Introduction
The incidence of neuroendocrine tumors (NETs) in the
United States increased 6.4 times between 1973 (1.09/100,000) and
2012 (6.98/100,000), however the overall 5-year survival rate of
NETs has also increased, the overall survival rate for all NETs
improved between 2000 and 2004 to 2009 and 2012 [hazard ratio (HR),
0.79; 95% confidence interval (CI), 0.73–0.85] (1). Currently, the majority of studies on
prognostic factors of digestive system NETs focus on those with
primary locations in the pancreas, stomach and small intestine.
Ter-Minassian et al (2)
reported that when distant metastases occur in NETs,
cancer-specific survival (CSS) time is shorter in male patients
with advanced age, primary pancreatic tumors and higher
histopathological grade. For patients with advanced pancreatic,
small bowel and other NETs, chromogranin A (CgA) is a highly useful
prognostic indicator (3). In
non-functioning pancreatic NETs, lymph node metastasis is an
independent factor of overall survival, and a tumor size <2 cm
is a protective factor for lymph node metastasis (4). Although the prognostic and predictive
factors for metastasis of NETs have been examined, the research
methods and findings of these studies vary, resulting in
considerable heterogeneity among the studies (5–8). To the
best of our knowledge, a limited number of studies have focused on
the prognostic and metastatic factors of colorectal NETs. They have
become the most common NETs in the digestive tract, as the
incidence of colorectal NETs has exceeded that of NETs in the
pancreas, small intestine and other parts of the digestive system
(1). The reason for the increased
incidence of digestive tract NETs may be due to the widespread
clinical application of high-resolution computed tomography and
colonoscopy (9). However, prognostic
and metastatic risk factors for colorectal NETs remain unclear.
In the present study, the Surveillance,
Epidemiology, and End Results (SEER) database was used to study
data from patients with colorectal NETs and to identify the
possible risk factors of NETs. Based on the examined risk factors,
a novel nomogram was proposed to predict the CSS of colorectal
NETs.
Materials and methods
Project design and medical record
selection
All medical records were obtained from the SEER
database (https://seer.cancer.gov), which provides
information on cancer statistics with the aim of reducing the
cancer burden among the US population (10). SEER is supported by the Surveillance
Research Program of the National Cancer Institute's Division of
Cancer Control and Population Sciences. The SEER*Stat software
(version 8.3.5; http://seer.cancer.gov/seerstat/) was used to obtain
detailed clinicopathological information from patients with
colorectal NETs. The criteria for the selection of medical records
were: i) The data was collected between 1973–2004, as some
covariates were introduced in the SEER database in 2004; ii) tumor
site was limited to colon and rectum; and iii) the
histopathological type code was used according to the NET
International Classification of Diseases codes for ‘carcinoid
tumors’ (8240/3), ‘enterochromaffin cell carcinoid’ (8241/3),
‘composite carcinoid’ (8244/3), ‘adenocarcinoid’ (8245/3),
‘neuroendocrine carcinoma’ (8246/3), ‘atypical carcinoid tumor’
(8249/3), ‘stromal carcinoid’ (9091/3), ‘islet cell carcinoma’
(8150/3) and ‘mixed islet cell/exocrine adenocarcinoma’ (8154/3)
(11). The records with the age of
diagnosis <18 years, unclear status of the 7th edition of the
American Joint Committee on Cancer (AJCC) staging (12) and incomplete information of CSS
status were excluded.
Variable selection
According to the rules of collection of SEER data,
the variables involved in the present study were classified based
on the type of data collected by SEER. Marital status was divided
into married and unmarried. The latter was divided into four
categories: i) Single (never married); ii) divorced; iii)
separated; and iv) widowed. The age grouping interception was
automatically calculated by SPSS version 22.0 (IBM Corporation),
which provided the best discrimination of cancer-specific survival.
Race/ethnicity was defined as Caucasian and other.
As the prognosis of patients with colorectal NETs
was distinctive from other colorectal tumors, the survival rate of
cecum, ascending colon, transverse colon, descending colon, sigmoid
colon and rectal NETs were previously assessed (Fig. S1). It was indicated that patients
with rectal NETs had the best survival rate, closely followed by
sigmoid colon. Survival analysis showed no statistically
significant difference between NETs at sigmoid colon and rectum.
NETs at the ascending, transverse and descending colon had the
worst survival, however results were also not statistically
significant. Combining with the conventional classification of
gastrointestinal tumors, which categorizes tumors based on the
anatomical location of the disease, such as the tumor originating
in the stomach was classified into gastric cancer, colorectal NETs
in the present study were classified into three categories: i)
Cecum; ii) ascending/transverse/descending colon; and iii) sigmoid
colon/rectum. Surgery was divided into two subgroups of ‘Yes’ or
‘No’. Tumor size was divided into two groups of >4 and ≤4 cm.
Histopathological grade was recorded as undifferentiated/poorly
differentiated, moderately differentiated and well differentiated
in the SEER database and using the 7th AJCC staging system these
were defined as low, intermediate and high histopathological
grades, respectively before analysis (10). In the SEER database, the specific
cause of death is an indicator of the CSS of a certain disease
(10). In the present study,
colorectal NETs were used as the specific cause of death.
Statistical analysis
All data was presented as mean ± standard deviation.
Statistical analysis was performed using SPSS version 22.0 (IBM
Corporation). The baseline demographic and clinicopathological
information were analyzed using the Descriptive Statistics function
in SPSS. Spearman's correlation analysis was used to initially
explore the potential correlation between the demographic and
clinicopathological factors, such as the correlation between 7th
AJCC stage (12) and primary tumor
site. Kaplan-Meier method and log-rank (Mantel-Cox) analysis were
used to visualize and compare the survival curves of each factor on
CSS, respectively. Receiver operating characteristic curve (ROC)
analysis was performed to reveal the risk prediction ability of
each potential factor for NETs distant metastasis, and to compare
the predictive power of the nomogram and clinical staging system to
the CSS of NETs. Univariate analysis was used to initially explore
a number of variables associated with CSS. Multivariate analysis
was used to screen variables with independent prognostic effects
and construct Cox proportional hazard regression models.
Crosstabulation and χ2 tests were used to analyze the
distribution of marital status in the age group and surgery group.
The nomogram was generated by R software (version 3.5.1; The R
Foundation for Statistical Computing). Binary logistic regression
analysis was used to obtain variables associated with distant
metastases. All P-values were two-sided. P<0.05 was considered
to indicate a statistically significant difference.
Results
Patient characteristics
Of the 3,093 patients, 48.9% were male and 51.1%
were female. The clinicopathological characteristics of patients
are presented in Table I. The median
age was 57.86±12.30 years. SPSS software automatically divided the
intercept points, and the age was divided into two groups: <70
and ≥70 years. A total of ~97.45% of the patients had insurance,
and there was a significant difference in the distribution of
insurance and no insurance (P<0.001). Of all patients, 62.75%
were married. The majority of the patients (92.79%) had undergone
surgical treatment. The primary tumor site was most commonly in the
sigmoid colon/rectum (71.71%). The total number of malignant tumors
did not exceed 2 in the majority of patients (96.50%), and 67.63%
of the patients had histopathological high-grade tumors. According
to the 7th edition of the AJCC staging system, 60.46% of the 3,093
patients were stage I, 7.73% were stage II, 17.85% were stage III
and 13.96% were stage IV. Correlation analysis was performed to
identify the factors that were likely to be relevant among these
clinicopathological factors; as a result, both clinical stage and
histopathological grade showed significant correlation with primary
tumor site, tumor size and surgery (Table II).
 | Table I.Baseline demographic and clinical
characteristics of 3,093 patients with colorectal neuroendocrine
tumors in Surveillance, Epidemiology and End Results databases. |
Table I.
Baseline demographic and clinical
characteristics of 3,093 patients with colorectal neuroendocrine
tumors in Surveillance, Epidemiology and End Results databases.
|
Characteristics | Total, n=3,093 |
|---|
| Age, years |
|
|
<70 | 2,579 |
|
≥70 | 514 |
| Sex |
|
|
Male | 1,512 |
|
Female | 1,581 |
| Race |
|
|
Caucasian | 1,964 |
|
Other | 1,129 |
| Insurance |
|
|
Yes | 3,014 |
| No | 79 |
| Marital status |
|
|
Married | 1,941 |
|
Unmarrieda | 1,152 |
| Tumor site |
|
| Sigmoid
colon/Rectum | 2,218 |
|
Cecum | 544 |
|
Asc/Transv/Desc colon | 331 |
| Surgery |
|
|
Yes | 2,870 |
| No | 223 |
| Tumor size, cm |
|
| ≤4 | 2,273 |
|
>4 | 820 |
| Malignant tumors
(n, ≤2 vs. >2) |
|
| ≤2 | 2,985 |
|
>2 | 108 |
| Histopathological
gradeb |
|
|
High | 2,092 |
|
Intermediate | 842 |
|
Low | 159 |
| 7th American Joint
Committee on cancer stagec |
|
| I | 1,870 |
| II | 239 |
|
III | 552 |
| IV | 432 |
 | Table II.Correlation analysis between
independent variables and the probable confounding variables
analyzed by Spearman's rho. |
Table II.
Correlation analysis between
independent variables and the probable confounding variables
analyzed by Spearman's rho.
| Interaction
factors | Correlation
coefficient | P-value |
|---|
| 7th American
Joint |
|
|
| Committee on cancer
stagea |
|
|
| Primary
tumor site | 0.693b | <0.001 |
| Tumor
size | 0.443b | <0.001 |
|
Surgery | 0.177b | <0.001 |
| Histopathological
gradec |
|
|
| Primary
tumor site | 0.409b | <0.001 |
| Tumor
size | 0.252b | <0.001 |
|
Surgery | 0.184b | <0.001 |
Prognostic and metastasis risk factor
screening and nomogram
Kaplan-Meier survival analysis was used to identify
the association between prognostic factors and CSS of patients with
colorectal NETs (Fig. 1). In the age
groups of <70 and ≥70 years, the CSS significantly decreased
with increasing age (P<0.001). For marital status, CSS rate of
the unmarried group was significantly lower compared with that of
the married group (P<0.001). The effect of marital status on CSS
of colorectal NETs was further studied; crosstabulation and
χ2 test were used to analyze the distribution of marital
status in the age (Table SI) and
surgery groups (Table SII). A
higher proportion of married patients in the age <70 group, and
a lower proportion in the age ≥70 group compared with unmarried
patients were observed (P<0.001; Table SI). In the surgery groups, compared
with unmarried patients, the proportion of married patients who
underwent surgical treatment was higher, and the proportion of
patients who did not receive surgical treatment was lower (P=0.001;
Table SII). The survival function
between the primary tumor site and CSS suggested that the CSS of
patients with colorectal NETs in the sigmoid colon/rectum was
higher compared with that of patients with NETs in the cecum or
ascending/transverse/descending colon (P<0.001). CSS reduced as
the total number of malignant tumors increased (P<0.001).
Fig. 1 also demonstrates that the
prognosis of patients that underwent surgery was improved compared
with that of patients who did not undergo surgery HR, 1.275; CI,
1.126–1.444; P<0.001. Patients with NETs with low
histopathological grade had shorter survival time compared with
those with high grade. The 7th edition of the AJCC staging system
successfully distinguished the survival conditions of the 3,093
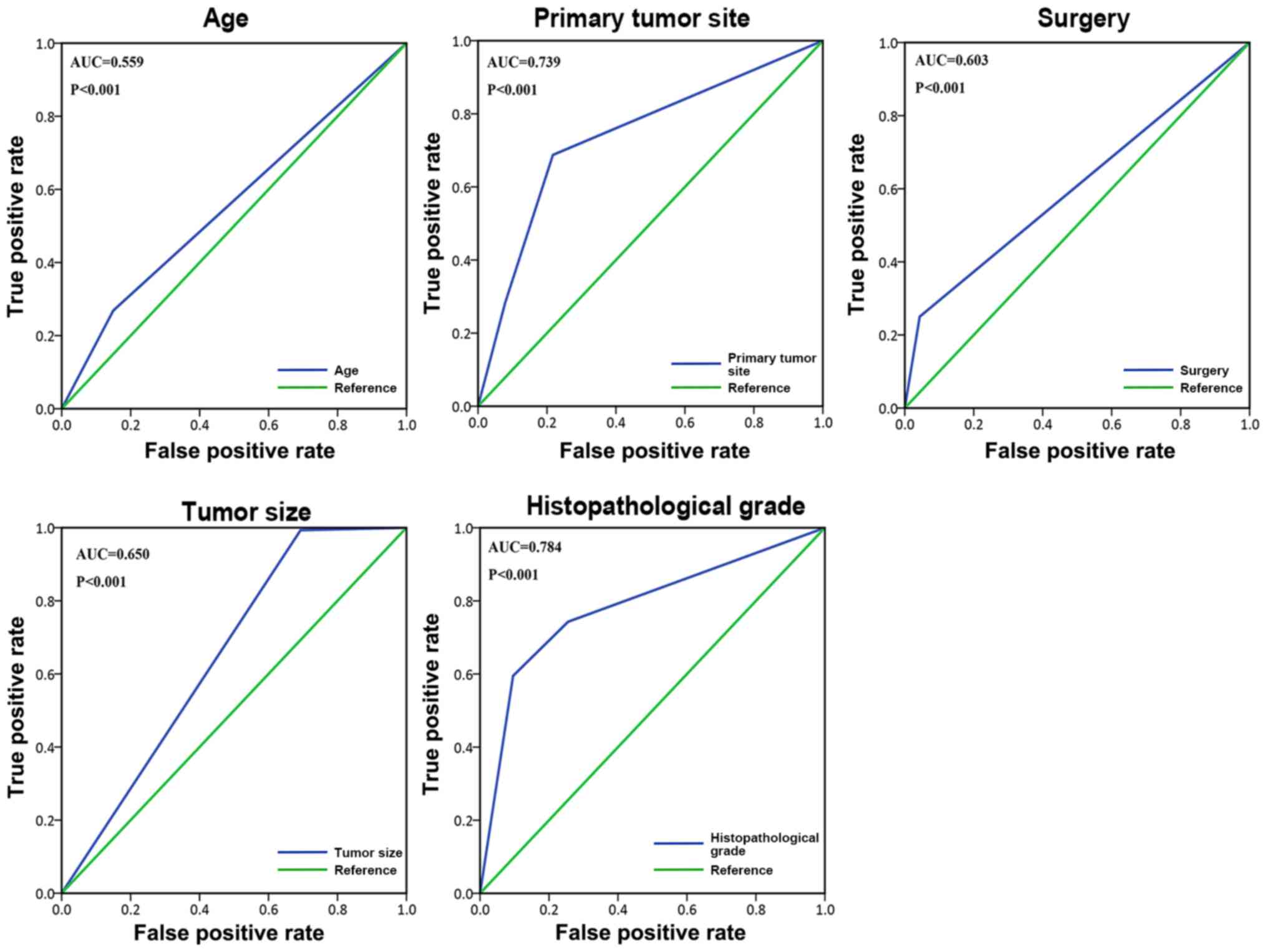
patients. The ROC curve (Fig. 2) was
used to demonstrate the value of the aforementioned five
metastasis-related variables: Age, primary tumor site, surgery,
tumor size, and histopathological grade. Among all the risk factors
for metastasis, histopathological grade [area under the curve
(AUC), 0.784; P<0.001] and age (AUC, 0.559; P<0.001)
exhibited the maximum and minimum AUC. Univariate analysis was used
to initially examine a number of variables associated with CSS
(Table III). Based on the results
of the univariate analysis, variables with P<0.05 were used as
statistically significant variables. Combining the results of the
univariate and correlation analyses, relevant variables were
incorporated into the Cox proportional hazard regression model to
obtain an unadjusted model (Table
IV). A total of five independent prognostic factors were
identified, including age, marital status, number of malignant
tumors, histopathological grade and clinical stage. HRs and 95% CIs
for each independent prognostic variable and two other adjusted Cox
proportional hazards regression models are presented in Table IV. After adjustment by demographic
and clinical pathological factors, the histopathological grade
(low) and 7th AJCC staging system in the adjusted model 2 all
showed a decrease in HR value and decreased by >10% compared
with the unadjusted model.
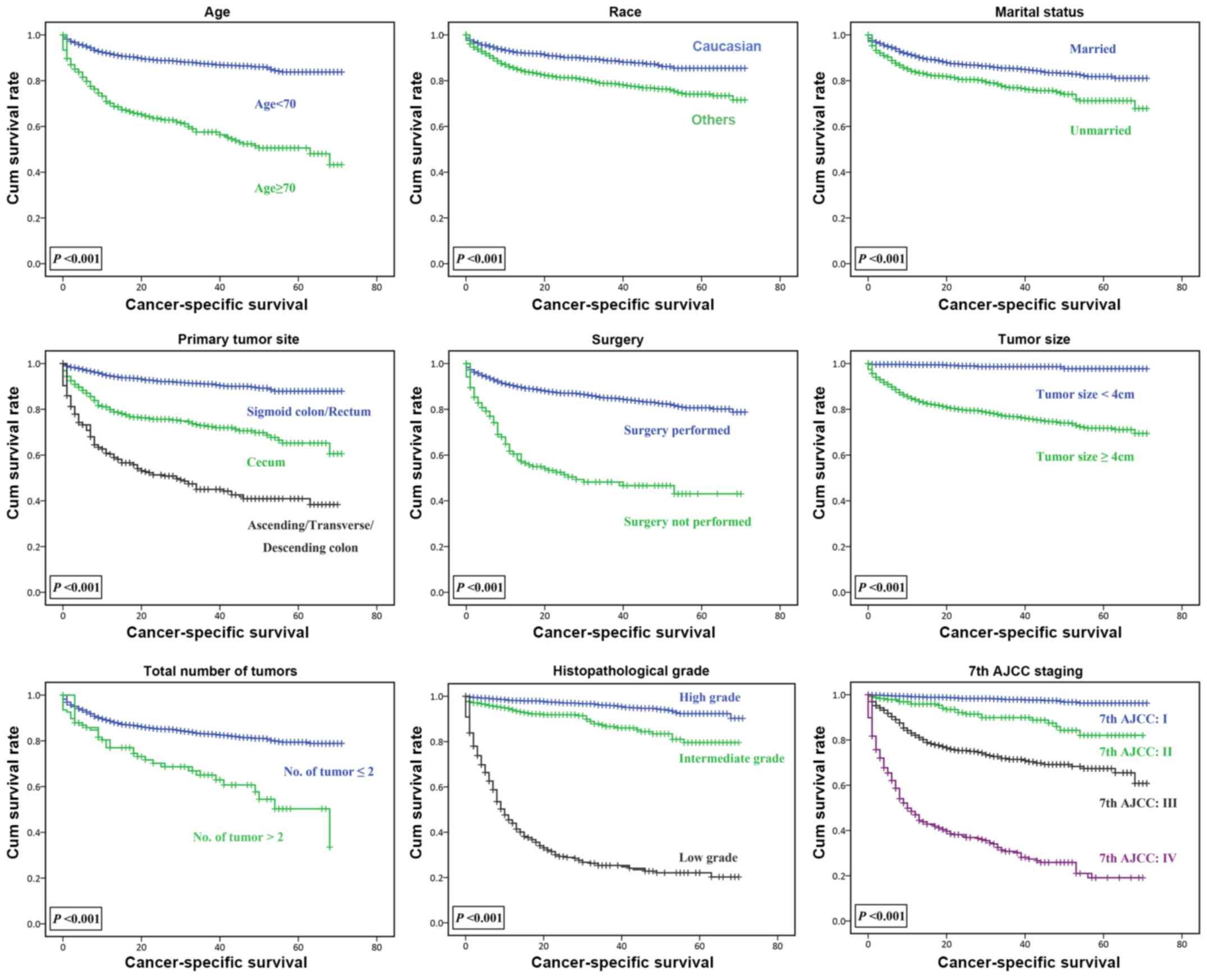 | Figure 1.Survival function reflecting the
association between age, race, marital status, primary tumor site,
surgery, tumor size, total number of tumors, histopathological
grade, clinical staging and cancer-specific survival. The 7th AJCC
staging system was used for the assessment of the size of the
primary tumor, local invasion, lymph node metastasis, and distant
metastasis. Histopathological grade is a grading system utilized to
assess the differentiation of tumors. Cumulative survival rate
refers to the cumulative probability of the survival rate after a
period of time. Cum survival, cumulative survival; AJCC, American
Joint Committee for Cancer. |
 | Table III.Univariate analysis with hazard
ratios of baseline characteristics for cancer-specific survival in
patients with colorectal neuroendocrine tumors. |
Table III.
Univariate analysis with hazard
ratios of baseline characteristics for cancer-specific survival in
patients with colorectal neuroendocrine tumors.
| Variable | Hazard ratio (95%
confidence interval) |
P-valuea |
|---|
| Age (<70 vs. ≥70
years) | 4.113
(3.407–4.966) | <0.001 |
| Sex (female vs.
male) | 1.152
(0.957–1.387) | 0.134 |
| Race (Caucasian vs.
other) | 1.976
(1.585–2.464) | <0.001 |
| Insurance (yes vs.
No) | 1.306
(0.767–2.225) | 0.325 |
| Marital status
(married vs. unmarriedb) | 1.682
(1.397–2.024) | <0.001 |
| Tumor site |
| <0.001 |
| Sigmoid
colon/rectum | Ref. |
|
|
Cecum | 3.590
(2.847–4.525) | <0.001 |
|
Asc/Transv/Desc colon | 8.477
(6.795–10.575) | <0.001 |
| Surgery (yes vs.
no) | 4.369
(3.474–5.494) | <0.001 |
| Tumor size (≤4 cm
vs. >4 cm) | 20.631
(10.253–41.513) | <0.001 |
| Malignant tumors
(n, ≤2 vs. >2) | 2.471
(1.765–3.459) | <0.001 |
| Histopathological
gradec |
| <0.001 |
|
High | Ref. |
|
|
Intermediate | 3.150
(2.191–4.529) | <0.001 |
|
Low | 31.652
(24.388–41.078) | <0.001 |
| 7th American Joint
Committee on cancer staged |
| <0.001 |
| I | Ref. |
|
| II | 5.304
(3.081–9.132) | <0.001 |
|
III | 15.255
(10.259–22.684) | <0.001 |
| IV | 58.410
(39.994–85.308) | <0.001 |
 | Table IV.Multivariate analysis with hazard
ratios for cancer-specific survival in patients with colorectal
neuroendocrine tumors. |
Table IV.
Multivariate analysis with hazard
ratios for cancer-specific survival in patients with colorectal
neuroendocrine tumors.
|
| Unadjusted
model | Adjusted model
1a | Adjusted model
2b |
|---|
|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
|
<70 | Ref. |
| Ref. |
| Ref |
|
|
≥70 | 1.667
(1.365–2.035) | <0.001 | 1.758
(1.436–2.151) | <0.001 | 1.693
(1.378–2.080) | <0.001 |
| Marital status |
|
Married | Ref. |
| Ref. |
| Ref. |
|
|
Unmarriedc | 1.736
(1.438–2.096) | <0.001 | 1.806
(1.488–2.190) | <0.001 | 1.811
(1.492–2.199) | <0.001 |
| Malignant tumors,
n |
| ≤2 | Ref. |
| Ref. |
| Ref. |
|
|
>2 | 2.177
(1.542–3.075) | <0.001 | 2.326
(1.641–3.297) | <0.001 | 2.273
(1.601–3.227) | <0.001 |
| Histopathological
graded |
| <0.001 |
| <0.001 |
| <0.001 |
|
High | Ref. |
| Ref. |
| Ref. |
|
|
Intermediate | 1.923
(1.329–2.783) | 0.001 | 1.957
(1.352–2.832) | 0.001 | 1.909
(1.319–2.763) | 0.001 |
|
Low | 9.977
(7.404–13.444) | <0.001 | 10.254
(7.596–13.842) | <0.001 | 8.771
(6.258–12.293) | <0.001 |
| 7th American
Joint |
| <0.001 |
| <0.001 |
| <0.001 |
| Committee on Cancer
stagee |
| I | Ref. |
| Ref. |
| Ref. |
|
| II | 1.542
(0.870–2.732) | 0.138 | 1.447
(0.814–2.574) | 0.208 | 1.094
(0.596–2.008) | 0.771 |
|
III | 4.525
(2.939–6.966) | <0.001 | 4.566
(2.958–7.048) | <0.001 | 3.266
(2.008–5.313) | <0.001 |
| IV | 16.343
(10.746–24.856) | <0.001 | 16.501
(10.845–25.105) | <0.001 | 11.490
(7.118–18.548) | <0.001 |
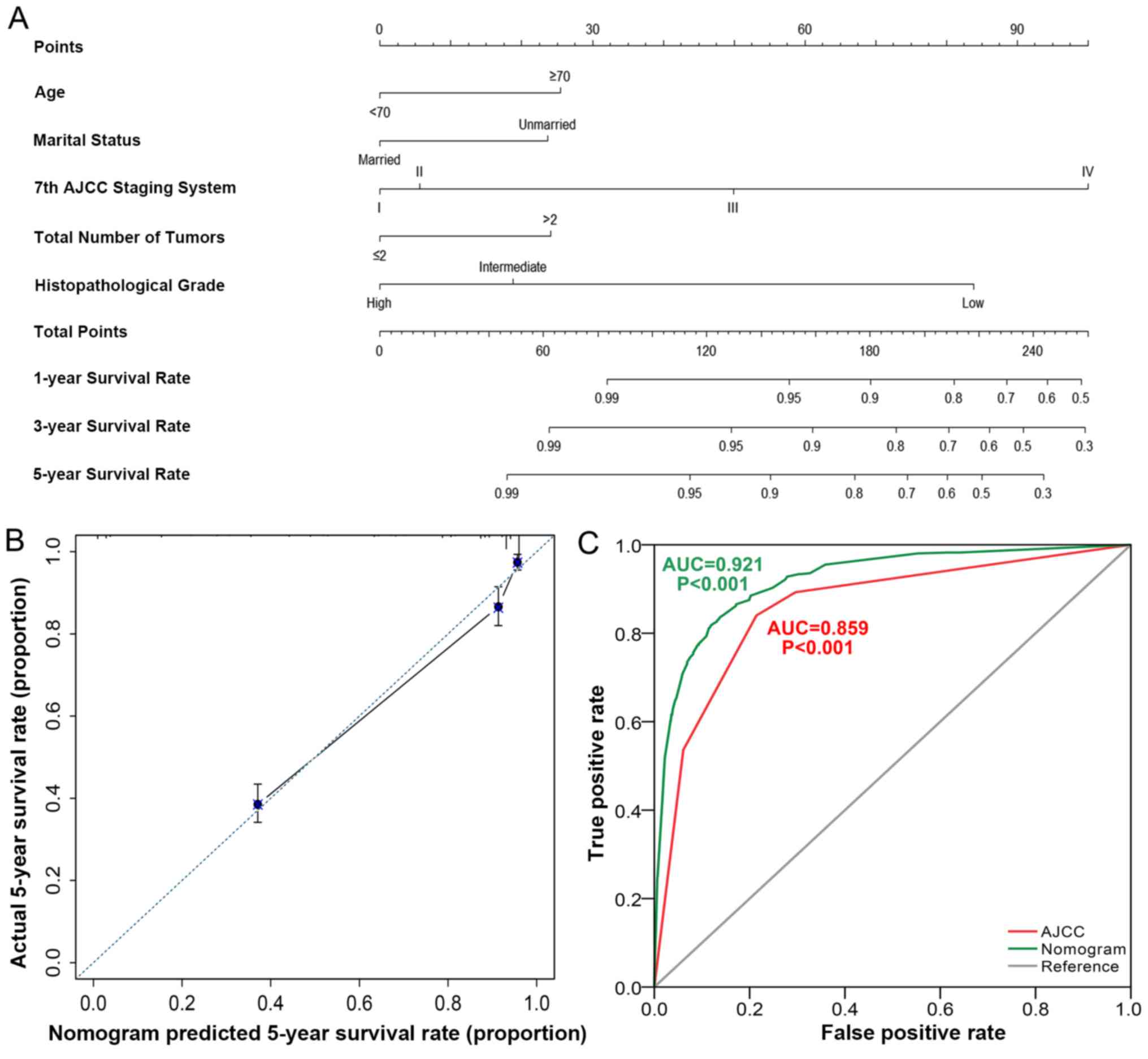
Based on these Cox proportional hazards regression
models, a nomogram containing five factors was constructed
(Fig. 3A). The calibration curve
(Fig. 3B) revealed moderate accuracy
of the nomogram, and the ROC curve (Fig.
3C) indicated that the nomogram (AUC, 0.921; P<0.001) had
higher predictive power compared with the 7th edition of AJCC
staging system (AUC, 0.850; P<0.001).
Nomogram scoring system
According to the nomogram calculation, when age is
<70 years, the score is 0, whereas when age is ≥70 years, the
score is 25 points (Fig. 3A).
Married patients are assigned 0 points, whereas unmarried patients
are assigned 24 points. AJCC stages I, II, III, IV are assigned
corresponding scores of 0, 6, 50 and 100, respectively. Total
number of tumors ≤2 is assigned 0 points, whereas >2 tumors gain
a score of 24. The scores of low, intermediate and high
histopathological grades are 84, 19 and 0, respectively.
For example, patient 1 aged 67 years, unmarried
(divorced), with AJCC stage III, had a total number of tumors >2
(three tumors), and the histopathological grade was intermediate
(moderately differentiated). The 1-, 3- and 5-year CSS predicted by
the nomogram was 90, 70 and 60%, respectively.
Risk factors for distant metastasis were analyzed
using logistic regression analysis. A total of five distant
metastasis relative risk factors were identified, including age,
primary tumor site, surgery, tumor size and histopathological
grade. This model was adjusted for demographics of race, sex,
insurance status, marital status and clinical characteristics of
the total number of malignant tumors (Table V).
 | Table V.Logistic regression analysis of
associated factors in colorectal neuroendocrine tumor
metastasis. |
Table V.
Logistic regression analysis of
associated factors in colorectal neuroendocrine tumor
metastasis.
| Variable | ORa (95% CI) | P-value | ORb (95% CI) | P-value |
|---|
| Age, years |
|
|
|
|
|
<70 | Ref. |
| Ref. |
|
|
≥70 | 0.670
(0.493–0.909) | 0.010 | 0.686
(0.503–0.937) | 0.018 |
| Tumor site |
|
|
| <0.001 |
| Sigmoid
colon/rectum | Ref. |
| Ref. |
|
|
Cecum | 6.246
(4.534–8.605) | <0.001 | 6.510
(4.683–9.049) | <0.001 |
|
Asc/transv/desc colon | 3.486
(2.390–5.085) | <0.001 | 3.555
(2.425–5.211) | <0.001 |
| Surgery |
|
|
|
|
|
Yes | Ref. |
| Ref. |
|
| No | 8.531
(5.686–12.799) | <0.001 | 8.374
(5.564–12.601) | <0.001 |
| Tumor size, cm |
|
|
|
|
| ≤4 | Ref. |
| Ref. |
|
|
>4 | 15.925
(5.004–50.683) | <0.001 | 16.340
(5.130–52.048) | <0.001 |
| Histopathological
gradec |
|
|
| <0.001 |
|
High | Ref. |
| Ref. |
|
|
Intermediate | 1.752
(1.227–2.501) | 0.002 | 1.761
(1.232–2.517) | 0.002 |
|
Low | 5.007
(3.530–7.102) | <0.001 | 5.042
(3.545–7.172) | <0.001 |
Discussion
Based on the 3,093 medical records from the SEER
database, age, marital status, number of malignant tumors,
histopathological grade and clinical stage were identified to be
independent prognostic factors for colorectal NETs. Therefore, a
novel nomogram to predict the survival of colorectal NETs was
proposed; the calibration and AUC curve indicated that the nomogram
had a higher predictive power compared with the AJCC staging
system. In addition, age, primary tumor site, surgery, primary
tumor size and histopathological grade were risk factors associated
with distant metastasis of colorectal NETs.
Multivariate analysis revealed that age, marital
status, number of malignant tumors, histopathological grade and
clinical stage were stable and independent variables of CSS.
Unadjusted model, adjusted model 1 and adjusted model 2 were
compared, which revealed that after adjusting for demographic
variables and demographic plus clinicopathological variables, the
HRs of the five variables mentioned above were roughly stable. Of
note, following adjustment, the HR of clinical stage and
histopathological grade (low) decreased by >10% compared with
unadjusted model. The results of the correlation analysis between
demographic and clinicopathological variables demonstrated that the
variables used to adjust the model were significantly correlated
with the clinical stage and histopathological grade, which may
explain why the HRs of these two factors decreased following
adjustment. In addition, patients with AJCC stage II exhibited no
statistically significant differences in CSS compared with patients
in stage I, which suggested that the prognostic discrimination of
stage I and stage II in the 7th edition of the AJCC staging system
may need to be improved. These results were consistent with a
previous study (13).
The association between marital status and disease
is currently an intriguing topic, as a number of researchers have
reported a protective effect of marriage on various malignancies,
such as bladder cancer and non-small cell lung cancer (14–16). As
marital status affects a wide range of diseases, it was
hypothesized that an association would exist between marital status
and colorectal NETs. In the present study, married patients with
NETs had longer CSS compared with unmarried patients. Although the
association between marital status and disease has been suggested
by a number of studies, to the best of our knowledge the possible
protective effect of marital status in colorectal NETs was proposed
for the first time in the present study. The role of marriage,
especially social support and its consistent and substantial impact
on colorectal NET treatment, intervention and survival was further
examined. These results indicate that investments in
social-targeted support for vulnerable populations, for example,
unmarried patients, may increase the possibility of treatment and
improve the survival of patients with colorectal NETs.
Marriage serves a protective role in a number of
diseases, including prostate and bladder cancer (14,15,17). A
number of studies have suggested that the protective effect of
marriage is likely associated with better access to care in married
patients compared with unmarried patients (18–20). The
encouragement from spouse and increased medication adherence are
the likely factors associated with the protective role of marital
status (21,22). In addition, married patients have a
more stable emotional state and are less prone to anxiety or
depression (23). Previous studies
have demonstrated that married patients exhibit lower levels of
cortisol and more stable circadian rhythms; factors beneficial for
CSS and overall survival rate (24–28).
A number of studies have suggested that that
cohabitation may bias the analysis of CSS based on the four marital
statuses registered in SEER (16,29,30). In
the present study, the marital status had a statistically
significant protective effect on CSS. As only married vs. unmarried
patients were compared, the effect of cohabitation in a limited
number of patients cannot be omitted, but the protective effect of
marriage existed in the present study.
No statistically significant association was
observed between marital status and distant metastasis of
colorectal NETs in the present study. However, Aizer et al
(31) reported that married patients
with any one of ten cancers (lung, colorectal, breast, pancreatic,
prostate, liver/intrahepatic bile duct, head/neck, ovarian and
esophageal cancers and non-Hodgkin's lymphoma) had a lower risk of
distant metastases compared with unmarried patients. As the present
study was conducted using data from only ~3,000 patients, a larger
cohort study may help explain this phenomenon.
Insurance has been associated with protective
effects in a number of cancer-associated studies, for example,
breast and lung cancer (32,33). However, in the present study, results
suggested that there was no statistically significant difference
between insurance status and overall survival of colorectal NETs.
The heterogeneity of data may account for this, as only 79 patients
(2.55%) were uninsured. In addition, the detailed information of
insurance classification was not recorded in SEER data. Therefore,
the corresponding hierarchical analysis of the types of insurance
was lacking in the current study. A larger and more targeted
designed cohort study is required to uncover the association
between insurance and the prognosis of colorectal NETs.
Based on the five factors, age, marital status, 7th
AJCC staging system, total number of tumors, and histopathological
grade, a novel nomogram of colorectal NETs with a high AUC was
proposed in the present study, which demonstrated increased
discrimination ability compared with the 7th AJCC staging system.
The nomogram integrated not only clinical stage, but also other
factors that may affect the CSS of patients with NETs, such as age,
total tumor number, histopathological grade and marital status. The
associations between age, total tumor number, histopathological
grade and CSS were linear; the older the age, the greater the
number of primary tumors and the lower the histopathological grade
of the tumor, the worse the overall survival of the patients. This
is common in cancers, such as breast cancer, leukemia and lymphoma
(34–36).
The possible limitations of this study should be
considered. First, data related to other clinical histopathological
indicators, such as CgA and Ki-67, are not available in SEER
database. However, the level of plasma CgA is usually normal in
poorly differentiated G3 NETs (37).
Second, with the exception of the basic records associated with
surgery, the medical records in the SEER database do not include
other information on patients, such as various health care
conditions or socioeconomic factors and the use of cigarettes and
alcohol, which may be associated with CSS.
Despite these limitations, the present study has
identified independent prognostic factors associated with CSS of
patients with colorectal NETs: Age, marital status, number of
malignant tumors, histopathological grade and clinical stage. The
protective effects of marital status in the CSS of colorectal NETs
were analyzed. Based on these five factors, a novel nomogram was
constructed to predict CSS. In addition, the risk factors
associated with distant metastasis of colorectal NETs were
identified: Age, primary tumor site, surgery, tumor size and
histopathological grade. Caregivers and medical professionals
concerned about patients with colorectal NETs should be aware of
the relative factors associated with the prognosis of this
population, and may use the nomogram to predict CSS to inform the
assessment of prognosis and the choice of coping strategies for the
disease.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81572396 and 81672408), the
Natural Science Foundation of Guangdong Province, China (grant nos.
2014A030313050 and 2018A030310227), the Specialized Research Fund
for the Doctoral Program of Higher Education (grant no.
20130171120093), the Program of Science and Technology Star of
Zhujiang Guangzhou City, China (grant no. 201610010078), the
Science and Technology Program of Guangzhou, China (grant no.
201508020013), the Science and Technology Planning Project of
Guangdong Province, China (grant no. 2013B021800233), the Key
Laboratory of Malignant Tumor Molecular Mechanism and Translational
Medicine of Guangzhou Bureau of Science and Information Technology
(grant no. 2013163), the Key Laboratory of Malignant Tumor Gene
Regulation and Target Therapy of Guangdong Higher Education
Institutes (grant no. KLB09001), the Medical Scientific Research
Foundation of Guangdong Province, China (grant no. A2016210), the
Fundamental Research Funds for the Central University (grant no.
18zxxt59) and the Medical Scientific Research Foundation of
Guangdong Province, China (grant no. A2018012).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the SEER database, https://seer.cancer.gov.
Authors' contributions
KH and YC conceived and designed the study, and
revised the manuscript. JZ, SC and GL prepared the manuscript and
completed statistical analysis. RL and YL acquired and processed
the data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NETs
|
neuroendocrine tumors
|
|
CSS
|
cancer-specific survival
|
|
SEER
|
Surveillance, Epidemiology and End
Results
|
|
CgA
|
chromogranin A
|
|
HR
|
hazard ratio
|
|
95% CI
|
95% confidence interval
|
|
AJCC
|
American Joint Committee on Cancer
|
|
ROC
|
receiver operating characteristic
|
|
AUC
|
area under the curve
|
References
|
1
|
Dasari A, Shen C, Halperin D, Zhao B, Zhou
S, Xu Y, Shih T and Yao JC: Trends in the incidence, prevalence,
and survival outcomes in patients with neuroendocrine tumors in the
united states. JAMA Oncol. 3:1335–1342. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ter-Minassian M, Chan JA, Frauenhoffer CS,
Hooshmand SM, Asomaning K, Lin X, Christiani DC and Kulke MH:
Prospective analysis of clinical outcomes and prognostic factors in
patients with neuroendocrine tumors (NETs). J Clin Oncol. 28:4044.
2010. View Article : Google Scholar
|
|
3
|
Ter-Minassian M, Chan JA, Hooshmand SM,
Brais LK, Daskalova A, Heafield R, Buchanan L, Qian ZR, Fuchs CS,
Lin X, et al: Clinical presentation, recurrence, and survival in
patients with neuroendocrine tumors: Results from a prospective
institutional database. Endocr Relat Cancer. 20:187–196. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Toste PA, Kadera BE, Tatishchev SF, Dawson
DW, Clerkin BM, Muthusamy R, Watson R, Tomlinson JS, Hines OJ,
Reber HA and Donahue TR: Nonfunctional pancreatic neuroendocrine
tumors <2 cm on preoperative imaging are associated with a low
incidence of nodal metastasis and an excellent overall survival. J
Gastrointest Surg. 17:2105–2113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scherubl H: Tumor biology and prognosis of
gastrointestinal carcinoids. J Clin Oncol. 26:6012–6013. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A and Evans
DB: One hundred years after ‘carcinoid’: Epidemiology of and
prognostic factors for neuroendocrine tumors in 35,825 cases in the
United States. J Clin Oncol. 26:3063–3072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Franko J, Feng W, Yip L, Genovese E and
Moser AJ: Non-functional neuroendocrine carcinoma of the pancreas:
Incidence, tumor biology, and outcomes in 2,158 patients. J
Gastrointest Surg. 14:541–548. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilson C: Cancer: Neuroendocrine
tumours-prognostic insights. Nat Rev Endocrinol. 9:1332013.
View Article : Google Scholar
|
|
9
|
Lawrence B, Gustafsson BI, Chan A, Svejda
B, Kidd M and Modlin IM: The epidemiology of gastroenteropancreatic
neuroendocrine tumors. Endocrinol Metab Clin North Am. 40:1–18.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
National Cancer Institute, . The
Surveillance, Epidemiology, and End Results (SEER) Program. Cancer
Statistics, SEER Data & Software, Registry Operations.
https://seer.cancer.govMay
30–2018
|
|
11
|
Fraenkel M, Kim MK, Faggiano A and Valk
GD: Epidemiology of gastroenteropancreatic neuroendocrine tumours.
Best Pract Res Clin Gastroenterol. 26:691–703. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
American Joint Committee on Cancer, . AJCC
7th Ed Cancer Staging Manual. https://cancerstaging.org/references-tools/deskreferences/Pages/default.aspxMay
30–2018
|
|
13
|
Gao S, Pu N, Liu L, Li C, Xu X, Wang X and
Lou W: The latest exploration of staging and prognostic
classification for pancreatic neuroendocrine tumors: A large
population-based study. J Cancer. 9:1698–1706. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nelles JL, Joseph SA and Konety BR: The
impact of marriage on bladder cancer mortality. Urol Oncol.
27:263–267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Neuman MD and Werner RM: Marital status
and postoperative functional recovery. JAMA Surg. 151:194–196.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jatoi A, Novotny P, Cassivi S, Clark MM,
Midthun D, Patten CA, Sloan J and Yang P: Does marital status
impact survival and quality of life in patients with non-small cell
lung cancer? Observations from the mayo clinic lung cancer cohort.
Oncologist. 12:1456–1463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nepple KG, Kibel AS, Sandhu GS, Kallogjeri
D, Strope SA and Grubb RL: Impact of marital status on prostate
cancer-specific mortality and overall mortality after radical
prostatectomy. J Clin Oncol. 30:73. 2012. View Article : Google Scholar
|
|
18
|
Vallgarda S: Addressing individual
behaviours and living conditions: Four Nordic public health
policies. Scand J Public Health. 39 (Suppl):6–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arntzen A and Nybo Andersen AM: Social
determinants for infant mortality in the Nordic countries,
1980–2001. Scand J Public Health. 32:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jakobsen L, Niemann T, Thorsgaard N,
Thuesen L, Lassen JF, Jensen LO, Thayssen P, Ravkilde J, Tilsted
HH, Mehnert F and Johnsen SP: Dimensions of socioeconomic status
and clinical outcome after primary percutaneous coronary
intervention. Circ Cardiovasc Interv. 5:641–648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aizer AA, Paly JJ, Zietman AL, Nguyen PL,
Beard CJ, Rao SK, Kaplan ID, Niemierko A, Hirsch MS, Wu CL, et al:
Multidisciplinary care and pursuit of active surveillance in
low-risk prostate cancer. J Clin Oncol. 30:3071–3076. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cohen SD, Sharma T, Acquaviva K, Peterson
RA, Patel SS and Kimmel PL: Social support and chronic kidney
disease: An update. Adv Chronic Kidney Dis. 14:335–344. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Uecker JE: Marriage and mental health
among young adults. J Health Soc Behav. 53:67–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goldzweig G, Andritsch E, Hubert A,
Brenner B, Walach N, Perry S and Baider L: Psychological distress
among male patients and male spouses: What do oncologists need to
know? Ann Oncol. 21:877–883. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sephton SE, Sapolsky RM, Kraemer HC and
Spiegel D: Diurnal cortisol rhythm as a predictor of breast cancer
survival. J Natl Cancer Inst. 92:994–1000. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weissman MM, Bland RC, Canino GJ,
Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK,
Lellouch J, et al: Cross-national epidemiology of major depression
and bipolar disorder. JAMA. 276:293–299. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sephton SE, Lush E, Dedert EA, Floyd AR,
Rebholz WN, Dhabhar FS, Spiegel D and Salmon P: Diurnal cortisol
rhythm as a predictor of lung cancer survival. Brain Behav Immun.
30 (Suppl):S163–S170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
DiMatteo MR, Lepper HS and Croghan TW:
Depression is a risk factor for noncompliance with medical
treatment: Meta-analysis of the effects of anxiety and depression
on patient adherence. Arch Intern Med. 160:2101–2107. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schaefer EW, Wilson MZ, Goldenberg D,
Mackley H, Koch W and Hollenbeak CS: Effect of marriage on outcomes
for elderly patients with head and neck cancer. Head Neck.
37:735–742. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lowery WJ, Stany MP, Phippen NT, Bunch KP,
Oliver KE, Tian C, Maxwell GL, Darcy KM and Hamilton CA: Survival
advantage of marriage in uterine cancer patients contrasts poor
outcome for widows: A surveillance, epidemiology and end results
study. Gynecol Oncol. 136:328–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aizer AA, Chen MH, McCarthy EP, Mendu ML,
Koo S, Wilhite TJ, Graham PL, Choueiri TK, Hoffman KE, Martin NE,
et al: Marital status and survival in patients with cancer. J Clin
Oncol. 31:3869–3876. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ellis L, Canchola AJ, Spiegel D, Ladabaum
U, Haile R and Gomez SL: Trends in cancer survival by health
insurance status in california from 1997 to 2014. JAMA Oncol.
4:317–323. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Halpern MT, Ward EM, Pavluck AL, Schrag
NM, Bian J and Chen AY: Association of insurance status and
ethnicity with cancer stage at diagnosis for 12 cancer sites: A
retrospective analysis. Lancet Oncol. 9:222–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shapiro CL: Cancer Survivorship. N Engl J
Med. 379:2438–2450. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie S and Hossain MJ: Survival differences
in childhood and young adult acute myeloid leukemia: A
cross-national study using us and england data. Cancer Epidemiol.
54:19–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Villano JL, Koshy M, Shaikh H, Dolecek TA
and McCarthy BJ: Age, gender, and racial differences in incidence
and survival in primary CNS lymphoma. Br J Cancer. 105:1414–1418.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oberg K, Knigge U, Kwekkeboom D and Perren
A; ESMO Guidelines Working Group, : Neuroendocrine
gastro-entero-pancreatic tumors: ESMO clinical practice guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 23
(Suppl):vii124–vii130. 2012. View Article : Google Scholar : PubMed/NCBI
|