Introduction
Gastric cancer (GC) is one of the most malignant
tumors worldwide, and for those diagnosed, the prognosis is still
poor (1). GC has recently been
ranked globally as the fourth most common malignancy, accounting
for >700,000 deaths each year (2), thus making it the second leading cause
of cancer-associated mortality worldwide. Due to the non-specific
symptoms of early-stage GC, the majority of patients are only
diagnosed at an advanced stage (3).
Current tumor biomarkers for GC, such as serum carcinoembryonic
antigen (CEA) and carbohydrate antigen 19-9 (CA199), are not ideal
due to their relatively low sensitivity and specificity when
regarding diagnosis and prognosis (4,5).
Therefore, it is necessary to identify novel sensitive and specific
biological markers for GC that can assist in early diagnosis, and
subsequently, determine a target therapy for prolonging the life of
patients with GC (6,7).
The pathogenesis of GC is complex and only partially
known. Previous studies primarily concentrated on the following
factors: Likelihood of Helicobacter pylori infection,
genetics, lifestyle and diet (8,9).
However, major medical advances in molecular technologies over the
past two decades have led to a deeper understanding of the
pathogenesis of GC. Identifying the biomarkers that are associated
with the development and metastasis of GC may assist clinicians in
tailoring therapies by identifying those patients who are at a high
risk of GC and thus, propose novel molecular targets for its
treatment (10). Previous studies
have investigated specific genes and proteins, as well as their
roles in GC in a search for reliable prognostic markers (8–11).
Studying novel molecular prognostic markers may contribute to the
elucidation of the molecular mechanisms that underlie GC. Nowadays,
tumor targeted therapy is the focus of current research; in
addition, the identification of prognostic tumor markers could be
used to develop new and effective targeted treatment strategies for
GC.
The gene for proteasome subunit α7 (PSMA7), also
known as XAPC7 in mammals, is located on the chromosomal anomaly
20q13.33. It has been demonstrated that the 20q region is amplified
in numerous types of tumor (11,12).
PSMA7 is an α-type subunit of the 20S proteasome core complex and
participates in the degradation of proteins through the
ubiquitin-proteasome pathway in eukaryotic cells. The protein
complex has a molecular mass of ~2,000 kDa and is comprised of an
associated 20S proteolytic core and one or two 19S regulatory
complexes (13,14). The core particle consists of two α-
and two β-rings, which form a cylindrical, four-layered αββα
structure (15,16). The α-ring is composed of seven
homologous but distinct subunits, α1-7 (17,18), and
the β-ring is also composed of seven homologous and distinct β
subunits, β1–7 (19–22). Previous studies have demonstrated
that PSMA7 serves a role in the retrogradation of a series of
proteins essential for the replication of Hepatitis B virus (HBV)
(23,24), Hepatitis C virus (25), and human immunodeficiency virus
(26). More studies have
demonstrated that PSMA7 interacts with a number of proteins
implicated in tumorigenesis, such as hypoxia-inducible factor-1α
(27), endothelial
monocyte-activating polypeptide-II (28), HBV X protein (23,24),
c-Abl and Arg tyrosine kinases (29), which are important for the regulation
of mammalian genes that are involved in angiogenesis, glucose
metabolism and apoptosis.
Previously, downregulation of PSMA7 was detected in
K-ras transformed AsPC-1 pancreatic cancer cells compared with
untransformed AsPC-1 cells (30),
while Richardson et al (31)
revealed that PSMA7 expression is elevated in testicular and breast
cancer. Romanuik et al (32)
observed that high or increased PSMA7 expression is also observed
in castration-recurrent prostate cancer. Shi et al (33) detected the overexpression of PSMA7 in
liver cancer. Scotto et al (34) and Hu et al (13) reported the overexpression of PSMA7 in
cervical cancer and colorectal cancer, respectively. Hu et
al (35) revealed that the
overexpression of PSMA7 correlated with certain clinical
pathological parameters of colorectal cancer and liver metastasis.
Honma et al (36)
demonstrated that PSMA7 is a potential target for RNA
interference-based therapeutics for colorectal cancer. However,
little is known about the expression and function of PSMA7 in the
development or metastasis of GC. In the present study, the
expression profile of PSMA7 was investigated in GC tissue samples,
as well as the association between PSMA7 expression and survival
rate among patients with GC.
Materials and methods
Human GC tissue samples and clinical
information
Tissue microarrays (TMA) were prepared using
paraffin-embedded blocks of malignant and benign gastric tissue
samples from 735 patients (age range, 19–84 years) and a tissue
array sample obtained from The Affiliated Hospital of Nantong
University (Nantong, China) between January 2003 and December 2010.
The tissue samples included 410 cancer and 212 matched
pericarcinomatous tissues: 24 high-grade intraepithelial neoplasia,
27 low-grade intraepithelial neoplasia, 28 intestinal metaplasia
and 34 chronic gastritis. The clinical information obtained
included sex, age, histological type, differentiation grade, tumor
invasion, lymph node metastasis, distant metastasis,
Tumor-Node-Metastasis (TNM) stage (37), preoperative serum CA19-9 levels and
CEA levels. The 7th edition of the TNM staging in malignant tumors
criteria was used to determine tumor stage (37). None of the patients received any form
of treatment, including radiotherapy, chemotherapy or
immunotherapy, prior to surgical resection. In addition, 60 paired
and freshly frozen GC and matching peritumoral tissues were
obtained from The Affiliated Xinghua People's Hospital of Yangzhou
University Medical College (Xinghua, China). The Ethics Committee
of Nanjing Medical University approved the study, and all the
following experiments were performed according to the relevant
guidelines and regulations. All participants provided written
informed consent.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was isolated from freshly frozen GC
tissues and matching peritumoral tissues using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol, and cDNA was synthesized using the
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd.).
RT-qPCR was performed using the Applied Biosystems StepOne and
StepOnePlus Real-Time PCR systems (Thermo Fisher Scientific, Inc.).
GAPDH was used for monitoring internal standards. Primers were
purchased from GenScript. Relative PSMA7 expression was analyzed
using the 2−ΔΔCq method (38). PCR primers were designed with Primer
Express Software (version 2.0), and were as follows: PSMA7 forward,
5′-AGTGCGGAAGATCTGTGCTT-3′ and reverse, 5′-TCCGTACGCGTTGTTGTAAT-3′;
GAPDH forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′. The reaction system (20 µl)
contained 2 µl of cDNA template, each primer 20 µM (0.4 ul), 7.2 µl
of 2X SYBR®-Green PCR Master Mix (High Rox Plus; Applied
Biosystems; Thermo Fisher Scientific, Inc.) and 10 ul aseptic
ultra-pure water. The following conditions were used for RT-qPCR:
Initial denaturation at 95°C for 5 min, followed by 40 cycles of
denaturation at 95°C for 10 sec, annealing and extension at 60°C
for 30 sec, and dissolution curve at 95°C for 15 sec, 60°C for 1
min and 95°C for 15 sec. The Cq-value for each sample was
calculated with the ΔΔCq method and relative results were analyzed
using 2−ΔΔCq (38). All
results were normalized to GAPDH expression and each experiment was
repeated three times.
TMA construction and
immunohistochemical (IHC) evaluation
Core tissue biopsies of 2 mm in diameter were
obtained from individual samples fixed with 10% formalin overnight
at 37°C and paraffin-embedded. IHC analysis of TMA slides was
applied to assess the expression of PSMA7, containing 735 gastric
tissues. Using the TMA System Quick Ray (UT06; UNITMA Co., Ltd.),
manual gastric TMAs were generated at the Affiliated Hospital of
Nantong University (Nantong, China).
IHC staining was performed using the Envision
technique (39). Briefly, TMA
sections (4 µm thick) were dewaxed in xylene and rehydrated in
graded alcohol, and then the sections were boiled in citrate
solution (0.01 M; pH 6.0) using a microwave for antigen retrieval.
Endogenous peroxidase activity was then blocked with 3%
H2O2 for 10 min at room temperature, and
sections were blocked in 5% bovine serum albumin (Sigma-Aldrich;
Merck KGaA) for 10 min at 37°C. Sections were then incubated with a
mouse anti-human PSMA7 monoclonal antibody (1:50; catalog no.
LS-C114781; LifeSpan Biosciences, Inc.) overnight at 4°C, and with
an Envision horseradish peroxidase-conjugated goat anti-mouse-IgG
monoclonal antibody at room temperature for 30 min (1:100; catalog
no. s35962; Dako; Agilent Technologies, Inc.). Diluted PBS replaced
the primary antibody for the negative control. All sections were
processed at the same time and under the same conditions.
Representative images were captured and analyzed using a light
microscope (Carl Zeiss AG). PSMA7 expression was assessed using the
semi-quantitative H-score method, which takes both the staining
intensity and the percentage of cells with that intensity into
account (40). Staining intensity
was scored using 4 grades, as follows; i) 0, no staining; ii) 1+,
weak staining; iii) 2+, moderate staining; and iv) 3+, intense
staining. For each case, the total score was calculated by
multiplying the percentage of positive cells by the staining
intensity score. The scores were computed, ranging from 0 to 300;
the minimum final staining score was 0 (no staining) and the
maximum score was 300 (100% of cells with 3+ staining intensity).
All scoring was evaluated by two experienced pathologists, who were
blinded to the experimental procedure.
Statistical analysis
All data were analyzed using SPSS (version 20.0; IBM
Corp.) and STATA (version 12.0; StataCorp) statistical software
applications. Continuous PSMA7 expression data from IHC were
initially converted into dichotic data (low vs. high) using
specific cut-off values. The cut-off values were selected to be
significant when taking into account overall survival (OS) using
the X-Tile software program (version 3.6.1) for TMA data analysis
(Rimm Lab, Yale University) (41,42). A
score of 0–130 was considered to indicate no or low expression,
while 131–300 was considered to indicate high expression. For
subsequent analyses, PSMA7 protein expression levels were
considered as either ‘No or Low’ or ‘High’ using these cut-off
points prior to analysis. Associations between clinical parameters
and the expression of PSMA7 were assessed using χ2
tests. Kaplan-Meier analysis was used to evaluate the associations
between the 5-year survival of patients with GC that were
exhibiting PSMA7 expression. The significance of the differences
between curves was analyzed using a log-rank test. The Cox
proportional hazards regression model was used to perform
univariate and multivariate analyses. A paired-samples Student's
t-test was also used. Each experiment was repeated three times.
P<0.05 was considered to indicate a statistically significant
difference, and 95% confidence intervals (CI) were used
throughout.
Results
Expression of PSMA7 mRNA in GC
tissues
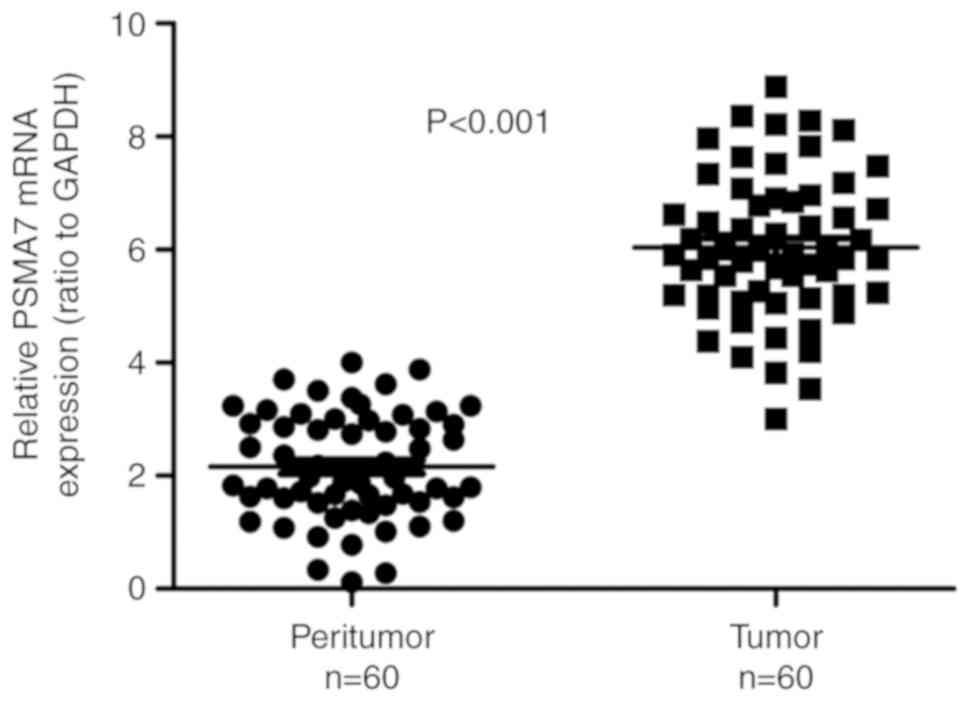
The PSMA7 expression at the mRNA level was evaluated
using RT-qPCR in 60 paired GC and matching peritumoral tissues. The
analysis demonstrated that PSMA7 mRNA expression in gastric
carcinoma tissues was significantly increased compared with in the
matching peritumoral tissues (P<0.001; Fig. 1).
Expression of PSMA7 protein in gastric
tissues
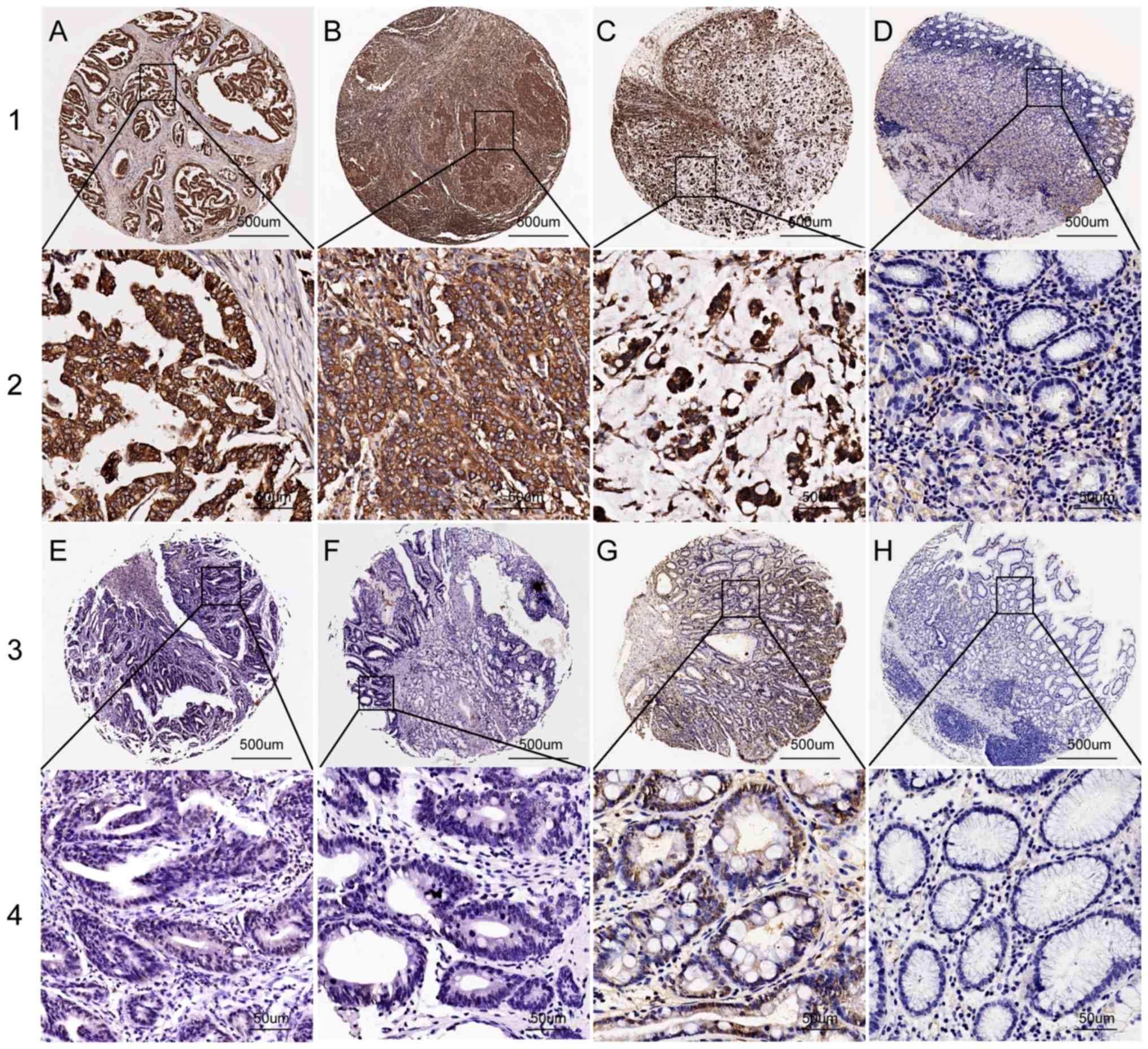
IHC was used to examine PSMA7 protein expression in
GC tissues, benign gastric disease tissues and matching peritumoral
tissues. PSMA7 protein expression was mostly present in the
cytoplasm and nucleus (Fig. 2). The
PSMA7 IHC data were scored using the semi-quantitative H-score
method, taking into account the staining intensity and the
percentage of cells at that intensity ranging from 0–300 (40). The cut-off point of PSMA7 expression
was ascertained using the X-tiles software program (41,42) for
clinical data analysis according to OS among patients with GC.
PSMA7 protein expression in GC tissues was higher compared with in
chronic gastritis, intestinal metaplasia, low-grade intraepithelial
neoplasia, high-grade intraepithelial neoplasia and matching
peritumoral tissues (all P<0.05; Table I).
 | Table I.PSMA7 expression in gastric
tissues. |
Table I.
PSMA7 expression in gastric
tissues.
|
|
| PSMA7 expression, n
(%) |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | Total, n | No or Low | High | χ2 | P-value |
|---|
| Gastric cancer | 410 | 159 (38.78) | 251 (61.22) | 75.3782 | <0.001 |
| Peritumoral
tissue | 212 | 154 (72.64) | 58 (27.36) | 64.0925 | <0.001 |
| High-grade
intraepithelial neoplasia | 24 | 15 (62.50) | 9 (37.50) | 5.3110 | 0.021 |
| Low-grade
intraepithelial neoplasia | 27 | 18 (66.67) | 9 (33.33) | 8.1745 | 0.004 |
| Intestinal
metaplasia | 28 | 17 (60.71) | 11 (39.29) | 5.2460 | 0.022 |
| Chronic
gastritis | 34 | 25 (73.53) | 9 (26.47) | 15.6220 | <0.001 |
Association of PSMA7 expression with
clinicopathological characteristics in patients with GC
The associations between PSMA7 expression and the
clinicopathological parameters of GC are presented as the following
(Table II): High PSMA7 positive
staining within the cytoplasm and nucleus was significantly
associated with tumor invasion (P=0.012), lymph node metastasis
(P=0.006), distant metastasis (P=0.006) and TNM stage (P<0.001).
PSMA7 expression was not associated with any other clinical
parameters, including sex, age, histological type, differentiation
and serum levels of CEA and CA199.
 | Table II.Association of PSMA7 expression with
clinicopathological characteristics of patients with gastric
cancer. |
Table II.
Association of PSMA7 expression with
clinicopathological characteristics of patients with gastric
cancer.
|
|
| PSMA7
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | Total, n | No or low (%) | High (%) | χ2 | P-value |
|---|
| Total | 410 | 159 (38.78) | 251 (61.22) |
|
|
| Sex |
|
|
| 2.0689 | 0.150 |
|
Male | 301 | 123 (40.86) | 178 (59.14) |
|
|
|
Female | 109 | 36 (33.03) | 73 (66.97) |
|
|
| Age, years |
|
|
| 0.0070 | 0.934 |
|
≤60 | 148 | 57 (38.51) | 91 (61.49) |
|
|
|
>60 | 262 | 102 (38.93) | 160 (61.07) |
|
|
| Histological
type |
|
|
| 5.7666 | 0.217 |
|
Tubular | 322 | 116 (36.02) | 206 (63.98) |
|
|
|
Mucinous | 29 | 13 (44.83) | 16 (55.17) |
|
|
| Mixed
(tubular and mucinous) | 10 | 6 (60.00) | 4 (40.00) |
|
|
| Signet
ring cell | 31 | 16 (51.61) | 15 (48.39) |
|
|
|
Othersa | 18 | 8 (44.44) | 10 (55.56) |
|
|
|
Differentiation |
|
|
| 6.6376 | 0.084 |
|
Well | 21 | 11 (52.38) | 10 (47.62) |
|
|
|
Moderate | 98 | 38 (38.78) | 60 (61.22) |
|
|
|
Poor | 252 | 89 (35.32) | 163 (64.68) |
|
|
|
Othersb | 39 | 21 (53.85) | 18 (46.15) |
|
|
| Tumor invasion |
|
|
| 12.7703 | 0.012 |
|
Tis | 30 | 18 (60.00) | 12 (40.00) |
|
|
| T1 | 53 | 27 (50.94) | 26 (49.06) |
|
|
| T2 | 70 | 29 (41.43) | 41 (58.57) |
|
|
| T3 | 222 | 74 (33.33) | 148 (66.67) |
|
|
| T4 | 35 | 11 (31.43) | 24 (68.57) |
|
|
| Lymph node
metastasis |
|
|
| 12.4718 | 0.006 |
| N0 | 163 | 80 (49.08) | 83 (50.92) |
|
|
| N1 | 81 | 28 (34.57) | 53 (65.43) |
|
|
| N2 | 89 | 28 (31.46) | 61 (68.54) |
|
|
| N3 | 77 | 23 (29.87) | 54 (70.13) |
|
|
| Distant
metastasis |
|
|
| 7.4160 | 0.006 |
| M0 | 386 | 156 (40.41) | 230 (59.59) |
|
|
| M1 | 24 | 3 (12.50) | 21 (87.50) |
|
|
| TNM stage |
|
|
| 24.1716 | <0.001 |
| 0 | 30 | 19 (63.33) | 11 (36.67) |
|
|
| I | 73 | 40 (54.79) | 33 (45.21) |
|
|
| II | 141 | 54 (38.30) | 87 (61.70) |
|
|
|
III | 142 | 40 (28.17) | 102 (71.83) |
|
|
| IV | 24 | 6 (25.00) | 18 (75.00) |
|
|
| Preoperative CEA,
ng/ml |
|
|
| 5.1842 | 0.075 |
| ≤5 | 177 | 62 (35.03) | 115 (64.97) |
|
|
|
>5 | 44 | 13 (29.55) | 31 (70.45) |
|
|
|
Unknown | 189 | 84 (44.44) | 105 (55.56) |
|
|
| Preoperative CA199,
ng/ml |
|
|
| 1.0034 | 0.605 |
|
≤37 | 191 | 79 (41.36) | 112 (58.64) |
|
|
|
>37 | 30 | 11 (36.67) | 19 (63.33) |
|
|
|
Unknown | 189 | 69 (36.51) | 120 (63.49) |
|
|
Upregulation of PSMA7 protein is
associated with poor prognosis of GC
Univariate and multivariate analyses were used to
investigate the prognostic value of PSMA7 expression in GC.
Univariate Cox regression analysis suggested that high PSMA7
expression [hazard ratio (HR), 1.005; 95% CI, 1.003–1.007;
P<0.001], age (HR, 3.135; 95% CI, 2.209–4.450; P<0.001),
histological differentiation (HR, 1.393; 95% CI, 1.131–1.715;
P=0.002), tumor invasion (HR, 2.059; 95% CI, 1.714–2.411;
P<0.001), lymph node metastasis (HR, 1.773; 95% CI, 1.576–1.995;
P<0.001), distant metastasis (HR, 3.316; 95% CI, 2.063–5.330;
P<0.001) and TNM stage (HR, 2.059; 95% CI, 1.764–2.402;
P<0.001) are positive prognostic factors (Table III). Multivariate Cox regression
analysis confirmed PSMA7 expression (HR, 0.995; 95% CI,
0.993–0.998; P<0.001), lymph node metastasis (HR, 1.592; 95% CI,
1.305–1.941; P<0.001) and TNM stage (HR, 1.592; 95% CI,
1.219–2.079; P<0.001) to be independent prognostic factors
(Table III). Kaplan-Meier survival
analysis revealed that patients with higher PSMA7 expression levels
showed poor OS and disease-free survival rates compared with those
with lower PSMA7 expression levels (P<0.001; Fig. 3A and B). In addition, patients with
deep tumor invasion, lymph node metastasis, distant metastasis and
poor TNM stage had significantly lower OS compared with patients
with shallow tumor invasion, no lymph node metastasis or distant
metastasis and good TNM stage (P<0.001; Fig. 3C-F).
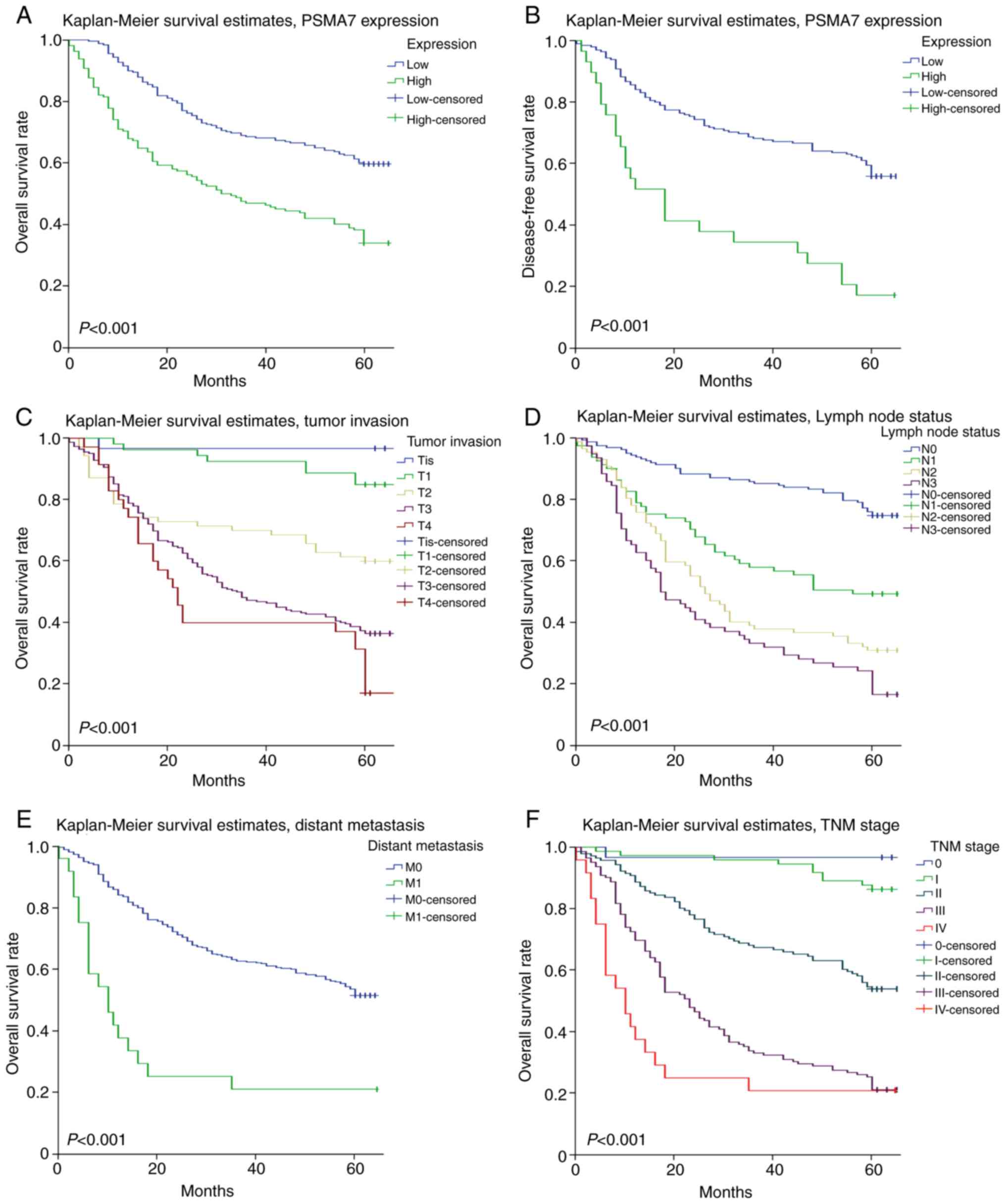 | Figure 3.Survival curves of gastric carcinoma
generated by the Kaplan-Meier method and the log-rank test. (A) OS
curves comparing PSMA7 expression levels. (B) Disease-free survival
curves comparing PSMA7 expression levels. (C) OS curves comparing
levels of tumor invasion. (D) OS curves comparing lymph node
metastasis status. (E) OS curves comparing distant metastasis
status. (F) OS curves comparing TNM stage. M0, metastasis-negative;
M1, metastasis-positive; N0, node-negative; N1, 1–2 nodes; N2, 3–6
nodes; N3, >6 nodes; OS, overall survival; PSMA7, proteasome
subunit α7; T1, lamina propria, submucosa; T2, muscularis propria;
T3, subserosa; T4, adjacent structures; Tis, carcinoma in
situ; TNM, Tumor-Node-Metastasis. |
 | Table III.Univariate and multivariate analysis
of prognostic factors for overall survival in gastric cancer. |
Table III.
Univariate and multivariate analysis
of prognostic factors for overall survival in gastric cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
| HR | P-value | 95% CI | HR | P-value | 95% CI |
|---|
| PSMA7
expression |
| High
vs. low or no | 1.005 | <0.001 | 1.003–1.007 | 0.995 | 0.001 | 0.993–0.998 |
| Age, years |
| ≤60 vs.
>60 | 3.135 | <0.001 | 2.209–4.450 |
|
|
|
| Sex |
| Male
vs. female | 1.109 | 0.903 | 0.747–1.390 |
|
|
|
| Histological type,
tubular vs. mucinous vs. mixed (tubular and mucinous) vs. signet
ring cell vs. othersa | 1.015 | 0.802 | 0.901–1.145 |
|
|
|
| Differentiation,
well vs. moderate vs. poor vs. othersb | 1.393 | 0.002 | 1.131–1.715 |
|
|
|
| Tumor invasion, Tis
vs. T1 vs. T2 vs. T3 vs. T4 | 2.059 | <0.001 | 1.714–2.411 |
|
|
|
| Lymph node
metastasis, N0 vs. N1 vs. N2 vs. N3 | 1.773 | <0.001 | 1.576–1.995 | 1.592 | <0.001 | 1.305–1.941 |
| Distant metastasis,
M0 vs. M1 | 3.316 | <0.001 | 2.063–5.330 |
|
|
|
| TNM stage, 0 vs. I
vs. II vs. III vs. IV | 2.059 | <0.001 | 1.764–2.402 | 1.592 | <0.001 | 1.219–2.709 |
| CEA, ≤5 vs.
>5 | 1.151 | 0.053 | 0.998–1.328 |
|
|
|
| CA199, ≤37 vs.
>37 | 1.133 | 0.081 | 0.985–1.303 |
|
|
|
Discussion
The majority of GC cases are characterized by late
clinical presentation, rapid progression and poor survival.
Considering the high morbidity and mortality rates of GC, a number
of studies have been dedicated to identifying available and new
prognostic markers. Several studies have demonstrated that PSMA7
serves a key role in the development of tumors (12,13).
However, other studies have indicated that PSMA7 expression is a
poor prognostic factor (14,35). Hu et al (13) highlighted the associations between
PSMA7 expression and the clinicopathological characteristics of
colorectal cancer and suggested that PSMA7 may be a molecular
target for colorectal cancer therapy. PSMA7 protein and mRNA levels
in GC tissues were measured by TMA, IHC and RT-qPCR in the present
study. The results of the present study indicated that the
expression of PSMA7 protein and mRNA is higher in GC tissues
compared with in benign tissues. These experimental results
preliminarily demonstrate that PSMA7 is a tumor-associated antigen,
which coincides with the findings of Shi et al (33). D'Errico et al (43) revealed that PSMA7 expression was
upregulated in gastric intestinal type adenocarcinoma and gastric
mixed adenocarcinoma compared with gastric mucosa samples. Li et
al (44) revealed that PSMA7 was
overexpressed in GC compared with normal tissues; however, only the
PSMA7 expression at the mRNA level was investigated. The results of
the present study confirmed that overexpression of PSMA7 predicted
poor prognosis at the mRNA and the protein level.
Based on the association between the PSMA7 protein
expression levels and the clinicopathological values in patients
with GC, data analysis indicated that high expression of PSMA7 in
GC was associated with tumor invasion, lymph node metastasis,
distant metastasis and TNM stage in the present study. These
results indicated that PSMA7 may be a potential biomarker for
prognosis of GC, which coincides with previous studies (13,35).
However, Tan et al (12)
verified that PSMA7 inhibits the proliferation, tumorigenicity and
invasion of A549 cells in vitro and in vivo. This may
be associated with the tissue specificity of PSMA7 expression in
different types of tumor cell (45).
For patients with GC, CEA and CA199 are the most commonly used
serum tumor markers. However, in the present study, high PSMA7
expression was not significantly associated with the serum levels
of CEA and CA199; this may be due to its relatively low sensitivity
and specificity for diagnosis and prognosis (4,5).
Univariate analyses indicated that PSMA7 expression
and six other factors (age, differentiation, tumor invasion, lymph
node metastasis, distant metastasis and TNM stage) have
statistically significant associations with OS. All seven factors
were included in the multivariate Cox proportional hazards model to
adjust for the effects of the covariates. Multivariate analysis
demonstrated that PSMA7 expression, lymph node metastasis and TNM
stage were independent risk factors in the prognosis of patients
with GC. Kaplan-Meier survival analysis indicated that elevated
expression of PSMA7 was closely associated with reduced OS and DFS
in patients with GCs as opposed to in those with low expression
levels. Hu et al (35)
revealed that the knockdown of PSMA7 by short hairpin RNA in the
RKO cell line inhibited their anchorage-independent growth, as well
as migration and invasion. Furthermore, PSMA7 depletion was able to
strongly suppress the tumorigenic ability of the RKO cells in
vivo. These results indicated that PSMA7 may serve as a novel
predictor of prognosis in patients with GC (35). However, Li et al (44) found that high mRNA expression of
PSMA7 was associated with improved OS, which is different from the
results of the present study. The sample size for biostatistics
should be expanded in future studies. There were some limitations
to the present study; although the expression and prognostic
significance of PSMA7 in GC have each been evaluated, the specific
functions and molecular mechanisms of PSMA7 in GC remain to be
further investigated in vivo and in vitro. In
subsequent studies the authors hope to identify the possible
signaling mechanisms involved in PSMA7 expression-triggered
proliferation, differentiation, invasion and metastasis in GC.
It is well known that a single gene cannot be
responsible for or directly connected with each step in the
occurrence and progression of GC. It has been reported previously
that certain factors are associated with PSMA7 in the colon
carcinoma cell line HCT116 and murine models, including
nucleotide-binding oligomerization domain-containing protein 1
(NOD1) (46), nuclear factor-kappa B
(NF-κB) (46), CD44 (13) and c-Abl (27,47),
among others. Yang et al (46) verified that PSMA7 regulates the
tumorigenesis of colorectal cancer through the inhibition of
NOD1-mediated apoptosis and NF-κB activation. Hu et al
(13) revealed that CD44 may be a
downstream element via PSMA7 depletion in colorectal cancer cell
tumorigenicity and metastasis. Future studies are required in order
to investigate these factors and their associations with PSMA7 in
GC, and to improve the current understanding of the molecular
mechanism underlying the role of PSMA7 in GC. In conclusion, to the
best of our knowledge, the present study proves for the first time
that PSMA7 is overexpressed at the mRNA and the protein level in GC
and that it is an independent prognostic factor for GC. PSMA7 may,
therefore, be a potential candidate for the diagnosis and targeted
therapy of patients with GC.
Acknowledgements
Not applicable.
Funding
This project was supported by the National Natural
Science Foundation of China (grant nos. 81201596, 81773100) and
Special Fund of Clinical Medicine in Jiangsu Province (grant no.
BL2013038).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding authors upon reasonable
request.
Authors' contributions
SX and QT were responsible for RNA extraction and
reverse transcription reaction. LZ and LJ performed polymerase
chain reaction. DW and PX prepared tissue sections. XW and XZ
conducted immunohistochemistry. LZ, LJ, GT and TY contributed to
the follow-up and analysis of patient's characteristics. SX drafted
the manuscript. ZF and LL made substantial contributions to article
design and manuscript revision, and gave final approval of the
version to be published. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Medical Ethics Committee of Xinghua People's Hospital (Xinghua,
China), and all the patients or their families provided written
informed consent
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shimizu D, Kanda M and Kodera Y: Review of
recent molecular landscape knowledge ofgastric cancer. Histol
Histopathol. 33:11–26. 2017.PubMed/NCBI
|
|
2
|
Daniyal M, Ahmad S, Ahmad M, Asif HM,
Akram M, Ur Rehman S and Sultana S: Risk factors and epidemiology
of gastric cancer in Pakistan. Asian Pac J Cancer Prev.
16:4821–4824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kanda M and Kodera Y: Recent advances in
the molecular diagnostics of gastric cancer. World J Gastroenterol.
21:9838–9852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mu JS, Huang WD, Tan ZJ, Li ML, Zhang L,
Ding QC, Wu XS, Lu JH, Liu YB, Dong Q and Xu HN: Dopamine receptor
D2 is correlated with gastric cancer prognosis. Oncol Lett.
13:1223–1227. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Emoto S, Ishigami H, Yamashita H,
Yamaguchi H, Kaisaki S and Kitayama J: Clinical significance of
CA125 and CA72-4 in gastric cancer with peritoneal dissemination.
Gastric Cancer. 15:154–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanda M, Fujii T, Takami H, Suenaga M,
Inokawa Y, Yamada S, Nakayama G, Sugimoto H, Koike M, Nomoto S and
Kodera Y: Combination of the serum carbohydrate antigen 19-9 and
carcinoembryonic antigen is a simple and accurate predictor of
mortality in pancreatic cancer patients. Surg Today. 44:1692–1701.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yong H, Zhu H, Zhang S, Zhao W, Wang W,
Chen C, Ding G, Zhu L, Zhu Z, Liu H, et al: Prognostic value of
decreased expression of RBM4 in human gastric cancer. Sci Rep.
6:282222016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng ZQ: The present situation and future
of personalized medicine of precise medicine. Acta Universitatis
Medicinalis Nanjing. 11:1283–1284. 2016.
|
|
9
|
Parkin DM: The global health burden of
infection-associated cancers in the year 2002. Int J Cancer.
118:3030–3044. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsugane S and Sasazuki S: Diet and the
risk of gastric cancer: Review of epidemiologica evidence. Gastric
Cancer. 10:75–83. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanda M, Shimizu D, Sueoka S, Nomoto S,
Oya H, Takami H, Ezaka K, Hashimoto R, Tanaka Y, Kobayashi D, et
al: Prognostic relevance of SAMSN1 expression in gastric cancer.
Oncol Lett. 12:4708–4716. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan JY, Huang X and Luo YL: PSMA7 inhibits
the tumorigenicity of A549 human lung adenocarcinoma cells. Mol
Cell Biochem. 366:131–137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu XT, Chen W, Wang D, Shi QL, Zhang FB,
Liao YQ, Jin M and He C: The proteasome subunit PSMA7 located on
the 20q13 amplicon is overexpressed and associated with liver
metastasis in colorectal cancer. Oncol Rep. 19:441–446.
2008.PubMed/NCBI
|
|
14
|
Du H, Huang X, Wang S, Wu Y, Xu W and Li
M: PSMA7, a potential biomarker of diseases. Protein Pept Lett.
16:486–489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pickart CM: Ubiquitin enters the new
millennium. Mol Cell. 8:499–504. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Glickman MH and Ciechanover A: The
ubiquitin-proteasome proteolytic pathway: Destruction for the sake
of construction. Physiol Rev. 82:373–428. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Groll M, Ditzel L, Löwe J, Stock D,
Bochtler M, Bartunik HD and Huber R: Structure of 20S proteasome
from yeast at 2.4 A resolution. Nature. 386:463–471. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Unno M, Mizushima T, Morimoto Y, Tomisugi
Y, Tanaka K, Yasuoka N and Tsukihara T: The structure of the
mammalian 20S proteasome at 2.75 A resolution. Structure.
10:609–618. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takagi K, Saeki Y, Yashiroda H, Yagi H,
Kaiho A, Murata S, Yamane T, Tanaka K, Mizushima T and Kato K:
Pba3-Pba4 heterodimer acts as a molecular matchmaker in proteasome
α-ring formation. Biochem Biophys Res Commun. 450:1110–1114. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tomko RJ Jr and Hochstrasser M: Molecular
architecture and assembly of the eukaryotic proteasome. Annu Rev
Biochem. 82:415–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hirano Y, Kaneko T, Okamoto K, Bai M,
Yashiroda H, Furuyama K, Kato K, Tanaka K and Murata S: Dissecting
beta-ring assembly pathway of the mammalian 20S proteasome. EMBO J.
27:2204–2213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishii K, Noda M, Yagi H, Thammaporn R,
Seetaha S, Satoh T, Kato K and Uchiyama S: Disassembly of the
self-assembled, double-ring structure of proteasome α7
homo-tetradecamer by α6. Sci Rep. 5:181672015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang J, Kwong J, Sun EC and Liang TJ:
Proteasome complex as a potential cellular target of hepatitis B
virus X protein. J Virol. 70:5582–5591. 1996.PubMed/NCBI
|
|
24
|
Zhang Z, Torii N, Furusaka A, Malayaman N,
Hu Z and Liang TJ: Structural and functional characterization of
interaction between hepatitis B virus X protein and the proteasome
complex. J Biol Chem. 275:15157–15165. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krüger M, Beger C, Welch PJ, Barber JR,
Manns MP and Wong-Staal F: Involvement of proteasome alpha-subunit
PSMA7 in hepatitis C virus internal ribosome entry site-mediated
translation. Mol Cell Biol. 21:8357–8364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Apcher GS, Heink S, Zantopf D, Kloetzel
PM, Schmid HP, Mayer RJ and Kruger E: Human immunodeficiency
virus-1 Tat protein interacts with distinct proteasomal alpha and
beta subunits. FEBS Lett. 553:200–204. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Semenza GL: O2-regulated gene expression:
Transcriptional control of cardiorespiratory physiology by HIF-1. J
Appl Physiol (1985). 96:1170–1172. 2004. View Article : Google Scholar
|
|
28
|
Tandle AT, Calvani M, Uranchimeg B, Zahavi
D, Melillo G and Libutti SK: Endothelial monocyte activating
polypeptide-II modulates endothelial cell responses by degrading
hypoxia- inducible factor-1alpha through interaction with PSMA7, a
component of the proteasome. Exp Cell Res. 315:1850–1859. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li D, Dong Q, Tao Q, Gu J, Cui Y, Jiang X,
Yuan J, Li W, Xu R, Jin Y, et al: c-Abl regulates proteasome
abundance by controlling the ubiquitin-proteasomal degradation of
PSMA7 subunit. Cell Rep. 10:484–496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ohnami S, Matsumoto N, Nakano M, Aoki K,
Nagasaki K, Sugimura T, Terada M and Yoshida T: Identification of
genes showing differential expression in antisense K-ras-transduced
pancreatic cancer cells with suppressed tumorigenicity. Cancer Res.
59:5565–5571. 1999.PubMed/NCBI
|
|
31
|
Richardson AL, Wang ZC, De Nicolo A, Lu X,
Brown M, Miron A, Liao X, Iglehart JD, Livingston DM and Ganesan S:
X chromosomal abnormalities in basal-like human breast cancer.
Cancer Cell. 9:121–132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Romanuik TL, Wang G, Morozova O, Delaney
A, Marra MA and Sadar MD: LNCaP Atlas: Gene expression associated
with in vivo progression to castration-recurrent prostate cancer.
BMC Med Genomics. 3:432010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi YY, Wang HC, Yin YH, Sun WS, Li Y,
Zhang CQ, Wang Y, Wang S and Chen WF: Identification and analysis
of tumour-associated antigens in hepatocellular carcinoma. Br J
Cancer. 92:929–934. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Scotto L, Narayan G, Nandula SV,
Arias-Pulido H, Subramaniyam S, Schneider A, Kaufmann AM, Wright
JD, Pothuri B, Mansukhani M and Murty VV: Identification of copy
number gain and overexpressed genes on chromosome arm 20q by an
integrative genomic approach in cervical cancer: Potential role in
progression. Genes Chromosomes Cancer. 47:755–765. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu XT, Chen W, Zhang FB, Shi QL, Hu JB,
Geng SM and He C: Depletion of the proteasome subunit PSMA7
inhibits colorectal cancer cell tumorigenicity and migration. Oncol
Rep. 22:1247–1252. 2009.PubMed/NCBI
|
|
36
|
Honma K, Takemasa I, Matoba R, Yamamoto Y,
Takeshita F, Mori M, Monden M, Matsubara K and Ochiya T: Screening
of potential molecular targets for colorectal cancer therapy. Int J
Gen Med. 2:243–257. 2009.PubMed/NCBI
|
|
37
|
Edge SB and Carolyn CC: The American Joint
Committee on cancer: The 7th edition of the AJCC cancer staging
manualand the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bai J, Yong HM, Chen FF, Mei PJ, Liu H, Li
C, Pan ZQ, Wu YP and Zheng JN: Cullin1 is a novel marker of poor
prognosis and a potential therapeutic target in human breast
cancer. Ann Oncol. 24:2016–2022. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tang Q, Liu YF, Zhu XJ, Li YH, Zhu J,
Zhang JP, Feng ZQ and Guan XH: Expression and prognostic
significance of the alpha B-crystallin gene in human hepatocellular
carcinoma. Hum Pathol. 40:300–305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao W, Zhu H, Zhang S, Yong H, Wang W,
Zhou Y, Wang B, Wen J, Qiu Z, Ding G, et al: Trop2 is overexpressed
in gastric cancer and predicts poor prognosis. Oncotarget.
7:6136–6145. 2016.PubMed/NCBI
|
|
42
|
Chen C, Wang X, Huang X, Yong H, Shen J,
Tang Q, Zhu J, Ni J and Feng Z: Nicotinamide N-methyltransferase: A
potential biomarker for worse prognosis in gastric carcinoma. Am J
Cancer Res. 6:649–663. 2016.PubMed/NCBI
|
|
43
|
D'Errico M, de Rinaldis E, Blasi MF, Viti
V, Falchetti M, Calcagnile A, Sera F, Saieva C, Ottini L, Palli D,
et al: Genome-wide expression profile of sporadic gastric cancers
with microsatellite instability. Eur J Cancer. 45:461–469. 2009.
View Article : Google Scholar
|
|
44
|
Li Y, Huang J, Sun J, Xiang S, Yang D,
Ying D, Lu M, Li H and Ren G: The transcription levels and
prognostic values of seven proteasome alpha subunits in human
cancers. Oncotarget. 8:4501–4519. 2017.PubMed/NCBI
|
|
45
|
Holmila R, Sklias A, Muller DC, Degli
Esposti D, Guilloreau P, McKay J, Sangrajrang S, Srivatanakul P,
Hainaut P, Merle P, et al: Targeted deep sequencing of plasma
circulating cell-free DNA reveals Vimentin and Fibulin 1 as
potential epigenetic biomarkers for hepatocellular carcinoma. PLoS
One. 12:e01742652017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang L, Tang Z, Zhang H, Kou W, Lu Z, Li
X, Li Q and Miao Z: PSMA7 directly interacts with NOD1 and
regulates its function. Cell Physiol Biochem. 31:952–959. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu X, Huang W, Li C, Li P, Yuan J, Li X,
Qiu XB, Ma Q and Cao C: Interaction between c-Abl and Arg tyrosine
kinases and proteasome subunit PSMA7 regulates proteasome
degradation. Mol Cell. 22:317–327. 2006. View Article : Google Scholar : PubMed/NCBI
|