoS One. 10:e01312252015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ashwal-Fluss R, Meyer M, Pamudurti NR,
Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N and
Kadener S: circRNA biogenesis competes with pre-mRNA splicing. Mol
Cell. 56:55–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P
and Yang BB: Foxo3 circular RNA retards cell cycle progression via
forming ternary complexes with p21 and CDK2. Nucleic Acids Res.
44:2846–2858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang D, Tatomer DC, Luo Z, Wu H, Yang L,
Chen LL, Cherry S and Wilusz JE: The output of protein-coding genes
shifts to circular RNAs when the Pre-mRNA processing machinery is
limiting. Mol Cell. 68:940–954.e3. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abe N, Hiroshima M, Maruyama H, Nakashima
Y, Nakano Y, Matsuda A, Sako Y, Ito Y and Abe H: Rolling circle
amplification in a prokaryotic translation system using small
circular RNA. Angew Chem Int Ed Engl. 52:7004–7008. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abe N, Matsumoto K, Nishihara M, Nakano Y,
Shibata A, Maruyama H, Shuto S, Matsuda A, Yoshida M, Ito Y and Abe
H: Rolling circle translation of circular RNA in living human
cells. Sci Rep. 5:164352015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Legnini I, Di Timoteo G, Rossi F, Morlando
M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade
M, et al: Circ-ZNF609 is a circular RNA that can be translated and
functions in myogenesis. Mol Cell. 66:22–37.e9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen LL and Yang L: Regulation of circRNA
biogenesis. RNA Biol. 12:381–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rybak-Wolf A, Stottmeister C, Glazar P,
Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss
R, et al: Circular RNAs in the mammalian brain are highly abundant,
conserved, and dynamically expressed. Mol Cell. 58:870–885. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Enuka Y, Lauriola M, Feldman ME, Sas-Chen
A, Ulitsky I and Yarden Y: Circular RNAs are long-lived and display
only minimal early alterations in response to a growth factor.
Nucleic Acids Res. 44:1370–1383. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
You X, Vlatkovic I, Babic A, Will T,
Epstein I, Tushev G, Akbalik G, Wang M, Glock C, Quedenau C, et al:
Neural circular RNAs are derived from synaptic genes and regulated
by development and plasticity. Nat Neurosci. 18:603–610. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li X, Yang L and Chen LL: The biogenesis,
functions and challenges of circular RNAs. Mol Cell. 71:428–442.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li S and Han L: Circular RNAs as promising
biomarkers in cancer: Detection, function, and beyond. Genome Med.
11:152019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao Y and Zhao F: Computational strategies
for exploring circular RNAs. Trends Genet. 34:389–400. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Glazar P, Papavasileiou P and Rajewsky N:
circBase: A database for circular RNAs. RNA. 20:1666–1670. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dong R, Ma XK, Li GW and Yang L: CIRCpedia
v2: An updated database for comprehensive circular RNA annotation
and expression comparison. Genomics Proteomics Bioinformatics.
16:226–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dudekula DB, Panda AC, Grammatikakis I, De
S, Abdelmohsen K and Gorospe M: CircInteractome: A web tool for
exploring circular RNAs and their interacting proteins and
microRNAs. RNA Biol. 13:34–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu YC, Li JR, Sun CH, Andrews E, Chao RF,
Lin FM, Weng SL, Hsu SD, Huang CC, Cheng C, et al: CircNet: A
database of circular RNAs derived from transcriptome sequencing
data. Nucleic Acids Res. 44:D209–D215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu SM, Liu H, Huang PJ, Chang IY, Lee CC,
Yang CY, Tsai WS and Tan BC: circlncRNAnet: An integrated web-based
resource for mapping functional networks of long or circular forms
of noncoding RNAs. Gigascience. 7:1–10. 2018.
|
|
35
|
Chen X, Han P, Zhou T, Guo X, Song X and
Li Y: circRNADb: A comprehensive database for human circular RNAs
with protein-coding annotations. Sci Rep. 6:349852016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fan C, Lei X, Fang Z, Jiang Q and Wu FX:
CircR2Disease: A manually curated database for experimentally
supported circular RNAs associated with various diseases. Database
(Oxford). Jan 1–2018.(Epub ahead of print). doi:
10.1093/database/bay044. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xia S, Feng J, Lei L, Hu J, Xia L, Wang J,
Xiang Y, Liu L, Zhong S, Han L and He C: Comprehensive
characterization of tissue-specific circular RNAs in the human and
mouse genomes. Brief Bioinform. 18:984–992. 2017.PubMed/NCBI
|
|
38
|
Xia S, Feng J, Chen K, Ma Y, Gong J, Cai
F, Jin Y, Gao Y, Xia L, Chang H, et al: CSCD: A database for
cancer-specific circular RNAs. Nucleic Acids Res. 46:D925–D929.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shen B, Wang Z, Li Z, Song H and Ding X:
Circular RNAs: An emerging landscape in tumor metastasis. Am J
Cancer Res. 9:630–643. 2019.PubMed/NCBI
|
|
40
|
Maia J, Caja S, Strano Moraes MC, Couto N
and Costa-Silva B: Exosome-based cell-cell communication in the
tumor microenvironment. Front Cell Dev Biol. 6:182018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J,
Chen D, Gu J, He X and Huang S: Circular RNA is enriched and stable
in exosomes: A promising biomarker for cancer diagnosis. Cell Res.
25:981–984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li J, Li Z, Jiang P, Peng M, Zhang X, Chen
K, Liu H, Bi H, Liu X and Li X: Circular RNA IARS (circ-IARS)
secreted by pancreatic cancer cells and located within exosomes
regulates endothelial monolayer permeability to promote tumor
metastasis. J Exp Clin Cancer Res. 37:1772018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang H, Deng T, Ge S, Liu Y, Bai M, Zhu
K, Fan Q, Li J, Ning T, Tian F, et al: Exosome circRNA secreted
from adipocytes promotes the growth of hepatocellular carcinoma by
targeting deubiquitination-related USP7. Oncogene. 38:2844–2859.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fanale D, Taverna S, Russo A and Bazan V:
Circular RNA in exosomes. Adv Exp Med Biol. 1087:109–117. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu H, Gong Z, Shen Y, Fang Y and Zhong S:
Circular RNA expression in extracellular vesicles isolated from
serum of patients with endometrial cancer. Epigenomics. 10:187–197.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huang ZK, Yao FY, Xu JQ, Deng Z, Su RG,
Peng YP, Luo Q and Li JM: Microarray expression profile of circular
RNAs in peripheral blood mononuclear cells from active tuberculosis
patients. Cell Physiol Biochem. 45:1230–1240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bahn JH, Zhang Q, Li F, Chan TM, Lin X,
Yong K, Wong DT and Xiao X: The landscape of MicroRNA,
Piwi-interacting RNA and circular RNA in human saliva. Clin Chem.
61:221–230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hu J, Han Q, Gu Y, Ma J, Mcgrath M, Qiao
F, Chen B, Song C and Ge Z: Circular RNA PVT1 expression and its
roles in acute lymphoblastic leukemia. Epigenomics. 10:723–732.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen J, Li Y, Zheng Q, Bao C, He J, Chen
B, Lyu D, Zheng B, Xu Y, Long Z, et al: Circular RNA profile
identifies circPVT1 as a proliferative factor and prognostic marker
in gastric cancer. Cancer Lett. 388:208–219. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Galasso M, Costantino G, Pasquali L,
Minotti L, Baldassari F, Corra F, Agnoletto C and Volinia S:
Profiling of the predicted circular RNAs in ductal in situ and
invasive breast cancer: A pilot study. Int J Genomics.
2016:45038402016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Su H, Lin F, Deng X, Shen L, Fang Y, Fei
Z, Zhao L, Zhang X, Pan H, Xie D, et al: Profiling and
bioinformatics analyses reveal differential circular RNA expression
in radioresistant esophageal cancer cells. J Transl Med.
14:2252016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Szymczyk A, Macheta A and Podhorecka M:
Abnormal microRNA expression in the course of hematological
malignancies. Cancer Manag Res. 10:4267–4277. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hassan S and Smith M: Acute myeloid
leukaemia. Hematology. 19:493–494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Estey E and Dohner H: Acute myeloid
leukaemia. Lancet. 368:1894–1907. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Short NJ, Rytting ME and Cortes JE: Acute
myeloid leukaemia. Lancet. 392:593–606. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dolnik A, Engelmann JC,
Scharfenberger-Schmeer M, Mauch J, Kelkenberg-Schade S, Haldemann
B, Fries T, Krönke J, Kühn MW, Paschka P, et al: Commonly altered
genomic regions in acute myeloid leukemia are enriched for somatic
mutations involved in chromatin remodeling and splicing. Blood.
120:e83–e92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wu S, Du Y, Beckford J and Alachkar H:
Upregulation of the EMT marker vimentin is associated with poor
clinical outcome in acute myeloid leukemia. J Transl Med.
16:1702018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yi YY, Yi J, Zhu X, Zhang J, Zhou J, Tang
X, Lin J, Wang P and Deng ZQ: Circular RNA of vimentin expression
as a valuable predictor for acute myeloid leukemia development and
prognosis. J Cell Physiol. 234:3711–3719. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Storlazzi CT, Fioretos T, Surace C, Lonoce
A, Mastrorilli A, Strombeck B, D'Addabbo P, Iacovelli F, Minervini
C, Aventin A, et al: MYC-containing double min in hematologic
malignancies: Evidence in favor of the episome model and exclusion
of MYC as the target gene. Hum Mol Genet. 15:933–942. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chinen Y, Sakamoto N, Nagoshi H, Taki T,
Maegawa S, Tatekawa S, Tsukamoto T, Mizutani S, Shimura Y,
Yamamoto-Sugitani M, et al: 8q24 amplified segments involve novel
fusion genes between NSMCE2 and long noncoding RNAs in acute
myelogenous leukemia. J Hematol Oncol. 7:682014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Qin S, Zhao Y, Lim G, Lin H and Zhang X
and Zhang X: Circular RNA PVT1 acts as a competing endogenous RNA
for miR-497 in promoting non-small cell lung cancer progression.
Biomed Pharmacother. 111:244–250. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kun-Peng Z, Xiao-Long M and Chun-Lin Z:
Overexpressed circPVT1, a potential new circular RNA biomarker,
contributes to doxorubicin and cisplatin resistance of osteosarcoma
cells by regulating ABCB1. Int J Biol Sci. 14:321–330. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Verduci L, Ferraiuolo M, Sacconi A, Ganci
F, Vitale J, Colombo T, Paci P, Strano S, Macino G, Rajewsky N and
Blandino G: The oncogenic role of circPVT1 in head and neck
squamous cell carcinoma is mediated through the mutant p53/YAP/TEAD
transcription-competent complex. Genome Biol. 18:2372017.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Panda AC, Grammatikakis I, Kim KM, De S,
Martindale JL, Munk R, Yang X, Abdelmohsen K and Gorospe M:
Identification of senescence-associated circular RNAs (SAC-RNAs)
reveals senescence suppressor CircPVT1. Nucleic Acids Res.
45:4021–4035. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen LL: The biogenesis and emerging roles
of circular RNAs. Nat Rev Mol Cell Biol. 17:205–211. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li W, Zhong C, Jiao J, Li P, Cui B, Ji C
and Ma D: Characterization of hsa_circ_0004277 as a new biomarker
for acute myeloid leukemia via circular RNA Profile and
bioinformatics analysis. Int J Mol Sci. 18(pii): E5972017.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hirsch S, Blätte TJ, Grasedieck S,
Cocciardi S, Rouhi A, Jongen-Lavrencic M, Paschka P, Krönke J,
Gaidzik VI, Döhner H, et al: Circular RNAs of the nucleophosmin
(NPM1) gene in acute myeloid leukemia. Haematologica.
102:2039–2047. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Okamoto M, Hirai H, Taniguchi K, Shimura
K, Inaba T, Shimazaki C, Taniwaki M and Imanishi J: Toll-like
receptors (TLRs) are expressed by myeloid leukaemia cell lines, but
fail to trigger differentiation in response to the respective TLR
ligands. Br J Haematol. 147:585–587. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Nagai Y, Garrett KP, Ohta S, Bahrun U,
Kouro T, Akira S, Takatsu K and Kincade PW: Toll-like receptors on
hematopoietic progenitor cells stimulate innate immune system
replenishment. Immunity. 24:801–812. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wu DM, Wen X, Han XR, Wang S, Wang YJ,
Shen M, Fan SH, Zhang ZF, Shan Q, Li MQ, et al: Role of circular
RNA DLEU2 in human acute myeloid leukemia. Mol Cell Biol. 38(pii):
e00259–18. 2018.PubMed/NCBI
|
|
71
|
Chen H, Liu T, Liu J, Feng Y, Wang B, Wang
J, Bai J, Zhao W, Shen Y, Wang X, et al: Circ-ANAPC7 is upregulated
in acute myeloid leukemia and appears to target the MiR-181 family.
Cell Physiol Biochem. 47:1998–2007. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Shang J, Chen WM, Wang ZH, Wei TN, Chen ZZ
and Wu WB: CircPAN3 mediates drug resistance in acute myeloid
leukemia through the miR-153-5p/miR-183-5p-XIAP axis. Exp Hematol.
70:42–54.e3. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
de Thé H and Chen Z: Acute promyelocytic
leukaemia: Novel insights into the mechanisms of cure. Nat Rev
Cancer. 10:775–783. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li S, Ma Y, Tan Y, Ma X, Zhao M, Chen B,
Zhang R, Chen Z and Wang K: Profiling and functional analysis of
circular RNAs in acute promyelocytic leukemia and their dynamic
regulation during all-trans retinoic acid treatment. Cell Death
Dis. 9:6512018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Huang R, Zhang Y, Han B, Bai Y, Zhou R,
Gan G, Chao J, Hu G and Yao H: Circular RNA HIPK2 regulates
astrocyte activation via cooperation of autophagy and ER stress by
targeting MIR124-2HG. Autophagy. 13:1722–1741. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hackanson B, Bennett KL, Brena RM, Jiang
J, Claus R, Chen SS, Blagitko-Dorfs N, Maharry K, Whitman SP,
Schmittgen TD, et al: Epigenetic modification of CCAAT/enhancer
binding protein alpha expression in acute myeloid leukemia. Cancer
Res. 68:3142–3151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kaleem B, Shahab S, Ahmed N and Shamsi TS:
Chronic myeloid leukemia-prognostic value of mutations. Asian Pac J
Cancer Prev. 16:7415–7423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhou T, Medeiros LJ and Hu S: Chronic
myeloid leukemia: Beyond BCR-ABL1. Curr Hematol Malig Rep.
13:435–445. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Okcanoğlu TB and Gündüz C: Circular RNAs
in leukemia. Biomed Rep. 1–5. 2019.
|
|
80
|
Guarnerio J, Bezzi M, Jeong JC, Paffenholz
SV, Berry K, Naldini MM, Lo-Coco F, Tay Y, Beck AH and Pandolfi PP:
Oncogenic role of fusion-circRNAs derived from cancer-associated
chromosomal translocations. Cell. 165:289–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Deininger MW, Goldman JM and Melo JV: The
molecular biology of chronic myeloid leukemia. Blood. 96:3343–3356.
2000.PubMed/NCBI
|
|
82
|
McGahon A, Bissonnette R, Schmitt M,
Cotter KM, Green DR and Cotter TG: BCR-ABL maintains resistance of
chronic myelogenous leukemia cells to apoptotic cell death. Blood.
83:1179–1187. 1994.PubMed/NCBI
|
|
83
|
Mauro MJ: Defining and managing imatinib
resistance. Hematol Am Soc Hematol Educ Program. 219–225. 2006.
View Article : Google Scholar
|
|
84
|
Pan Y, Lou J, Wang H, An N, Chen H, Zhang
Q and Du X: CircBA9.3 supports the survival of leukaemic cells by
up-regulating c-ABL1 or BCR-ABL1 protein levels. Blood cells Mol
Dis. 73:38–44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Liu J, Kong F, Lou S, Yang D and Gu L:
Global identification of circular RNAs in chronic myeloid leukemia
reveals hsa_circ_0080145 regulates cell proliferation by sponging
miR-29b. Biochem Biophy Res Commun. 504:660–665. 2018. View Article : Google Scholar
|
|
86
|
Musolino C, Oteri G, Allegra A, Mania M,
D'Ascola A, Avenoso A, Innao V, Allegra AG and Campo S: Altered
microRNA expression profile in the peripheral lymphoid compartment
of multiple myeloma patients with bisphosphonate-induced
osteonecrosis of the jaw. Ann Hematol. 97:1259–1269. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Du XJ, Lu JM and Sha Y: MiR-181a inhibits
vascular inflammation induced by ox-LDL via targeting TLR4 in human
macrophages. J Cell Physiol. 233:6996–7003. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Litwińska Z and Machaliński B: miRNAs in
chronic myeloid leukemia: Small molecules, essential function. Leuk
Lymphoma. 58:1297–1305. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zenz T, Mertens D, Kuppers R, Döhner H and
Stilgenbauer S: From pathogenesis to treatment of chronic
lymphocytic leukaemia. Nat Rev Cancer. 10:37–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Rai KR and Jain P: Chronic lymphocytic
leukemia (CLL)-then and now. Am J Hematol. 91:330–340. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Xia L, Wu L, Bao J, Li Q, Chen X, Xia H
and Xia R: Circular RNA circ-CBFB promotes proliferation and
inhibits apoptosis in chronic lymphocytic leukemia through
regulating miR-607/FZD3/Wnt/β-catenin pathway. Biochem Biophys Res
Commun. 503:385–390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Franiak-Pietryga I, Maciejewski H, Ziemba
B, Appelhans D, Voit B, Robak T, Jander M, Treliński J, Bryszewska
M and Borowiec M: Blockage of Wnt/β-catenin signaling by
nanoparticles reduces survival and proliferation of CLL cells in
vitro-preliminary study. Macromol Biosci. 172017.doi:
10.1002/mabi.201700130.
|
|
93
|
Dahl M, Daugaard I, Andersen MS, Hansen
TB, Grønbæk K, Kjems J and Kristensen LS: Enzyme-free digital
counting of endogenous circular RNA molecules in B-cell
malignancies. Lab Invest. 98:1657–669. 2018. View Article : Google Scholar : PubMed/NCBI
|