Introduction
Factor that binds to the inducer of short
transcripts of the human immunodeficiency virus-1 (FBI-1) plays an
important role in gene expression. FBI-1 is also known as
leukemia/lymphoma-related factor or POK erythroid myeloid ontogenic
factor (pokemon) (1–3). FBI-1 is an essential regulator of
genes, and previous studies have reported that FBI-1 is
overexpressed in various types of cancer, including ovarian cancer,
hepatocellular carcinoma (HCC), non-small-cell lung cancer,
endometrial carcinoma, nasopharyngeal carcinoma, prostate cancer
and breast cancer (4–11). Although most cancers exhibit
FBI-1-overexpression, a previous study reported that FBI-1 is
significantly downregulated in oral squamous cell carcinoma
(12), which indicates its complex
role in tumorigenesis.
Previous studies have investigated the protein and
mRNA expression of FBI-1 in small sample size populations of
colorectal cancer (CRC; 46–66 CRC samples). The results
demonstrated that FBI-1 is overexpressed in CRC tissues compared
with normal mucosa and that FBI-1-silencing inhibits the
proliferation, cell cycle and apoptosis of CRC cells (13,14). In
addition, higher FBI-1 expression is associated with lymph node
metastasis and higher Duke's stage (14). Furthermore, a study reported that
low-dose radiation under hypoxic conditions downregulates FBI-1
expression in rat pheochromocytoma PC12 cells (15). Preoperative radiotherapy (RT) is
recommended for patients with locally advanced RC and is associated
with a decreased rate of local recurrence and an improved survival
(16,17). Although numerous studies have
reported that FBI-1 is an important gene regulator in CRC (13,14), the
association of FBI-1 expression with the prognosis and RT of
patients with RC require further investigation.
The present study used immunohistochemistry and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) to determine whether FBI-1 was associated with the
response to RT, and with clinicopathological variables and
biological factors, including p53, Wrap53, p73, peroxisome
proliferator-activated receptor δ (PPARδ), lysyl oxidase (LOX) and
Ki-67, in patients with RC.
Materials and methods
Patient samples
Samples were collected from a total of 139 patients
who underwent RC resection planned between March 1987 and February
1990 and were recruited at the Swedish clinical trial (86151) of
preoperative RT. Patients included 85 men and 54 women, with an age
range of 36–85 years (mean, 66 years old). Patients were randomly
assigned either to 1 week of preoperative RT followed by surgery
within the next week (RT + surgery group), or surgery with no
preoperative RT (surgery alone group).
RC tissues following resection were immediately
fixed, paraffin-embedded and stored at 4°C at the Linköping
University until further use. These formalin-fixed
paraffin-embedded sections were used for immunohistochemistry
(IHC). IHC was also performed on 118 distant normal mucosa
specimens taken from the resection margins, which were
histologically free from tumor cells (105 normal mucosa samples had
matched primary tumors from the same patient, whereas 13 samples
did not have matched tumor samples). Amongst the 139 patients, 77
underwent surgery only and 62 received RT followed by tumor
resection. RT was administered as 25 Gy in five fractions within a
median of 7 days (range, 4–12 days). Surgery was then performed at
a median of 3 days (range, 0–11 days) following RT. Information on
tumor-node-metastasis (TNM) stage (American Joint Committee on
Cancer staging manual, 4th edition) (18), differentiation, local and distant
recurrence, disease-free survival (DFS) and overall survival (OS)
was obtained from surgical and pathological hospital records. Tumor
differentiation was graded as improved (including well-and
moderately differentiated tumors) or poorer (including poor,
mucinous and signet-ring cell tumors). The mean follow-up period
was 100 months (range, 0–309 months). The characteristics of
patients and tumors are presented in Table I.
 | Table I.Characteristics of patients with
rectal cancer. |
Table I.
Characteristics of patients with
rectal cancer.
|
| Non-radiotherapy | Radiotherapy |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Number | % | Number | % | P-value |
|---|
| Sex |
|
|
|
| 0.465 |
|
Male | 45 | 58.4 | 40 | 64.5 |
|
|
Female | 32 | 41.6 | 22 | 35.5 |
|
| Age (years) |
|
|
|
| 0.929 |
|
≤66 | 28 | 36.4 | 23 | 37.1 |
|
|
>66 | 49 | 63.6 | 39 | 62.9 |
|
| TNM stage |
|
|
|
| 0.270 |
| I | 20 | 26 | 17 | 27.4 |
|
| II | 19 | 24.7 | 22 | 35.5 |
|
|
III | 34 | 44.1 | 18 | 29.0 |
|
| IV | 4 | 5.2 | 5 | 8.1 |
|
|
Differentiation |
|
|
|
| 0.765 |
|
High | 2 | 2.6 | 1 |
1.6 |
|
|
Moderate | 61 | 79.2 | 47 | 75.8 |
|
|
Poor | 14 | 18.2 | 14 | 22.6 |
|
| Number of
tumors |
|
|
|
| 0.392 |
|
Single | 64 | 83.1 | 51 | 82.3 |
|
|
Multiplea | 11 | 14.3 | 11 | 17.7 |
|
|
Unknown | 2 | 2.6 | 0 | 0 |
|
| Resection
margin |
|
|
|
| 0.677 |
|
Negative | 72 | 93.5 | 59 | 95.2 |
|
|
Positive | 5 | 6.5 | 3 |
4.8 |
|
Additional samples were collected from another
cohort of 55 patients with RC treated at the Linköping University
hospital between April 1990 and February 2003. Patients were aged
between 51–88 years old, comprising 35 men and 20 females, and had
not received any treatment prior to RC resection. These samples
were collected to detect FBI-1 mRNA expression. For each patient,
samples from the primary tumor and corresponding normal mucosa were
collected. All specimens were immediately flash-frozen in liquid
nitrogen following RC resection and stored at −80°C.
Expression of p53, Wrap53, p73, PPARδ, LOX and Ki-67
were examined by IHC on the normal mucosa and RC samples from the
non-RT and RT groups, as previously described (19–22).
The present study was approved by the Medical Ethics
Committee of Linköping University approval, and written informed
consent was obtained from each patient.
IHC
FBI-1 IHC was performed on 5-µm paraffin-embedded
tissue sections. Sections were incubated at 60°C for 12 h prior to
being deparaffinized in xylene and hydrated in descending
concentrations of ethanol (99.5%, for 5 min twice; 95%, for 5 min;
70%, for 5 min) at room temperature. Sections were placed in
Tris-EDTA buffer (pH, 9.0), heated to 125°C for 30 sec and cooled
down to 90°C for 10 sec in a high-pressure cooker to allow antigen
retrieval. Sections were then washed in PBS (pH 7.4). Endogenous
peroxidase activity of the sections was blocked with 3% hydrogen
peroxide dissolved in 99.9% methanol for 10 min at room
temperature. Sections were washed three times with PBS. Blocking of
non-specific interactions was then performed with 1.5% blocking
serum (Dako; Agilent Technologies, Inc.) in PBS for 10 min at room
temperature. Sections were incubated overnight at 4°C with the
primary antibody against FBI-1 (cat. no. ab70208; Abcam) diluted in
antibody diluent (1:200; Dako; Agilent Technologies, Inc.). The
sections were then incubated with a horseradish
peroxidase-conjugated anti-rabbit secondary antibody (1:100; cat.
no. P0397; Dako; Agilent Technologies, Inc.) for 45 min at 37°C and
stained with 3,3′-diaminobenzidine tetrahydrochloride (Dako;
Agilent Technologies, Inc.) at room temperature for 5 min. The
sections were eventually counterstained with haematoxylin.
Immunoglobulin G1 (1:800; cat. no. SAB5500149; Sigma-Aldrich; Merck
KGaA) was used as negative control.
Sections were examined with a light microscope
(CX43; Olympus Corporation) at ×400 magnification and scored
independently by Dr Wang and Professor Zhang, who were
double-blinded to the clinicopathological and biological data
(including p53, Wrap53, p73, PPARδ, LOX and Ki-67 data). The
nuclear staining intensity in epithelial or tumor cells was scored
as negative, weak, moderate or strong staining, for <5, 5–24,
25–50 or >50% cells positively stained, respectively. The
staining patterns were recorded as cytoplasmic or nuclear. Cases of
scoring discrepancy were re-evaluated individually until the two
investigators agreed on the scoring. The remaining cases were
re-examined by the two investigators together using a dual-headed
microscope in order to reach a consensus score. To avoid artifacts,
areas with necrosis or poor morphology and sections margins were
excluded.
RT-qPCR
The relative expression level of FBI-1 was
determined by RT-qPCR in a 7900HT Fast Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and normalized
to GAPDH. Primers and probes were included in the
TaqMan™® Gene Expression assays for FBI-1
(Hs00252415_s1) and GAPDH (4352934E; both Applied Biosystems;
Thermo Fisher Scientific, Inc.).
According to the manufacturer's instructions, total
RNA of RC and the corresponding normal mucosa samples was extracted
using the TRIzol reagent (Sigma-Aldrich; Merck KGaA) and RNeasy
extraction kit (Qiagen, Inc.). The concentration, purity, and
integrity of RNA were measured by NanoDrop (Thermo Fisher
Scientific, Inc.) and Bioanalyzer Agilent (Agilent Technologies,
Inc.). RT was conducted according to the manufacturer's protocol of
the High Capacity cDNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The relative
expression levels of FBI-1 were determined by RT-qPCR and
normalized to GAPDH. The PCR mix included 1 µl RT product, 7.5 µl
TaqMan Fast Universal PCR Master mix (2X No AmpErase UNG), 0.75 µl
2X TaqMan gene Assay and 5.75 µl nuclease-free water. The reactions
were incubated in a 96-well plate at 95°C for 20 sec, followed by
40 circles of 95°C for 3 sec and 60°C for 30 sec. In addition,
double-distilled H2O was analyzed as the no-template
control for every plate. All reactions, including the no-template
control, were performed in triplicates. The relative expression
level of FBI-1 was calculated using the 2−ΔΔCq method
(23).
Statistical analysis
The values for FBI-1 mRNA level were transformed to
log2 values and data were normally distributed. The data
are expressed as the mean ± standard deviation of at least three
independent experiments. Paired t-test or McNemar's test were used
to determine the differences in FBI-1 mRNA or protein level between
normal mucosa and primary RC respectively. χ2 test was used to
analyze unpaired data, and to determine the association between
FBI-1 protein level in primary RC and clinicopathological
variables. Spearman's correlation test was used to analyze the
correlations between the protein expression of FBI-1 and other
molecular markers, including p53, Wrap53, p73, PPARδ, LOX and
Ki-67, from previous studies performed on the same samples
(19–22). Cox's proportional hazard model was
used to determine the association between FBI-1 protein expression
and patient survival. The Kaplan-Meier method was used to generate
survival curves. All statistical analyses were carried out by using
STATISTICA software package (version 7.0; StatSoft, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of FBI-1 in normal mucosa
and RC samples in non-RT and RT groups
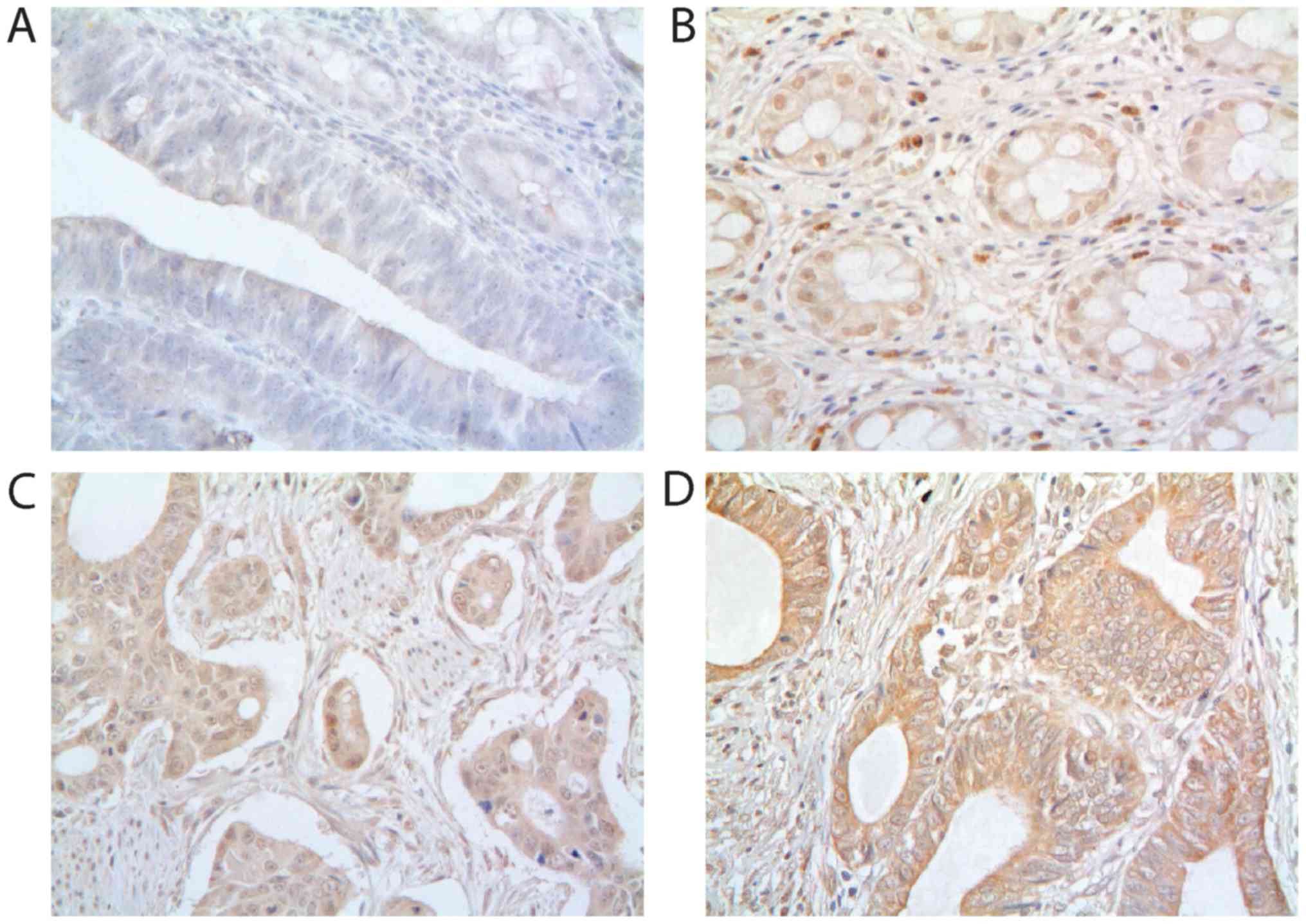
FBI-1 protein expression was detected in the
cytoplasm and nucleus of normal mucosa and primary cancer cells
(Fig. 1). The intensity in
epithelial cells or tumor cells was scored as negative, weak (light
yellow), moderate (yellow brown) and strong staining (brown). The
negative control showed no staining (Fig. 1A). Moderate cytoplasmic and strong
nuclear staining of FBI-1 was observed in normal mucosa (Fig. 1B) and primary tumor (Fig. 1C). Strong cytoplasmic staining of
FBI-1 was observed in the primary tumor (Fig. 1D). Subsequently, samples were
separated into subgroups based on the expression level of FBI-1,
including low-expression (negative and weak staining intensity) and
high-expression (moderate and strong staining intensities).
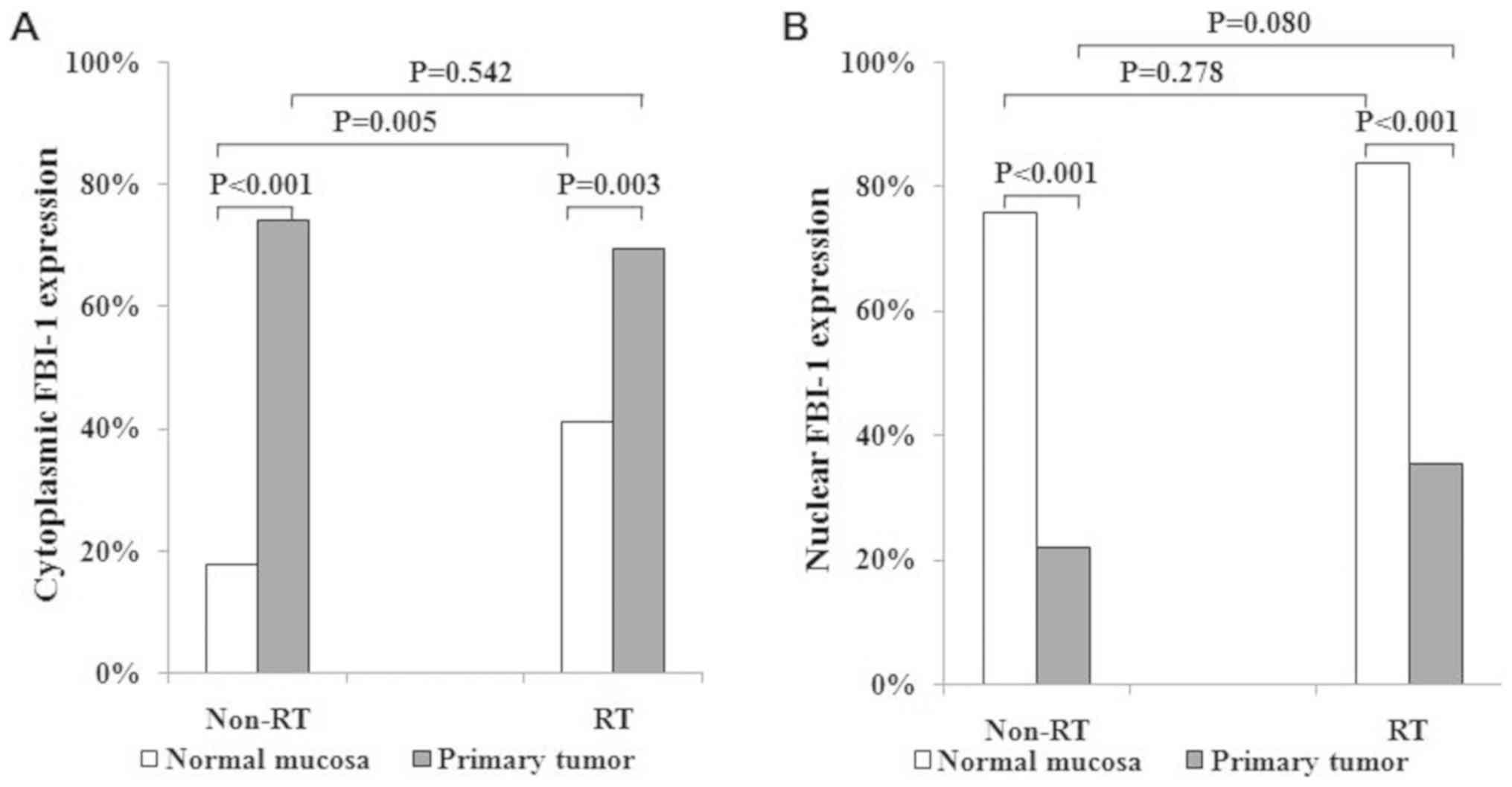
Cytoplasmic staining of FBI-1 in RC samples was significantly
upregulated in the non-RT and RT groups (17.7 vs. 74.0%; 41.1 vs.
69.4%; Fig. 2A) compared with the
corresponding normal mucosa. However, nuclear staining of FBI-1 was
significantly downregulated in the non-RT and RT groups of RC
samples (75.8 vs. 22.1 and 83.9 vs. 35.5%, respectively; Fig. 2B) compared with the corresponding
normal mucosa. Furthermore, RT resulted in markedly increased FBI-1
expression in the cytoplasm of normal mucosa compared with non-RT
(41.1 vs. 17.7%, respectively; P=0.005; Fig. 2A), but not in the nucleus (83.9 vs.
75.8%, respectively; P=0.278; Fig.
2B). In addition, cytoplasmic and nuclear staining of FBI-1 in
RC cells were similar between the non-RT and RT groups
(cytoplasmic, 74.0 vs. 69.4%; P=0.542; Fig. 2A; nuclear, 22.1 vs. 35.5%; P=0.080;
Fig. 2B). The non-RT and RT groups
were therefore combined for further analyses.
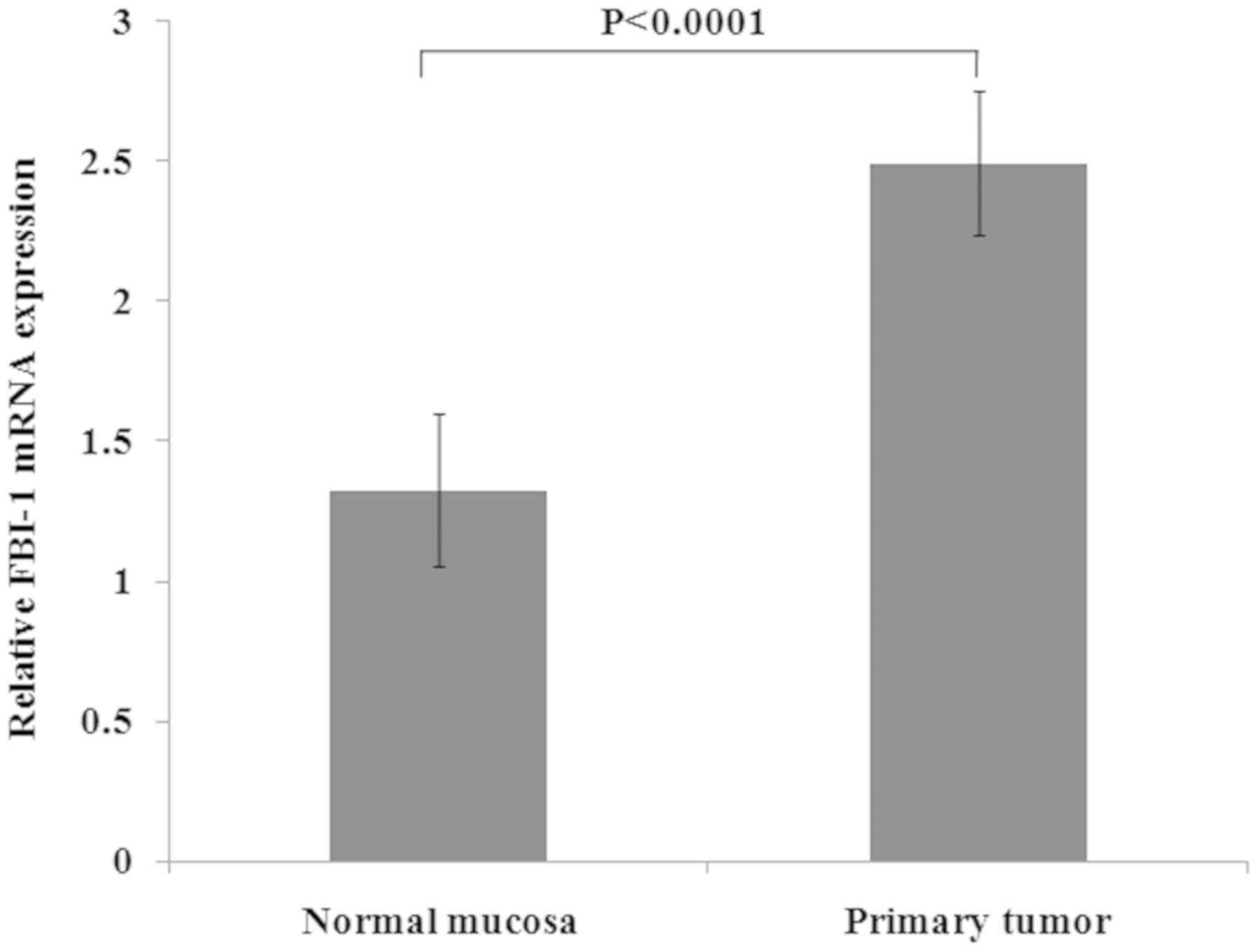
In the additional cohort of 55 patients with RC,
FBI-1 mRNA expression was significantly upregulated in RC tissues
(2.491±0.257 vs. 1.325±0.270; P<0.0001; Fig. 3) compared with the corresponding
normal mucosa.
Association between nuclear FBI-1
expression and clinicopathological characteristics
The present study analyzed the association between
cytoplasmic and nuclear FBI-1 expression and clinicopathological
characteristics, including sex, age, RC stage, differentiation,
growth pattern and local and distant recurrence. As presented in
Table II, the high expression of
nuclear FBI-1 was significantly higher in patients with stage
III+IV RC compared with patients with stage I+II RC (41.0 vs.
17.9%, respectively; P=0.003). Furthermore, nuclear FBI-1
expression in patients with distant recurrence exhibited a higher
positive staining compared with patients without distant recurrence
(39.7 vs. 19.8%, respectively; P=0.010). In addition, FBI-1
expression exhibited a trend for higher expression in patients with
RC and invasive growth pattern, compared with patients with RC and
expanding growth pattern (37.8 vs. 22%, respectively; P=0.070).
Nuclear FBI-1 expression was not associated with other
characteristics analyzed in the present study, including sex, age,
differentiation and local recurrence.
 | Table II.Association between nuclear
expression of FBI-1 and clinicopathological characteristics of
patients with rectal cancer. |
Table II.
Association between nuclear
expression of FBI-1 and clinicopathological characteristics of
patients with rectal cancer.
|
| Nuclear
expression |
|
|---|
|
|
|
|
|---|
|
Characteristics | Low (%) | High (%) | P-value |
|---|
| Sex |
|
| 0.222 |
|
Male | 58 (68.2) | 27 (31.8) |
|
|
Female | 42 (77.8) | 12 (22.2) |
|
| Age (years) |
|
| 0.292 |
|
≤66 | 34 (66.7) | 17 (33.3) |
|
|
>66 | 66 (75.0) | 22 (25.0) |
|
| TNM stage |
|
| 0.003 |
|
I+II | 64 (82.1) | 14 (17.9) |
|
|
III+IV | 36 (59.0) | 25 (41.0) |
|
|
Differentiation |
|
| 0.139 |
|
High/moderate | 83 (74.8) | 28 (25.2) |
|
|
Poor | 17 (60.7) | 11 (39.3) |
|
| GP |
|
| 0.070 |
|
Expanding | 64 (78.0) | 18 (22.0) |
|
|
Invasive | 23 (62.2) | 14 (37.8) |
|
| LR |
|
| 0.544 |
| No | 83 (70.9) | 34 (29.1) |
|
|
Yes | 17 (77.3) | 5
(22.7) |
|
| DR |
|
| 0.010 |
| No | 65 (80.2) | 16 (19.8) |
|
|
Yes | 35 (60.3) | 23 (39.7) |
|
Cytoplasmic FBI-1 expression and FBI-1 mRNA
expression were also compared to the clinicopathological
characteristics; however, no significant association was observed
(data not shown).
Nuclear FBI-1 expression as a
prognostic factor in primary cancer
Among the 139 patients with RC included in the
present study, 37, 41, 52 and 9 had stage I, II, III and IV RC,
respectively. The present study conducted a survival analysis that
only included patients with stage I, II and III RC, due to the poor
survival of patients with stage IV RC. The results demonstrated
that, in patients with stage I, II or III RC, upregulated nuclear
FBI-1 expression in RC samples correlated with poorer DFS and OS
times (P=0.017 and P=0.029, respectively; Fig. 4A and B).
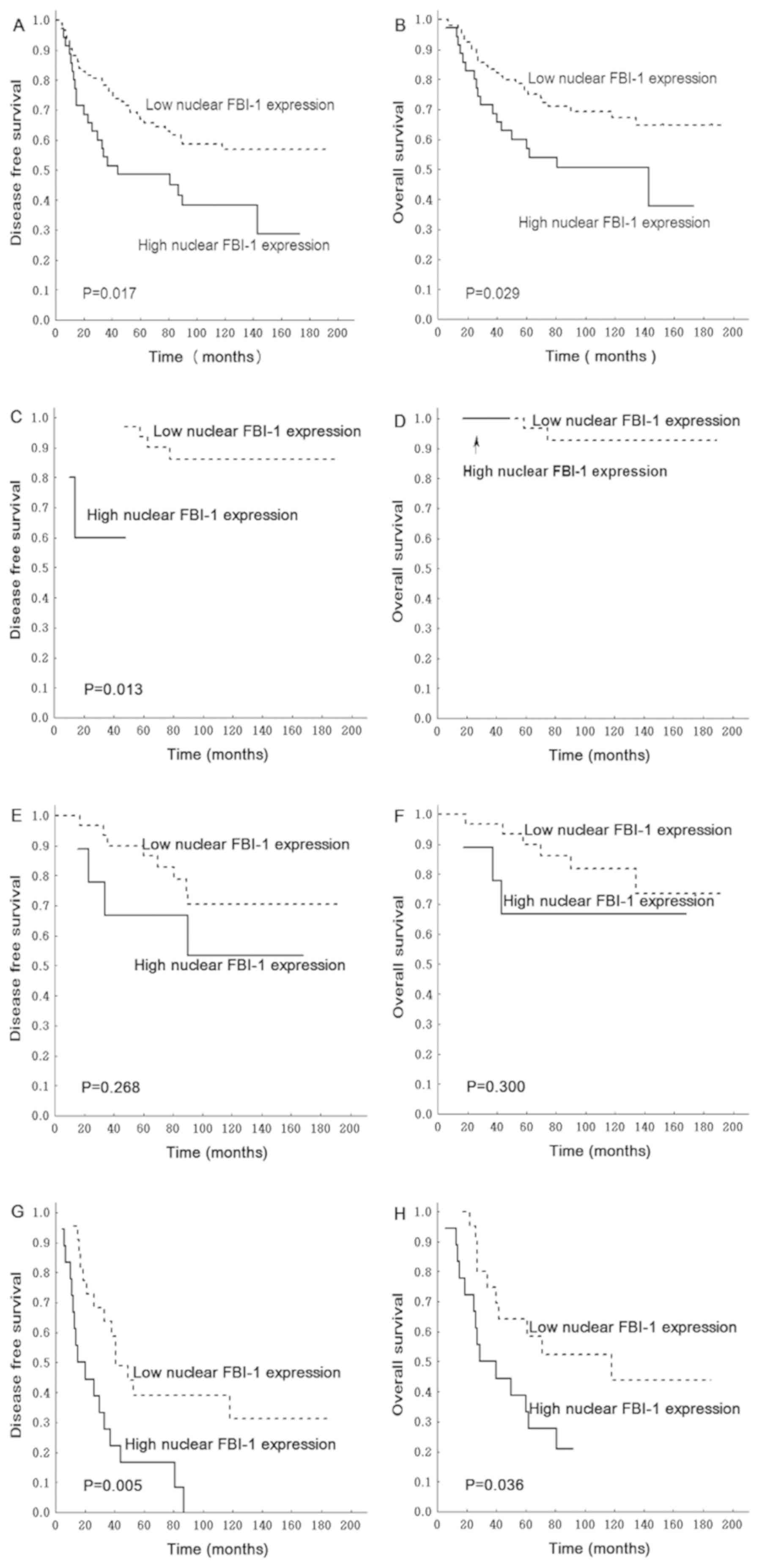 | Figure 4.Association between
tumor-node-metastasis stage of RC, and DFS and OS. Patients with
stage I, II and III RC and high nuclear FBI-1 expression exhibited
poorer (A) DFS and (B) OS. In patients with stage I RC, high
nuclear FBI-1 was significantly associated with poorer (C) DFS, but
no significance was observed with (D) OS. In stage II patients,
nuclear FBI-1 expression was not associated with (E) DFS or (F) OS.
In patients with stage III RC, high nuclear FBI-1 expression was
significantly associated with poorer (G) DFS and (H) OS. DFS,
disease-free survival; FBI-1, factor that binds to the inducer of
short transcripts of the human immunodeficiency virus-1; OS,
overall survival; RC, rectal cancer. |
A subgroup analysis in patients with TNM stages I,
II or III RC was also performed. In patients with stage I RC, high
nuclear FBI-1 expression was significantly associated with poorer
DFS time (P=0.013; Fig. 4C);
however, no significance was observed following analysis of high
nuclear FBI-1 and OS time (Fig. 4D).
This could be due to the low positive rate and small sample size,
since only 5 samples presented high nuclear FBI-1 staining in this
subgroup of patients. In patients with stage II RC, high nuclear
FBI-1 expression was associated with poor DFS time (P=0.268;
Fig. 4E) and OS time (P=0.300;
Fig. 4F); however the differences
were not significant. In patients with stage III RC, high nuclear
FBI-1 expression was significantly associated with poor DFS time
(P=0.005; Fig. 4G) and OS time
(P=0.036; Fig. 4H). However,
cytoplasmic FBI-1 expression was not associated with DFS and OS
times (P=0.645 and P=0.982, respectively; data not shown).
Following multivariable analysis, the significance
remained following adjusting for sex, age, growth pattern,
differentiation and RT [DFS time hazard ratio (HR), 1.934, 95%
confidence interval (CI), 1.055–3.579; OS time HR, 2.174, 95% CI,
1.102–4.290; Table III].
 | Table III.Multivariate analysis of the
association between nuclear FBI-1 expression and survival of
patient with rectal cancer. |
Table III.
Multivariate analysis of the
association between nuclear FBI-1 expression and survival of
patient with rectal cancer.
|
| Disease-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
| Variables | Number | HR | 95% CI | P-value | Number | HR | 95% CI | P-value |
|---|
| Nuclear FBI-1 |
|
|
| 0.033 |
|
|
| 0.025 |
|
Low | 82 | 1 |
|
| 82 | 1 |
|
|
|
High | 29 | 1.934 | 1.055–3.579 |
| 29 | 2.174 | 1.102–4.290 |
|
| Sex |
|
|
| 0.358 |
|
|
| 0.447 |
|
Male | 65 | 1 |
|
| 65 | 1 |
|
|
|
Female | 46 | 0.764 | 0.429–1.360 |
| 46 | 0.780 | 0.411–1.479 |
|
| Age, years |
|
|
| 0.066 |
|
|
| 0.018 |
|
≤66 | 42 | 1 |
|
| 42 | 1 |
|
|
|
>66 | 69 | 1.770 | 0.963–3.256 |
| 69 | 2.356 | 1.158–4.792 |
|
| GP |
|
|
| 0.113 |
|
|
| 0.077 |
|
Expanding | 76 | 1 |
|
| 76 | 1 |
|
|
|
Invasive | 35 | 1.598 | 0.895–2.855 |
| 35 | 1.787 | 0.938–3.404 |
|
|
Differentiation |
|
|
| 0.508 |
|
|
| 0.828 |
|
High/moderate | 88 | 1 |
|
| 88 | 1 |
|
|
|
Poor | 23 | 0.788 | 0.388–1.597 |
| 23 | 0.921 | 0.437–1.942 |
|
| Radiotherapy |
|
|
| 0.077 |
|
|
| 0.083 |
| No | 67 | 1 |
|
| 67 | 1 |
|
|
|
Yes | 44 | 0.586 | 0.324–1.061 |
| 44 | 0.554 | 0.284–1.081 |
|
Correlation between nuclear FBI-1 and
biological variables
The present study analyzed the correlation between
the expression of nuclear FBI-1 expression and of biological
factors, including p53, Wrap53, p73, PPARδ, LOX and Ki-67, which
were previously investigated in the same patient cohort (19–22). In
the non-RT group, FBI-1 nuclear expression was positively
correlated with the expression of p73 [Spearman's correlation
coefficient (rs)=0.332] and LOX (rs=0.234),
but negatively correlated with Wrap53 expression
(rs=−0.425) (Table IV).
In the RT group, FBI-1 nuclear expression was negatively correlated
with PPARδ expression (rs=−0.294; Table IV). FBI-1 nuclear expression was
neither correlated with Ki-67 or p53 expression in the non-RT and
RT groups (Table IV).
 | Table IV.Association between expressions of
nuclear FBI-1 protein and other proteins. |
Table IV.
Association between expressions of
nuclear FBI-1 protein and other proteins.
|
| Non-radiotherapy
group | Radiotherapy
group |
|---|
|
|
|
|
|---|
| Biological
variables | Number | rs | P-value | Number | rs | P-value |
|---|
| p53 | 73 | −0.120 | 0.311 | 59 | −0.079 | 0.550 |
| Wrap53 | 74 | −0.425 | <0.001 | 60 | 0.002 | 0.987 |
| P73 | 65 | 0.332 | 0.007 | 46 | −0.048 | 0.754 |
| PPAR δ | 74 | −0.132 | 0.261 | 57 | −0.294 | 0.026 |
| LOX | 75 | 0.234 | 0.043 | 59 | 0.213 | 0.106 |
| Ki-67 | 62 | −0.138 | 0.284 | 46 | −0.264 | 0.077 |
Discussion
The present study investigated FBI-1 expression in
patients with RC who underwent or not preoperative RT, and
determined its association with patients' clinicopathological
variables and biological factors. The results demonstrated that,
compared with normal mucosa, FBI-1 was expressed in the cytoplasm
and nucleus of RC samples, and that cytoplasmic FBI-1 expression
was upregulated in RC samples from the non-RT and RT groups;
however, nuclear staining was downregulated in these two groups. RT
may therefore have no effect on FBI-1 expression. Furthermore,
nuclear FBI-1 was positively associated with TNM stage (P=0.003)
and distance recurrence (P=0.010). In patients with stage I, II or
III RC, increased nuclear FBI-1 expression was associated with
poorer DFS and OS times independent of sex, age, growth pattern,
differentiation and RT.
With regards to the localization of FBI-1 protein in
CRC, the results are contradictory. A previous study reported that
FBI-1 is mainly expressed in the nucleus of colon cancer cells
(24); however, other studies
demonstrated that FBI-1 is predominantly expressed in the cytoplasm
and partly in the nucleus of CRC cells (13,14). The
results from the present study provided further evidence that FBI-1
protein was localized in the cytoplasm and the nucleus of RC cells.
This was also observed in other types of cancer, including HCC
where FBI-1 is mostly expressed in the cytoplasm and rarely in the
nucleus (25). The present study
analyzed cytoplasmic and nuclear FBI-1 staining separately, and
reported that, compared with normal mucosa, the expression of
cytoplasmic FBI-1 and FBI-1 mRNA were upregulated, whereas nuclear
FBI-1 expression was downregulated in RC cells. A possible reason
for this discrepancy may be the sample investigated, since all
samples from the present study were RC samples; however previous
studies have included rectal and colon cancer samples. Increasing
evidence reported that RC possesses numerous molecular markers and
some characteristics of colon cancer (26,27).
Another possible reason may be the aberrant transport process of
FBI-1 protein from the cytoplasm to the nucleus, which requires
further investigation.
The results from the present study demonstrated that
preoperative RT had no effect on FBI-1 expression in RC. The non-RT
and RT groups were therefore combined in the further analyses.
FBI-1 nuclear staining was positively associated with TNM stage and
distant recurrence. Furthermore, patients with stage III and IV RC
exhibited higher FBI-1 expression compared with patients with stage
I and II RC, and patients with distant recurrence exhibited higher
FBI-1 expression compared with those without distant recurrence
(Table II). These results were in
accordance with a previous study reporting that FBI-1 expression is
positively associated with Dukes' stage in CRC (14). Although FBI-1 expression could be
used as a prognostic biomarker in HCC and lung cancer (5,6), no
studies reported its prognostic significance in RC. To the best of
our knowledge, the present study is the first to demonstrate that
nuclear FBI-1 expression may serve as a prognostic factor in
patients with RC, in particular in patients with stage III RC.
As one of the most important oncogene regulators,
FBI-1 has been implicated in numerous signaling pathways, including
the ARF-MDM2-p53 (28),
phosphoinositide 3-kinase/Akt signaling (29), Smad4 and TGF-β (30), and myocyte enhancer factor 2D
pathways (31). However, the pathway
associated with FBI-1 in RC remains unclear. Zhao et al
(14) reported that FBI-1 and p14ARF
or p53 are not correlated, and that FBI-1-knockdown in the colon
cancer LoVo cell line did not affect p14ARF expression, which
demonstrated that FBI-1 functions independently of the
p14ARF-MDM2-p53 pathway. In the present study, no correlation was
observed between FBI-1 and p53 expression, which further suggested
that FBI-1 may exert its activity independently of the
p14ARF-MDM2-p53 pathway. Of note, a negative correlation between
FBI-1 and Wrap53 was reported, combined with a positive correlation
between p73 and LOX in the non-RT group and a positive correlation
with PPARδ in the RT group. These results suggested that FBI-1 may
regulate multiple genes and therefore affect RC development and
progression. The underlying mechanisms of this pathway require
further investigation.
Although the present study provided evidence that
FBI-1 may participate in RC, there were a few limitations. Firstly,
FBI-1 expression with or without preoperative RT should also be
determined in RC cell lines. Secondly, the molecular mechanism
underlying the role of FBI-1 in RC requires further examination.
Further investigation using an RC cell line and animal model
experiments is therefore required in order to explore the FBI-1
signaling network in RC.
In conclusion, the present study demonstrated that
FBI-1 was highly expressed in RC samples and that nuclear FBI-1 may
be an independent prognostic factor in patients with RC. Since
FBI-1 was positively correlated with several biological factors,
its role in RC may be complex.
Acknowledgements
The authors would like to thank Dr Sebastian Gnosa
and Dr Johannes Stratmann (Linköping University, Sweden) for their
technical help.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. U1204818; 2013), the
Projects of Science and Technology in Henan Province (grant nos.
172102310064 and 201702195; 2017) and the Swedish Cancer Foundation
(grant no. 3610-B06-11XCC; 2007).
Availability of data and materials
The datasets generated and analyzed during the
present study are available from the corresponding author upon
reasonable request.
Authors' contributions
CJW, HZ and XFS designed the study. CJW, CRC, HML
and GA performed the experiments, data analysis and interpretation.
YYZ, GA and IJ collected the samples and performed the clinical
characterization of patients. CJW and XFS wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Linköping University. Written informed consent was
obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang Y, Li Y, Di F, Cui J, Wang Y and
David Xu ZQ: Pokemon decreases the transcriptional activity of RARα
in the absence of ligand. Biol Chem. 398:331–340. 2016. View Article : Google Scholar
|
|
2
|
Maeda T, Hobbs RM, Merghoub T, Guernah I,
Zelent A, Cordon-Cardo C, Teruya-Feldstein J and Pandolfi PP: Role
of the proto-oncogene Pokemon in cellular transformation and ARF
repression. Nature. 433:278–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang L, Li Q, Ye Z and Qiao B:
Pokemon/miR-137 auto-regulatory circuit promotes the progression of
renal carcinoma. Oncol Res. Apr 19–2018.doi:
10.3727/096504018X15231148037228, (Epub ahead of print).
|
|
4
|
Jiang L, Siu MK, Wong OG, Tam KF, Lam EW,
Ngan HY, Le XF, Wong ES, Chan HY and Cheung AN: Overexpression of
proto-oncogene FBI-1 activates membrane type 1 matrix
metalloproteinase in association with adverse outcome in ovarian
cancers. Mol Cancer. 9:3182010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang F, Yang L, Tao Y and Qin W: FBI-1
promotes cell proliferation and enhances resistance to chemotherapy
of hepatocellular carcinoma in vitro and in vivo. Cancer.
118:134–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao ZH, Wang SF, Yu L, Wang J, Chang H,
Yan WL, Zhang J and Fu K: Overexpression of Pokemon in non-small
cell lung cancer and foreshowing tumor biological behavior as well
as clinical results. Lung Cancer. 62:113–119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao Z, Wang J, Wang S, Chang H, Zhang T
and Qu J: LncRNA CCAT2 promotes tumorigenesis by over-expressed
Pokemon in non-small cell lung cancer. Biomed Pharmacother.
87:692–697. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yi TJ and Wang P: The expression of
Pokemon in endometrial carcinoma tissue and the correlation with
mutant p53. Sichuan Da Xue Xue Bao Yi Xue Ban. 47:321–325. 2016.(In
Chinese). PubMed/NCBI
|
|
9
|
Jiao W, Liu F, Tang FZ, Lan J, Xiao RP,
Chen XZ, Ye HL and Cai YL: Expression of the Pokemon proto-oncogene
in nasopharyngeal carcinoma cell lines and tissues. Asian Pac J
Cancer Prev. 14:6315–6319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aggarwal H, Aggarwal A, Hunter WJ III,
Yohannes P, Khan AU and Agrawal DK: Expression of leukemia/lymphoma
related factor (LRF/Pokemon) in human benign prostate hyperplasia
and prostate cancer. Exp Mol Pathol. 90:226–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zu X, Ma J, Liu H, Liu F, Tan C, Yu L,
Wang J, Xie Z, Cao D and Jiang Y: Pro-oncogene Pokemon promotes
breast cancer progression by upregulating survivin expression.
Breast Cancer Res. 13:R262011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sartini D, Lo Muzio L, Morganti S, Pozzi
V, Di Ruscio G, Rocchetti R, Rubini C, Santarelli A and Emanuelli
M: Pokemon proto-oncogene in oral cancer: Potential role in the
early phase of tumorigenesis. Oral Dis. 21:462–469. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu M, Li M, Zhang F, Feng F, Chen W, Yang
Y, Cui J, Zhang D and Linghu E: FBI-1 enhances ETS-1 signaling
activity and promotes proliferation of human colorectal carcinoma
cells. PLoS One. 9:e980412014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Y, Yao YH, Li L, An WF, Chen HZ, Sun
LP, Kang HX, Wang S and Hu XR: Pokemon enhances proliferation, cell
cycle progression and anti-apoptosis activity of colorectal
independently of p14ARF-MDM2-p53 pathway. Med Oncol. 31:2882014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim SW, Yu K, Shin KS, Kwon K, Hwang TS
and Kwon OY: Low-dose radiation suppresses Pokemon expression under
hypoxic conditions. Z Naturforsch C. 69:68–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Rosa M, Pace U, Rega D, Costabile V,
Duraturo F, Izzo P and Delrio P: Genetics, diagnosis and management
of colorectal cancer (Review). Oncol Rep. 34:1087–1096. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Zheng B, Lu X, Bai R, Feng L, Wang
Q, Zhao Y and He S: Preoperative short-course radiotherapy and
long-course radiochemotherapy for locally advanced rectal cancer:
Meta-analysis with trial sequential analysis of long-term survival
data. PLoS One. 13:e02001422018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hermanek P and Sobin LH: TNM
Classification of Malignant Tumours4th. Geneva: International Union
Against Cancer; 1987
|
|
19
|
Pfeifer D, Gao J, Adell G and Sun XF:
Expression of the p73 protein in rectal cancers with or without
preoperative radiotherapy. Int J Radiat Oncol Biol Phys.
65:1143–1148. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang L, Zhang H, Zhou ZG, Yan H, Adell G
and Sun XF: Biological function and prognostic significance of
peroxisome proliferator-activated receptor δ in rectal cancer. Clin
Cancer Res. 17:3760–3770. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang H, Wang DW, Adell G and Sun XF:
WRAP53 is an independent prognostic factor in rectal cancer- a
study of Swedish clinical trial of preoperative radiotherapy in RC
patients. BMC Cancer. 12:2942012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu N, Cox TR, Cui W, Adell G, Holmlund B,
Ping J, Jarlsfelt I, Erler JT and Sun XF: Nuclear expression of
lysyl oxidase enzyme is an independent prognostic factor in rectal
cancer patients. Oncotarget. 8:60015–60024. 2016.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi WI, Jeon BN, Yun CO, Kim PH, Kim SE,
Choi KY, Kim SH and Hur MW: Proto-oncogene FBI-1 represses
transcription of p21CIP1 by inhibition of transcription activation
by p53 and Sp1. J Biol Chem. 284:12633–12644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin CC, Zhou JP, Liu YP, Liu JJ, Yang XN,
Jazag A, Zhang ZP, Guleng B and Ren JL: The silencing of Pokemon
attenuates the proliferation of hepatocellular carcinoma cells in
vitro and in vivo by inhibiting the PI3K/Akt pathway. PLoS One.
7:e519162012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ichimasa K, Kudo SE, Miyachi H, Kouyama Y,
Hayashi T, Wakamura K, Hisayuki T, Kudo T, Misawa M, Mori Y, et al:
Comparative clinicopathological characteristics of colon and rectal
T1 carcinoma. Oncol Lett. 13:805–810. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tamas K, Walenkamp AM, de Vries EG, van
Vugt MA, Beets-Tan RG, van Etten B, de Groot DJ and Hospers GA:
Rectal and colon cancer: Not just a different anatomic site. Cancer
Treat Rev. 41:671–679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maeda T, Hobbs RM and Pandolfi PP: The
transcription factor Pokemon: A new key player in cancer
pathogenesis. Cancer Res. 65:8575–8578. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mak VC, Wong OG, Siu MK, Wong ES, Ng WY,
Wong RW, Chan KK, Ngan HY and Cheung AN: FBI-1 is overexpressed in
gestational trophoblastic disease and promotes tumor growth and
cell aggressiveness of choriocarcinoma via PI3K/Akt signaling. Am J
Pathol. 185:2038–2048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Y, Cui J, Xue F, Zhang C, Mei Z, Wang
Y, Bi M, Shan D, Meredith A, Li H and Xu ZQ: Pokemon (FBI-1)
interacts with Smad4 to repress TGF-β-induced transcriptional
responses. Biochim Biophys Acta. 1849:270–281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kong J, Liu X, Li X, Wu J, Wu N, Chen J
and Fang F: Pokemon promotes the invasiveness of hepatocellular
carcinoma by enhancing MEF2D transcription. Hepatol Int.
10:493–500. 2016. View Article : Google Scholar : PubMed/NCBI
|