Introduction
Esophageal squamous cell carcinoma (ESCC) is one of
the most common and deadliest malignancies worldwide, with the
incidence and mortality rates increasing annually (1,2). Despite
rapid advances in multiple therapies, the prognosis of ESCC remains
poor due to its late diagnosis and extensive metastases (3). Dietary, lifestyle habits and genetic
polymorphisms are currently considered as major factors affecting
the occurrence and development of ESCC (4). For instance, alcohol intake can
interact with functional genetic polymorphisms of aldehyde
dehydrogenase and alcohol dehydrogenase to increase ESCC risk
(5). In addition, genetic
polymorphisms in the autophagy related 5 gene predict survival and
recurrence in patients with early-stage ESCC (6). However, the potential molecular
mechanisms underlying ESCC progression have not been fully
elucidated.
Cell proliferation is crucial for cancer
progression. A critical tumor suppressor gene, p53, is typically
found to harbor mutations or deletions in several human
malignancies, including ESCC (7,8); it can
inhibit cell cycle progression by inducing p21 (9). Cyclin D1, a key regulator of
G1-to-S-phase transition, is overexpressed and amplified in a many
cancers, and it has been previously demonstrated that cyclin D1
enables progression from G1 to S phase of the cell cycle by binding
and sequestering p21 (10).
Invasion and metastasis are hallmarks of cancer.
Epithelial-to-mesenchymal transition (EMT) is a process during
which epithelial cells lose their polarity and acquire a
mesenchymal phenotype, which is a pivotal step towards cancer
invasion and metastasis (11,12).
Activation of EMT is associated with aberrant expression of a
variety of genes. It is commonly characterized by downregulation of
E-cadherin (E-cad), which is a key epithelial marker, accompanied
by upregulation of N-cadherin (N-cad), Vimentin and Slug, which are
crucial mesenchymal marker genes (13–15). The
aforementioned changes lead to the induction of invasive and
migratory properties in cancer cells. It has been well documented
that transforming growth factor-β (TGF-β) is one of the crucial
factors that regulate the initiation and maintenance of EMT in a
number of cancers (16). In
addition, accumulating evidence shows that TGF-β is implicated in
EMT in several human malignancies, such as lung, gastric and
ovarian cancer, as well as ESCC (16–19).
Muscle relaxants, including rocuronium and
cisatracurium (Cis), effectively block the activation of muscles by
nerves. Rocuronium has been shown to promote the invasion, adhesion
and growth of MDA-231 breast cancer cells (20). It was previously reported that
propofol, which is one of the most common intravenous anesthetic
agents used during cancer resection surgery, suppresses
proliferation and invasion by downregulating ERK-vascular
endothelial growth factor/matrix metallopeptidase-9 signaling in
ECA-109 ESCC cells (21). Cis has
been shown to inhibit the proliferation, invasion and migration of
gastric cancer cells (22). In
addition, emerging evidence indicates that Cis can suppress cancer
cell proliferation, invasion and migration via upregulation of p53,
and inhibits the aggressiveness of colorectal cancer (23). However, the role of Cis in the
progression of ESCC has not been clearly determined.
The aim of the present study was to investigate the
role of Cis in ESCC. The results revealed that exposure of ESCC
cells to Cis inhibited TGF-β-induced EMT, and reduced cell invasion
and metastasis through the TGF-β/Smad signaling pathway.
Materials and methods
Cell culture
Human ECA-109 cells were purchased from the Type
Culture Collection of the Chinese Academy of Science and incubated
in DMEM (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2.
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8) assay (OBiO
Technology, Corp., Ltd.) was employed to evaluate the proliferation
response of ECA-109 cells treated with different concentrations of
Cis (10, 20 and 30 mM; Nimbex; GlaxoSmithKline plc) (24), in accordance with the manufacturer's
protocol. ECA-109 cells were seeded in 96-well plates
(5×103 cells/well) and incubated at 37°C with 5%
CO2 for 24 h. Subsequently, the cells were exposed to
Cis at 10, 20 and 30 mM for 24, 48 and 72 h. At the end of the
exposure periods, 10 µl CCK-8 solution was added to each well.
Following incubation at 37°C for 1 h, absorbance rate was measured
at 490 nm using a plate reader.
Wound healing assay
For the scratch wound healing assay, Cis (20 mM)
alone or Cis (20 mM) combined with TGF-β (1 ng/ml; Sigma-Aldrich;
Merck KGaA)-treated ECA-109 cells were plated in 12-well plates at
a density of 1×105 cells/well. Once cells reached 80%
confluence, medium was replaced by serum-free DMEM and cells were
incubated at 37°C overnight before initiating the experiment. A
wound was created on the surface of the cell monolayer using a
200-µl pipette tip. The cells were then rinsed twice with
serum-free medium in order to remove free-floating cells and
debris. An inverted microscope (magnification, ×20; BX51; Olympus
Corporation) was used to monitor cells at the edges of the scratch.
The percentage of wound closure was determined according to the
following equation: [(Ai-At)/Ai] × 100%, where Ai represents the
initial area of the wound at 0 h and At represents the area of the
wound after 24 h.
Cell invasion assay
To assess the effect of Cis on invasion of ECA-109
cells, 24-well Transwell plates (Corning, Inc.) with 8-µm pore
inserts coated with Matrigel (BD Biosciences). A total of 200 µl
serum-free medium ECA-109 cell suspension containing
5×105 cells/ml was added to the upper chamber and 600 µl
DMEM containing 10% FBS was added to the lower compartment. After
24 h of incubation at 37°C with 5% CO2, the Matrigel and
the cells remaining in the upper chamber were removed by a
cotton-tipped swab. The filters were fixed in 4% formaldehyde for
10 min at room temperature and stained with 0.1% crystal violet
solution for 30 min at room temperature. The cells in five random
fields (magnification, ×20) were observed under an inverted
microscope (Olympus Corporation).
Flow cytometry assay
The cell cycle distribution was examined using the
Cell Cycle Analysis kit (Beyotime Institute of Biotechnology),
according to the manufacturer's protocols. ECA-109 cells treated
with Cis (20 mM) or Cis (20 mM) combined with TGF-β (1 ng/ml) for
48 h, were treated with 70% cold ethanol at 4°C overnight to
increase cell membrane penetrability. Subsequently, 100 µg/ml RNase
(Nanjing KeyGen Biotech Co., Ltd.) was used to treat cells at 37°C
for 20 min. Following staining with 30 µg/ml propidium iodide
(Nanjing KeyGen Biotech Co., Ltd.) for 30 min at room temperature
in the dark, the cell cycle was analyzed using a Gallios Flow
Cytometer (Beckman Coulter, Inc.). The flow cytometry results were
evaluated using a Cell Quest kit (BD CellQuest™ Pro software
version 6.1; BD Biosciences), according to the manufacturer's
protocols.
Cell apoptosis assay
ECA-109 cells were seeded into 6-well plates
(5×105/well) and incubated overnight at 37°C with 5%
CO2. Cell apoptosis was determined by using the Annexin V-PE/7AAD
Staining Cell Apoptosis Detection kit (Nanjing KeyGen Biotech Co.,
Ltd.), according to the manufacturer's protocols, and analyzed
using FlowJo software (version10.5.2; Becton, Dickinson and
Company).
Western blot analysis
ECA-109 cells were collected and lysed with RIPA
lysis buffer (Beyotime Institute of Biotechnology) and incubated
for 30 min on ice. Then proteins were detected using a BCA protein
assay kit (Bio-Rad Laboratories, Inc.). A total of 40 µg protein
was loaded onto 10% SDS-polyacrylamide gels to separate various
proteins, which were subsequently transferred to PVDF membranes.
The membranes were blocked with 10% skimmed milk for 2 h at room
temperature, followed by incubation with primary antibodies
overnight at 4°C. Subsequently, the membranes were incubated with
goat anti-rabbit horseradish peroxidase-conjugated IgG secondary
antibodies (1:2,000; cat. no. 4414s) at room temperature for 1 h.
The signals were detected using enhanced chemiluminescence reagent
(GE Healthcare) and Image J software (version 146; National
Institutes of Health) was used to analyze the fold-changes of
protein levels. Anti-p-Smad2/3 (1:1,000; cat. no. 8828S),
anti-Smad2/3 (1:1,000; cat. no. 8685S), anti-cyclin D1 (1:1,000;
cat. no. 2978T), anti-p53 (1:1,000; cat. no. 2527T), anti-p21
(1:1,000; cat. no. 2947S), anti-E-cad (1:1,000; cat. no. 3195S),
anti-N-cad (1:1,000; cat. no. 13116S), anti-Vimentin (1:1,000; cat.
no. 5741S), anti-TGF-β (1:1,000; cat. no. 3711s), anti-Slug
(1:1,000; cat. no. 9585T), anti-β-actin (1:1,000; cat. no. 3700s)
and anti-GAPDH (1:1,000; cat. no. 5174S) antibodies were obtained
from Cell Signaling Technology, Inc.
Statistical analysis
Statistical data analysis was performed with SPSS
22.0 (IBM Corp.) and GraphPad Prism 5.0 (GraphPad Software, Inc.).
All experimental results are expressed as mean ± standard
deviation. Each experiment was repeated at least three times. Data
were analyzed using either a ANOVA followed by a Tukey's post-hoc
test for comparison of multiple groups or an independent Student's
t-test for comparison of two groups. P<0.05 was considered to
indicate statistically significant differences.
Results
Cis treatment decreases the expression
levels of TGF-β and Smad2/3 in ESCC cells
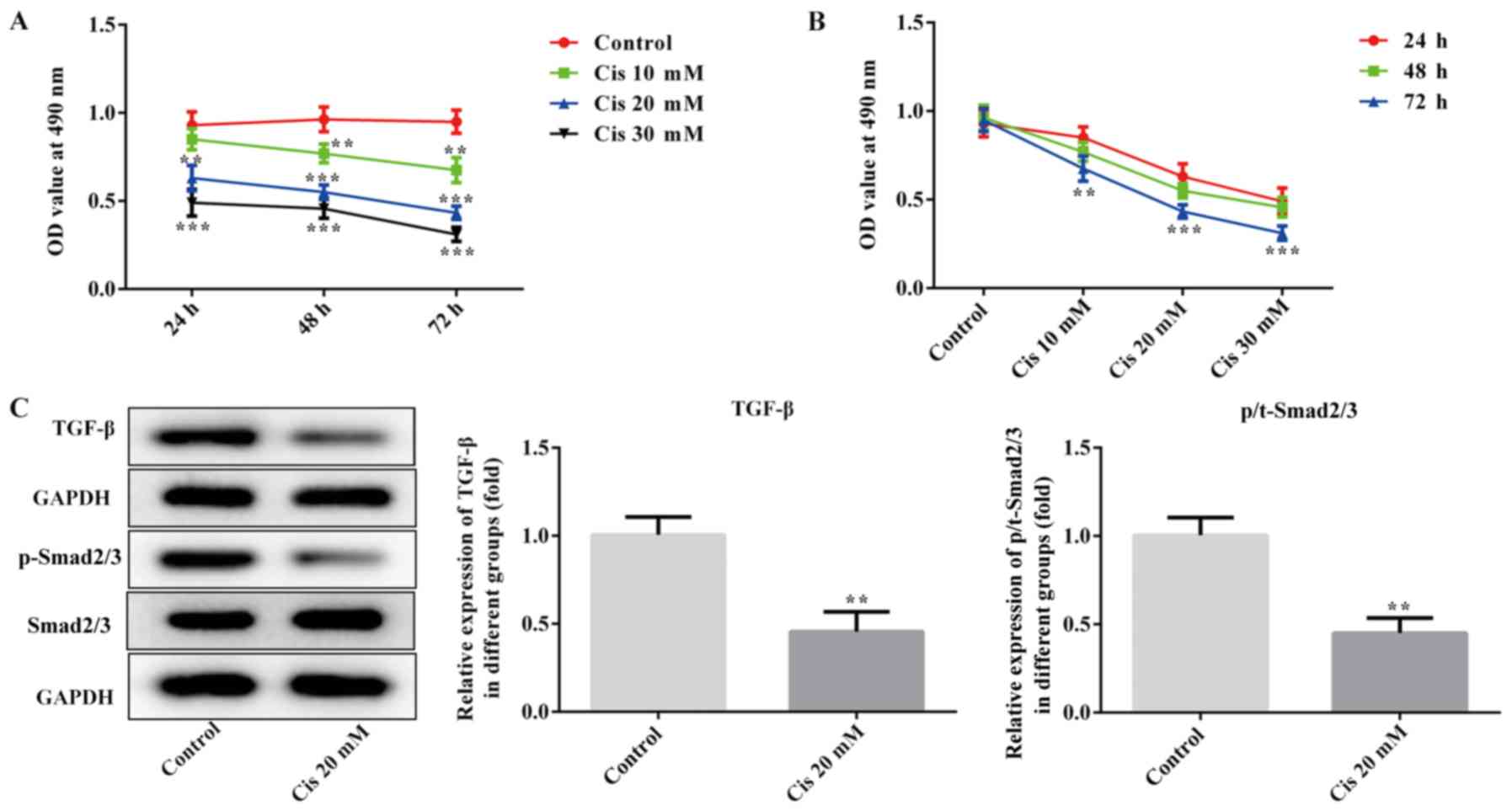
The CCK-8 assay was used to determine the optimal
time and concentration of Cis treatment. As shown in Fig. 1A and B, the ability of ESCC cells to
proliferate decreased with the increase in the concentration of
Cis. When ECA-109 cells were treated with 20 mM Cis for 48 h, cell
viability was reduced by ~50%, therefore this dose was selected for
further experiments. In addition, the expression of TGF-β and
p-Smad2/3 was significantly downregulated in the Cis 20 mM group
compared with the control (P<0.01; Fig. 1C). These results indicated that Cis
inhibited the proliferation of ESCC cells and regulated TGF-β/Smad
signaling.
Cis treatment inhibits proliferation
and promotes apoptosis in TGF-β-treated ESCC cells
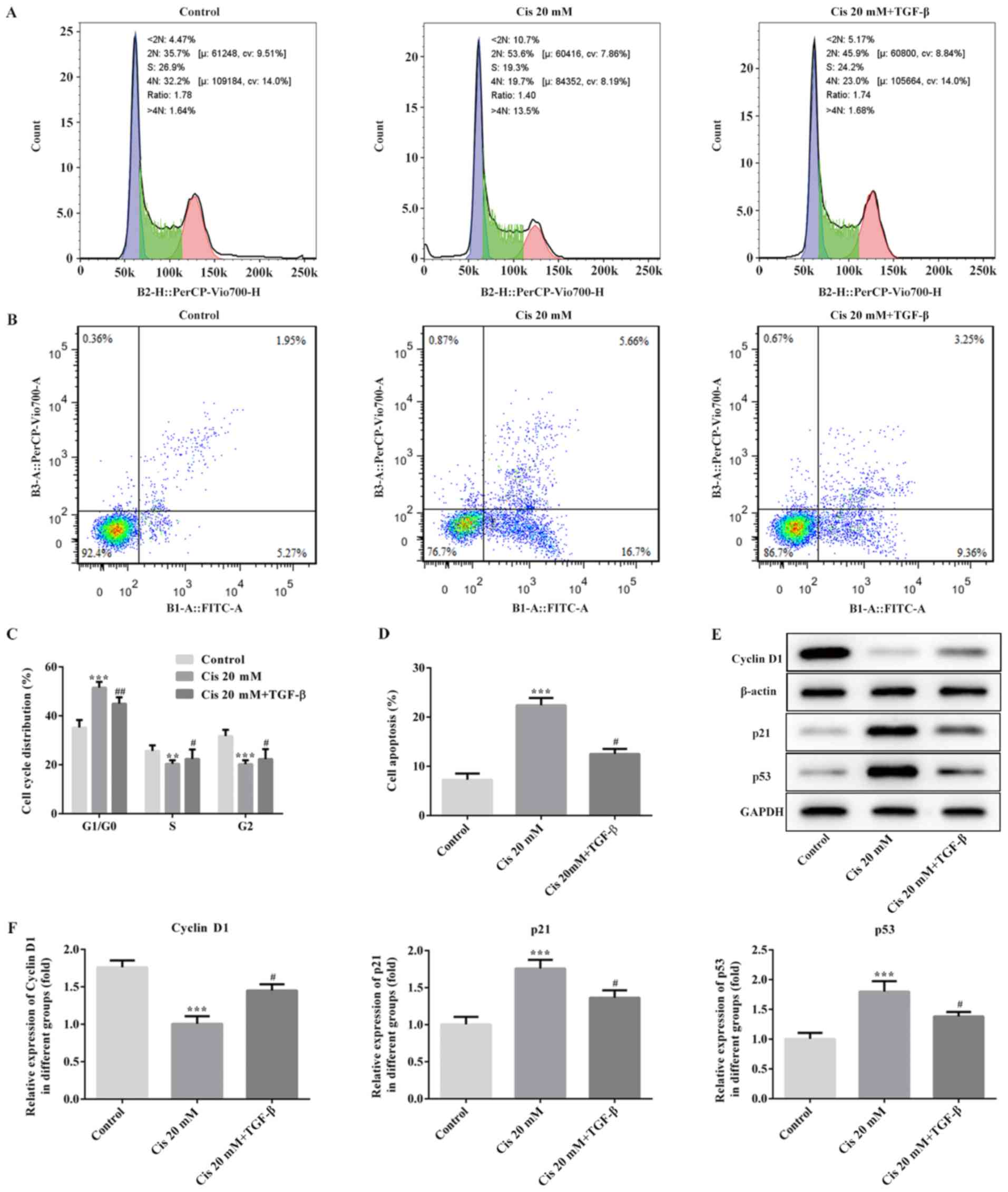
To further investigate the role of Cis in the
proliferation and apoptosis of TGF-β-treated ESCC cells, flow
cytometry was applied. The results suggested that the percentage of
cells in the S phase in the Cis 20 mM group was lower compared with
that of the untreated group, while the opposite results were
observed for the G0/G1 phase (Fig. 2A
and C). After treating ESCC cells with 20 mM Cis plus TGF-β,
the number of cells in the S phase increased, whereas that in the
G0/G1 phase was notably decreased compared with treatment with Cis
alone (Fig. 2A and C). Furthermore,
the rate of cell apoptosis increased following Cis treatment,
whereas the apoptotic cell number decreased following intervention
with both Cis and TGF-β (Fig. 2B and
D). In addition, the expression of cyclin D1 was downregulated,
accompanied by upregulated expression of p53 and p21 in the Cis 20
mM group, while the level of cyclin D1 was increased, along with
decreased levels of p53 and p21 following treatment with 20 mM Cis
plus TGF-β (Fig. 2E and F). These
data indicated that Cis treatment suppressed proliferation and
promoted apoptosis in ESCC cells.
Cis treatment suppresses invasion and
migration in TGF-β-treated ESCC cells
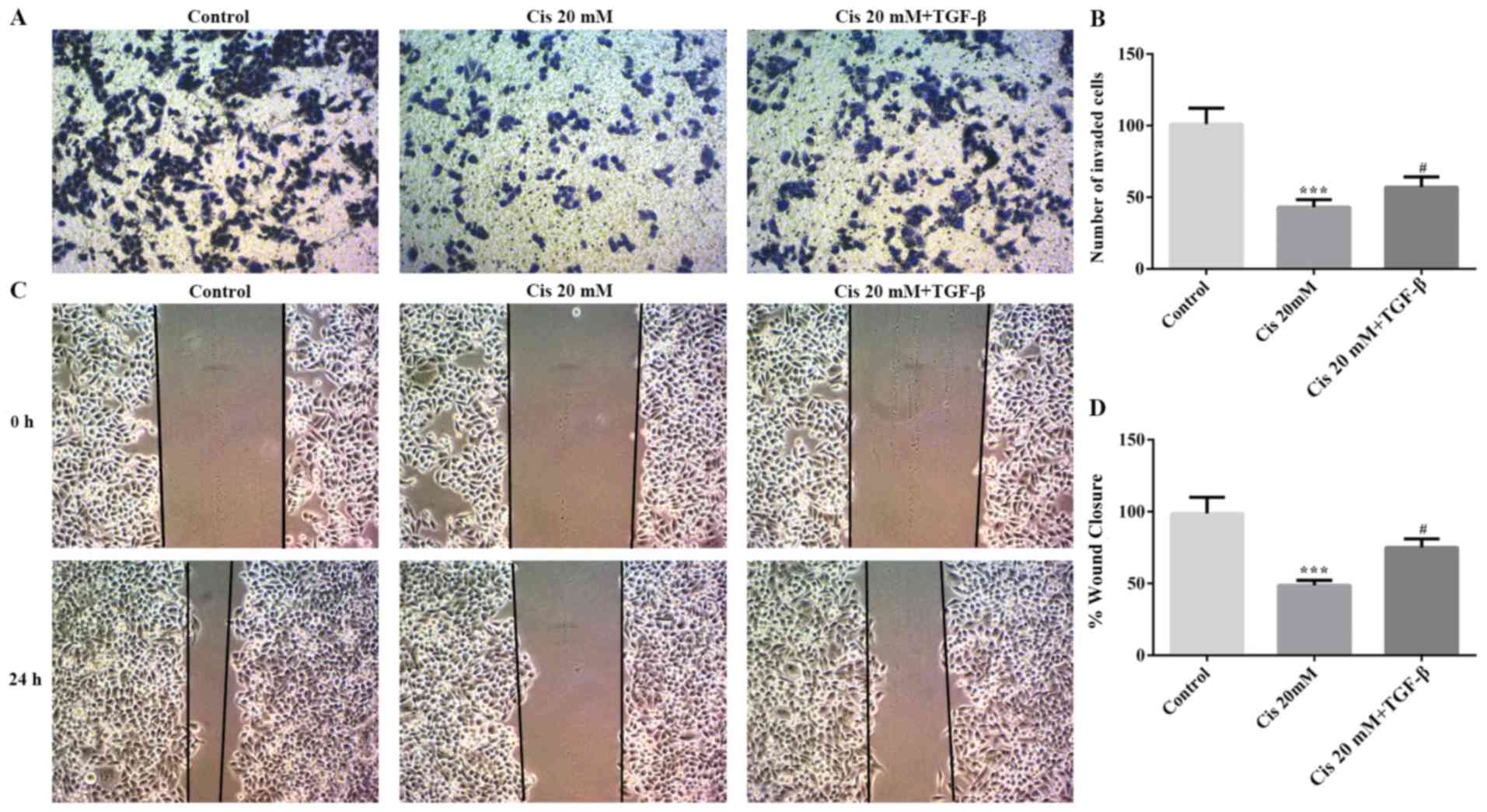
To evaluate invasion and migration in TGF-β-treated
ESCC cells, the wound healing and Transwell assays were used in the
present study. As shown in Fig. 3A and
B, the ability of cell invasion was decreased by Cis compared
with the control group; however, cell invasion was increased
following treatment with both Cis and TGF-β. Moreover, the change
in cell migration was consistent with the results on invasion
(Fig. 3C and D). Therefore, Cis
treatment inhibited invasion and migration in ESCC cells.
Cis treatment inhibits EMT in
TGF-β-treated ESCC cells
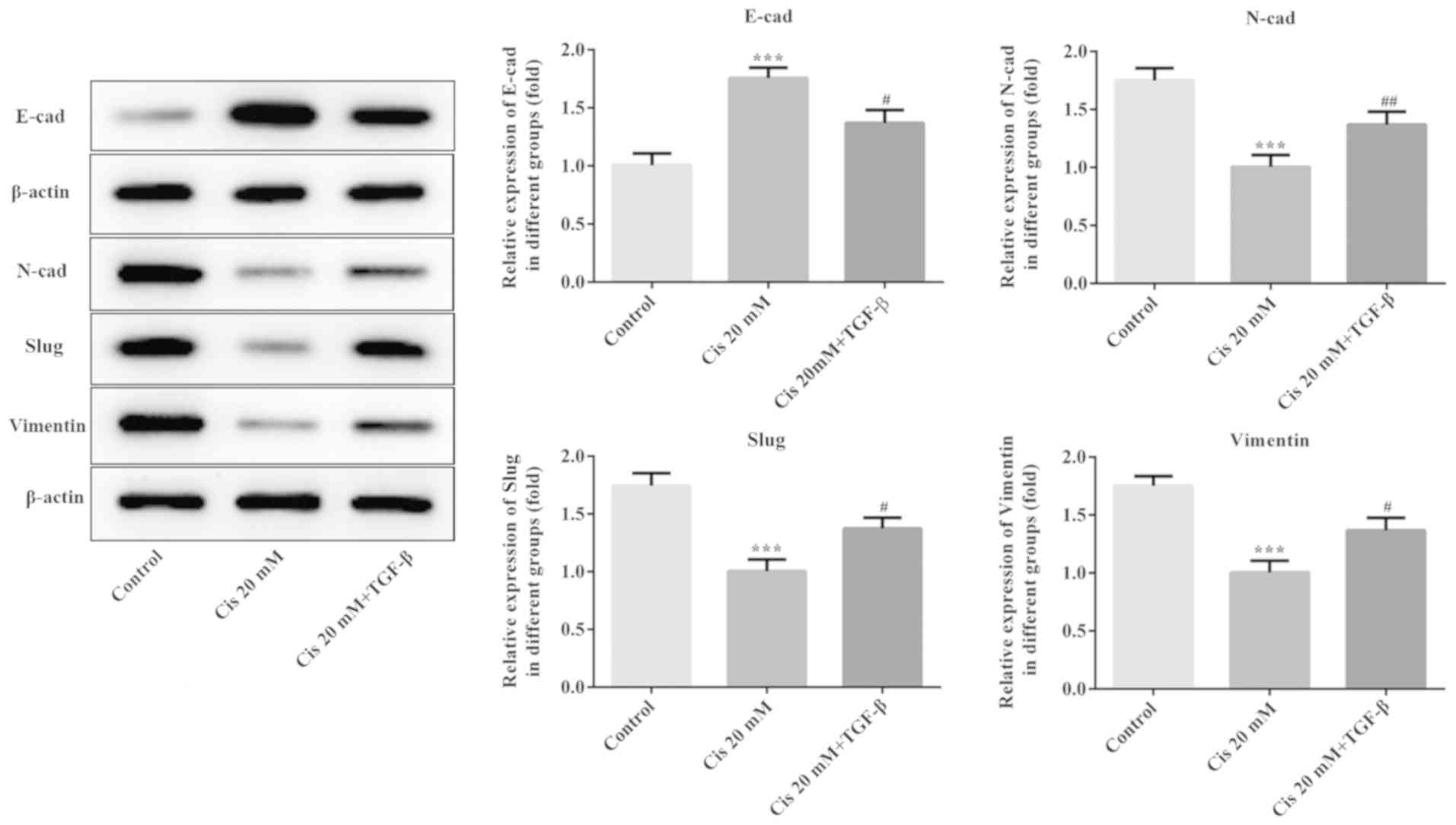
The expression of EMT-related proteins was evaluated
using western blot analysis. As shown in Fig. 4, the expression level of E-cad was
increased, whereas the expression levels of N-cad, Slug and
Vimentin decreased when ESCC cells were treated with Cis alone.
Following treatment with TGF-β plus Cis, the E-cad expression was
reduced, accompanied by increased expression of N-cad, Slug and
Vimentin compared with Cis treatment alone. These results indicated
that Cis suppressed EMT in TGF-β-treated ESCC cells.
Discussion
Esophageal cancer is one of the most common cancers
worldwide, and the majority of cases of esophageal cancer are ESCC,
which is particularly common in China (25). Despite the advances in surgery,
chemotherapy and radiotherapy over the past decades, current
medical interventions are not satisfactory in the clinical setting,
and the overall prognosis of ESCC is poor due to the late diagnosis
and lack of effective therapies (26,27).
Anesthesia plays a crucial role in surgery, ensuring patient safety
(28); however, to the best of our
knowledge, there are very few studies on the effects of anesthetic
agents on cancer. Emerging evidence supports the hypothesis that
certain anesthetic drugs may affect cancer cell proliferation,
angiogenesis and apoptosis (29). In
the present study, the results demonstrated that Cis inhibited the
proliferation, invasion, migration and EMT induced by TGF-β in ESCC
cells.
The cell cycle is a well-regulated intracellular
program that maintains normal cell division and growth; however,
deregulation of the cell cycle machinery frequently occurs various
human malignancies, promoting abnormal and uncontrollable cell
growth, as well as acquisition of aggressive metastatic features
(30). Cyclin D1 is known to
regulate transition through the G1-S phase (31,32).
During G1 phase arrest, cyclin-dependent kinase inhibitors,
including p21, bind to cyclin complexes, thereby inhibiting cell
proliferation (33). Previous
studies have revealed that the transcription factor cellular tumor
antigen p53 regulates the expression of numerous genes involved in
the cell cycle and induces cell cycle arrest (8,34). p21,
a general G1 phase cell cycle inhibitor, was the first p53-effector
gene to be identified (35,36). In the present study, the
proliferation of ESCC cells was inhibited, accompanied a decrease
in cyclin D1 expression and increase in p53 and p21 expression
following treatment with Cis compared with the control group.
However, after exposure to both Cis and TGF-β, there was a
promotion of ESCC cell proliferation and the expression of cyclin
D1, whereas the opposite results were observed for p53 and p21 with
Cis treatment alone.
Mounting evidence has demonstrated that the
TGF-β/Smad pathway is implicated in the progression of cancer
through regulation of the expression of related genes (37–39).
TGF-β has the ability to induce invasion, migration and EMT in a
number of cancer cell types, including glioblastoma and breast
cancer (40,41). Novel TGF-β1 inhibitors have been used
to block TGF-β1-induced EMT in human A549 lung cancer cells
(42). A recent study reported that
CD82 suppresses invasion and metastasis of ESCC via TGF-β1
(43). In addition, accumulating
evidence has demonstrated that inhibition of EMT contributes to
improved treatment and prognosis of ESCC (44–46). The
findings of the present study revealed that treatment with Cis
downregulated the expression of TGF-β and phosphorylation of
Smad2/3. In addition, Cis inhibited invasion and migration of ESCC
cells. Furthermore, Cis increased the expression of E-cad and
decreased N-cad, Slug and Vimentin expression, which are
well-established EMT-associated proteins. These results indicated
that Cis inhibited EMT in ESCC cells. The aforementioned findings
were in accordance with those of previous studies using other
anesthetics, such as rocuronium and fentanyl (20,47).
In conclusion, this mechanistic study is, to the
best of our knowledge, the first to reveal that Cis suppresses the
proliferation, invasion, migration and EMT in ESCC cells, which may
prove to be of value in guiding clinical diagnosis and treatment.
However, the use of a single ESCC cell line is a limitation of this
study and therefore, a comprehensive analysis of more cell lines is
required in the future to understand the effects of Cis in
ESCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL wrote the manuscript, analyzed the data and
revised the manuscript. JW and SZ searched the literature, designed
the study and performed experiments. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
D'Amico TA: Outcomes after surgery for
esophageal cancer. Gastrointest Cancer Res. 1:188–196.
2007.PubMed/NCBI
|
|
4
|
Dotto GP and Rustgi AK: Squamous cell
cancers: A unified perspective on biology and genetics. Cancer
Cell. 29:622–637. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suo C, Yang Y, Yuan Z, Zhang T, Yang X,
Qing T, Gao P, Shi L, Fan M, Cheng H, et al: Alcohol intake
interacts with functional genetic polymorphisms of aldehyde
dehydrogenase (ALDH2) and alcohol dehydrogenase (ADH) to increase
esophageal squamous cell cancer risk. J Thorac Oncol. 14:712–725.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang PW, Hsieh MS, Chang YH, Huang PM and
Lee JM: Genetic polymorphisms of ATG5 predict survival and
recurrence in patients with early-stage esophageal squamous cell
carcinoma. Oncotarget. 8:91494–91504. 2017.PubMed/NCBI
|
|
7
|
Cheng YW, Liao LD, Yang Q, Chen Y, Nie PJ,
Zhang XJ, Xie JJ, Shan BE, Zhao LM, Xu LY and Li EM: The histone
deacetylase inhibitor panobinostat exerts anticancer effects on
esophageal squamous cell carcinoma cells by inducing cell cycle
arrest. Cell Biochem Funct. 36:398–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kastan MB, Canman CE and Leonard CJ: P53,
cell cycle control and apoptosis: Implications for cancer. Cancer
Metastasis Rev. 14:3–15. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z, Zhang Y, Gu JJ, Davitt C, Reeves R
and Magnuson NS: Pim-2 phosphorylation of p21(Cip1/WAF1) enhances
its stability and inhibits cell proliferation in HCT116 cells. Int
J Biochem Cell Biol. 42:1030–1038. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang H, Han Y, Yang X, Li M, Zhu R, Hu J,
Zhang X, Wei R, Li K and Gao R: HNRNPK inhibits gastric cancer cell
proliferation through p53/p21/CCND1 pathway. Oncotarget.
8:103364–103374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ghasri P, Admani S, Petelin A and Zachary
CB: Treatment of actinic cheilitis using a 1,927-nm thulium
fractional laser. Dermatol Surg. 38:504–507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Quan J, Elhousiny M, Johnson NW and Gao J:
Transforming growth factor-β1 treatment of oral cancer induces
epithelial-mesenchymal transition and promotes bone invasion via
enhanced activity of osteoclasts. Clin Exp Metastasis. 30:659–670.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Creighton CJ, Chang JC and Rosen JM:
Epithelial-mesenchymal transition (EMT) in tumor-initiating cells
and its clinical implications in breast cancer. J Mammary Gland
Biol Neoplasia. 15:253–260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang Y and Massague J:
Epithelial-mesenchymal transitions: Twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bai X, Li YY, Zhang HY, Wang F, He HL, Yao
JC, Liu L and Li SS: Role of matrix metalloproteinase-9 in
transforming growth factor-β1-induced epithelial-mesenchymal
transition in esophageal squamous cell carcinoma. Onco Targets
Ther. 10:2837–2847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang C, Hao Y, Wang Y, Xu J, Teng Y and
Yang X: TGF-β/SMAD4-regulated LncRNA-LINP1 inhibits
epithelial-mesenchymal transition in lung cancer. Int J Biol Sci.
14:1715–1723. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li F, Shi J, Xu Z, Yao X, Mou T, Yu J, Liu
H and Li G: S100A4-MYH9 Axis promote migration and invasion of
gastric cancer cells by inducing TGF-β-mediated
epithelial-mesenchymal transition. J Cancer. 9:3839–3849. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu X, Zhao J, Ruan Y, Sun L, Xu C and
Jiang H: Sialyltransferase ST3GAL1 promotes cell migration,
invasion, and TGF-β1-induced EMT and confers paclitaxel resistance
in ovarian cancer. Cell Death Dis. 9:11022018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang A, Zhao H, Cai J and Jiang WG:
Possible effect of muscle-relaxant anaesthetics on invasion,
adhesion and migration of breast cancer cells. Anticancer Res.
36:1259–1265. 2016.PubMed/NCBI
|
|
21
|
Xu YB, Du QH, Zhang MY, Yun P and He CY:
Propofol suppresses proliferation, invasion and angiogenesis by
down-regulating ERK-VEGF/MMP-9 signaling in Eca-109 esophageal
squamous cell carcinoma cells. Eur Rev Med Pharmacol Sci.
17:2486–2494. 2013.PubMed/NCBI
|
|
22
|
Jiang A, Zhao H, Liu X, Yu M, Chen J and
Jiang WG: Comparison of different muscle-relaxant anesthetics on
growth, migration and invasion of gastric cancer cells. Anticancer
Res. 37:4371–4378. 2017.PubMed/NCBI
|
|
23
|
Yabasin IB, Sanches JGP, Ibrahim MM,
Huidan J, Williams W, Lu ZL and Wen Q: Cisatracurium retards cell
migration and invasion upon upregulation of p53 and inhibits the
aggressiveness of colorectal cancer. Front Physiol. 9:9412018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yabasin IB, Lu Z and Yu JC:
Cisatracurium-induced proliferation impairment and death of
colorectal cancer cells, HCT116 is mediated by p53 dependent
intrinsic apoptotic pathway in vitro. Biomed Pharmacother.
43:320–329. 2017. View Article : Google Scholar
|
|
25
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsuda S, Takeuchi H, Kawakubo H, Ando N
and Kitagawa Y: Current advancement in multidisciplinary treatment
for resectable cStage II/III esophageal squamous cell carcinoma in
Japan. Ann Thorac Cardiovasc Surg. 22:275–283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baba Y, Watanabe M, Yoshida N and Baba H:
Neoadjuvant treatment for esophageal squamous cell carcinoma. World
J Gastrointest Oncol. 6:121–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ciechanowicz SJ and Ma D: Anaesthesia for
oncological surgery-can it really influence cancer recurrence?
Anaesthesia. 71:127–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Santamaria LB, Schifilliti D, La Torre D
and Fodale V: Drugs of anaesthesia and cancer. Surg Oncol.
19:63–81. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Besson A, Dowdy SF and Roberts JM: CDK
inhibitors: Cell cycle regulators and beyond. Dev Cell. 14:159–169.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sherr CJ and Roberts JM: CDK inhibitors:
positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Santarius T, Shipley J, Brewer D, Stratton
MR and Cooper CS: A census of amplified and overexpressed human
cancer genes. Nat Rev Cancer. 10:59–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen G, Xu C, Chen C, Hebbar V and Kong
AN: p53-independent G1 cell cycle arrest of human colon carcinoma
cells HT-29 by sulforaphane is associated with induction of p21CIP1
and inhibition of expression of cyclin D1. Cancer Chemother
Pharmacol. 57:317–327. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mo J, Lin M, He B, Tan K, Jin C, Jiang H,
Pan X and Lin W: Recombinant human adenovirus-p53 improves the
outcome of mid-late stage pancreatic cancer via arterial infusion.
Oncol Lett. 14:6829–6832. 2017.PubMed/NCBI
|
|
35
|
Huang X, Qiao Y, Zhou Y, Ruan Z, Kong Y,
Li G, Xie X and Zhang J: Ureaplasma spp. Lipid-associated membrane
proteins induce human monocyte U937 cell cycle arrest through
p53-independent p21 pathway. Int J Med Microbiol. 308:819–828.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Y, Qiu C, Lu N, Liu Z, Jin C, Sun C,
Bu H, Yu H, Dongol S and Kong B: FOXD1 is targeted by miR-30a-5p
and miR-200a-5p and suppresses the proliferation of human ovarian
carcinoma cells by promoting p21 expression in a p53-independent
manner. Int J Oncol. 52:2130–2142. 2018.PubMed/NCBI
|
|
37
|
Liu L, Liu H, Zhou Y, He J, Liu Q, Wang J,
Zeng M, Yuan D, Tan F, Zhou Y, et al: HLTF suppresses the migration
and invasion of colorectal cancer cells via TGF β/SMAD signaling in
vitro. Int J Oncol. 53:2780–2788. 2018.PubMed/NCBI
|
|
38
|
Liu Y, Wang JX, Huang D, Wang B, Li LL, Li
XX, Ni P, Dong XL, Xia W, Yu CX, et al: PMLIV overexpression
promotes TGF-β-associated epithelial-mesenchymal transition and
migration in MCF-7 cancer cells. J Cell Physiol. 233:9575–9583.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li L, Lv Y and Yan D: Inhibition of Ep3
attenuates migration and promotes apoptosis of non-small cell lung
cancer cells via suppression of TGF-β/Smad signaling. Oncol Lett.
16:5645–5654. 2018.PubMed/NCBI
|
|
40
|
Luo D, Xu X, Li J, Chen C, Chen W, Wang F,
Xie Y and Li F: The PDK1/c-Jun pathway activated by TGF-β induces
EMT and promotes proliferation and invasion in human glioblastoma.
Int J Oncol. 53:2067–2080. 2018.PubMed/NCBI
|
|
41
|
Wu W, Chen F, Cui X, Yang L, Chen J, Zhao
J, Huang D, Liu J, Yang L, Zeng J, et al: LncRNA NKILA suppresses
TGF-β-induced epithelial-mesenchymal transition by blocking NF-κB
signaling in breast cancer. Int J Cancer. 143:2213–2224. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ko H, So Y, Jeon H, Jeong MH, Choi HK, Ryu
SH, Lee SW, Yoon HG and Choi KC: TGF-β1-induced
epithelial-mesenchymal transition and acetylation of Smad2 and
Smad3 are negatively regulated by EGCG in human A549 lung cancer
cells. Cancer Lett. 335:205–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zeng TD, Zheng B, Zheng W and Chen C:
CD82/KAI1 inhibits invasion and metastasis of esophageal squamous
cell carcinoma via TGF-β1. Eur Rev Med Pharmacol Sci. 22:5928–5937.
2018.PubMed/NCBI
|
|
44
|
Thar Min AK, Okayama H, Saito M, Ashizawa
M, Aoto K, Nakajima T, Saito K, Hayase S, Sakamoto W, Tada T, et
al: Epithelial-mesenchymal transition-converted tumor cells can
induce T-cell apoptosis through upregulation of programmed death
ligand 1 expression in esophageal squamous cell carcinoma. Cancer
Med. 2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hong D, Liu T, Huang W, Liao Y, Wang L,
Zhang Z, Chen H, Zhang X and Xiang Q: Gremlin1 delivered by
mesenchymal stromal cells promoted epithelial-mesenchymal
transition in human esophageal squamous cell carcinoma. Cell
Physiol Biochem. 47:1785–1799. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sato F, Kubota Y, Natsuizaka M, Maehara O,
Hatanaka Y, Marukawa K, Terashita K, Suda G, Ohnishi S, Shimizu Y,
et al: EGFR inhibitors prevent induction of cancer stem-like cells
in esophageal squamous cell carcinoma by suppressing
epithelial-mesenchymal transition. Cancer Biol Ther. 16:933–940.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang N, Zhang Z and Lv J: Fentanyl
inhibits proliferation and invasion via enhancing miR-302b
expression in esophageal squamous cell carcinoma. Oncol Lett.
16:459–466. 2018.PubMed/NCBI
|