Introduction
Pancreatic cancer is one of the most aggressive
malignancies worldwide, with a mortality rate that is nearly equal
to its incidence rate (1).
Pancreatic cancer is expected to become the second leading cause of
cancer-associated mortality in the US by 2030 (2). Despite advances in diagnostic and
therapeutic strategies over the past years, the prognosis of
patients with pancreatic cancer remains unsatisfactory, with a
5-year survival rate of <8.5% (3). Pancreatic cancer has become one of the
major public health concerns worldwide. In order to improve the
treatment of pancreatic cancer, it is necessary to elucidate the
pathological mechanism underlying its development, identify a
drug-based framework and investigate the functional mechanisms
involved.
Arsenic trioxide (ATO) has been reported to be an
effective therapeutic agent for acute promyelocytic leukemia (APL)
(4). It has been reported that ATO
can induce molecular remission, as well as prolong the overall
survival of patients with APL (5,6).
Combined treatment with retinoic acid (RA) and ATO has been
demonstrated to be curative for the majority of patients with APL
(7–9). Furthermore, an increasing number of
studies have proven the anti-carcinogenic properties of ATO in
different tumors, including in gastric cancer (10), lymphoma (11), bladder cancer (12), head and neck cancer (13), and ovarian cancer (14). Although it has been reported that ATO
may inhibit the progression of pancreatic cancer (15), the potential targets and molecular
mechanisms of action of ATO remain unclear.
In recent years, with the continuous improvement and
development of high-throughput sequencing technology, multi-omics
research (such as genomics, transcriptomics, proteomics and
metabolomics) has gradually improved and has been used to identify
disease-associated genes, which may uncover the molecular
mechanisms underlying disease development and enable effective
treatment. DrugBank is a richly annotated web-based bioinformatics
tool that contains multiple drug data with comprehensive drug
targets information (16). It
provides detailed, step-by-step information on the results of
previous studies on drugs, drug targets and drug effects, allowing
researchers to search, view and export data, text and image
(17). Furthermore, Search Tool for
Interactions of Chemicals (STITCH) is an online database of the
interaction networks of chemicals and proteins (18).
In the present study, DrugBank and STITCH were used
to identify the targets of ATO, in order to construct an ATO-target
interaction network. Protein-protein interaction (PPI) network
construction and Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway analysis demonstrated that ATO is associated with several
types of malignancies, including pancreatic cancer. In total, 6
genes (AKT1, CCND1, CDKN2A, IKBKB, MAPK1 and MAPK3) were identified
as the target genes of ATO in pancreatic cancer. The
interconnection of these 6 ATO target genes in pancreatic cancer
was then analyzed using the Search Tool for the Retrieval of
Interacting Genes (STRING) database. The cBioPortal website was
further used to analyze the genomics data of these 6 target genes,
while online Kaplan-Meier (K-M) plotter was used to analyze the
prognosis of pancreatic cancer. In addition, the Oncomine website
was used to reveal the expression levels of ATO target genes in
pancreatic cancer tissues. Taken together, the findings of the
bioinformatics analysis performed in the present study may help
determine the gene targets of ATO and provide new therapeutic
approaches for pancreatic cancer.
Materials and methods
Search for target genes of ATO
DrugBank, version 5.0 (https://www.drugbank.ca), is a richly annotated
web-based bioinformatics tool that contains multiple drug data with
comprehensive drug target information. In the present study, the
DrugBank 5.0 and STITCH (http://stitch.embl.de/) databases were used to
identify the ATO-target interactions in order to obtain an
ATO-target network. The information on ATO target genes was then
used to construct a visualization table and analyzed by STRING for
further analysis. The search term ‘arsenic trioxide’ was used to
search the DrugBank 5.0 and STITCH databases, and the targets of
ATO in these two websites were identified. A total of 19 targets
were recorded and included in subsequent analyses.
KEGG pathway and Gene Ontology
analyses, and PPI network construction of ATO targets
The STRING database (https://string-db.org/) gathers and provides known and
predicted PPI data for a large number of organisms, including
humans (19,20). Cytoscape is a general-purpose
software platform used for integrating bimolecular interaction
networks (21). CluePedia, a plugin
of Cytoscape software, can be used for searching potential genes
associated with the reported signaling pathway by calculating
linear and non-linear statistical dependencies from experimental
data (22). In the present study,
the KEGG pathway and Gene Ontology (GO) analyses were conducted
using the STRING database. Subsequently, the STRING website and
Cytoscape software were utilized to establish a PPI network of the
ATO target genes.
Genomics information of ATO targets in
pancreatic cancer
The cBioPortal for Cancer Genomics (http://www.cbioportal.org/) is a free platform that is
used for finding multidimensional cancer genomics data (23). OncoPrint is an online tool, which can
visualize alterations of tumor samples from gene arrays. The
alterations of 6 ATO target genes associated with pancreatic cancer
(AKT1, CCND1, CDKN2A, IKBKB, MAPK1 and MAPK3) were visualized using
cBioPortal and OncoPrint (24), and
the genomic alteration frequency within the specific cancer study,
listed below, was applied as a filter. In the present study, a
total of 850 pancreatic cancer samples were analyzed in this
section, which included International Cancer Genome Consortium
(ICGC; n=99), Queensland Centre for Medical Genomics (QCMG2016;
n=456), The Cancer Genome Atlas (TCGA; n=186) and University of
Texas Southwestern Medical Center (UTSW; n=109) datasets (25–28).
Overall survival analysis of ATO
target genes in patients with pancreatic cancer
The K-M plotter (http://kmplot.com/analysis/) is a free online tool
that can be used to assess the association of multiple genes with
the survival of patients with cancer using different cancer samples
(29). According to mRNA expression
data from the aforementioned 6 ATO genes, the patients with
pancreatic cancer were divided into the high- and low-expression
groups based on the median expression value, and then K-M survival
curves were constructed. In the present study, the information of
177 patients with pancreatic cancer was acquired from the K-M
website, and the K-M survival curves were used to analyze the
association between the expression of ATO target genes (AKT1,
CCND1, CDKN2A, IKBKB, MAPK1 and MAPK3) and the prognosis of 177
patients with pancreatic cancer.
Determination of the expression of the
3 ATO target genes in pancreatic cancer
Oncomine (https://www.oncomine.org/) is an online cancer
microarray website that facilitates the discovery of genome-wide
expression analyses (30). In the
present study, differential gene expression was detected by
Oncomine when comparing cancer tissues with normal pancreatic
tissues. The studies by Ishikawa et al (31), Iacobuzio-Donahue et al
(32), Pei et al (33), Badea et al (34), Logdons et al (35) and Segara et al (36) were used to reveal the expression of 3
ATO target genes in pancreatic cancer. Differences in
transcriptional expression were compared using the Student's
t-test. P<0.01 was used to indicate a statistically significant
difference and fold change was set to 2.
Results
Evaluation of ATO target genes
ATO is a chemotherapeutic agent of idiopathic
function used to treat leukemia that is unresponsive to first-line
agents. It is suspected that ATO induces cancer cells to undergo
apoptosis (11). In the current
study, DrugBank and STITCH tools were used to identify the target
genes of ATO. As shown in Table I, a
total of 19 target genes were identified, including IKBKB, TXNRD1,
JUN, CCND1, MAPK3, MAPK1, AKT1, CDKN1A, HDAC1, PML, RARA, ESR1,
CDKN2A, ABCC1, SP1, DNMT3B, DNMT1, HSPA4 and ABCB1.
 | Table I.Target genes (n=19) of arsenic
trioxide identified using DrugBank 5.0 and STITCH. |
Table I.
Target genes (n=19) of arsenic
trioxide identified using DrugBank 5.0 and STITCH.
| Target gene | Source |
|---|
| IKBKB | Drugbank 5.0 |
| TXNRD1 | Drugbank 5.0 |
| JUN | Drugbank 5.0 |
| CCND1 | Drugbank 5.0 |
| MAPK3 | Drugbank 5.0 |
| MAPK1 | Drugbank 5.0 |
| AKT1 | Drugbank 5.0 |
| CDKN1A | Drugbank 5.0 |
| PML | Drugbank 5.0 and
STITCH |
| HDAC1 | STITCH |
| RARA | STITCH |
| ESR1 | STITCH |
| CDKN2A | STITCH |
| ABCC1 | STITCH |
| SP1 | STITCH |
| DNMT3B | STITCH |
| DNMT1 | STITCH |
| HSPA4 | STITCH |
| ABCB1 | STITCH |
Gene Ontology (GO) analysis
The online STRING software was used to determine
functional enrichments based on 19 ATO target genes. GO analysis
revealed a significant difference in biological processes, which
included ‘response to lipid’, ‘DNA-templated transcription’,
‘initiation, regulation of transcription from RNA polymerase II
promoter’, ‘positive regulation of gene expression’, and
‘transcription initiation from RNA polymerase II promoter,’ among
others. The molecular function terms identified included ‘enzyme
binding’, ‘transcription factor binding’, ‘sequence-specific DNA
binding’, ‘cyclin-dependent protein serine/threonine kinase
regulator activity’ and ‘transcription corepressor activity’, among
others. As regards the area of the cell components, the 19 ATO
target genes were enriched in ‘chromatin’, ‘nucleoplasm’, ‘nuclear
chromatin’, ‘chromosome’ and ‘nuclear lumen’. The top 5 terms for
biological processes, molecular function and cellular components
were shown in Table SI.
Association between ATO targets and
pancreatic cancer
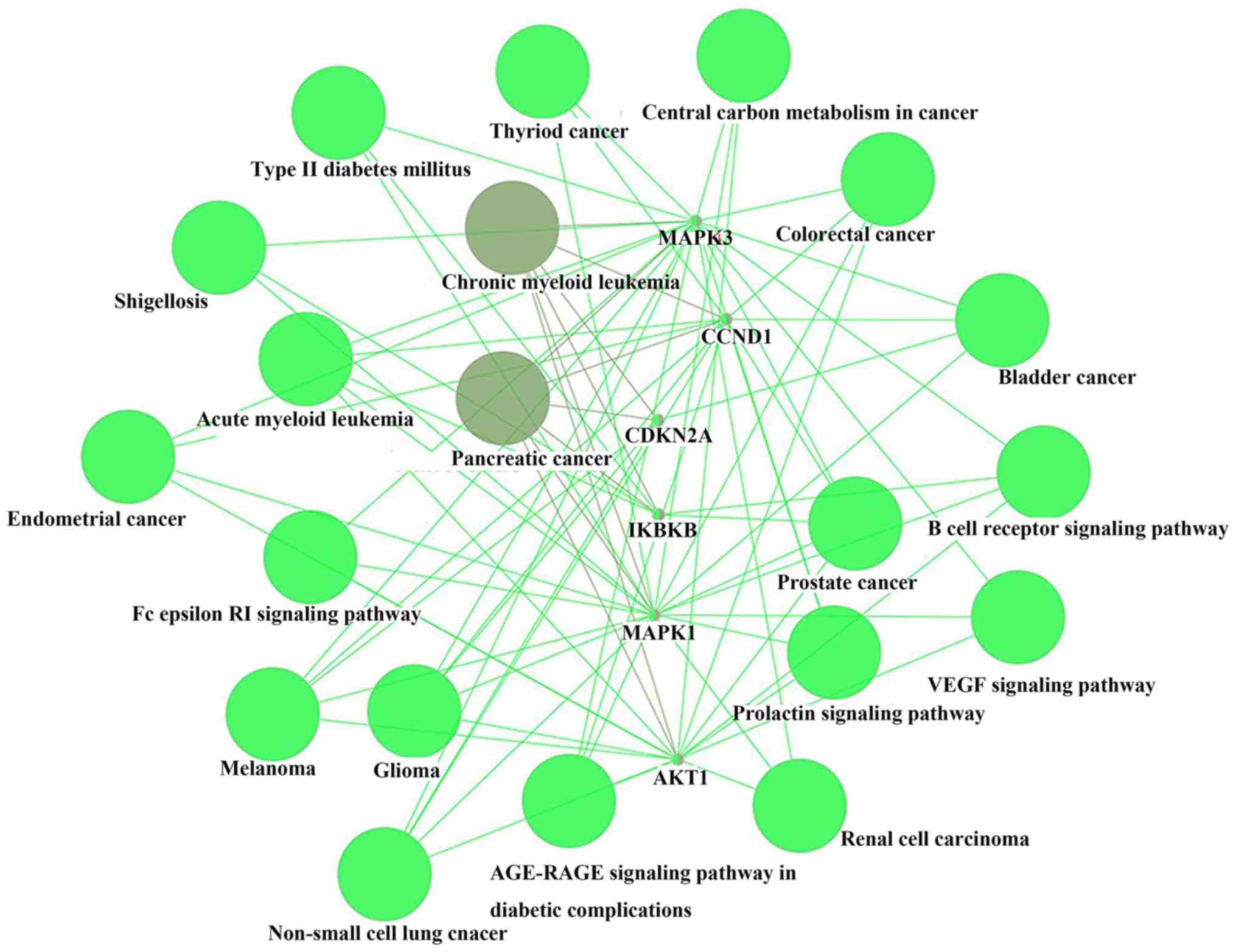
The PPI network (Fig.
1) and KEGG pathway analysis for the 19 ATO target genes
(Table II) were obtained from the
STRING database. The top 10 signaling pathways associated with the
ATO target genes were as follows: MicroRNAs in cancer, pathways in
cancer, chronic myeloid leukemia, acute myeloid leukemia,
pancreatic cancer, glioma, melanoma, hepatitis B, pancreatic cancer
and viral carcinogenesis. The most common solid tumor was
pancreatic cancer (P=6.88×10−10). A total of 6 genes,
namely AKT1, CCND1, CDKN2A, IKBKB, MAPK1 and MAPK3, were found to
be associated with pancreatic cancer. Subsequently, CluePedia
software was used to verify and visualize the association of ATO
target genes with pancreatic cancer, and the results were
consistent with the STRING analysis results (Fig. 2).
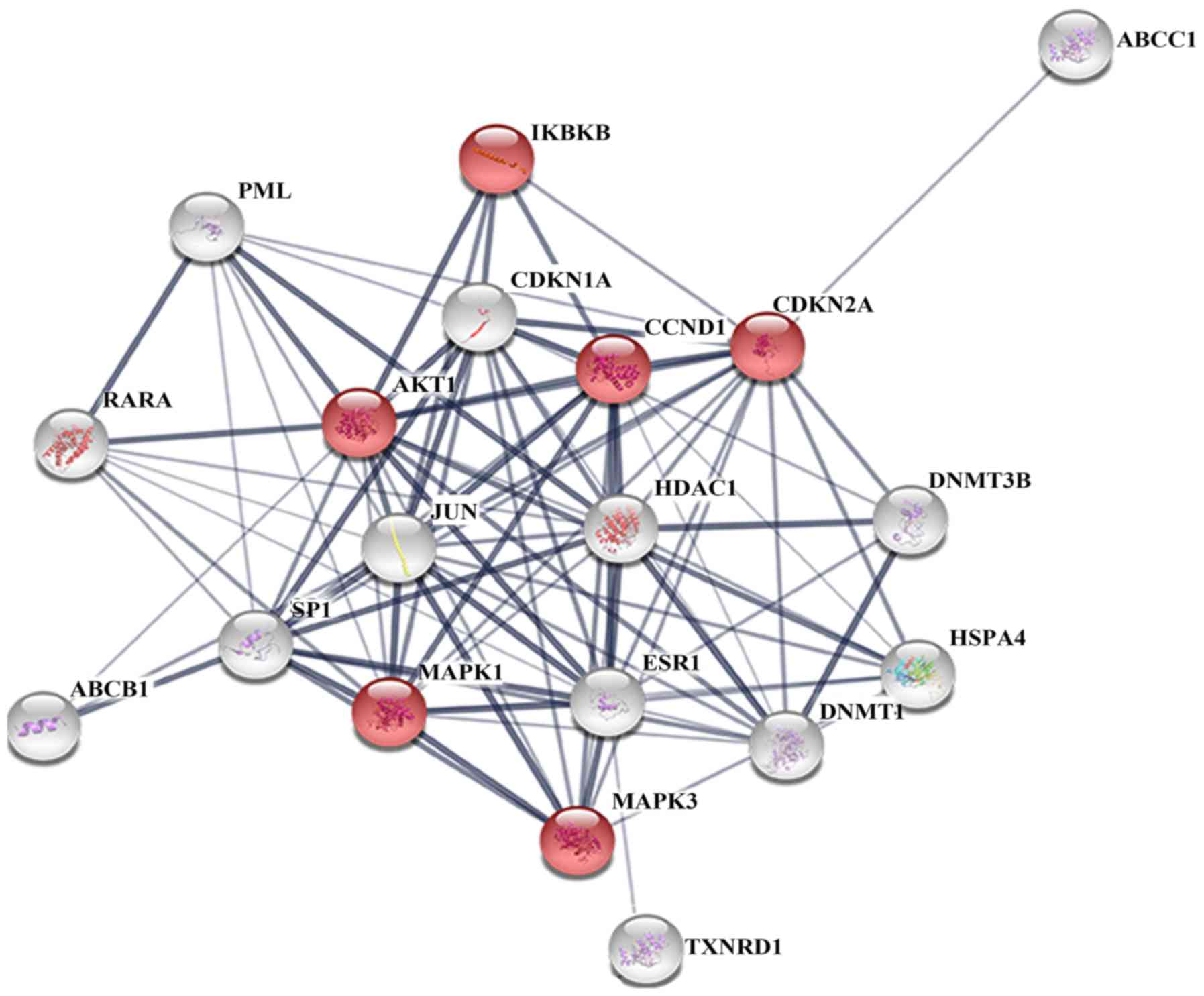 | Figure 1.Protein-protein interaction network
of arsenic trioxide target genes, including IKBKB, TXNRD1, JUN,
CCND1, MAPK3, MAPK1, AKT1, CDKN1A, HDAC1, PML, RARA, ESR1, CDKN2A,
ABCC1, SP1, DNMT3B, DNMT1, HSPA4 and ABCB1. Red color indicates
association with pancreatic cancer. |
 | Table II.Kyoto Encyclopedia of Genes and
Genomes pathways associated with the arsenic trioxide target
genes. |
Table II.
Kyoto Encyclopedia of Genes and
Genomes pathways associated with the arsenic trioxide target
genes.
| Pathway | Count | False discovery
rate | Genes |
|---|
| MicroRNAs in
cancer | 10 |
5.09×10−15 | ABCB1, ABCC1,
CCND1, CDKN1A, CDKN2A, DNMT1, DNMT3B, HDAC1, IKBKB, MAPK1 |
| Pathways in
cancer | 11 |
7.73×10−14 | AKT1, CCND1,
CDKN1A, CDKN2A, HDAC1, IKBKB, JUN, MAPK1, MAPK3, PML, RARA |
| Chronic myeloid
leukemia | 8 |
7.73×10−14 | AKT1, CCND1,
CDKN1A, CDKN2A, HDAC1, IKBKB, MAPK1, MAPK |
| Acute myeloid
leukemia | 7 |
2.41×10−12 | AKT1, CCND1, IKBKB,
MAPK1, MAPK3, PML, RARA |
| Pancreatic
cancer | 6 |
6.88×10−10 | AKT1, CCND1,
CDKN2A, IKBKB, MAPK1, MAPK3 |
| Glioma | 6 |
6.88×10−10 | AKT1, CCND1,
CDKN1A, CDKN2A, MAPK1, MAPK3 |
| Hepatitis B | 7 |
1.11×10−9 | AKT1, CCND1,
CDKN1A, IKBKB, JUN, MAPK1, MAPK3 |
| Melanoma | 6 |
1.11×10−9 | AKT1, CCND1,
CDKN1A, CDKN2A, MAPK1, MAPK3 |
| Prostate
cancer | 6 |
3.56×10−9 | AKT1, CCND1,
CDKN1A, IKBKB, MAPK1, MAPK3 |
| Viral
carcinogenesis | 7 |
4.54×10−9 | CCND1, CDKN1A,
CDKN2A, HDAC1, JUN, MAPK1, MAPK3 |
Genetic data of ATO target genes in
pancreatic cancer
The genetic information of these 6 genes in patients
with pancreatic cancer were subsequently evaluated using the
cBioPortal. The genomic alterations and clinical expression
characteristics of AKT1, CCND1, CDKN2A, IKBKB, MAPK1 and MAPK3 in
the aforementioned datasets ICGC (n=99), QCMG2016 (n=456), TCGA
(n=186) and UTSW (n=109) were investigated (25–28). As
shown in Fig. 3, the alteration rate
of the 6 genes in pancreatic cancer varied between 0 and 63%. AKT1
exhibited an alteration rate between 0 and 18%, CCDN1 had an
alteration rate between 0 and 10%, CDKN2A had an alteration between
0 and 42%, IKBKB had an alteration of 0–7%, MAPK1 had an alteration
between 0 and 8%, and MAPK3 had an alteration between 0 and 9%.
Furthermore, AKT1, IKBKB and MAPK1 exhibited amplification, deep
deletion and missense mutations. The alterations of CDKN2A included
deep deletion, truncating, missense, inframe mutations and other
mutations. The alterations of CCND1 and MAPK3 included
amplification.
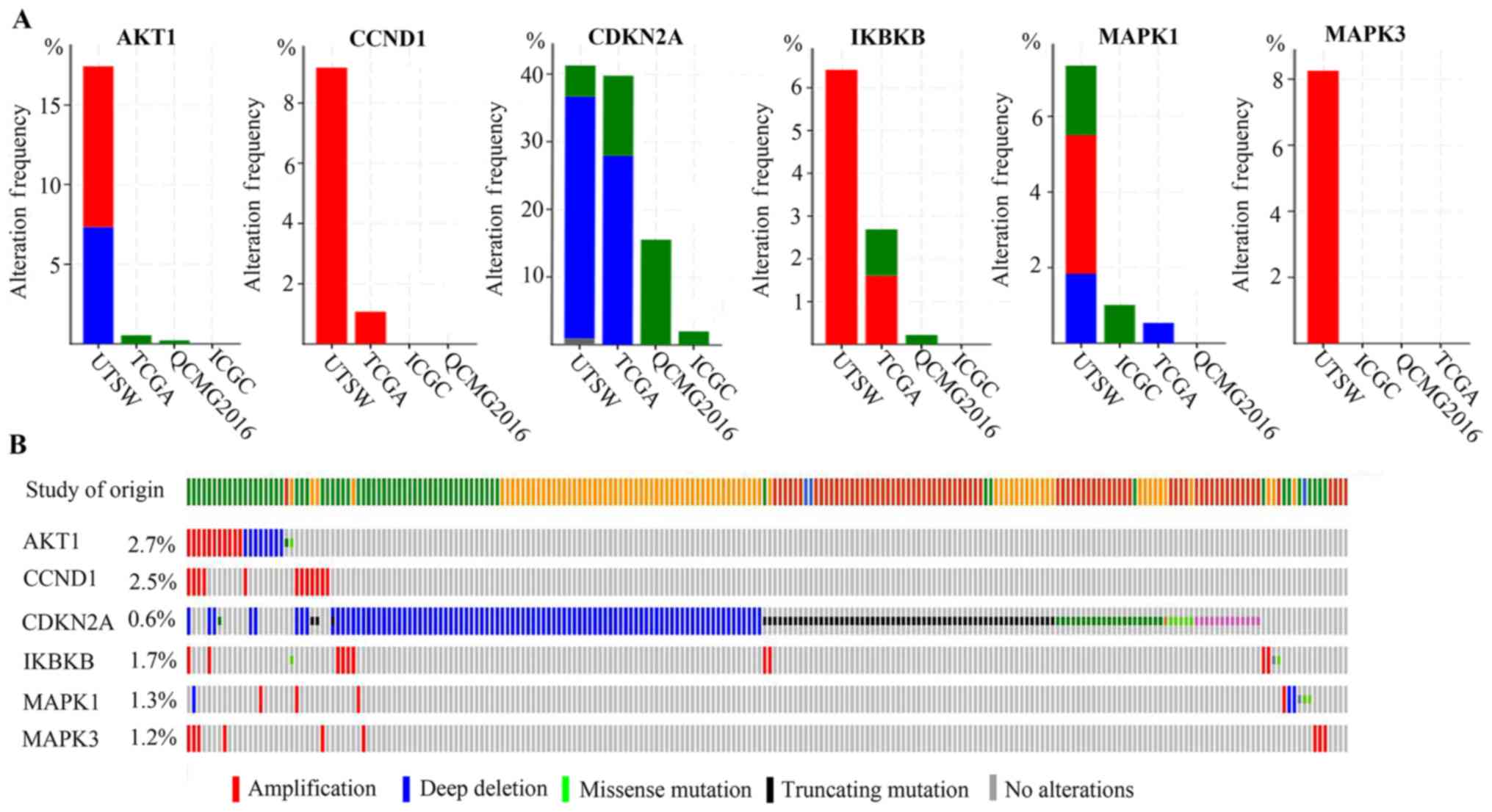 | Figure 3.Genetic alterations of arsenic
trioxide target genes (AKT1, CCND1, CDKN2A, IKBKB, MAPK1 and MAPK3)
in four pancreatic cancer studies, embedded in the cBioPortal for
Cancer Genomics. ICGC, QCMG2016, TCGA and UTSW represent the
different origin of the studies. (A) Overview of changes on genes
(AKT1, CCND1, CDKN2A, IKBKB, MAPK1 and MAPK3) in genomics datasets
available in four studies. (B) OncoPrint, providing a visual
summary of alteration across a set of pancreatic cancer samples
based on a query of the 6 genes. There were a total of 850 samples
in the four studies included in the analysis. |
Interconnection of the 6 ATO target
genes in pancreatic cancer
STRING analysis performed in the current study
revealed that the 6 ATO target genes (AKT1, CCND1, CDKN2A, IKBKB,
MAPK1 and MAPK3) were involved in chronic myeloid leukemia,
pancreatic cancer, non-small cell lung cancer, acute myeloid
leukemia, melanoma, glioma, prostate cancer, endocrine resistance,
FoxO signaling and hepatitis B (Table
SII). A PPI network was constructed to analyze the genes
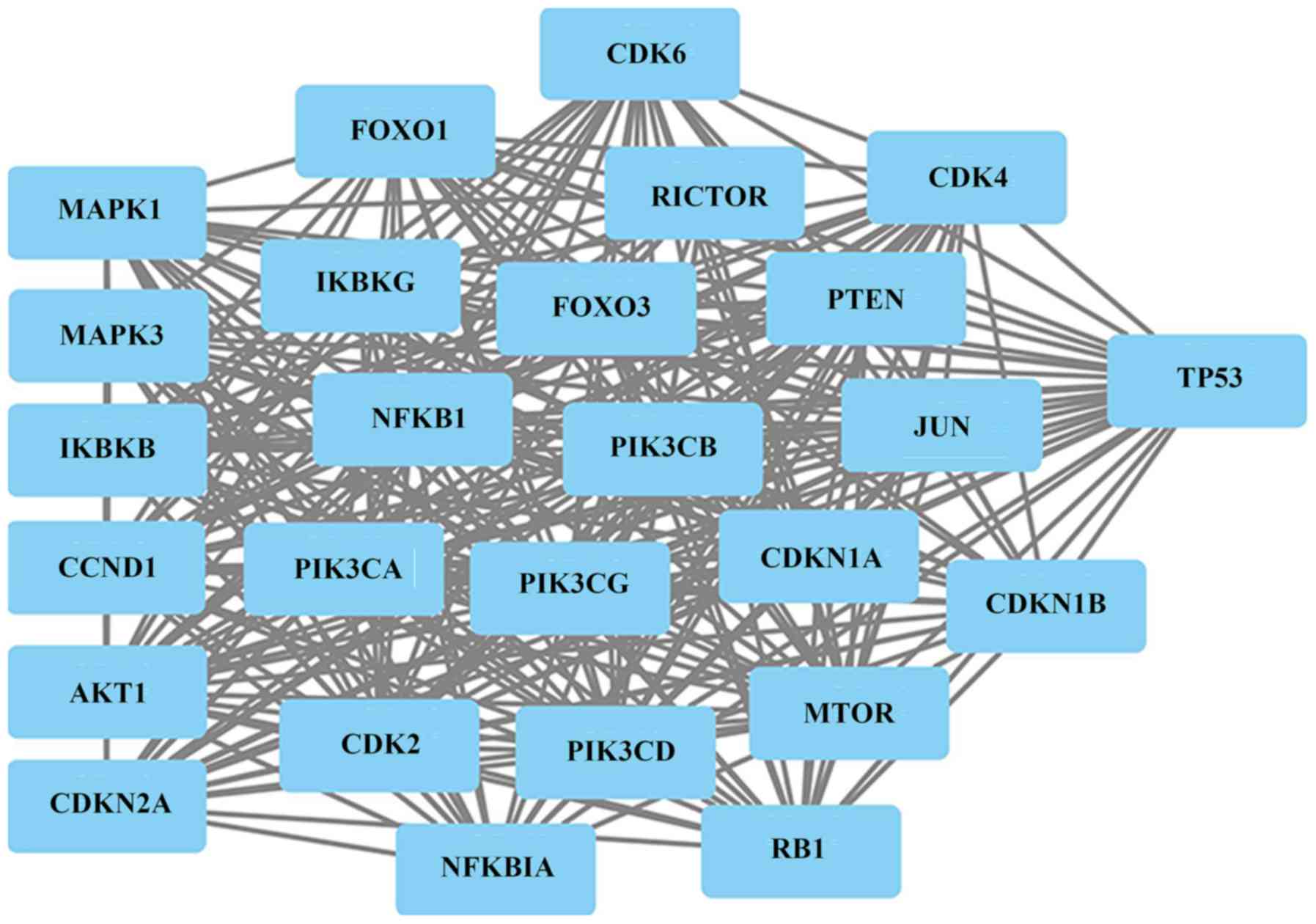
interconnected with AKT1, CCND1, CDKN2A, IKBKB, MAPK1 and MAPK3. A
total of 20 ATO interacting genes (FOXO1, CDK6, CDK4, RICTOR,
IKBKG, FOXO3, PTEN, NFKB1, PIK3CB, JUN, TP53, PIK3CA, PIK3CG,
CDKN1A, CDKN1B, CDK2, PIK3CD, MTOR, NFKBIA and RB1) were identified
and were mainly involved in hepatitis B, prostate cancer, pathways
in cancer, glioma and chronic myeloid leukemia (Fig. 4 and Table III). The results may indicate a new
therapeutic strategy for pancreatic cancer.
 | Table III.Kyoto Encyclopedia of Genes and
Genomes pathways in the protein-protein interaction network of the
arsenic trioxide target genes interacted 20 genes. |
Table III.
Kyoto Encyclopedia of Genes and
Genomes pathways in the protein-protein interaction network of the
arsenic trioxide target genes interacted 20 genes.
| Pathway | Count | False discovery
rate | Gene name |
|---|
| Hepatitis B | 13 |
4.42×10−23 | CDK4, CDK6, CDKN1A,
CDKN1B, JUN, NFKB1, PIK3CA, PIK3CB, PIK3CD, PIK3CG, PTEN, RB1,
TP53 |
| Prostate
cancer | 12 |
4.42×10−23 | CDKN1A, CDKN1B,
FOXO1, MTOR, NFKB1, PIK3CA, PIK3CB, PIK3CD, PIK3CG, PTEN, RB1,
TP53 |
| Pathways in
cancer | 15 |
4.43×10−23 | CDK4, CDK6, CDKN1A,
CDKN1B, FOXO1, JUN, MTOR, NFKB1, PIK3CA, PIK3CB, PIK3CD, PIK3CG,
PTEN, RB1, TP53 |
| Glioma | 11 |
1.43×10−22 | CDK4, CDK6, CDKN1A,
MTOR, PIK3CA, PIK3CB, PIK3CD, PIK3CG, PTEN, RB1, TP53 |
| Chronic myeloid
leukemia | 11 |
4.99×10−22 | CDK4, CDK6, CDKN1A,
CDKN1B, NFKB1, PIK3CA, PIK3CB, PIK3CD, PIK3CG, RB1, TP53 |
| Small cell lung
cancer | 11 |
4.21×10−21 | CDK4, CDK6, CDKN1B,
NFKB1, PIK3CA, PIK3CB, PIK3CD, PIK3CG, PTEN, RB1, TP53 |
| Viral
carcinogenesis | 12 |
1.16×10−19 | CDK4, CDK6, CDKN1A,
CDKN1B, JUN, NFKB1, PIK3CA, PIK3CB, PIK3CD, PIK3CG, RB1, TP53 |
| Melanoma | 10 |
1.48×10−19 | CDK4, CDK6, CDKN1A,
PIK3CA, PIK3CB, PIK3CD, PIK3CG, PTEN, RB1, TP53 |
| PI3K-AKT signaling
pathway | 13 |
1.27×10−18 | CDK4, CDK6, CDKN1A,
CDKN1B, FOXO3, MTOR, NFKB1, PIK3CA, PIK3CB, PIK3CD, PIK3CG, PTEN,
TP53 |
| Non-small cell lung
cancer | 9 |
4.22×10−18 | CDK4, CDK6, FOXO3,
PIK3CA, PIK3CB, PIK3CD, PIK3CG, RB1, TP53 |
Survival prediction of the 6 ATO
target genes in patients with pancreatic cancer
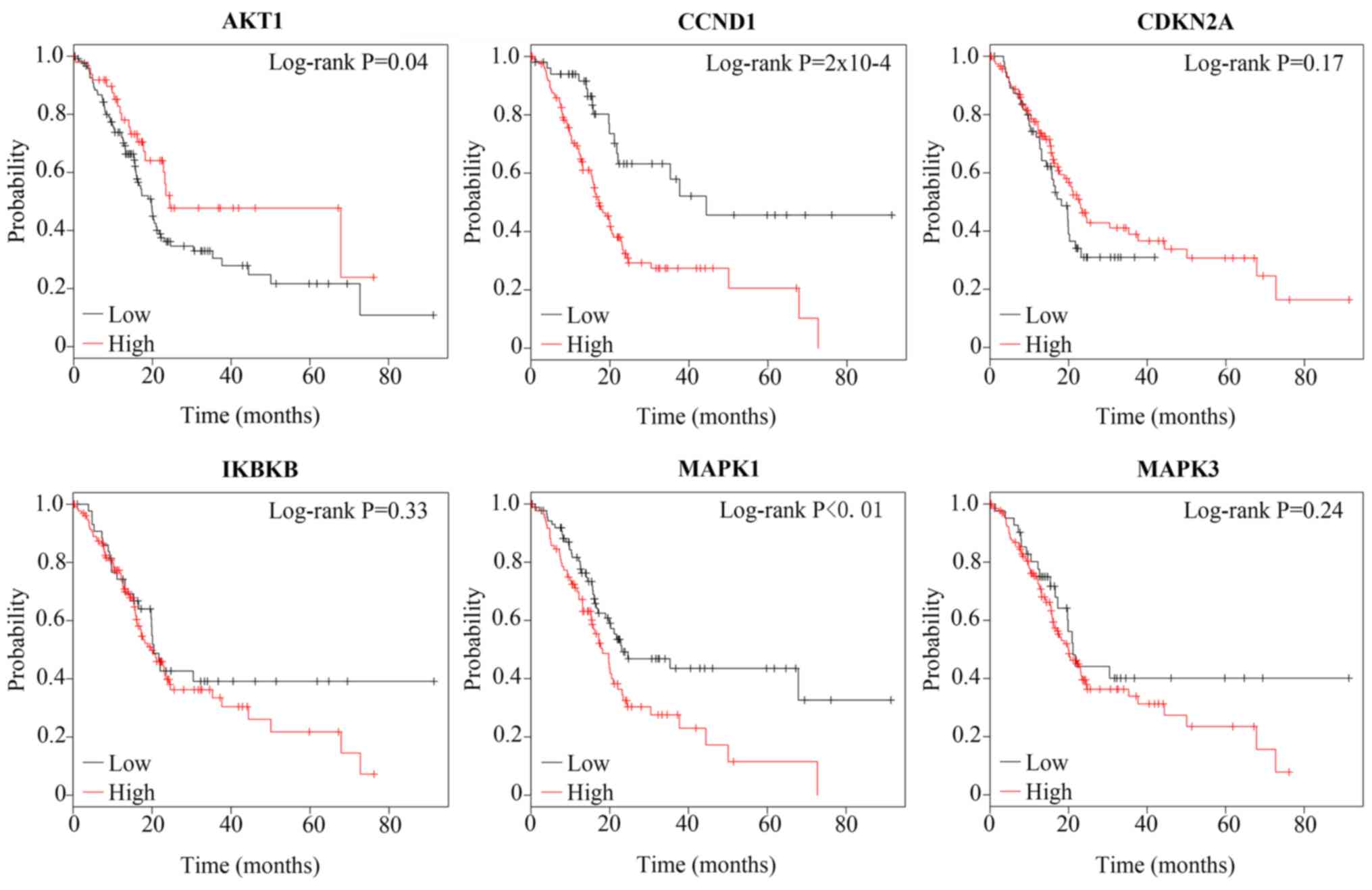
After the 6 ATO target genes associated with
pancreatic cancer were identified, the K-M plotter was used to
analyze the association of these genes with the overall survival of
177 patients with pancreatic cancer. As shown in Fig. 5, a low AKT1 level indicated poorer
prognosis in patients with pancreatic cancer [hazard ratio
(HR)=0.60; P=0.04]. Furthermore, a high level of CCND1 was
correlated with poor prognosis of patients with pancreatic cancer
(HR=2.71; P=2×10−4). MAPK1 may also serve as a
prognostic factor for patients with pancreatic cancer (HR=1.74;
P<0.01), with high expression levels indicating poor prognosis.
However, CKDN2A (P=0.17), IKBKB (P=0.33) and MAPK3 (P=0.24) were
not found to be prognostic factors for patients with pancreatic
cancer.
Differential expression of the 3 ATO
target genes in pancreatic cancer
As aforementioned, AKT1, CCND1 and MAPK1 were
demonstrated to be associated with the prognosis of pancreatic
cancer. Differential expression analysis of these three ATO target
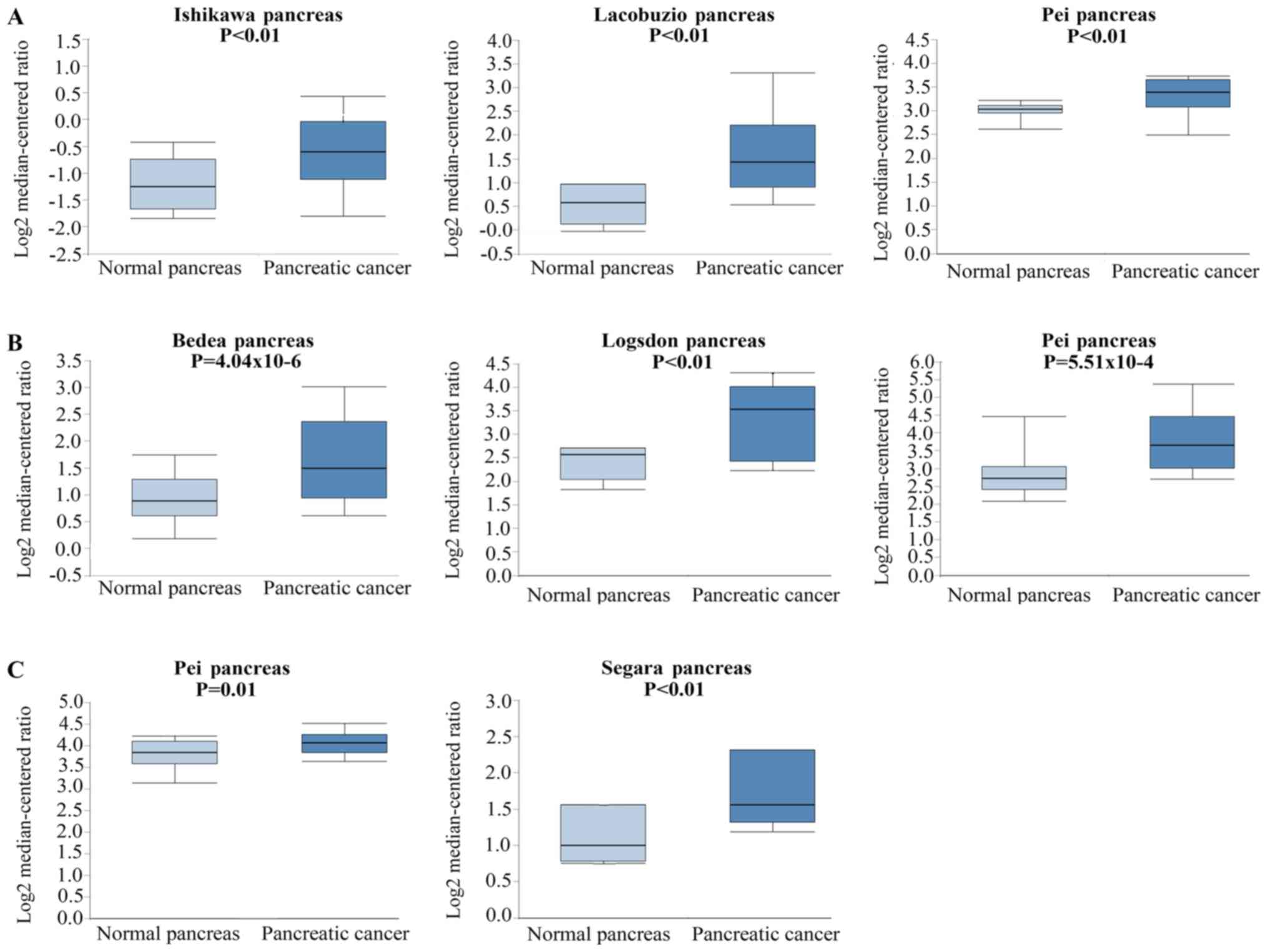
genes was then conducted using the Oncomine website. As shown in
Fig. 6, AKT1 was observed to be
overexpressed in pancreatic cancer compared with normal pancreatic
tissue in the studies of Ishikawa et al (31), Iacobuzio-Donahue et al
(32) and Pei et al (33) (P<0.01, P<0.01 and <0.01,
respectively). Badea et al (34), Logdons et al (35) and Pei et al (33) also reported that CCND1 was more
highly expressed in pancreatic cancer as compared with the normal
pancreas (P=4.04×10−6, P<0.01 and
P=5.51×10−4, respectively). Furthermore, MAPK1 was
reported to be overexpressed in pancreatic cancer by Pei et
al (33) and Segara et al
(36) (P=0.01 and P<0.01,
respectively).
Discussion
Pancreatic cancer is one of the most aggressive
malignancies and the fourth cause of cancer-associated mortalities
worldwide. This cancer is frequently diagnosed at an advanced
stage, and most tumors are unresectable at clinical presentation
(37), which leads to a poor
prognosis and high mortality rates among patients with pancreatic
cancer. Despite the advances in diagnostic and treatment methods,
there have been no significant improvements in the prognosis of
patients with pancreatic cancer in the past years. Therefore, more
effective treatment methods for pancreatic cancer are urgently
required.
The antitumor properties of ATO have been identified
in a number of tumors (10–14). ATO has also been shown to inhibit the
viability of pancreatic cancer stem cells in vitro and in
vivo (38). In addition, Gao
et al (15) reported that ATO
exerted anticancer effects on pancreatic cancer. The combination of
ATO with hypoxia-inducible factor-1 inhibitor was also found to be
effective (39). However, the
potential targets and molecular mechanisms of action of ATO in
pancreatic cancer remain elusive.
In the present study, the molecular targets and
associated genes of ATO were investigated by bioinformatics
analysis. First, the 19 target genes of ATO were identified by
DrugBank 5.0 and STITCH, which included IKBKB, TXNRD1, JUN, CCND1,
MAPK3, MAPK1, AKT1, CDKN1A, HDAC1, PML, RARA, ESR1, CDKN2A, ABCC1,
SP1, DNMT3B, DNMT1, HSPA4 and ABCB1. Next, the STRING website was
used to construct the PPI network and conduct KEGG pathway analysis
for the ATO target genes. A total of 6 target genes were
demonstrated to be associated with pancreatic cancer, including
AKT1, CCND1, CDKN2A, IKBKB, MAPK1 and MAPK3. Subsequently, the
cBioPortal database was used to detect the alterations of these 6
genes, and the results were as follows: AKT1, IKBKB and MAPK1 were
found to display amplification, deep deletion and missense
mutations; CDKN2A exhibited deep deletion, truncating, missense,
inframe and other mutations; and CCND1 and MAPK3 exhibited
amplification. Furthermore, 20 genes that are interconnected with
the 6 ATO target genes in pancreatic cancer were identified. These
genes were mainly involved in hepatitis B, pancreatic cancer,
pathways in cancer, glioma and chronic myeloid leukemia. Finally,
the K-M plotter was used to analyze the association of the 6 target
genes with the overall survival of patients with pancreatic cancer
and Oncomine was used to analyze differential expression of these
genes in pancreatic cancer. The results demonstrated that CCND1 and
MAPK1 were overexpressed in pancreatic cancer and may be of
prognostic value for patients with pancreatic cancer.
By conducting bioinformatics analysis, 6 ATO target
genes were identified in the current study. Among these 6 genes,
AKT1 is a member of the PI3K/AKT pathway and serves an important
role in multiple types of cancer, including pancreatic cancer
(40). CCND1, also referred to as
cyclin D1, forms a complex with CDK4, thus phosphorylating and
inhibiting RB1, and regulating the cell cycle during G1/S
transition. The activation of the CCND1/CDK4 complex also promotes
pancreatic cancer cell growth (41).
Furthermore, it is well-established that the increased risk of
pancreatic cancer is partly due to germline mutation carrier of
CDKN2A (42). A previous study
demonstrated that nuclear factor (NF)-κB promoted the proliferation
and growth, and inhibited the apoptosis of pancreatic cancer cells
(43). Inhibition of NF-κB also
blocked metastasis in pancreatic cancer-derived xenograft tumors
(44). In another study, the
MAPK/ERK signaling pathway was revealed to be correlated with
cancer cell growth, proliferation and apoptosis, including
pancreatic cancer (45). Hu et
al (46) used integrative
analysis and identified MAPK3 as a significant hub gene in
pancreatic cancer. In the present study, AKT1, CCND1 and MAPK1 were
found to be overexpressed in pancreatic cancer, while high CCND1
and MAPK1 levels indicated poor prognosis.
STRING analysis performed in the current study
revealed that the 6 ATO target genes (AKT1, CCND1, CDKN2A, IKBKB,
MAPK1 and MAPK3) were involved in chronic myeloid leukemia,
pancreatic cancer, non-small cell lung cancer, acute myeloid
leukemia, melanoma, glioma, prostate cancer, endocine resistance
FoxO signaling and hepatitis B. The PPI network was also used to
analyze the genes interconnected with the 6 ATO target genes, and
the 20 interconnected genes were observed to be involved in
hepatitis B, prostate cancer, pathways in cancer, glioma and
chronic myeloid leukemia. These results may help develop new
therapeutic strategies for cancer.
However, there were certain limitations to the
present study. Firstly, this research was based on bioinformatics
analysis, and experimental results are required to support the
conclusions. Secondly, since the NF-κB pathway serves an important
role in multiple cancer development, a significant association of
IKBKB with poor prognosis of patients with pancreatic cancer was
not observed by Kaplan-Meier analysis. Furthermore, the key genes
implicated in pancreatic cancer should also be analyzed to confirm
the reliability of the results. The present study showed that 6 ATO
target genes (AKT1, CCND1, CDKN2A, IKBKB, MAPK1 and MAPK3) were
associated with pancreatic cancer, and that high CCND1 and MAPK1
levels indicated poor prognosis. However, since tissue samples were
not collected, these 6 proteins could not be verified in pancreatic
cancer samples. Next, immunohistochemistry will be used to validate
the expression of these proteins in pancreatic cancer and evaluate
their association with the prognosis of patients with pancreatic
cancer.
In conclusion, the bioinformatics analysis was used
to elucidate the molecular mechanism of action of ATO in pancreatic
cancer. The results not only suggest that ATO may be an effective
drug for pancreatic cancer, but also provide a novel approach for
identifying potential treatments for other diseases by
bioinformatics analysis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81672921) and the
Innovation Capacity Support Plan of Shaanxi Province (no.
2018TD-002).
Availability of data and materials
The datasets generated and analyzed during the
present study are available in the public databases.
Authors' contributions
QZ and SXH designed the study. CYZ acquired and
analyzed the data, drafted the manuscript and revised it. LYG was
involved in data analysis and revised the manuscript. RL, NNW, YYF,
YPD, HZ, YQZ and YYZ performed the data analysis. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ATO
|
arsenic trioxide
|
|
PPI
|
protein-protein interaction
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saad AM, Turk T, Al-Husseini MJ and
Abdel-Rahman O: Trends in pancreatic adenocarcinoma incidence and
mortality in the United States in the last four decades; a
SEER-based study. BMC Cancer. 18:6882018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soignet SL, Frankel SR, Douer D, Tallman
MS, Kantarjian H, Calleja E, Stone RM, Kalaycio M, Scheinberg DA,
Steinherz P, et al: United States multicenter study of arsenic
trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol.
19:3852–3860. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mathews V, George B, Chendamarai E,
Lakshmi KM, Desire S, Balasubramanian P, Viswabandya A, Thirugnanam
R, Abraham A, Shaji RV, et al: Single-agent arsenic trioxide in the
treatment of newly diagnosed acute promyelocytic leukemia:
Long-term follow-up data. J Clin Oncol. 28:3866–3871. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghavamzadeh A, Alimoghaddam K, Rostami S,
Ghaffari SH, Jahani M, Iravani M, Mousavi SA, Bahar B and Jalili M:
Phase II study of single-agent arsenic trioxide for the front-line
therapy of acute promyelocytic leukemia. J Clin Oncol.
29:2753–2757. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lo-Coco F, Avvisati G, Vignetti M, Thiede
C, Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona
E, et al: Retinoic acid and arsenic trioxide for acute
promyelocytic leukemia. N Engl J Med. 369:111–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burnett AK, Russell NH, Hills RK, Bowen D,
Kell J, Knapper S, Morgan YG, Lok J, Grech A, Jones G, et al:
Arsenic trioxide and all-trans retinoic acid treatment for acute
promyelocytic leukaemia in all risk groups (AML17): Results of a
randomised, controlled, phase 3 trial. Lancet Oncol. 16:1295–1305.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seftel MD, Barnett MJ, Couban S, Leber B,
Storring J, Assaily W, Fuerth B, Christofides A and Schuh AC: A
Canadian consensus on the management of newly diagnosed and
relapsed acute promyelocytic leukemia in adults. Curr Oncol.
21:234–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Liu L, Zhan S, Chen L, Wang Y,
Zhang Y, Du J, Wu Y and Gu L: Arsenic trioxide suppressed migration
and angiogenesis by targeting FOXO3a in gastric cancer cells. Int J
Mol Sci. 19(pii): E37392018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhong L, Xu F and Chen F: Arsenic trioxide
induces the apoptosis and decreases NF-KB expression in lymphoma
cell lines. Oncol Lett. 16:6267–6274. 2018.PubMed/NCBI
|
|
12
|
Mao MH, Huang HB, Zhang XL, Li K, Liu YL
and Wang P: Additive antitumor effect of arsenic trioxide combined
with intravesical bacillus Calmette-Guerin immunotherapy against
bladder cancer through blockade of the IER3/Nrf2 pathway. Biomed
Pharmacother. 107:1093–1103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du S, Liu K, Gao P, Li Z and Zheng J:
Differential anticancer activities of arsenic trioxide on head and
neck cancer cells with different human papillomavirus status. Life
Sci. 212:182–193. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo D, Zhang X, Du R, Gao W, Luo N, Zhao
S, Li Y, Chen R, Wang H, Bao Y, et al: Low dosage of arsenic
trioxide (As2O3) inhibits angiogenesis in
epithelial ovarian cancer without cell apoptosis. J Biol Inorg
Chem. 23:939–947. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao JK, Wang LX, Long B, Ye XT, Su JN, Yin
XY, Zhou XX and Wang ZW: Arsenic Trioxide inhibits cell growth and
invasion via Down-Regulation of Skp2 in pancreatic cancer cells.
Asian Pac J Cancer Prev. 16:3805–3810. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang YA, You ZH and Chen X: A systematic
prediction of drug-target interactions using molecular fingerprints
and protein sequences. Curr Protein Pept Sci. 19:468–478. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wishart DS, Knox C, Guo AC, Cheng D,
Shrivastava S, Tzur D, Gautam B and Hassanali M: DrugBank: A
knowledgebase for drugs, drug actions and drug targets. Nucleic
Acids Res. 36:D901–D906. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuhn M, von Mering C, Campillos M, Jensen
LJ and Bork P: STITCH: Interaction networks of chemicals and
proteins. Nucleic Acids Res. 36:D684–D688. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szklarczyk D, Morris JH, Cook H, Kuhn M,
Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al:
The STRING database in 2017: Quality-controlled protein-protein
association networks, made broadly accessible. Nucleic Acids Res.
45:D362–D368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bindea G, Galon J and Mlecnik B: CluePedia
Cytoscape plugin: Pathway insights using integrated experimental
and in silico data. Bioinformatics. 29:661–663. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Biankin AV, Waddell N, Kassahn KS, Gingras
MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J,
et al: Pancreatic cancer genomes reveal aberrations in axon
guidance pathway genes. Nature. 491:399–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bailey P, Chang DK, Nones K, Johns AL,
Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC,
et al: Genomic analyses identify molecular subtypes of pancreatic
cancer. Nature. 531:47–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sanchez-Vega F, Mina M, Armenia J, Chatila
WK, Luna A, La KC, Dimitriadoy S, Liu DL, Kantheti HS, Saghafinia
S, et al: Oncogenic signaling pathways in the cancer genome atlas.
Cell. 173:321–337.e10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Witkiewicz AK, McMillan EA, Balaji U, Baek
G, Lin WC, Mansour J, Mollaee M, Wagner KU, Koduru P, Yopp A, et
al: Whole-exome sequencing of pancreatic cancer defines genetic
diversity and therapeutic targets. Nat Commun. 6:67442015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lanczky A, Nagy A, Bottai G, Munkácsy G,
Szabó A, Santarpia L and Győrffy B: miRpower: A web-tool to
validate survival-associated miRNAs utilizing expression data from
2178 breast cancer patients. Breast Cancer Res Treat. 160:439–446.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rhodes DR, Kalyana-Sundaram S, Mahavisno
V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ,
Kincead-Beal C, Kulkarni P, et al: Oncomine 3.0: Genes, pathways,
and networks in a collection of 18,000 cancer gene expression
profiles. Neoplasia. 9:166–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ishikawa M, Yoshida K, Yamashita Y, Ota J,
Takada S, Kisanuki H, Koinuma K, Choi YL, Kaneda R, Iwao T, et al:
Experimental trial for diagnosis of pancreatic ductal carcinoma
based on gene expression profiles of pancreatic ductal cells.
Cancer Sci. 96:387–393. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iacobuzio-Donahue CA, Maitra A, Olsen M,
Lowe AW, van Heek NT, Rosty C, Walter K, Sato N, Parker A, Ashfaq
R, et al: Exploration of global gene expression patterns in
pancreatic adenocarcinoma using cDNA microarrays. Am J Pathol.
162:1151–1162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pei H, Li L, Fridley BL, Jenkins GD,
Kalari KR, Lingle W, Petersen G, Lou Z and Wang L: FKBP51 affects
cancer cell response to chemotherapy by negatively regulating Akt.
Cancer Cell. 16:259–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Badea L, Herlea V, Dima SO, Dumitrascu T
and Popescu I: Combined gene expression analysis of whole-tissue
and microdissected pancreatic ductal adenocarcinoma identifies
genes specifically overexpressed in tumor epithelia.
Hepatogastroenterology. 55:2016–2027. 2008.PubMed/NCBI
|
|
35
|
Logsdon CD, Simeone DM, Binkley C,
Arumugam T, Greenson JK, Giordano TJ, Misek DE, Kuick R and Hanash
S: Molecular profiling of pancreatic adenocarcinoma and chronic
pancreatitis identifies multiple genes differentially regulated in
pancreatic cancer. Cancer Res. 63:2649–2657. 2003.PubMed/NCBI
|
|
36
|
Segara D, Biankin AV, Kench JG, Langusch
CC, Dawson AC, Skalicky DA, Gotley DC, Coleman MJ, Sutherland RL
and Henshall SM: Expression of HOXB2, a retinoic acid signaling
target in pancreatic cancer and pancreatic intraepithelial
neoplasia. Clin Cancer Res. 11:3587–3596. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chari ST: Detecting early pancreatic
cancer: Problems and prospects. Semin Oncol. 34:284–294. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han JB, Sang F, Chang JJ, Hua YQ, Shi WD,
Tang LH and Liu LM: Arsenic trioxide inhibits viability of
pancreatic cancer stem cells in culture and in a xenograft model
via binding to SHH-Gli. Onco Targets Ther. 6:1129–1138. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lang M, Wang X, Wang H, Dong J, Lan C, Hao
J, Huang C, Li X, Yu M, Yang Y, et al: Arsenic trioxide plus PX-478
achieves effective treatment in pancreatic ductal adenocarcinoma.
Cancer Lett. 378:87–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Michl P and Downward J: Mechanisms of
disease: PI3K/AKT signaling in gastrointestinal cancers. Z
Gastroenterol. 43:1133–1139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yan L, Wang Y, Wang ZZ, Rong YT, Chen LL,
Li Q, Liu T, Chen YH, Li YD, Huang ZH and Peng J: Cell motility and
spreading promoted by CEACAM6 through cyclin D1/CDK4 in human
pancreatic carcinoma. Oncol Rep. 35:418–426. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
McWilliams RR, Wieben ED, Chaffee KG,
Antwi SO, Raskin L, Olopade OI, Li D, Highsmith WE Jr, Colon-Otero
G, Khanna LG, et al: CDKN2A Germline rare coding variants and risk
of pancreatic cancer in minority populations. Cancer Epidemiol
Biomarkers Prev. 27:1364–1370. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liptay S, Weber CK, Ludwig L, Wagner M,
Adler G and Schmid RM: Mitogenic and antiapoptotic role of
constitutive NF-kappaB/Rel activity in pancreatic cancer. Int J
Cancer. 105:735–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fujioka S, Sclabas GM, Schmidt C,
Frederick WA, Dong QG, Abbruzzese JL, Evans DB, Baker C and Chiao
PJ: Function of nuclear factor kappaB in pancreatic cancer
metastasis. Clin Cancer Res. 9:346–354. 2003.PubMed/NCBI
|
|
45
|
Hu Y, Yang H, Lu XQ, Xu F, Li J and Qian
J: ARHI suppresses pancreatic cancer by regulating MAPK/ERK 1/2
pathway. Pancreas. 44:342–343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hu B, Shi C, Jiang HX and Qin SY:
Identification of novel therapeutic target genes and pathway in
pancreatic cancer by integrative analysis. Medicine (Baltimore).
96:e82612017. View Article : Google Scholar : PubMed/NCBI
|