Introduction
NK/T cell lymphoma is a type of aggressive
non-Hodgkin lymphoma and mainly occurs in the extranodal organs
with short survival time and poor response to therapy. It always
occurs in adults at 44–54 years of age, and it is more common in
males than in females. Asian and indigenous populations of Mexico,
central and south of American have the highest incidence rates for
this disease, which is associated with Epstein Barr virus infection
(1,2). The nose is a common site for the
development of this type of lymphoma; however, it can also develop
in the skin, digestive tract, testis and lung (3,4).
Histologically, classical extranodal NK/T cell lymphoma includes
the following morphological features: Neoplastic cells infiltrate
diffusely, often invading and destroying the vascular wall,
accompanied by massive tissue necrosis. Tumor cells vary in size,
being primarily mixed with small-to-medium-sized lymphocytes. Large
cells could often be observed in varying numbers and there are a
large number of mixed inflammatory cells in the background.
Immunohistochemical staining demonstrates that tumor cells usually
express CD3ε, CD56, Cytotoxic markers, such as Granzyme B, and TIA,
while B cell markers have not been detected. Epstein Barr
virus-Epstein Barr virus encoded RNA (EBV-EBER) is often detected
via in situ hybridization staining (5–7).
Mucosa-associated lymphoid tissue lymphoma (MALToma), which is
different to NK/T cell lymphoma, is one kind of indolent B cell
lymphoma with neoplastic cells of the same size. In the present
study, a rare case of NK/T cell lymphoma with consistent small
neoplastic cells resembling MALToma in histological morphology is
reported. The specific clinical presentation, imaging and
pathological data were retrospectively analyzed in order to
investigate the clinicopathological characteristics of this type of
lymphoma.
Case report
Patient and methods
Clinical data collection
In the present study, the case diagnosed as NK/T
cell lymphoma in October 11, 2017 was obtained from the Department
of Pathology, Yantai Yuhuangding Hospital (Shandong, China). Biopsy
was taken via nasal endoscopy and the clinical data were obtained
via CT scan, MRI scan, PET-CT scan, lumbar puncture, bone marrow
biopsy, blood tests and follow-up.
Sample processing and morphological
observation
The samples were immersed in 10% buffered formalin
for complete fixation at room temperature after surgery. The ratio
of fixative solution volume to tissue was 10:1, and fixation time
was 12 h. Subsequently, tissue dehydration and paraffin embedding
were performed. Sections 4 µm thick were cut from tissue blocks for
hematoxylin and eosin staining at room temperature for 90 min.
Tissue morphology was observed under a light microscope (Olympus
BX45; Olympus Corporation).
Immunohistochemical staining
EnVision two-step method was adopted using an
automatic immunostainer (Ventana Medical Systems, Inc.) for
immunohistochemical staining and subsequent DAB staining (DAB
secondary antibody kit; Ventana Medical Systems Inc.). Endogenous
peroxidase activity was removed by incubation with 0.3%
H2O2 for 4 min at 37°C. The duration of
incubations in primary and secondary antibodies was 32 min at 37°C.
Hematoxylin was used for counterstaining at room temperature for 1
min, and each section was stained with known positive tissues as
the positive control, while the negative control sample used PBS
instead of primary antibody. The antibodies used in the current
study were purchased from Beijing Zhongshan Jinqiao Biological Co.
(Table I). SPN-9001 Histostain™-SP
kit was used as the secondary antibody (OriGene Technologies,
Inc.). A microscope (Olympus B45) was used to assess the tissues,
2×10, 4×10, 10×10, 20×10 and 40×10 magnification.
 | Table I.Primary antibodies used in
immunohistochemistry. |
Table I.
Primary antibodies used in
immunohistochemistry.
| Antibody | Catalog no. | Dilution |
|---|
| Anti-CD2 | ZM-0278 | 1:100 |
| Anti-CD3 | ZM-0417 | 1:100 |
| Anti-CD4 | ZM-0418 | 1:100 |
| Anti-CD5 | ZM-0280 | 1:100 |
| Anti-CD7 | ZA-0589 | 1:100 |
| Anti-CD8 | ZA-0508 | 1:100 |
| Anti-CD56 | ZM-0057 | 1:100 |
| Anti-CD20 | ZA-0293 | 1:100 |
| Anti-BCL-2 | ZA-0536 | 1:100 |
| Anti-Granzyme
B | ZA-0599 | 1:100 |
| Anti-Ki67 | ZM-0166 | 1:100 |
| Anti-cyclin D1 | ZM-0366 | 1:200 |
| Anti-BCL-6 | ZM-0011 | 1:100 |
| Anti-CD23 | ZM-0273 | 1:100 |
| Anti-CD10 | ZM-0283 | 1:100 |
|
Anti-Synaptophysin | ZA-0263 | 1:100 |
|
Anti-cytokeratin | ZM-0069 | 1:100 |
| Anti-Desmin | ZA-0610 | 1:100 |
| Anti-Epithelial
membrane antigen | ZM-0095 | 1:50 |
| Anti-CD21 | ZM-0040 | 1:100 |
| Anti-TP53 | ZM-0408 | 1:100 |
In situ hybridization detection
Tissue sections were dewaxed, dehydrated and
digested by proteinase K for 20 min at 37°C. EBER probe
hybridization solution (100 ng/ml) was added after the slice was
dried using absolute ethanol and then incubation at 37°C for 30
min. Tissues were incubated with horseradish peroxidase-labeled
digoxin for 30 min at 37°C; rinsed three times with PBS buffer for
2 min each time; rinsed with deionized water and 0.2 ml DAB to each
slide for 15 min in the dark. Epstein Barr virus probe was
purchased from Beijing Zhongshan Jinqiao Biological Co., Ltd (Probe
cat. no. A500P.9900; Probe sequence:
5′-CTCCTCCCTACCAAAACCCTCACCACCCCC-3′).
Results
Clinical data
A 43-year-old female patient suffered from
persistent congestion in the left nasal cavity for ten days. The
nasal congestion was persistent, and was accompanied by decreased
olfactory sensation and purulent sputum. No improvement in symptoms
was obtained with oral cephalosporin treatment, at which point the
patient was referred to Yantai Yuhuangding Hospital. A physical
examination gave the following measurements: Temperature, 36°C;
Pulse, 79 beats/min; Rate, 18 beats/min; blood pressure, 136/90
mmHg; Eastern Cooperative Oncology Group performance score, 0; and
weight, 70.0 kg. The nasal septum was in the middle. The mucosa
inflamed, causing congestion, and was purulent on the surface in
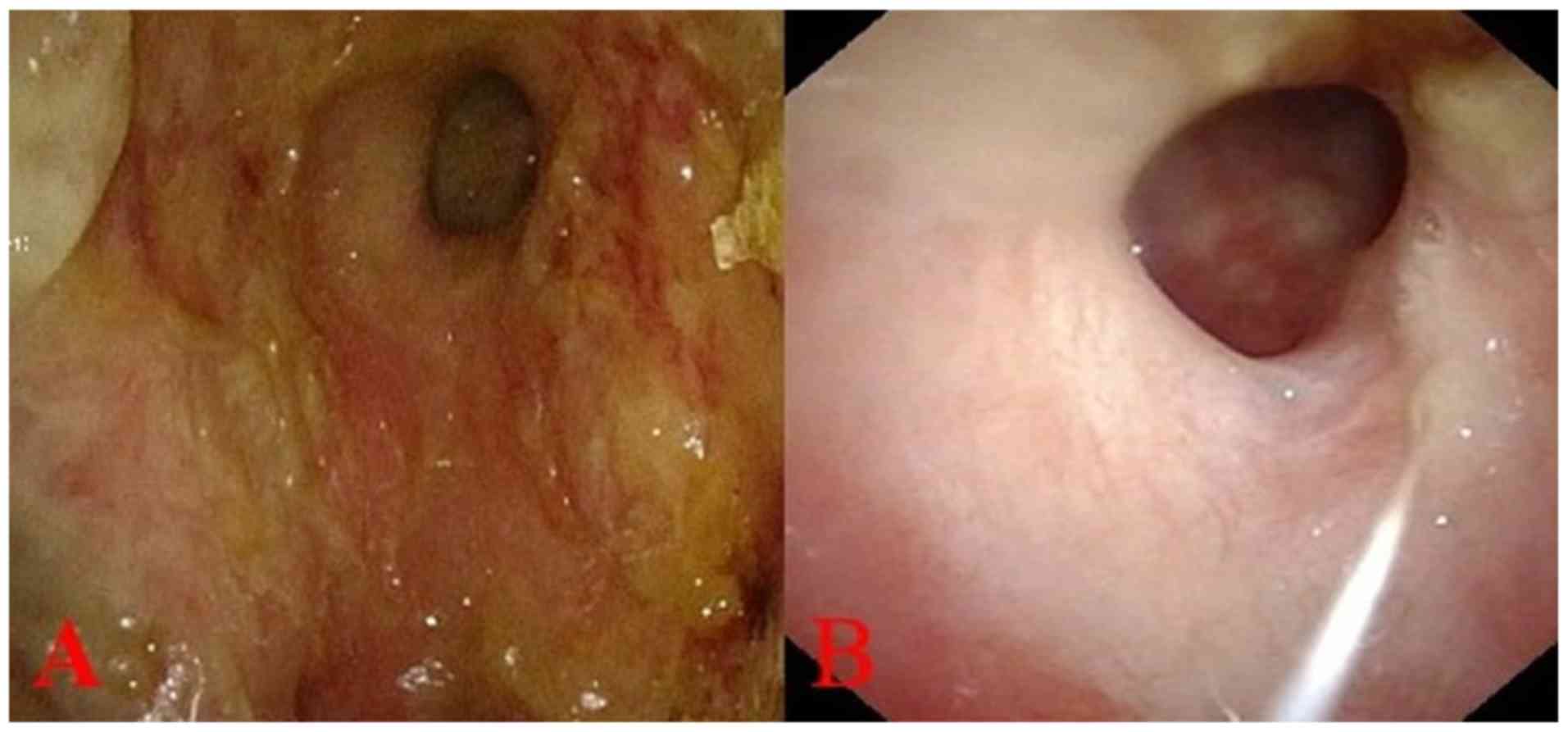
the left nasal cavity, as observed by nasal endoscopy (Fig. 1A). The nasal mucosa on the right side
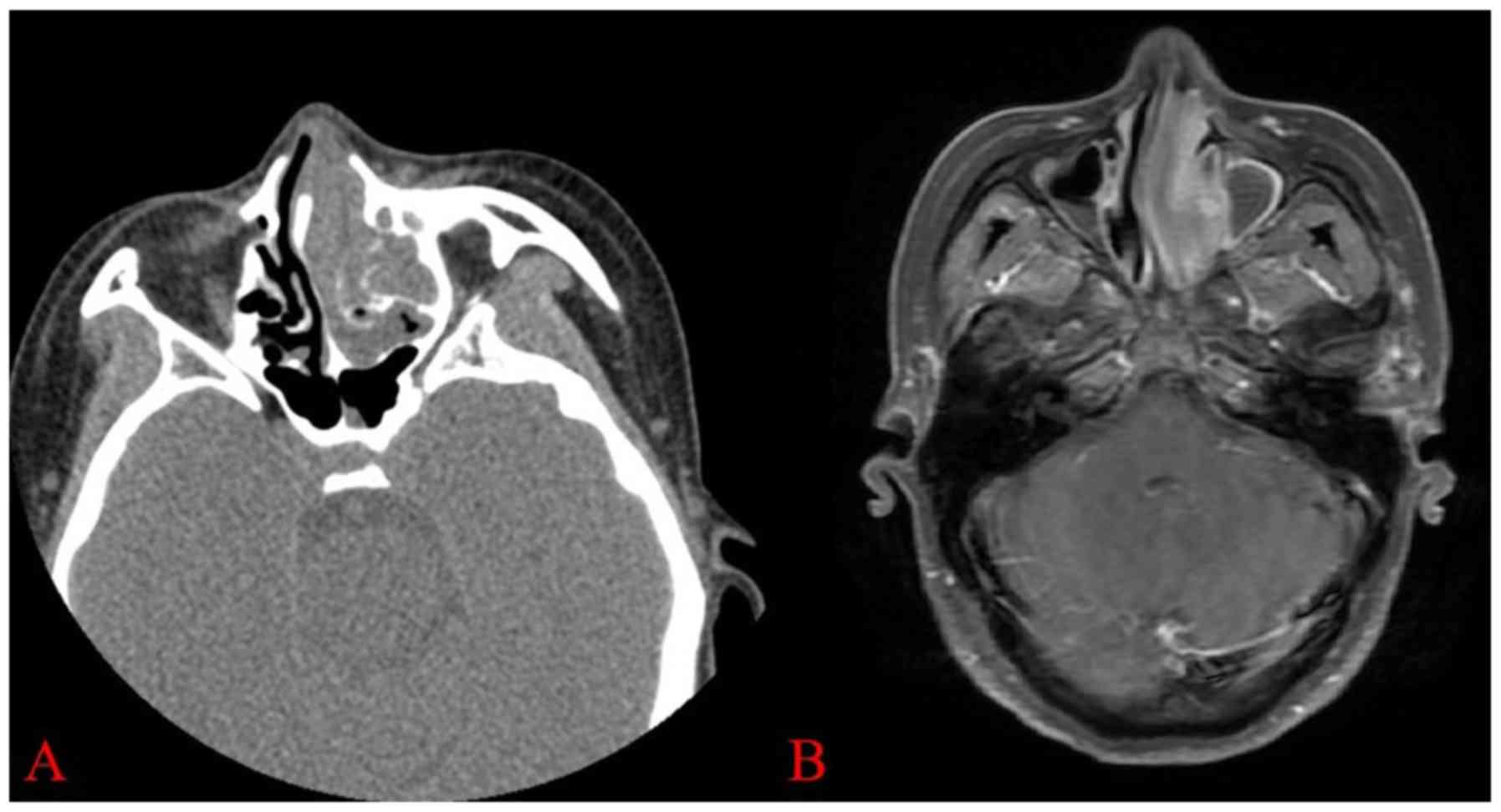
was smooth and no mass was observed. A CT scan identified
obstruction, inflammatory lesions and granuloma formation in the
left nasal cavity (Fig. 2A). MRI
revealed that the mucosa was thickened in the left nasal cavity,
and the lesion was considered to be inflammatory (Fig. 2B). Whole blood counts were as
follows: White blood cells, 7.97×109/l; red blood cells,
3.47×1012/l; hemoglobin, 102 g/l; Platelet,
363×109/l; serum lactate dehydrogenase levels, 133 U/l;
and C-reactive protein level was normal. Blood tests for liver and
renal function were normal. Peripheral blood EBV DNA copy number
was <3,000 copies/ml. Positron Emission Tomography-CT (PET-CT)
scan, lumbar puncture, bone marrow biopsy and aspiration did not
show evidence of extra-nasal tumor metastasis. The patient received
resection of left nasal mucosa and septum under endoscope in
September 29, 2017.
Gross examination
The sample was obtained after surgery under nasal
endoscopy. The gray and white tissue was irregular, and was
2.5×2×0.5 cm in size. The surface of some parts of the tissue was
smooth.
Histological findings
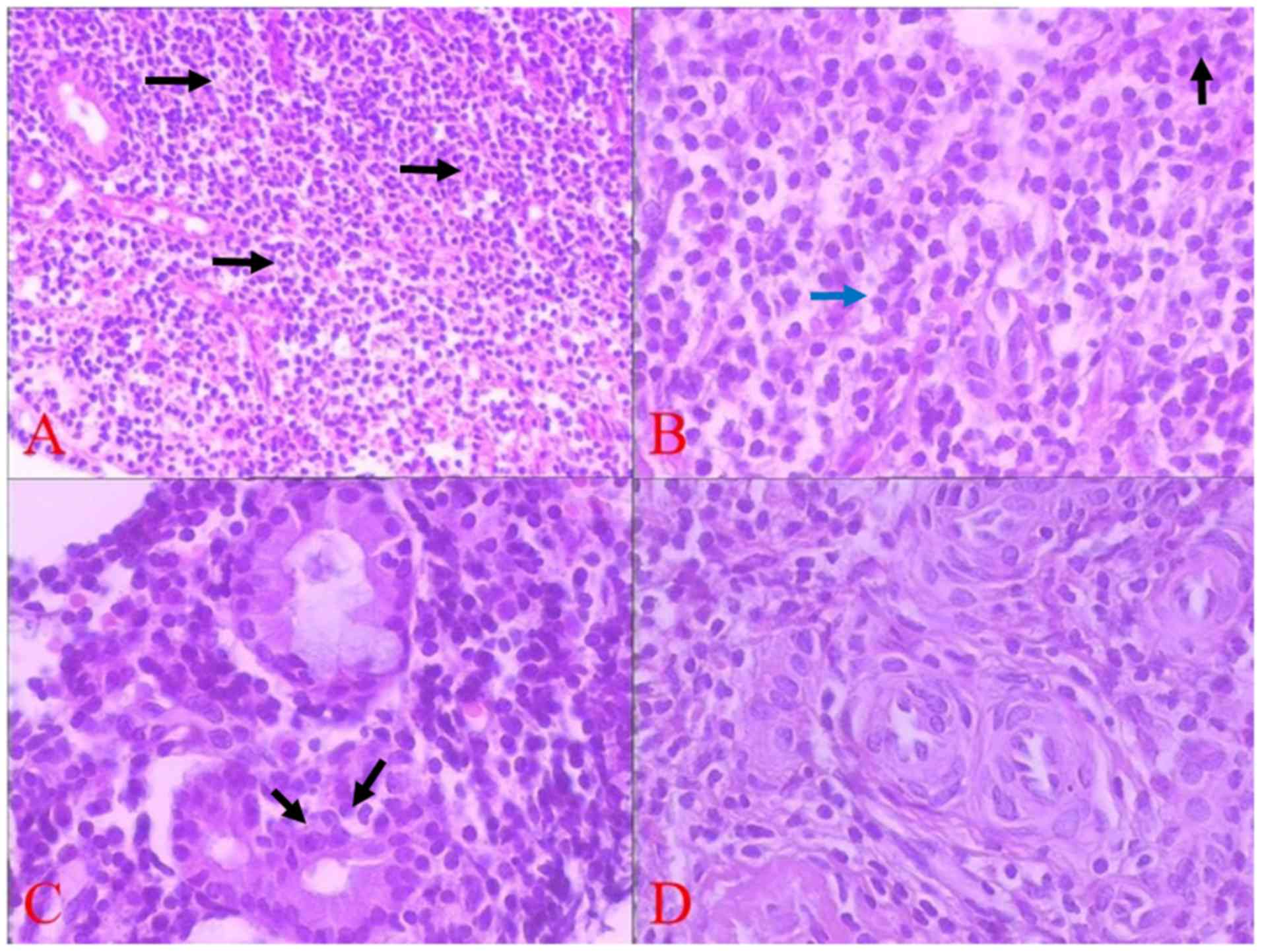
Microscopically, the tissue was covered by
pseudostratified ciliated columnar epithelium and intrinsic glands
were atrophic. Monomorphic tumor cells of a small volume had
infiltrated into the interstitial space (Fig. 3A). Under high magnification, the
neoplastic cells showed slight dysplasia with unclear boundary and
translucent or pink cytoplasm. The nuclei were small in size and
slightly irregular in shape, with evenly distributed chromatin. The
nucleolus was not obvious, and mitosis was observed occasionally
(Fig. 3B). The epithelium of mucosal
glands was invaded by tumor cells, and lymphoid epithelial lesions
were found locally (Fig. 3C).
Mitotic division was ~3/10 under high power field (light
microscope, 40×10 magnification). No blood vessel invasion or
coagulative necrosis were observed (Fig.
3D).
Immunohistochemical staining
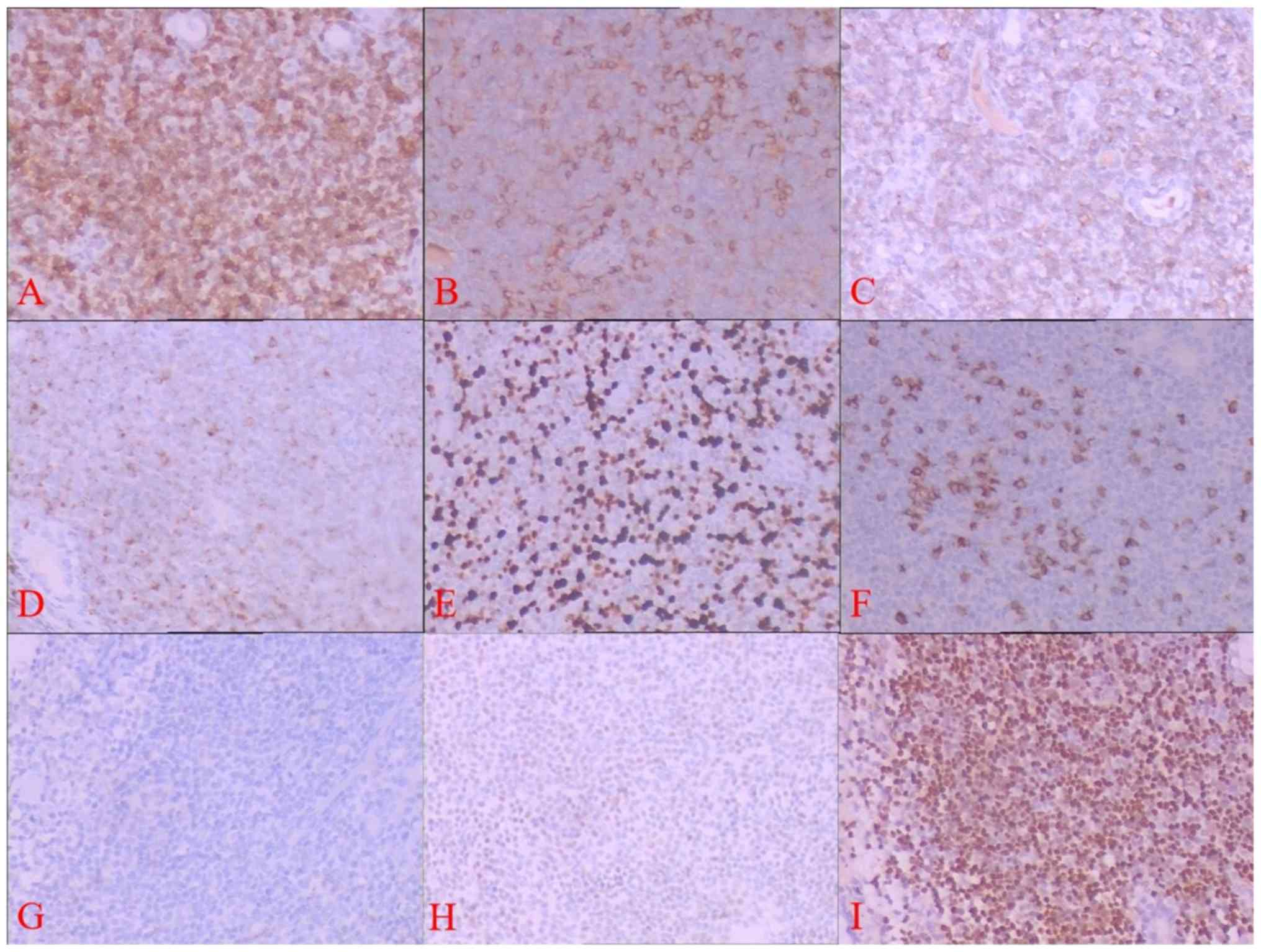
Immunohistochemical staining showed tumor cells were
positive for CD3, CD4, CD56, Bcl-2 and Granzyme B, and the Ki67
positive rate was <20%. No expression of CD2, CD5, CD7, CD8,
CD20, Cyclin D1, CD10, Bcl-6, CD23, Synaptophysin (Syn),
Cytokeratin, Desmin and epithelial membrane antigen (EMA) was
observed. Follicular dendritic cell network was not observed by
CD21 staining and 5% of tumor cells expressed TP53 weakly (Fig. 4A-H).
In situ hybridization detection
A positive EBV-EBER signal was diffusely and
consistently detected by in situ hybridization (Fig. 4I).
Diagnosis
On the basis of histological features,
immunohistochemical staining and results of in situ
hybridization, a diagnosis of left nasal NK/T cell lymphoma was
made after ruling out other small cell neoplasm such as MALToma,
Pseudomalignant NK-cell proliferation, Chronic active EBV infection
of NK cells, Peripheral T cell lymphoma, small cell carcinoma,
melanoma, embryonic rhabdomyosarcoma. This monomorphic type of NK/T
cell lymphoma was rare, however special staining supported the
diagnosis. As patient was young, there was low proliferation of
tumor cells and there was a lack of TP53 mutation, a good prognosis
was expected.
Treatment and follow-up
The disease was classified as clinical stage IE,
with a prognostic index for NK/T-cell lymphoma (PINK) score of 0
and PINK-EBV score of 0 after comprehensive evaluation. The patient
received ‘sandwich’ chemoradiation. The chemotherapy regimen
consisted of 3 cycles of intravenous GELOX regimen (gemcitabine,
1.2 g/m2 d1, 8; oxaliplatin, 160 mg/m2 d1;
pegaspargase, 3,200 IU/m2 d3, q21d) between October 19,
2017 and February 20, 2018. Nasopharynx radiotherapy was performed
between December 11, 2017 and January 7, 2018 [planning target
volume (PTV) 54 Gy/27 f]. Two cycles of LOP chemotherapy program
(pegaspargase, 3,200 IU/m2 d1; vindesine, 4
mg/m2 d1; dexamethasone, 10 mg/m2 d1-5) was
adapted during radiotherapy. After radiotherapy, the patient
received another cycle of GELOX for a total of 6 cycles of
chemotherapy. The patient also received two lumbar intrathecal
injections of cytarabine (50 mg/m2), dexamethasone (5
mg/m2) and methotrexate (10 mg/m2). All
chemotherapy was done by intravenous. An endoscopy on March 26,
2018 revealed the left nasal mucosa was smooth (Fig. 1B), and PET-CT showed the metabolic
value of the left nasal was lower than before (decrease from 2.1 to
4.3). No residual tumor cells were observed by mucosal biopsy. The
response to therapy was complete remission. The patient was
followed-up for 16 months after surgery, at which point she was in
good condition with no relapse.
Discussion
Classical nasal NK/T cell lymphoma includes three
main morphological characteristics: Pleomorphic tumor cells,
vascular invasion and coagulation necrosis (8). The case described in the present study
was unusual as it lacked the typical histological features
mentioned above and presented with monomorphic features similar to
MALToma. However, immunohistochemical markers and in situ
hybridization staining results supported the diagnosis of NK/T cell
lymphoma (9). A potential
differential diagnosis is neoplasm showing monomorphic shape, which
mimics MALToma, and should be ruled out. MALToma consists of small
lymphoid cells with relatively uniform size, and lymphoid
epithelial lesions and follicular implantation may also be present
(10). MALToma expressed CD20, but
not CD3, and the expression of restricted immunoglobulin light
chain could be helpful for differential diagnosis with NK/T cell
lymphoma (11). Pseudomalignant
NK-cell proliferation is considered as a pseudomalignant disease
because it spontaneously regresses without any treatment. The
atypical cells in pseudomalignant NK-cell proliferation disease
infiltrated diffusely and the glandular epithelium could be
involved occasionally. These cells showed the immunophenotype that
CD3, CD56 and cytotoxic molecule-associated proteins, such as
Granzyme B, were usually positive which was similar with NK/T cell
lymphoma. However, large eosinophilic cytoplasmic granules observed
in pseudomalignant NK-cell proliferation and negative pattern of
EBV-EBER were not consistent with NK/T-cell lymphoma (12). Chronic active EBV (CAEBV) infection
of NK cells occurs most often in children and adolescents according
to WHO classification (13). CAEBV
has the following diagnostic criteria: Increased EBV DNA
(>102.5 copies/mg) in peripheral blood; infectious
mononucleosis-like symptoms persisting for >3 months;
histological evidence of organ disease; and demonstration of EBV
RNA or viral protein in affected tissues in patients without known
immunodeficiency, malignancy or autoimmune disorders. The most
common sites of CAEBV are bone marrow, lymph nodes, liver, spleen
and skin (14). Although CAEBV and
NK/T cell lymphoma both originate from NK cells, clinical
presentation and laboratory tests distinguish the diseases from
each other (15,16). Peripheral T cell lymphoma also
presents with small tumor cells that express markers of T
lymphocytes such as CD2, CD3, CD4, CD5, CD7 and CD8, but negative
expression of CD56, cytotoxic markers, and no EBV-EBER detection
which could be helpful to differentiate it from NK/T cell lymphoma
(17).
Tumor cells in small cell carcinoma have significant
atypia, widespread mitosis and necrosis (18). Cytokeratin and neuroendocrine markers
such as Syn and chromograninA are positively expressed in small
cell carcinoma; however, these markers are negative in NK/T cell
lymphoma (19). The nasal cavity is
also a common site for malignant melanoma, which is aggressive and
can result in destruction of the skull (20). Melanoma could also present with small
cell in morphology. Positive expression of S-100, and melanoma
markers, HMB45 and melan-A, can be used to distinguish melanoma
from lymphoma (21). Embryonal
rhabdomyosarcoma usually occurs in children and young adults. This
type of tumor is composed of small cells with significant
eosinophilic cytoplasm and deviated nuclei, showing ‘tadpole-like’
or ‘racket-like’ cells, with the nucleus towards one side of the
cell (22). Myogenic markers such as
Desmin and myogenin were positive in embryonal rhabdomyosarcoma,
which could distinguish from NK/T cell lymphoma (23,24).
The clinical features of the patient described in
the present study were unusual. No clear mass was observed by nasal
endoscopy, and only mucosal inflammation and purulent secretion
were observed. Imaging studies lead to the misdiagnosis of chronic
inflammation. Obtaining a biopsy earlier in the disease stage could
be useful for early diagnosis. Mutant TP53 is considered to be an
important protein for the progression of NK/T cell lymphoma
(25–27). Previous studies on NK/T cell lymphoma
in small cell types have not reported mutant TP53 (9,28,29). In
the present study, mutant TP53 was found to be expressed lowly in
the tumor cells and ~5% of tumor cells were weakly positive. Ki67
expression, a marker of cell proliferation, was <20%, which is
much lower compared with Ki67 expression reported in NK/T cell
lymphoma with classic morphology (30). Combining the clinical characteristics
and laboratory results of this case, the conclusion was drawn that
small cell type of NK/T cell lymphoma may be indolent.
Radiotherapy and chemotherapy are two main
treatments for NK/T cell lymphoma (31); however, the prognosis of the majority
of patients is poor (32). The
patient in the present study received radiotherapy and
chemotherapy. Complete remission was obtained after 6 months, and
the patient achieved a good prognosis. This may be related to the
early clinical stage at which the disease was diagnosed. The
patient refused hematopoietic stem cell transplantation, and
prognosis remains to be followed up in the future.
NK/T cell lymphoma with small cell morphology is
unusual, and may be a type of indolent lymphoma with particular
characteristics of clinical presentation, imaging, pathology and
prognosis. Early biopsy would aid in obtaining the correct
diagnosis. More cases are required for further study, and attention
should be paid to this kind of NK/T cell lymphoma by radiologists,
pathologists and hematologists to prevent misdiagnosis in the
future.
Acknowledgements
Not applicable.
Funding
The present study was supporting by the Yantai Key
Research and Development Project (grant no. 2017WS101).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
GY designed the study, performed the histological
evaluation and drafted the manuscript. XL, LA, HL, SW and YL were
involved in the literature search and preparing of the material. HZ
and XP performed the histological diagnosis and revised the
manuscript. GQ and XC were involved in immunohistochemical
evaluation and amending the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Yantai
Yuhuangding Hospital Ethics Committee.
Patient consent for publication
Written informed consent was obtained from the
patient for participation in the present study and for publication
of this article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yamaguchi M, Suzuki R and Oguchi M:
Advances in the treatment of extranodal NK/T-cell lymphoma, nasal
type. Blood. 131:2528–2540. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan JKC, Quintanilla-Martinez L and Ferry
JA: WHO classification of tumor of hematopoietic and lymphoid
tissues (Revised 4th edition)IARC; Lyon: pp. 368–371. 2017
|
|
3
|
Kang DH, Huh J, Lee JH, Jeong YK and Cha
HJ: Gastrosplenic fistula occurring in lymphoma patients:
Systematic review with a new case of extranodal NK/T-cell lymphoma.
World J Gastroenterol. 23:6491–6499. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liang P, Ren XC and Gao JB: Radiological
and clinical features of primary NK/T-cell lymphoma involving the
whole length of the esophagus: A case report. Oncol Lett.
14:2147–2152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haverkos BM, Pan Z, Gru AA, Freud AG,
Rabinovitch R, Xu-Welliver M, Otto B, Barrionuevo C, Baiocchi RA,
Rochford R and Porcu P: Extranodal NK/T cell lymphoma, nasal type
(ENKTL-NT): An update on epidemiology, clinical presentation, and
natural history in North American and European cases. Curr Hematol
Malig Rep. 11:514–527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tse E and Kwong YL: Diagnosis and
management of extranodal NK/T cell lymphoma nasal type. Expert Rev
Hematol. 9:861–871. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zanelli M, Mengoli MC, Del Sordo R, Cagini
A, De Marco L, Simonetti E, Martino G, Zizzo M and Ascani S:
Intravascular NK/T-cell lymphoma, Epstein-Barr virus positive with
multiorgan involvement: A clinical dilemma. BMC Cancer.
18:11152018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sinkovics JG and Horvath JC: Human natural
killer cells: A comprehensive review. Int J Oncol. 27:5–47.
2005.PubMed/NCBI
|
|
9
|
Li S, Feng X, Li T, Zhang S, Zuo Z, Lin P,
Konoplev S, Bueso-Ramos CE, Vega F, Medeiros LJ and Yin CC:
Extranodal NK/T-cell lymphoma, nasal type: A report of 73 cases at
MD Anderson cancer center. Am J Surg Pathol. 37:14–23. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cook JR, Isaacson PG, Chott A, Nakamura S,
Muller-Hermelink HK, Harris NL and Swerdlow SH: WHO classification
of tumor of hematopoietic and lymphoid tissues (Revised 4th
edition)IARC; Lyon: pp. 259–262. 2017
|
|
11
|
Coha B, Vucinic I, Mahovne I and
Vukovic-Arar Z: Extranodal lymphomas of head and neck with emphasis
on NK/T-cell lymphoma, nasal type. J Craniomaxillofac Surg.
42:149–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takeuchi K, Yokoyama M, Ishizawa S, Terui
Y, Nomura K, Marutsuka K, Nunomura M, Fukushima N, Yagyuu T,
Nakamine H, et al: Lymphomatoid gastropathy: A distinct
clinicopathologic entity of self-limited pseudomalignant NK-cell
proliferation. Blood. 116:5631–5637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM,
et al: WHO classification of tumours of hematopoietic and lymphoid
tissues, revised 4th editionIARC; Lyon: 2017
|
|
14
|
Kimura H and Cohen JI: Chronic active
Epstein-Barr virus disease. Front Immunol. 8:18672017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu LM, Takata K, Miyata-Takata T, Asano N,
Takahashi E, Furukawa K, Miyoshi H, Satou A, Kohno K, Kosugi H, et
al: Clinicopathological analysis of 12 patients with Epstein-Barr
virus-positive primary intestinal T/natural killer-cell lymphoma
(EBV+ ITNKL). Histopathology. 70:1052–1063. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kimura H: EBV in T-/NK-cell tumorigenesis.
Adv Exp Med Biol. 1045:459–475. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uemura Y, Isobe Y, Uchida A, Asano J,
Nishio Y, Sakai H, Hoshikawa M, Takagi M, Nakamura N and Miura I:
Expression of activating natural killer-cell receptors is a
hallmark of the innate-like T-cell neoplasm in peripheral T-cell
lymphomas. Cancer Sci. 109:1254–1262. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hourdequin KC, Lefferts JA, Brennick JB,
Ernstoff MS, Tsongalis GJ and Pipas JM: Merkel cell polyomavirus
and extrapulmonary small cell carcinoma. Oncol Lett. 6:1049–1052.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ding W, Wang J, Zhao S, Yang Q, Sun H, Yan
J, Gao L, Yao W, Zhang W and Liu W: Clinicopathological study of
pulmonary extranodal nature killer/T-cell lymphoma, nasal type and
literature review. Pathol Res Pract. 211:544–549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ganti A, Raman A, Shay A, Kuhar HN, Auger
SR, Patel T, Kuan EC, Diaz AZ, Batra PS and Tajudeen BA: Treatment
modalities in sinonasal mucosal melanoma: A national cancer
database analysis. Laryngoscope. Apr 25–2019.(Epub ahead of print).
View Article : Google Scholar
|
|
21
|
Bourne TD, Bellizzi AM, Stelow EB, Loy AH,
Levine PA, Wick MR and Mills SE: p63 Expression in olfactory
neuroblastoma and other small cell tumors of the sinonasal tract.
Am J Clin Pathol. 130:213–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Y, Li C, Zhong Y, Guo W and Ren G: Head
and neck rhabdomyosarcoma in adults. J Craniofac Surg. 25:922–925.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiménez-Heffernan JA, González-Peramato P,
Perna C, Alvarez-Ferreira J, López-Ferrer P and Viguer JM:
Fine-needle aspiration cytology of extranodal natural killer/T-cell
lymphoma. Diagn Cytopathol. 27:371–374. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wooff JC, Weinreb I, Perez-Ordonez B,
Magee JF and Bullock MJ: Calretinin staining facilitates
differentiation of olfactory neuroblastoma from other small round
blue cell tumors in the sinonasal tract. Am J Surg Pathol.
35:1786–1793. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aozasa K, Takakuwa T, Hongyo T and Yang
WI: Nasal NK/T-cell lymphoma: Epidemiology and pathogenesis. Int J
Hematol. 87:110–117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang HS, Liao CK, Liu TT, You HL, Wang MC
and Huang WT: TP53 mutations in peripheral mature T and NK cell
lymphomas: A whole-exome sequencing study with correlation to p53
expression. Hum Pathol. 80:145–151. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou J, Zhang C, Sui X, Cao S, Tang F, Sun
S, Wang S and Chen B: Histone deacetylase inhibitor chidamide
induces growth inhibition and apoptosis in NK/T lymphoma cells
through ATM-Chk2-p53-p21 signalling pathway. Invest New Drugs.
36:571–580. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuo TT, Shih LY and Tsang NM: Nasal NK/T
cell lymphoma in Taiwan: A clinicopathologic study of 22 cases,
with analysis of histologic subtypes, Epstein-Barr virus LMP-1 gene
association, and treatment modalities. Int J Surg Pathol.
12:375–387. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McKelvie PA, Climent F, Krings G,
Hasserjian RP, Abramson JS, Pilch BZ, Harris NL, Ferry JA,
Zukerberg LR and Sohani AR: Small-cell predominant extranodal NK/T
cell lymphoma, nasal type: Clinicopathological analysis of a series
of cases diagnosed in a Western population. Histopathology.
69:667–679. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Au WY, Weisenburger DD, Intragumtornchai
T, Nakamura S, Kim WS, Sng I, Vose J, Armitage JO and Liang R;
International Peripheral T-Cell Lymphoma Project, : Clinical
differences between nasal and extranasal natural killer/T-cell
lymphoma: A study of 136 cases from the International peripheral
T-Cell lymphoma project. Blood. 113:3931–3937. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moon JH, Lee BH, Kim JA, Lee YJ, Chae YS,
Yhim HY, Kwak JY, Do YR, Park Y, Song MK, et al: Clinical impact of
induction treatment modalities and optimal timing of radiotherapy
for the treatment of limited-stage NK/T cell lymphoma. Leuk Res.
49:80–87. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamaguchi M and Miyazaki K: Current
treatment approaches for NK/T-cell lymphoma. J Clin Exp Hematop.
57:98–108. 2017. View Article : Google Scholar : PubMed/NCBI
|