Introduction
Gastric subepithelial neoplasms usually present as
protuberances covered by normal-appearing mucosa on endoscopy
(1). These protuberances vary in
different tumor types which include gastrointestinal stromal tumors
(GISTs), lipomas, leiomyomas and liposarcomas (2). In western countries, subtotal
gastrectomy or laparoscopic surgery is usually considered the
mainstay treatment (3).
Recently, endoscopic submucosal dissection (ESD) has
become a minimally invasive approach for gastric subepithelial
lesions. The technique was first reported in 1988 for treating
early gastric cancers in Japan (4),
and now it has been widely used in Asian countries as an effective
and relatively safe method for removing gastric tumors.
Nonetheless, controversies still exist because this technique is
also associated with high rates of resection failure,
intraoperative perforation and excessive haemorrhage (5,6),
especially in cases of large tumors growing around the gastric
cardia (3,7). The gastric cardia is an anatomically
narrow region in which the most proximal part of the stomach is
attached to the esophagus (8). Due
to its anatomic features, the cardia is often considered as a
contraindicated area for endoscopic resections (9). In the routine ESD procedure,
circumferential mucosal incision is initially applied, and lesion
tissue will be subsequently retrieved (9,10). If
the tumor is large, it would be more technically difficult to
finish endoscopic closure of the residual tissues, and operative
time would be elongated accordingly.
Herein, we report an advanced ESD procedure which
may facilitate the endoscopic resection techniques of removing
large subepithelial tumors, especially for those in the gastric
cardia region. We analyzed the short-term clinical outcomes to
determine the feasibility of this approach.
Patients and methods
Study design and patients
The present study was performed according to the
principles of the Declaration of Helsinki (1989). A prospective
case series was conducted at the First Affiliated Hospital of
Nanjing Medical University (Nanjing, China) from October 2015 to
October 2016. Patients (3 males and 5 females) were enrolled to
receive ESD using the advanced procedures if they met the following
criteria: i) individuals aged 18–75 years, ii) no contracted signs
for endoscopical resection, iii) gastric subepithelial tumor
invades the submucosal or the muscularis propria layer, iv) the
diameter of tumor ranges from 4–8 cm, and v) tumor located in the
subcardia or fundus region and vi) no extra-gastrointestinal
metastasis. Exclusion criteria were: i) patients with severe heart,
liver and kidney dysfunction, ii) patients with abnormal
coagulation function, iii) patients with tumor metastasis and iv)
patients who refused endoscopic treatment. The diagnosis was
established on the findings of gastroscopy and endoscopic
ultrasonography (EUS). The maximum diameter of tumor and layer of
tumor origin were carefully evaluated by two experienced GI
endoscopists. Tumors were classified to have muscularis propria
origin if they were connected with the underlying fourth layer in
EUS (8). Patients underwent routine
laboratory examinations in addition to electrocardiography and
computed tomography (CT) scan before the ESD procedure. If the
tumor was incompletely resected, patient would be referred to
receive surgical interventions. The data of therapeutic outcomes of
ESD such as the complete resection, bleeding, perforation and the
operating time were analyzed.
The study was approved by the Ethics Committee of
Nanjing Medical University. All enrolled patients signed an
informed consent after receiving explanations of possible treatment
options and potential complications regarding endoscopic
treatments.
ESD procedures
All patients were in the left-lateral position and
were under general anesthesia with an intravenous injection of
remifentanil (0.2 mg/h) and propofol (0.5 mg/kg). ESD was performed
by two experienced endoscopists. A single-channel video endoscope
was applied (Olympus GIT-H260) along with a transparent cap
(Olympus D-201-12704) on the tip, providing a good view during the
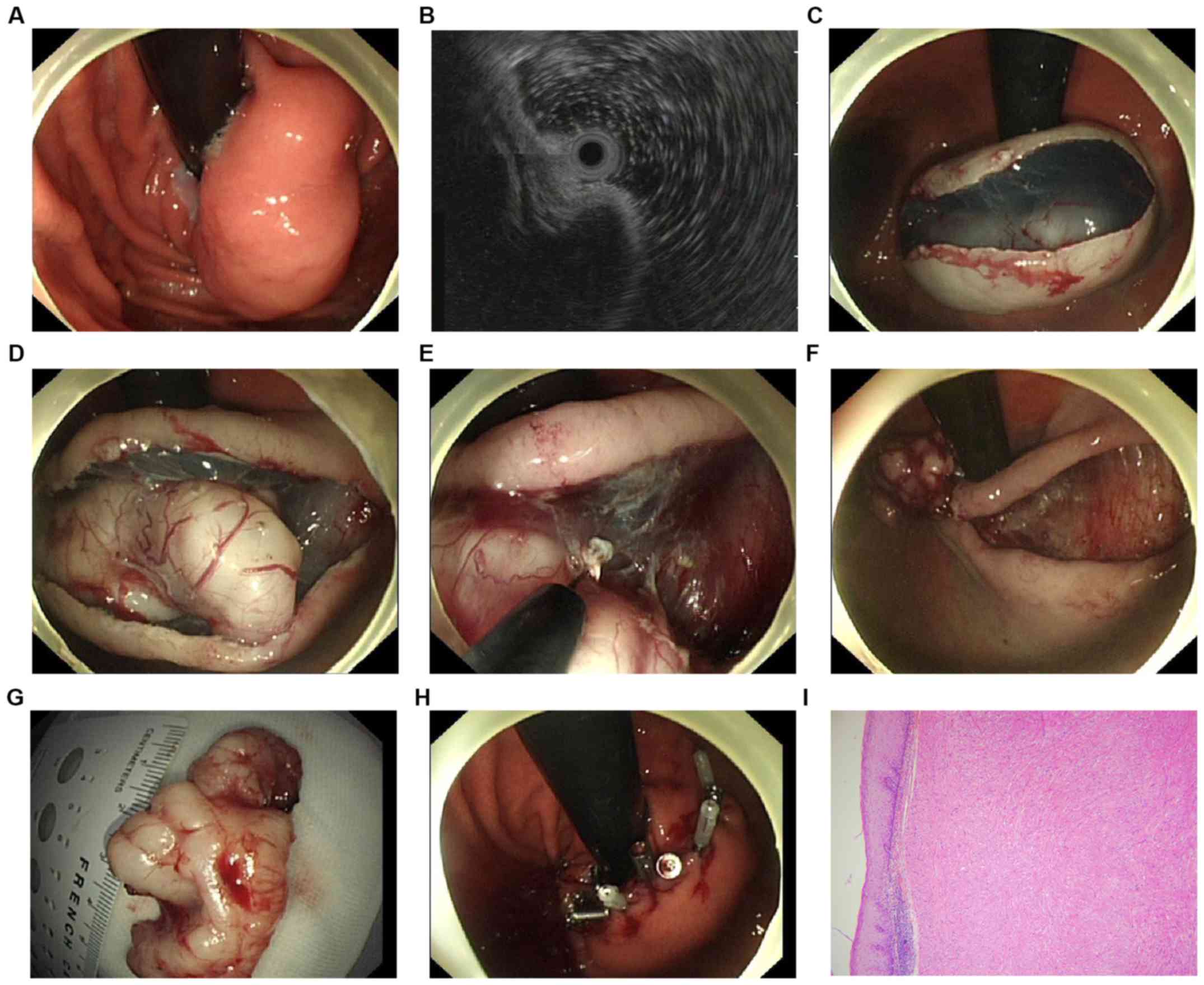
procedure. Fig. 1 summarizes the
whole procedure of the method. The marking step was skipped in this
procedure. To separate the mucosa and the tumor, a solution mixed
with 0.9% saline, methylene blue and diluted epinephrine (1:10,000)
was injected into the submucosal layer in position with the
central, superficial mucosa area of the tumor. A hook-knife (model
KD-620LR; Olympus) was directly used to make a median linear
incision of the mucosa in the same position above. Next, the en
bloc resection was carried out with the hook knife, or an
insulated-tip knife (ITknife2 model KD-611L; Olympus) until the
tumor was completely dissected: i) First, the submucosal tissues
were dissected gradually from both sides of the incision; ii) next,
a hook knife or an insulated-tip knife was used to carefully
dissect the tumor at the bottom from its original layer. Muscular
fibers or stalks were also resected to separate the tumor and the
muscularis propria. If necessary, an additional submucosal
injection was carried out repeatedly during the dissection
procedures. Endoscopic full-thickness resection (EFR) was applied
in cases where the tumor was attached tightly to the muscularis
propria layer or the serosa (10):
i) An active perforation was made around the tumor by the needle
knife (KD-10Q-1, Olympus), ii) the full-thickness incision was
applied by the insulated-tip (IT) knife (KD-610L, Olympus) and iii)
if necessary, the forceps within the Dual-channel gastroscope were
applied to prevent the tumor from dropping into the peritoneal
cavity. After the tumor was dissected, the residual defect was
closed using titanium endoclips (Sureclip, Micro-Tech). Hot
hemostatic forceps (Olympus) were applied for hemostasis. When
necessary, endoclips were used to stop bleeding. If a tension
pneumoperitoneum developed due to the perforation, a 20-gauge
needle was applied to make an immediate decompression of the
peritoneal cavity. In general, patients were allowed liquids after
24 h of ESD. The daily use of phosgel and proton pump inhibitor
were administered for two months after the procedure. The ESD
related complications were recorded in all patients to evaluate the
efficacy and safety of this advanced ESD method.
Histopathology
Resected samples were immersed in 10% formalin and
then embedded in the paraffin with eosin and hematoxylin staining.
Histopathological characteristics including the tumor size,
resection margin status and invasion depth were carefully evaluated
by two distinct pathologists. The complete resection was defined as
resection margins free of any tumor tissues. Immunohistochemical
staining was performed in all patients. GIST was diagnosed by the
positive results of SMA, c-KIT (CD117), CD34 and DOG-1. The
malignancy potential of GIST was accessed on the basis of tumor
size and mitotic index under the 50 high power fields (HPF).
Leiomyoma was diagnosed by the positive reaction of smooth muscle
actin and desmin.
Statistical analysis
The follow-up endoscopy was performed at the 1st and
3rd month after the procedure, in order to record any ESD-related
complications, tumor residue, recurrence or metastasis. Statistical
analyses were conducted using SPSS 21.0 statistics software (IBM
Corp.). All data were analyzed as the means with SD (standard
deviation).
Results
Baseline characteristics of eligible
patients
Eight consecutive patients (median age: 53.5 years;
range: 22.0–78.0 years) fulfilled the entry criteria and underwent
the advanced method of ESD. There were 3 males and 5 females. Three
lesions were located in subcardia region and 5 were in the fundus.
There was no sign of metastasis in these patients. The EUS findings
showed that all tumors had a connection with the fourth EUS layer,
indicating that these tumors originated from the muscularis
propria.
Outcomes of endoscopical
resection
ESD using the advanced procedure was carried out
successfully in all eight patients with a mean operation time of
83±13 min (range: 60–100 min). The mean tumor size was 45.6±7.5 mm
(range: 40.0–65.0 mm). The en bloc resection rate was 100% in these
patients, with 87.5% complete resection rates. The average numbers
of forceps were 12 (range: 7–20). Although perforations occurred in
5 out of 8 patients, all of them were positive perforations by EFR
technique, and all were successfully closed with endoclips. No
patient presented tension pneumoperitoneum, and all patients with
perforations received gastrointestinal decompression after the
procedure. The estimated blood loss was 20–100 ml, and no patient
required blood transfusion. None of the patients needed further
gastrectomy and no other complications were recorded. Table I summarizes the baseline
characteristics and the outcomes of the endoscopic resection.
 | Table I.Baseline characteristics and outcomes
of endoscopic resection. |
Table I.
Baseline characteristics and outcomes
of endoscopic resection.
| Patient sex | Age (years) | Follow-up
(months) | Symptoms 0-none,
1-pain, 2-bleeding | Tumor location | Tumor size (mm) | Layer of origin | Complete
resection | En bloc
resection | EFR | Complications 0-none,
1-bleeding, 2-perforations | Length of
hospitalization (days) |
|---|
| M | 22 | 8 | 0 | Fundus | 5.0 | Deep MP | Yes | Yes | Yes | 2 | 5 |
| F | 51 | 10 | 1 | Subcardia | 4.0 | Superficial MP | Yes | Yes | No | 0 | 6 |
| F | 44 | 12 | 0 | Fundus | 4.0 | Deep MP | No | Yes | Yes | 2 | 6 |
| F | 78 | 12 | 1 | Fundus | 4.5 | Superficial MP | Yes | Yes | No | 0 | 6 |
| F | 56 | 4 | 0 | Subcardia | 4.5 | Deep MP | Yes | Yes | No | 2 | 8 |
| M | 64 | 15 | 1 | Fundus | 4.0 | Deep MP | Yes | Yes | Yes | 2 | 7 |
| F | 64 | 21 | 0 | Fundus | 4.0 | Superficial MP | Yes | Yes | No | 0 | 6 |
| M | 38 | 30 | 0 | Subcardia | 6.5 | Deep MP | Yes | Yes | Yes | 2 | 8 |
Histopathological findings
The average size of lesions were 48.5±8.0 mm (range:
40.0–66.0 mm) in diameter. Six of the patients were diagnosed as
leiomyomas, and two were GI stromal tumors. No patients had lymph
node metastasis. The immunohistochemistry staining revealed that
the positive rates of c-KIT (CD117), CD34 and DOG-1, SMA, Desmin
were 25, 25, 25, 75, 75%, respectively. One patient was at low risk
of malignant potential, none was at intermediate risk and one
patient was at high risk. Table II
summarizes the histopathological evaluation of the resected
specimens.
 | Table II.Histopathological characteristics of
gastric subepithelial tumors. |
Table II.
Histopathological characteristics of
gastric subepithelial tumors.
| Patients | No. |
|---|
| Size
(pathology) |
|
| <4.5
cm | 2 |
| 4.5–5.5
cm | 5 |
| >5.5
cm | 1 |
| Pathological
diagnosis |
|
|
Leiomyomas | 6 |
| GI
stromal tumors | 2 |
| Risk
classification |
|
| Low
risk | 1 |
|
Moderate risk | 0 |
| High
risk | 1 |
|
Immunohistochemistry data |
|
|
CD117+ | 2 |
|
CD34+ | 2 |
|
DOG-1+ | 2 |
|
SMA+ | 6 |
|
Desmin+ | 6 |
The median follow-up was 36 months (range: 28–54
months), and no patient developed local recurrence or distant
metastasis. The average length of inpatient hospital stay was 10
days (range: 6–16 days).
Discussion
Most subepithelial tumors are asymptomatic and are
usually diagnosed incidentally during endoscopy. There are various
types of tumors, including GIST, lipomas, leiomyomas, schwannomas
and non-GIST sarcomas (2,11). GISTs are the most common gastric
mesenchymal neoplasms, but some might have been previously
misdiagnosed as leiomyomas during endoscopical examination
(12). Among the 8 patients in our
study, 2 cases were misclassified as leiomyomas but were finally
confirmed as GISTs according to histopathological results. This
emphasizes the importance of immunohistochemistry staining in the
final diagnosis.
Over the past decades, open surgery has been the
major treatment option of removing subepithelial tumors (3,13,14),
especially for those with large tumors in the subcardia region.
Although surgery can provide satisfactory clinical outcomes, the
proximal resection of the stomach might results in severe
gastroesophageal reflux disease and stenosis, which, in the long
run, reduces the quality of life among patients (15,16).
Laparoscopic surgery and endoscopic resection have
emerged in recent years as alternative treatments. According to the
latest guidelines in Asian countries (17,18),
laparoscopic technique is only recommended for small GISTs (no more
than 5 cm) and is associated with the possibility of abdominal
metastasis (19). As a newly
developed treatment, endoscopic resection (ER) has become a
minimally invasive treatment for gastric subepithelial tumors
(5–7,9–11). The implementation of this technique
facilitates the preservation of organ and functions in the GI
tract, however, the safety and endoscopical outcomes still remain
uncertain.
Due to the possibility of procedure-related
complications and some technical limitations especially for tumors
in the subcardia region (5,6,20) and to
the anatomic feature of the cardia where the His angle is very
sharp and the blood supply is abundant, it might be technically
difficult to perform endoscopical treatment even for experienced
endoscopists. Traditional ESD procedure includes the
circumferential cutting of the mucosa surrounding the tumor
followed by submucosal dissection (5,9,10). If a tumor is large, all the overlying
mucosa will be removed after resection, which makes the closure of
residual tissue much more difficult (6,21).
Moreover, formation of the scar will result in preventricular
stenosis, which influences the function of the gastric cardia.
To improve the feasibility of ESD in removing large
SMTs around gastric cardia, we made the following technical
adjustments: firstly, different from the conventional ESD
procedure, our method omitted the step of marking the outside
borders of the tumor, and a saline solution was directly injected
around the lesion to lift it off the muscular layer. This
simplified the whole procedure and relatively reduced the operation
time; secondly, instead of circumferential incision around the
lesion, we initially cut along the mucosa with a median linear
incision in central region of the tumor. The purpose of this step
was to reserve the overlying mucosa and thus guarantee the
successful closure of residual defect after tumor dissection.
Furthermore, after the median linear incision of the mucosa was
made in the central area of the tumor, the submucosal tissues were
dissected gradually from both sides of the incision, this
facilitated the exposure of the tumor and thus made the dissection
easier than before.
The major advantage of our procedure was that it
simplified the traditional ESD method and facilitated the
application of ESD in large SMTs. In general, perforation was
considered a severe ESD-related complication, which mainly
restricted the application of ESD. However, with the development of
endoscopic full-thickness resection (EFR) (11,22,23), the
positive perforation facilitated the removal of tumors, especially
for those with a deep invasion of the muscularis propria layer or
even the serosa. In our study, although the perforation rate was
75%, all of them were positive perforations and tumors were
successfully removed using the EFR.
The closure technique remains one of the most
important steps after dissection of tumors. Endoclips are generally
used in traditional EFR for small defects. If the tumor is large in
size, air suction will be applied to reduce the defect (24,25).
Although new techniques such as the omental patch method and string
suture have been used to close large defects, they are
time-consuming and require greater technical skills than
endoclips-assisted closure. Differently, in our method, the closure
step is easily conducted by using several endoclips because we
initially preserved the overlying mucosa. Neither omental patch
method nor string suture is needed in our procedure, and there is
no necessity to perform the ‘suction-clip-suture’, which
dramatically simplifies the closure procedure. During the
short-term follow-up, no remarkable complications were recorded in
any of the patients.
In conclusion, the advanced method of ESD is a
feasible and safe procedure for tumors especially for large tumors
located in subcardia region. It could be applied as a novel
technique for treating patient without performing open gastrectomy
or any laparoscopic-assisted techniques.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL wrote the manuscript. HC and WZ collected and
analyzed the general data of patients. BL and GZ were responsible
for the analysis of the histopathology results. All the authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
First Affiliated Hospital of Nanjing Medical University (Nanjing,
China). Patients who participated in this research had complete
clinical data. Signed informed consents were obtained from the
patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Salah W and Faigel DO: When to puncture,
when not to puncture: Submucosal tumors. Endosc Ultrasound.
3:98–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Janeway KA and Weldon CB: Pediatric
gastrointestinal stromal tumor. Semin Pediatr Surg. 21:31–43. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nishida T, Blay JY, Hirota S, Kitagawa Y
and Kang YK: The standard diagnosis, treatment, and follow-up of
gastrointestinal stromal tumors based on guidelines. Gastric
Cancer. 19:3–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirao M, Masuda K, Asanuma T, Naka H, Noda
K, Matsuura K, Yamaguchi O and Ueda N: Endoscopic resection of
early gastric cancer and other tumors with local injection of
hypertonic saline-epinephrine. Gastrointest Endosc. 34:264–269.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee IL, Lin PY, Tung SY, Shen CH, Wei KL
and Wu CS: Endoscopic submucosal dissection for the treatment of
intraluminal gastric subepithelial tumors originating from the
muscularis propria layer. Endoscopy. 38:1024–1028. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY,
Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ, et al: Therapeutic
outcomes in 1000 cases of endoscopic submucosal dissection for
early gastric neoplasms: Korean ESD Study Group multicenter study.
Gastrointest Endosc. 69:1228–1235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jang YS, Lee BE, Kim GH, Park DY, Jeon HK,
Baek DH, Kim DU and Song GA: Factors associated with outcomes in
endoscopic submucosal dissection of gastric cardia tumors: A
retrospective observational study. Medicine (Baltimore).
94:e12012015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McColl KE: Cancer of the gastric cardia.
Best Pract Res Clin Gastroenterol. 20:687–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kakushima N, Yahagi N, Fujishiro M,
Kodashima S, Nakamura M and Omata M: Efficacy and safety of
endoscopic submucosal dissection for tumors of the esophagogastric
junction. Endoscopy. 38:170–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Białek A, Wiechowska-Kozłowska A,
Pertkiewicz J, Polkowski M, Milkiewicz P, Karpińska K, Ławniczak M
and Starzyńska T: Endoscopic submucosal dissection for treatment of
gastric subepithelial tumors (with video). Gastrointest Endosc.
75:276–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD,
Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW, et al: Endoscopic
full-thickness resection without laparoscopic assistance for
gastric submucosal tumors originated from the muscularis propria.
Surg Endosc. 25:2926–2931. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang HC, Menias CO, Gaballah AH, Shroff S,
Taggart MW, Garg N and Elsayes KM: Beyond the GIST: Mesenchymal
tumors of the stomach. Radiographics. 33:1673–1690. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishida T, Hirota S, Yanagisawa A, Sugino
Y, Minami M, Yamamura Y, Otani Y, Shimada Y, Takahashi F and Kubota
T; GIST Guideline Subcommittee, : Clinical practice guidelines for
gastrointestinal stromal tumor (GIST) in Japan: English version.
Int J Clin Oncol. 13:416–430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roggin KK and Posner MC: Modern treatment
of gastric gastrointestinal stromal tumors. World J Gastroenterol.
18:6720–6728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kong SH and Yang HK: Surgical treatment of
gastric gastrointestinal stromal tumor. J Gastric Cancer. 13:3–18.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JW, Yoon H, Kong SH, Kim JS, Paeng JC,
Lee HJ, Lee KU and Yang HK: Analysis of esophageal reflux after
proximal gastrectomy measured by wireless ambulatory 24-hr
esophageal pH monitoring and TC-99m diisopropyliminodiacetic acid
(DISIDA) scan. J Surg Oncol. 101:626–633. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
ESMO/European Sarcoma Network Working
Group, : Gastrointestinal stromal tumours: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 25
(Suppl 3):iii21–iii26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang YK, Kang HJ, Kim KM, Sohn T, Choi D,
Ryu MH, Kim WH and Yang HK; Korean GIST Study Group (KGSG), :
Clinical practice guideline for accurate diagnosis and effective
treatment of gastrointestinal stromal tumor in Korea. Cancer Res
Treat. 44:85–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim MD, Kang DH, Park JH, Lee JH, Choi CW,
Kim DH, Kim HW and Kim GH: Abdominal wound metastasis after
laparoscopic surgery of gastrointestinal stromal tumor. Gut Liver.
4:283–286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kakushima N, Fujishiro M, Kodashima S,
Muraki Y, Tateishi A, Yahagi N and Omata M: Technical feasibility
of endoscopic submucosal dissection for gastric neoplasms in the
elderly Japanese population. J Gastroenterol Hepatol. 22:311–314.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Imagawa A, Okada H, Kawahara Y, Takenaka
R, Kato J, Kawamoto H, Fujiki S, Takata R, Yoshino T and Shiratori
Y: Endoscopic submucosal dissection for early gastric cancer:
Results and degrees of technical difficulty as well as success.
Endoscopy. 38:987–990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi Q, Chen T, Zhong YS, Zhou PH, Ren Z,
Xu MD and Yao LQ: Complete closure of large gastric defects after
endoscopic full-thickness resection, using endoloop and metallic
clip interrupted suture. Endoscopy. 45:329–334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schlag C, Wilhelm D, von Delius S,
Feussner H and Meining A: EndoResect study: Endoscopic
full-thickness resection of gastric subepithelial tumors.
Endoscopy. 45:4–11. 2013.PubMed/NCBI
|
|
24
|
Chiu PW, Phee SJ, Wang Z, Sun Z, Poon CC,
Yamamoto T, Penny I, Wong JY, Lau JY and Ho KY: Feasibility of
full-thickness gastric resection using master and slave
transluminal endoscopic robot and closure by overstitch: A
preclinical study. Surg Endosc. 28:319–324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mori H, Kobara H, Rafiq K, Nishiyama N,
Fujihara S, Kobayashi M, Oryu M, Fujiwara M, Suzuki Y and Masaki T:
New flexible endoscopic full-thickness suturing device: A
triple-arm-bar suturing system. Endoscopy. 45:649–654. 2013.
View Article : Google Scholar : PubMed/NCBI
|