Introduction
Pituitary adenoma is one of the most common
neuroendocrine tumors (1). The
incidence rate is 3.47/100,000 in the United States in 2015, which
corresponds to 10–25% of tumors of the central nervous system
(1,2). Although most pituitary adenomas are
histologically benign, 34–60% of the lesions that remain destroy
the dura, periosteum and even the bone, exhibiting malignant and
invasive characteristics (3). The
invasion and migration features of pituitary adenomas result in
difficulties in surgical treatment and postoperative recurrence
(4). The diagnosis of invasive
pituitary adenoma (IPA) is based on comprehensive imaging,
intraoperative investigation and postoperative pathology. At
present, there is a lack of specific molecular biological
indicators, which is a difficult issue to overcome in clinical
treatment (5).
A previous study demonstrated that CCNB1 protein,
encoded by the CCNB1 gene, belongs to the cyclin superfamily and is
expressed in almost all tissues in humans (6). CCNB1 can form a complex with p34, also
known as cdc2, forming the maturation-promoting factor, and is
necessary for proper control of the G2/M transition
phase of the cell cycle (7). In
normal cells, CCNB1 is primarily expressed in the late S stage,
increases significantly in the G2 phase, and reaches its
peak value at M stage (8,9). CCNB1 is highly expressed in numerous
different types of human tumors, including breast cancer, cervical
cancer, lung cancer, esophageal squamous cell carcinoma and
melanoma (10–15), indicating its potential role in
cancer transformation and progression. In line with previous
findings, CCNB1 identified in the integrated analysis was closely
related to the tumorigenesis of pituitary adenomas (16). Further research found that
upregulation of cyclin B1 has potential roles in the invasiveness
of pituitary adenomas (17).
However, the specific molecular mechanism underlying CCNB1 function
remains unclear.
EMT is supposed to be responsible for increased
invasion and metastasis in epithelial cancer cells (18). The activation of EMT facilitates
cancer invasion and metastasis, and accelerates cancer progression
(19,20). It has been reported that CCNB1 may be
involved in the processes of EMT and metastasis (21). Therefore, there may be a certain
association between the cyclin family and EMT activation in
pituitary tumor cell transformation and tumor progression.
Resveratrol (RES) is a common drug used to inhibit
the proliferation of a number of cancers. In addition, RES can
affect cell cycle and apoptosis by interacting with various key
genes (22–24). RES may inhibit the expression of
CCNB1 and CDK1 in tumor cells, and can induce cells to stop at the
G2/M stage (25).
Therefore, due to its potential in suppressing cell proliferation,
RES can be used as a cell cycle inhibitor.
The aim of the present study was to further
investigate the role and potential regulatory mechanism of CCNB1 in
the proliferation and apoptosis of pituitary adenomas. In the
present study, the expression level of CCNB1 was found to influence
the EMT process and cell function was found to be affected
following lentiviral-mediated CCNB1 knockdown in GH3 and MMQ cell
lines. RES was found to act as an inhibitor of proliferation, and
RES treatment was able to affect the expression levels of CCNB1 in
pituitary tumor cells.
Materials and methods
Patients and specimens
A total of 24 samples were included in the present
study. Twenty-two pituitary adenoma specimens were obtained during
neurological surgery between September 2016 and December 2017 from
patients at the Beijing Tiantan Hospital, Capital Medical
University (Beijing, China). In addition, two normal pituitary
glands that were donated by two subjects (deceased for other
causes) were used as control. A total of 10 women and 12 men with
pituitary adenoma were included in the present study. The mean age
of the patients was 40.2 years (range, 15–74 years) at the time of
surgery. One of the normal pituitary glands was from a 49-year-old
female and the other was from a 35-year-old male. Patients were
divided into invasive pituitary adenoma (IPA) and non-invasive
pituitary adenoma (NIPA) groups according to Hardy-Wilson (26,27) and
Knosp classification (28).
According to the Knosp classification, 0–1 grade pituitary adenoma
belongs to the NIPA groups and 2–4 grade pituitary adenoma belongs
to IPA groups. In the Hardy-Wilson classification, pituitary
adenoma of grade I–II and stage A-B belongs to the NIPA groups,
grade III–IV and stage C-E belongs to the IPA groups. Based on the
preoperative endocrinological tests and postoperative pathological
results, which were derived from routine pathological examination
of pituitary adenomas by pathologist Professor Gui-Lin Li
(Department of Pathology, Beijing Tiantan Hospital), all the
specimens were considered as non-functional adenomas.
First, the expression level of CCNB1 was
investigated in 14 pituitary adenoma tissue specimens and a normal
pituitary gland. This group was studied to detect the expression
level of CCNB1 in normal pituitary and pituitary adenoma. In
addition, four NIPA and four IPA tissue specimens with a normal
pituitary were examined, which were not included in the 14
aforementioned samples. This group was examined to detect the
expression of CCNB1 in IPA and NIPA samples. The pituitary adenoma
samples were quickly frozen in liquid nitrogen within 30 min of
surgery. All patients enrolled were followed up for 1–2 years. The
present study was approved by the Beijing Tiantan Hospital Ethics
Committee, and written informed consent was obtained from every
participant.
Cell culture
Rat pituitary tumor cell lines GH3 and MMQ
(Institute of Basic Medical Sciences, Chinese Academy of Sciences)
were maintained in F-12 (Gibco; Thermo Fisher Scientific, Inc.),
supplemented with 15% horse serum (HS; Beijing Solarbio Science and
Technology Co., Ltd.), 2.5% FBS (Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin under 5%
CO2, at 37°C in a humidified incubator. Cells were
regularly passaged to maintain exponential growth.
Lentiviral CCNB1 short hairpin RNA
(shRNA) recombinant vector production and transfection
Based on the gene sequence of CCNB1 in GenBank (Gene
ID, 25203; http://www.ncbi.nlm.nih.gov/gene/25203), primers of
CCNB1 shRNA (0.5 µM) and negative control (0.5 µM) were cloned into
a pGMLV-SC5 vector (Genomeditech Co., Ltd.), which carried the
green fluorescent protein (GFP) gene. The sequences used to
construct the short hairpin targeting CCNB1 (CCNB1-shRNA) and the
respective negative control are presented in Table I.
 | Table I.Primers for CCNB1-shRNA. |
Table I.
Primers for CCNB1-shRNA.
|
Oligonucleotide | Sequence
(5′-3′) |
|---|
| CCNB1-shRNA | F:
gatccGCCTGAGCCTGAACCTGTTATTTCAAGAGAATAACAGGTTCAGGCTCAGGCTTTTTTg |
|
| R:
aattcAAAAAAGCCTGAGCCTGAACCTGTTATTCTCTTGAAATAACAGGTTCAGGCTCAGGCg |
| Negative
control | F:
gatcTGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTTc |
|
| R:
aattgAAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAACa |
The 293T cells (American Type Culture Collection)
were selected for packaging and titer measurement of the
lentivirus. GH3 and MMQ cells were transferred to each well of a
6-well culture plate 24 h before transfection. When cells reached
70–80% confluence, the constructed lentiviral vector and its
auxiliary packaging original vector plasmid for control were
co-transfected into 293T cells using HG transgene reagent
(Genomeditech Co., Ltd.). The following groups were analyzed: i)
non-infected (Con); ii) negative control shRNA-infected (NC); iii)
CCNB1 shRNA-infected (shRNA). After cell culture for 5 days, the
number of fluorescent cells in the wells was observed under a
fluorescence microscope (×100 magnification) and the infection rate
(the number of fluorescent cells/by the total number of cells) was
calculated using Image Pro Plus software (v6.0; Media Cybernetics,
Inc). CCNB1-shRNA lentivirus titer was set in 5×108
TU/ml, and normal control-shRNA lentivirus titer was in
6×108 TU/ml.
Reverse transcription-quantitative PCR
(RT-qPCR) assay
Total RNA was extracted from the cell lines and
pituitary adenoma tissue samples using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), and RNA was reverse
transcribed to cDNA using the High-Capacity cDNA Reverse
Transcription Kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The reverse transcription conditions were:
25°C for 10 min, 37°C for 120 min and 85°C for 5 min. Primers were
designed using Primer software (version 5.0; Premier Biosoft
International) based on gene sequences from the Genbank database
for CCNB1 (Table II). The qPCR was
performed using PowerUp™ SYBR™ green master mix on a Pharmaceutical
Analytics QuantStudio™ 5 Real-Time PCR System (both Thermo Fisher
Scientific, Inc.). PCR conditions were as follows: Initial
denaturation at 95°C for 2 min, followed by 40 cycles of 95°C for 1
sec and 60°C for 30 sec. The PCR results were verified by the
melting curve. The data were normalized to the housekeeping gene
GAPDH and counted using the 2−∆∆Cq method (29).
 | Table II.Primers used for reverse
transcription-quantitative PCR and their respective sequences. |
Table II.
Primers used for reverse
transcription-quantitative PCR and their respective sequences.
| Gene | Sequence
(5′-3′) |
|---|
| CCNB1 | F:
CTTAGACAAATTCTGAACTAGTGTACA |
|
| R:
ATTCTTGACAACGGTGAAT |
| E-cadherin | F:
GAGTCATCAGTGTGGTCACC |
|
| R:
GGGGGCATCAGCATCAGTCA |
| N-cadherin | F:
AAGTGGGTTGAAGCGTATCACA |
|
| R:
ACACCGAGCTCAGCTACACTTG |
| p120-catenin | F:
GCCAACTCAGCAGACATATCAC |
|
| R:
CCATCAAGAACCTACAGACTCC |
| GAPDH | F:
GGCAGTGATGGCATGGACTGT |
|
| R:
CCTTCATTGACCTCAACTACA |
Protein extraction and western blot
analysis
The tissues and treated GH3 and MMQ cells were lysed
with RIPA buffer containing a 50X protease inhibitor (both Applygen
Technologies Inc.) cocktail. The concentration of protein was
determined using a BCA Protein Assay kit (Beijing Solarbio Science
& Technology, Co., Ltd.). Proteins (30 µg per lane) were
separated via SDS-PAGE (10% gel) and transferred to PVDF membranes
(EMD Millipore). The membranes were incubated with the primary
antibodies anti-CCNB1 (1:1,000; cat. no. 12231), anti-β-actin
(1:1,000; cat. no. 4970) and anti-GAPDH (1:1,000; cat. no. 5174)
(all from Cell Signaling Technology, Inc.). After washing with TBST
three times, the membrane was incubated with anti-rabbit IgG
HRP-linked antibody (1:4,000; cat. no. 7074; Cell Signaling
Technology, Inc.) for 1 h at room temperature and then washed with
TBST three times. The ECL chemiluminescence reagent (Santa Cruz
Biotechnology, Inc.) was used for detection. β-actin and GAPDH were
used as internal controls and the grey values of the protein bands
were quantified with the ImageJ software (version 1.8; National
Institutes of Health).
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was analyzed with a CCK-8 kit
(Dojindo Molecular Technologies, Inc.) according to the
manufacturer's protocol. Following treatment, GH3 and MMQ cells
were seeded and grown in 96-well plates. A solution containing 90
µl fresh F-12 medium [(Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 15% HS (Beijing Solarbio Science and Technology
Co., Ltd.), 2.5% FBS (Gibco; Thermo Fisher Scientific, Inc.)] and
10 µl CCK-8 solution were added to each sample at 24, 48, 72 and 96
h. Subsequently, cells were incubated at 37°C for 1–4 h. The
optical density value was measured using a microplate reader
(Varioskan Flash; Thermo Fisher Scientific, Inc.) at the wavelength
of 450 nm.
Flow cytometric analysis of cell cycle
distribution
To analyze cell cycle distribution, the treated
cells were digested by pancreatin and washed with ice-cold PBS,
then suspended in 1 ml ice-cold 70% ethanol. The cells were washed
with ice-cold PBS prior to staining and resuspended in 1 ml of
Vindelov's propidium iodide (PI) at room temperature for 30 min in
the dark. The DNA content of cells was analyzed using a flow
cytometer (BD Accuri™ C6; BD Biosciences). Cell cycle distribution
was analyzed as a typical DNA content histogram using BD CellQuest™
cell cycle analysis software (version 5.1; BD Biosciences).
Annexin V-FITC/PI staining
Cellular apoptosis was evaluated via Annexin V-FITC
apoptosis detection kit (Sigma-Aldrich; Merck KGaA). The treated
cells were collected by centrifugation (37°C for 5 min at 500 × g)
and suspended in 500 µl 10X binding buffer (Sigma-Aldrich; Merck
KGaA), and 5 µl Annexin V-FITC and 5 µl PI were added. The cells
were incubated at room temperature for 30 min in the dark. The
cells stained with Annexin V-FITC and PI were detected using a flow
cytometer (BD FACS Calibur; BD Biosciences). Flow cytometry of
Annexin V-FITC/PI apoptosis assay was determined to quantify
necrotic, early apoptotic, late apoptotic and viable cells. The
cytometric data were analysed using WinMDI version 2.9 (The Scripps
Research Institute).
EMT-associated marker assay
In order to evaluate the activation of the EMT by
CCNB1, the associated markers were analyzed via RT-qPCR. The
epithelial cell markers tested were E-cadherin and p120-catenin,
and the mesothelial cell marker was N-cadherin. The treated GH3 and
MMQ cells were seeded at a density of 2×104 cells/well
in 6-well plates and treated with or without shRNA or inhibitor for
24, 48 and 72 h. The primers used are listed in Table II. The RT-qPCR was performed
following the aforementioned protocol.
RES inhibition assay
For the in vitro CCNB1 inhibitor experiments,
RES (Beijing Solarbio Science & Technology, Co., Ltd.) was
dissolved in dimethyl sulfoxide (DMSO; Beijing Solarbio Science
& Technology, Co., Ltd.) and added to the F-12 culture medium,
based on the research by Joe et al (30). Briefly, cells were treated with 0.2%
DMSO (negative control) and RES inhibitor (100 and 300 µM) at 37°C
in a humidified incubator with 5% CO2. After 48 h of
treatment, both adherent and floating cells were harvested for
further examinations.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism (version 7.0; GraphPad Software, Inc.). All quantitative data
are presented as the mean ± standard deviation. Differences between
groups were determined using one-way ANOVA test with Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference. All experiments were repeated three
times.
Results
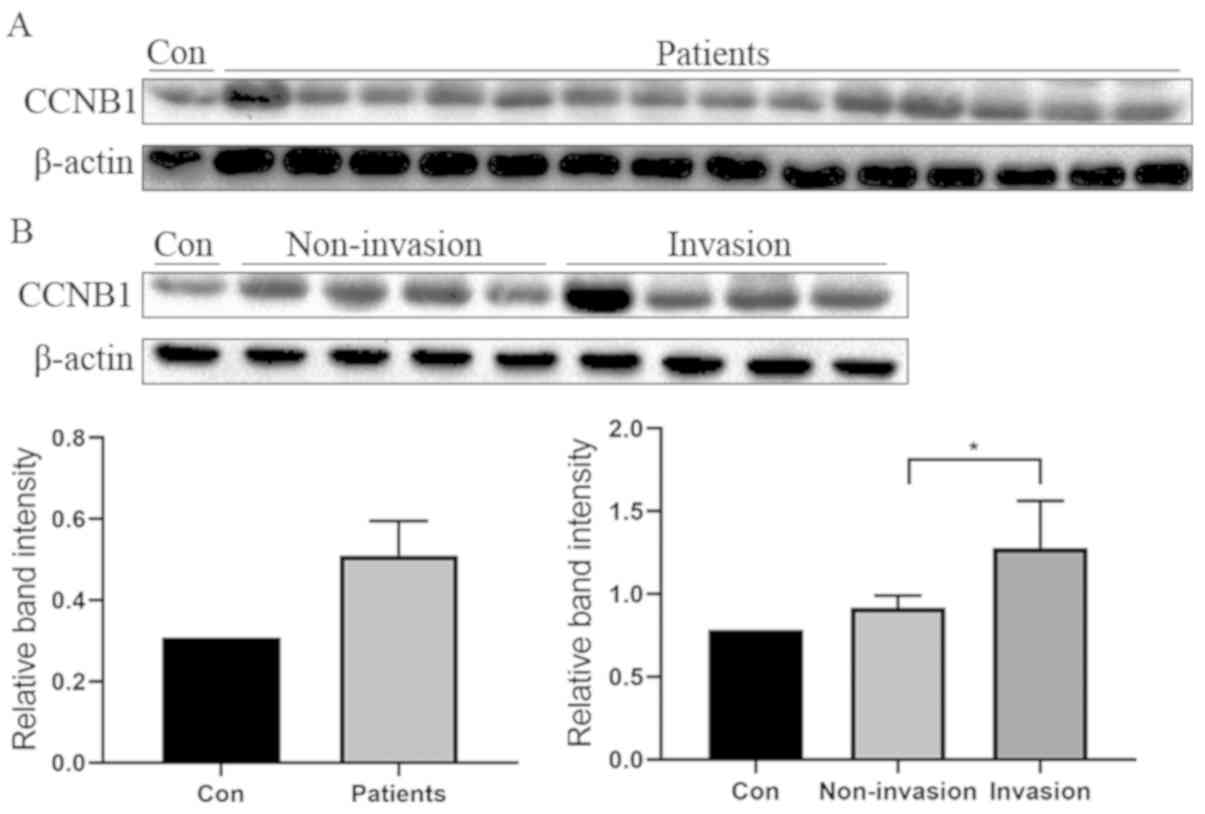
CCNB1 expression is upregulated in
pituitary adenomas and is higher in the invasive group
The present results revealed that the expression of
CCNB1 was upregulated in tumor samples compared with the normal
control (Fig. 1A). In addition, the
experimental results of another group of tumor specimens proved
that the expression of CCNB1 was markedly higher in the invasive
group compared with the non-invasive group (Fig. 1B).
sh-CCNB1 downregulates the expression
of CCNB1
Furthermore, it was demonstrated that CCNB1
expression was affected by the lentiviral-mediated shRNA infection.
The infection effect was observed using fluorescent imaging of
GFP-positive GH3 and MMQ cells following lentivirus transfection
(Fig. 2A-F). The cells were
transfected with the shRNA and after 72 h, the transfection rates
of shRNA-CCNB1 group and shRNA-NC group were both ~80%. The
interference effect was validated via RT-qPCR and western blotting.
These results revealed that CCNB1 was significantly decreased both
at the mRNA and protein levels compared with the control and NC
groups (Fig. 2G and H).
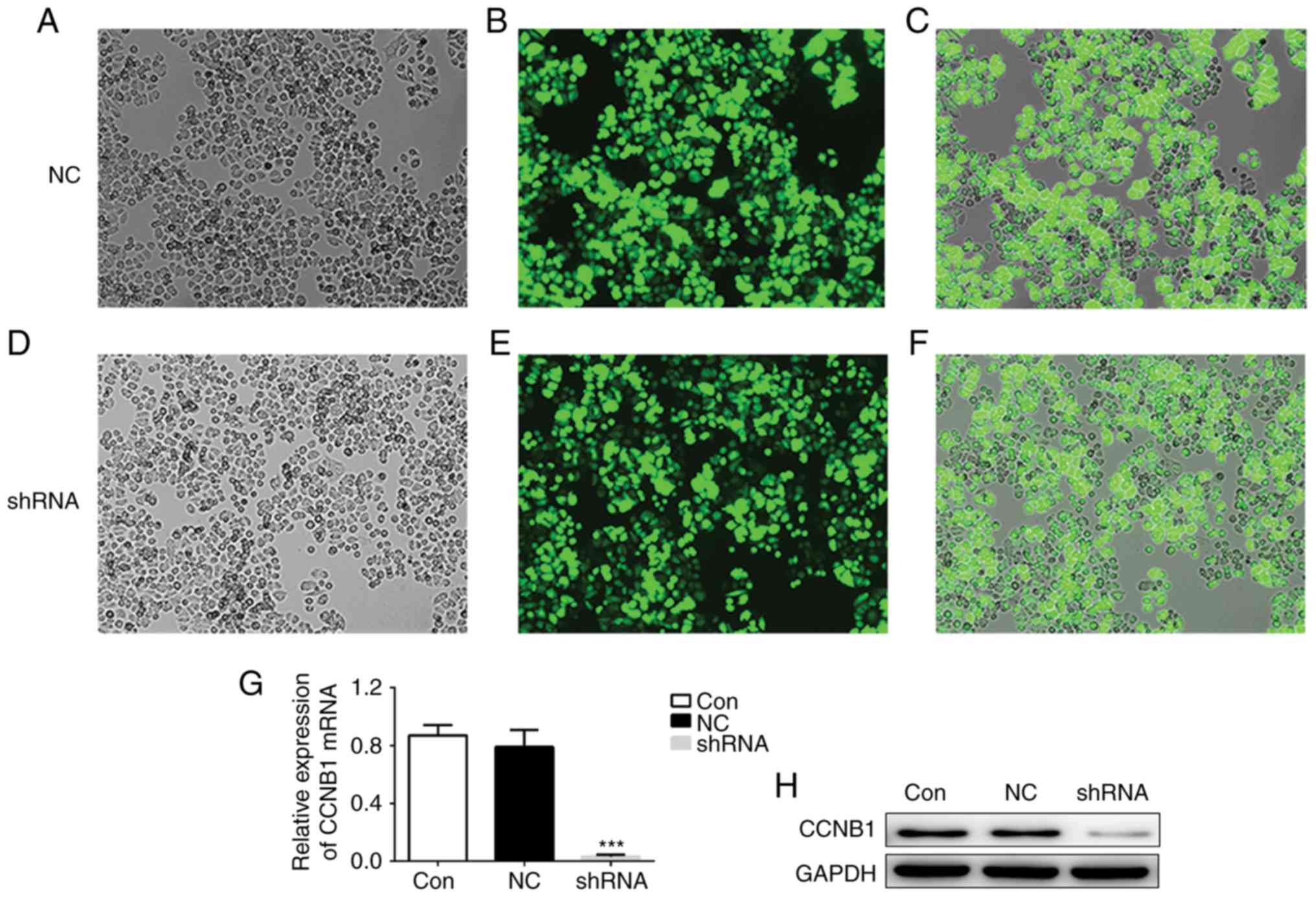 | Figure 2.Effect of shRNA on CCNB1 expression,
proliferation, cell cycle, apoptosis and EMT markers. (A)
Brightfield, (B) fluorescent and (C) merged images of the NC group
after 72 h. (D) Brightfield, (E) fluorescent and (F) merged images
of the CCNB1 shRNA group after 72 h. (G) Quantification of mRNA
expression levels of CCNB1 following lentiviral transfection by
RT-qPCR. (H) Western blot analysis of the protein expression levels
of CCNB1 in the CCNB1 shRNA group. (I) Proliferation of lentiviral
transfected cells. (J) Percentage of cells in G1,
G2/M or S phase was assessed after lentivirus
transfection. (K) Annexin V-FITC/PI double staining flow cytometry
was performed to evaluate apoptosis. (L) Quantification of mRNA
expression levels via RT-qPCR in the shRNA group. **P<0.01,
***P<0.001 vs. NC. CCNB1, cyclin B1; RT-qPCR, reverse
transcription-quantitative PCR; Con, blank control group; NC,
negative control group; shRNA, short hairpin RNA lentivirus
infection group; N-cad, N-cadherin; E-cad, E-cadherin; p120,
p120-catenin; PI, propidium iodide. |
Knockdown of CCNB1 suppresses the
proliferation of GH3 and MMQ cells
In order to investigate the function of CCNB1 in the
growth of pituitary adenoma, loss-of-function studies were
performed with a CCK-8 assay for 3 days following
lentiviral-mediated CCNB1 knockdown. GH3 and MMQ cells growth were
inhibited in the shRNA-CCNB1 group in comparison with the shRNA-NC
group (Fig. 2I). The present results
indicated that downregulation of CCNB1 could inhibit the
proliferation of pituitary adenoma cells.
Downregulation of CCNB1 induces cell
cycle arrest and promotes apoptosis
Annexin V-FITC/PI flow cytometry was performed to
investigate the rate of apoptosis. The results of the flow
cytometry analysis revealed that the percentage of cells in the
G0/G1 phase in the shRNA-CCNB1 group was
significantly decreased, while a larger percentage of these cells
were arrested at the G2/M phase compared with the NC
group (Fig. 2J). In addition,
compared with the NC group, the rate of apoptosis in the
shRNA-CCNB1 group was significantly increased (0.08 vs. 54.81%,
respectively; Fig. 2K). The present
results suggested that shRNA-CCNB1-mediated inhibition of cell
proliferation was partially due to cell apoptosis and abnormal cell
cycle progression.
Downregulation of CCNB1 inhibits the
EMT
The present study further investigated whether
changes in CCNB1 influenced the EMT using RT-qPCR. The results
demonstrated that the mRNA expression of the epithelial cell
markers E-cadherin and p120-catenin were significantly increased,
and in contrast, the mesothelial cell marker N-cadherin was
significantly decreased between the sh-CCNB1 group and the normal
control (Fig. 2L). These results
suggested that CCNB1 is involved in the process of EMT in pituitary
adenoma cells.
RES inhibits the expression of
CCNB1
The effect of RES on CCNB1 was analyzed using
RT-qPCR and western blot analysis. The present results revealed
that CCNB1 was significantly decreased following treatment with RES
at different concentrations (100 and 300 µM), in line with the
RT-qPCR results (Fig. 3A and B,
respectively).
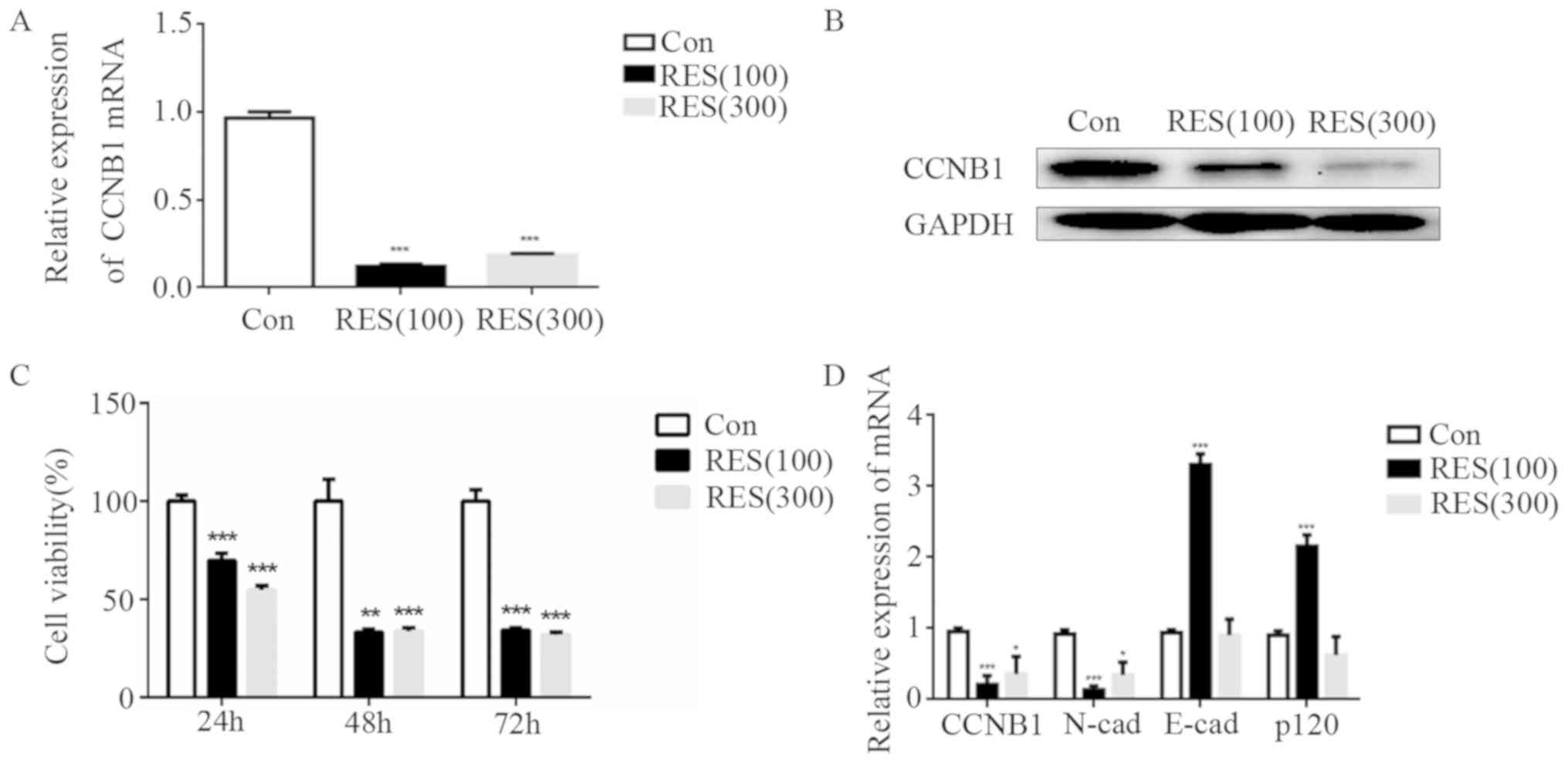 | Figure 3.Effect of RES on CCNB1 expression,
cell proliferation and EMT markers. (A) Quantification of mRNA
expression levels of CCNB1 in the RES treatment group via RT-qPCR.
(B) Western blotting images of the protein expression levels of
CCNB1 in the RES treatment group. (C) Downregulation of CCNB1 by
RES suppressed the cell proliferation following RES treatment. (D)
Expression levels of mRNA of E-cad and p120 were increased, and the
N-cad was decreased when comparing the RES group and control group
using RT-qPCR. *P<0.05; **P<0.01 and ***P<0.001 vs. Con.
RES, resveratrol; CCNB1, cyclin B1; RT-qPCR, reverse
transcription-quantitative PCR; Con, blank control group; RES(100),
100 µM RES group; RES(300), 300 µM RES group; p120, p120-catenin;
E-cad, E-cadherin; N-cad, N-cadherin. |
RES suppresses cell proliferation and
inhibits EMT
Downregulation of CCNB1 mediated by RES treatment
significantly suppressed the growth rate of GH3 cells compared with
the control group (Fig. 3C). The
mRNA expression levels of the epithelial cell markers E-cadherin
(P<0.001) and p120-catenin (P<0.001) were increased, and the
mesothelial cell marker N-cadherin (P<0.001) was decreased in
the 100 µM RES group. However, E-cadherin and p120-catenin
expression is similar between the 300 µM RES group and the control
group (Fig. 3D).
Discussion
CCNB1, encoded by the CCNB1 gene, belongs to the
cyclin superfamily (6). In a
previous study, it was revealed that CCNB1 exhibited abnormal
expression levels in tumor tissues through a meta analysis
(16). Other previous studies have
demonstrated that CCNB1 was closely associated with tumor
progression and was highly expressed in tumor tissues and cells
(31). Song et al (21) revealed that the high expression
levels of CCNB1 promoted the proliferation of esophageal cells,
enhanced cell migration, and led to a significant increase in the
invasiveness of esophageal squamous cell carcinoma cells (21). In addition, abnormal expression
levels of CCNB1 were demonstrated to be associated with tumor
invasion, metastasis and prognosis in other studies (32,33).
Thus, CCNB1 was indicated as a pivotal target gene to promote the
malignant phenotype and proliferation of tumors (34,35).
However, the molecular mechanism underlying CCNB1-mediated
promotion for tumor cell invasion remains unknown. Therefore, the
present study aimed to examine the role of CCNB1 in the development
of pituitary adenomas. Using clinical samples, it was revealed that
the expression levels of CCNB1 increased in tumor samples
exhibiting increasing tumor invasion potential, and the levels of
CCNB1 were significantly higher compared with the non-invasive
group, which was consistent with a previous study (17). The present results indicated that the
high expression levels of CCNB1 were associated with the invasion
degree of pituitary adenoma. In addition, knockdown of CCNB1 using
a lentivirus-packaged shRNA designed in the present study could
arrest the proliferation of pituitary adenoma cells at the
G2/M phase, and also induce cell apoptosis. The present
results suggested that CCNB1 was associated with pituitary adenoma
cell proliferation and mediated cell cycle.
EMT is a biological process characterized by a loss
of polarization and cell-cell adhesion, and a gain of fibroblastoid
phenotype and increased cell motility (36,37).
Recent studies have demonstrated that EMT plays an important role
in tumor invasiveness. Previous research has reported that cyclin
A2 deficiency promoted cell invasion in fibroblasts, and they also
revealed that silencing of cyclin A2 in normal breast cancer
epithelial cells significantly promoted EMT (38,39).
Wang et al (19) reported
that ADAM12 can induce the invasion, migration and EMT of pituitary
tumor cells through the EGFR/ERK pathway. The EMT process was
regulated by key EMT mediators and resulted in a switch from
E-cadherin to N-cadherin (40).
p120-catenin is a multifunctional protein that is bound to
E-cadherin on the cell membrane, and its dissociation leads to
E-cadherin degradation (41). Jia
et al (42) examined the
expression levels of EMT biomarkers in 95 human pituitary tumors
and revealed that E-cadherin and N-cadherin were valuable
biomarkers in assessing the clinical course of pituitary adenomas.
In the present study, it was revealed that knockdown of CCNB1
significantly decreased the expression levels of the mesothelial
cell marker N-cadherin, whereas the expression of epithelial cell
markers E-cadherin and p120-catenin were significantly increased
following lentivirus transfection. The present results demonstrated
that CCNB1 downregulation inhibited EMT in pituitary adenomas.
Therefore, the present results suggested that CCNB1 may activate
EMT.
A previous study has demonstrated that RES inhibits
cell proliferation and decreased prolactin level via estrogen
receptors (43). A number of studies
have demonstrated that RES oligomers can be used for the prevention
and treatment of different types of cancer, for example, lung
cancer, colon cancer and hepatoma (44–46). In
the present study, the expression levels of CCNB1 were
significantly decreased with the addition of RES at different
concentrations (100 and 300 µM). Furthermore, downregulation of
CCNB1 mediated by RES significantly suppressed the growth and
proliferation of GH3 and MMQ cells. These results demonstrated that
RES could downregulate the expression levels of CCNB1, affecting
the cell cycle. These results were consistent with the lentiviral
transfection that inhibited cell proliferation. Therefore, RES
could be used as an inhibitor to suppress the proliferation of
pituitary tumor cells. The mRNA expression levels of the epithelial
cell markers E-cadherin and p120-catenin were increased whereas the
mesothelial cell marker N-cadherin was decreased in the 100 µM RES
group. In addition, E-cadherin and p120-catenin expression were
similar between the 300 µM RES group and the control group. The
results indicate that 100 µM RES can affect the EMT process by
reducing the expression of CCNB1. However, we hypothesize that
excessive RES may directly affect the EMT process, in addition to
inhibiting the expression of CCNB1. In future research, the
specific mechanisms by which resveratrol affects the EMT process of
pituitary tumors will be explored.
In summary, the present study suggested that
suppressing the CCNB1 gene may regulate the proliferation and
apoptosis of pituitary tumor cells and activate EMT. The present
results may improve the current understanding of the biological
mechanisms of CCNB1 in the development and progression of pituitary
adenoma. However, further studies are required in order to confirm
these results and to investigate the potential pathway involved in
the development of pituitary adenomas. It was also observed that
RES inhibited the expression levels of CCNB1 in pituitary tumor
cells, affecting cell proliferation and EMT. The present results
suggested that RES may suppress the expression level of CCNB1 and
may represent a novel clinical treatment for pituitary
adenomas.
Acknowledgements
The authors would like to thank Ms Hong-Yun Wang
(Department of Cell and Biology, Beijing Neurosurgical Institute)
for the collection of the pituitary adenoma samples and Professor
Gui-Lin Li for the pathology results (Department of Pathology,
Beijing Tiantan Hospital).
Funding
The present study was funded by the Beijing Natural
Science Foundation (grant no. 7162034).
Availability of data and materials
All data generated or analyzed during the present
study are included within this published article.
Authors' contributions
PZ designed and supervised the project. BL and HBZ
performed the majority of the experiments and drafted the
manuscript. GDS and JHC performed the experiments, acquired the
data and wrote the article. CZL and YZZ designed the experiments
and revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Beijing Tiantan Hospital, Capital Medical University
(Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McNeill KA: Epidemiology of brain tumors.
Neurol Clin. 34:981–998. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scoazec JY, Couvelard A and Reseau T:
Classification of pancreatic neuroendocrine tumours: Changes made
in the 2017 WHO classification of tumours of endocrine organs and
perspectives for the future. Ann Pathol. 37:444–456. 2017.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raverot G, Burman P, McCormack A, Heaney
A, Petersenn S, Popovic V, Trouillas J and Dekkers OM; European
Society of Endocrinology, : European society of endocrinology
clinical practice guidelines for the management of aggressive
pituitary tumours and carcinomas. Eur J Endocrinol. 178:G1–G24.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lenders N and McCormack A: Malignant
transformation in non-functioning pituitary adenomas (pituitary
carcinoma). Pituitary. 21:217–229. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lopes MBS: The 2017 World Health
Organization classification of tumors of the pituitary gland: A
summary. Acta Neuropathol. 134:521–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyazaki T and Arai S: Two distinct
controls of mitotic cdk1/cyclin B1 activity requisite for cell
growth prior to cell division. Cell Cycle. 6:1419–1425. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takizawa CG and Morgan DO: Control of
mitosis by changes in the subcellular location of cyclin-B1-Cdk1
and Cdc25C. Curr Opin Cell Biol. 12:658–665. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strauss B, Harrison A, Coelho PA, Yata K,
Zernicka-Goetz M and Pines J: Cyclin B1 is essential for mitosis in
mouse embryos, and its nuclear export sets the time for mitosis. J
Cell Biol. 217:179–193. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mussnich P, Raverot G, Jaffrain-Rea ML,
Fraggetta F, Wierinckx A, Trouillas J, Fusco A and D'Angelo D:
Downregulation of miR-410 targeting the cyclin B1 gene plays a role
in pituitary gonadotroph tumors. Cell Cycle. 14:2590–2597. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pandey JP, Kistner-Griffin E, Namboodiri
AM, Iwasaki M, Kasuga Y, Hamada GS and Tsugane S: Higher levels of
antibodies to the tumour-associated antigen cyclin B1 in
cancer-free individuals than in patients with breast cancer. Clin
Exp Immunol. 178:75–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nimeus-Malmström E, Koliadi A, Ahlin C,
Holmqvist M, Holmberg L, Amini RM, Jirström K, Wärnberg F,
Blomqvist C, Fernö M and Fjällskog ML: Cyclin B1 is a prognostic
proliferation marker with a high reproducibility in a
population-based lymph node negative breast cancer cohort. Int J
Cancer. 127:961–967. 2010.PubMed/NCBI
|
|
12
|
Kreis NN, Sanhaji M, Krämer A, Sommer K,
Rödel F, Strebhardt K and Yuan J: Restoration of the tumor
suppressor p53 by downregulating cyclin B1 in human papillomavirus
16/18-infected cancer cells. Oncogene. 29:5591–5603. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshida T, Tanaka S, Mogi A, Shitara Y and
Kuwano H: The clinical significance of Cyclin B1 and Wee1
expression in non-small-cell lung cancer. Ann Oncol. 15:252–256.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nozoe T, Korenaga D, Kabashima A, Ohga T,
Saeki H and Sugimachi K: Significance of cyclin B1 expression as an
independent prognostic indicator of patients with squamous cell
carcinoma of the esophagus. Clin Cancer Res. 8:817–822.
2002.PubMed/NCBI
|
|
15
|
Kedinger V, Meulle A, Zounib O, Bonnet ME,
Gossart JB, Benoit E, Messmer M, Shankaranarayanan P, Behr JP,
Erbacher P, et al: Sticky siRNAs targeting survivin and cyclin B1
exert an antitumoral effect on melanoma subcutaneous xenografts and
lung metastases. BMC Cancer. 13:3382013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao P, Hu W, Wang H, Yu S, Li C, Bai J,
Gui S and Zhang Y: Identification of differentially expressed genes
in pituitary adenomas by integrating analysis of microarray data.
Int J Endocrinol. 2015:1640872015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao P, Zhang PF, Hu W, Wang H, Yu G, Wang
Z, Li C, Bai J and Zhang Y: Upregulation of cyclin B1 plays
potential roles in the invasiveness of pituitary adenomas. J Clin
Neurosci. 43:267–273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vu T and Datta PK: Regulation of EMT in
colorectal cancer: A culprit in metastasis. Cancers (Basel).
9:E1712017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang JW, Zhang Z, Li R, Mao F, Sun W, Chen
J, Zhang H, Bartsch JW, Shu K and Lei T: ADAM12 induces EMT and
promotes cell migration, invasion and proliferation in pituitary
adenomas via EGFR/ERK signaling pathway. Biomed Pharmacother.
97:1066–1077. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Forte E, Chimenti I, Rosa P, Angelini F,
Pagano F, Calogero A, Giacomello A and Messina E: EMT/MET at the
crossroad of stemness, regeneration and oncogenesis: The Ying-Yang
equilibrium recapitulated in cell spheroids. Cancers. 9:E982017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song Y, Zhao C, Dong L, Fu M, Xue L, Huang
Z, Tong T, Zhou Z, Chen A, Yang Z, et al: Overexpression of cyclin
B1 in human esophageal squamous cell carcinoma cells induces tumor
cell invasive growth and metastasis. Carcinogenesis. 29:307–315.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh SK, Banerjee S, Acosta EP, Lillard
JW and Singh R: Resveratrol induces cell cycle arrest and apoptosis
with docetaxel in prostate cancer cells via a p53/p21 (WAF1/CIP1)
and p27(KIP1) pathway. Oncotarget. 8:17216–17228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Medina-Aguilar R, Marchat LA, Arechaga
Ocampo E, Gariglio P, García Mena J, Villegas Sepúlveda N, Martínez
Castillo M and López-Camarillo C: Resveratrol inhibits cell cycle
progression by targeting Aurora kinase A and Polo-like kinase 1 in
breast cancer cells. Oncol Rep. 35:3696–3704. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Freitas Silva M, Coelho LF, Guirelli
IM, Pereira RM, Ferreira-Silva GÁ, Graravelli GY, Horvath RO,
Caixeta ES, Ionta M and Viegas C: Synthetic resveratrol-curcumin
hybrid derivative inhibits mitosis progression in estrogen positive
MCF-7 breast cancer cells. Toxicol In Vitro. 50:75–85. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang G, Guo X, Chen H, Lin T, Xu Y, Chen
Q, Liu J, Zeng J, Zhang XK and Yao X: A resveratrol analog,
phoyunbene B, induces G2/M cell cycle arrest and apoptosis in HepG2
liver cancer cells. Bioorg Med Chem Lett. 22:2114–2118. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hardy J and Vezina JL: Transsphenoidal
neurosurgery of intracranial neoplasm. Adv Neurol. 15:261–273.
1976.PubMed/NCBI
|
|
27
|
Wilson CB: A decade of pituitary
microsurgery. The Herbert Olivecrona lecture. J Neurosurg.
61:814–833. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Knosp E, Steiner E, Kitz K and Matula C:
Pituitary adenomas with invasion of the cavernous sinus space: A
magnetic resonance imaging classification compared with surgical
findings. Neurosurgery. 33:610–618. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Joe AK, Liu H, Suzui M, Vural ME, Xiao D
and Weinstein IB: Resveratrol induces growth inhibition, S-phase
arrest, apoptosis, and changes in biomarker expression in several
human cancer cell lines. Clin Cancer Res. 8:893–903.
2002.PubMed/NCBI
|
|
31
|
Zhou SL, Yue WB, Fan ZM, Du F, Liu BC, Li
B, Han XN, Ku JW, Zhao XK, Zhang P, et al: Autoantibody detection
to tumor-associated antigens of P53, IMP1, P16, cyclin B1, P62,
C-myc, Survivn and Koc for the screening of high-risk subjects and
early detection of esophageal squamous cell carcinoma. Dis
Esophagus. 27:790–797. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fang Y, Liang X, Jiang W, Li J, Xu J and
Cai X: Cyclin B1 suppresses colorectal cancer invasion and
metastasis by regulating E-cadherin. PLoS One. 10:e01268752015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee JH, Lee HJ, Sim DY, Jung JH, Kim KR
and Kim SH: Apoptotic effect of lambertianic acid through
AMPK/FOXM1 signaling in MDA-MB231 breast cancer cells. Phytother
Res. 32:1755–1763. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li L, Yang Y, Wu M, Yu Z, Wang C, Dou G,
He H, Wang H, Yang N, Qi H and Xu X: β-asarone induces apoptosis
and cell cycle arrest of human glioma U251 cells via suppression of
HnRNP A2/B1-mediated pathway in vitro and in vivo. Molecules.
23:E10722018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu M, Breyssens H, Salter V, Zhong S, Hu
Y, Baer C, Ratnayaka I, Sullivan A, Brown NR, Endicott J, et al:
Restoring p53 function in human melanoma cells by inhibiting MDM2
and Cyclin B1/CDK1-phosphorylated nuclear iASPP. Cancer Cell.
30:822–823. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Bio. 15:178–196. 2014. View Article : Google Scholar
|
|
37
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bendris N, Cheung CT, Leong HS, Lewis JD,
Chambers AF, Blanchard JM and Lemmers B: Cyclin A2, a novel
regulator of EMT. Cell Mol Life Sci. 71:4881–4894. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arsic N, Bendris N, Peter M, Begon-Pescia
C, Rebouissou C, Gadéa G, Bouquier N, Bibeau F, Lemmers B and
Blanchard JM: A novel function for Cyclin A2: Control of cell
invasion via RhoA signaling. J Cell Biol. 196:147–162. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rastogi I, Rajanna S, Webb A, Chhabra G,
Foster B, Webb B and Puri N: Mechanism of c-Met and EGFR tyrosine
kinase inhibitor resistance through epithelial mesenchymal
transition in non-small cell lung cancer. Biochem Bioph Res Commun.
477:937–944. 2016. View Article : Google Scholar
|
|
41
|
Kourtidis A, Ngok SP and Anastasiadis PZ:
p120 Catenin: An essential regulator of cadherin stability,
adhesion-induced signaling, and cancer progression. Prog Mol Biol
Transl Sci. 116:409–432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jia W, Zhu JK, Martin TA, Jiang AH,
Sanders AJ and Jiang WG: Epithelial-mesenchymal Transition (EMT)
markers in human pituitary adenomas indicate a clinical course.
Anticancer Res. 35:2635–2643. 2015.PubMed/NCBI
|
|
43
|
Wang C, Hu ZQ, Chu M, Wang Z, Zhang WG,
Wang LZ, Li CG and Wang JS: Resveratrol inhibited GH3 cell growth
and decreased prolactin level via estrogen receptors. Clin Neurol
Neurosur. 114:241–248. 2012. View Article : Google Scholar
|
|
44
|
Xue YQ, Di JM, Luo Y, Cheng KJ, Wei X and
Shi Z: Resveratrol oligomers for the prevention and treatment of
cancers. Oxid Med Cell Longev. 2014:7658322014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yousef M, Vlachogiannis IA and Tsiani E:
Effects of resveratrol against lung cancer: In vitro and in vivo
studies. Nutrients. 9(pii): E12312017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nana AW, Chin YT, Lin CY, Ho Y, Bennett
JA, Shih YJ, Chen YR, Changou CA, Pedersen JZ, Incerpi S, et al:
Tetrac downregulates-catenin and HMGA2 to promote the effect of
resveratrol in colon cancer. Endocr Relat Cancer. 25:279–293. 2018.
View Article : Google Scholar : PubMed/NCBI
|