Introduction
Osteosarcoma (OS) is a bone malignancy that
primarily affects adolescents and young adults. OS accounts for
~20% of all primary bone cancer cases in Europe and is the second
highest cause of cancer-associated mortality in children (1). Despite advances in medical treatments,
including surgery, transplantation and radiation, effective
treatments for postsurgical recurrence and metastasis are not
currently available, thus accounting for the poor patient outcome
for OS (2). Although therapy for OS
has improved, the overall survival rate of patients with OS has not
substantially increased, and 35% of patients with OS die within 5
years of diagnosis (3). Furthermore,
>20% of young patients with OS present with distant metastases
at the time of diagnosis, and 40% of advanced stage cases progress
to metastasis during therapy (4,5). To the
best of our knowledge, the underlying molecular mechanism of
metastasis in patients with OS has not yet been elucidated
(6,7). It is therefore crucial to identify
novel biomarkers and therapeutic strategies for the better
management of OS.
Long non-coding RNAs (lncRNAs) are polyadenylated
RNA polymerase II-transcribed RNAs that are ≥200 nucleotides in
length and do not present obvious open reading frames to encode
proteins (8–10). An increasing number of studies have
demonstrated that lncRNAs serve crucial roles in tumor initiation,
progression, metastasis, drug-resistance and recurrence (11–13). A
previous study reported that epigenetic activation of the
lncRNA-maternally expressed gene 3 (MEG3) and/or inactivation of
the mesenchymal-epithelial transition factor could represent a
therapeutic strategy in pancreatic neuroendocrine tumor and
insulinoma treatment (14). lncRNAs
affect a variety of biological processes, including cellular
proliferation, differentiation, migration, the immune response and
apoptosis, which are all associated with tumorigenesis (15,16).
However, the functional roles of lncRNAs remain commonly unknown.
lncRNAs have been found to act as tumor suppressors or oncogenes
(17–19). It has been reported that Ewing
sarcoma-associated transcript 1 (EWSAT1) facilitates the growth and
metastasis of OS cells via MEG3 regulation, which suggests that the
EWSAT1-MEG3 axis may represent a promising target for OS treatment
(20,21). A meta-analysis indicated that MEG3
may be considered as a potential novel biomarker for the clinical
outcome of certain types of human cancer (22), including bladder cancer (23), cervical carcinoma (24), hepatocellular cancers (25) and meningiomas (26). AWPPH is a novel IncRNA that has been
reported to be involved in the development of several types of
cancer and to have oncogenic functions (27,28). For
examples, a recent study reported that AWPPH is overexpressed in
hepatoma cells, where it can promote proliferation and migration of
liver cancer cells, and stimulate the growth and metastasis of
tumor cells by interacting with Y-box binding protein 1 (YBX1)
(29). However, it remains unclear
whether AWPPH could be considered as a critical regulator in OS
that could predict patient prognosis.
The present study aimed to evaluate the effects of
AWPPH on OS cell proliferation, migration and apoptosis.
Furthermore, this study will investigate the role of AWPPH on the
regulation of phosphorylated (p)-PI3K, p-AKT, Bcl-2 and
cleaved-caspase-3 expressions, in order to determine the underlying
mechanisms of AWPPH in OS.
Materials and methods
Patients
A total of 30 pairs of OS tissues and paracancerous
tissues were obtained from 30 patients (17 men and 13 women)
undergoing tumor resection at the Changhai Hospital (Shanghai,
China) between April 2010 and October 2017. The average age of the
patients was 22.34 years (age range, 10–59 years). All tissues (4–8
cm thickness) were immediately snap-frozen in liquid nitrogen and
stored at −80°C until further use. Written informed consent was
obtained from all patients and the study protocol was approved by
the Ethics Committee of the Changhai Hospital.
Cells and reagents
The OS MG-63 and U2OS, and the osteoblast hFOB1.19
cell lines were purchased from the American Type Culture Collection
(ATCC). Fetal bovine serum (FBS), Dulbecco's modified Eagle's
medium (DMEM) and the MTT assay were purchased from Sigma-Aldrich;
Merck KGaA. Penicillin, streptomycin, glutamine and
TRIzol® were obtained from Invitrogen; Thermo Fisher
Scientific, Inc. Antibodies against p-PI3K (cat. no. 4228S), PI3K
(cat. no. 4249S), p-Akt (cat. no. 4060S), Akt (cat. no. 9272S),
Bcl-2 (cat. no. 15071S), Bax (cat. no. 2774) cleaved-caspase-3
(cat. no. 9661S), caspase-3 (cat. no. 9662), cleaved-caspase-9
(cat. no. 20750) and caspase-9 (cat. no. 9508) were obtained from
Cell Signaling Technology, Inc. and used at 1:500 dilution
according to the manufacturer's instructions. Antibody against
GAPDH, (cat. no. sc-32233); mouse anti-rabbit IgG-HRP antibody,
(cat. no. sc-2357) and m-IgGκ BP-HRP antibody (cat. no. sc-516102)
were purchased from Santa Cruz Biotechnology, Inc. and used at
1:5,000 dilution.
Cell culture
The MG-63 and U2OS cell lines were cultured in DMEM
supplemented with 10% FBS, 100 U/ml penicillin, 100 mg/ml
streptomycin and 2 mM glutamine, and placed at 37°C in a humidified
incubator containing 5% CO2.
The hFOB1.19 cell line was cultured as previously
described (30) and according to
ATCC procedures. For in vitro proliferation, the hFOB1.19
cells were maintained in a non-differentiation medium that
consisted of 1:1 DMEM/F-12 (GE Healthcare Life Sciences) containing
10% FBS (Sigma-Aldrich; Merck KGaA) and 0.3 mg/l G418
(Sigma-Aldrich; Merck KGaA), and cultured in a humidified incubator
with 5% CO2 at 34°C. The medium was replaced every 2–3
days.
Short hairpin (sh)RNA
transfection
The AWPPH shRNA used in the MG-63 and U2OS cell
lines was purchased from Guangzhou RiboBio Co., Ltd. Cells were
transfected using Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. The sequence of the AWPPH shRNA was
5′-CTGGATGGTCGCTGCTTTTTA-3′. AWPPH shRNA (100 nM) was inserted into
the shRNA expression vector pGPH1/Neo (40 nM; Clontech
Laboratories, Inc.) and packaged using Platinum-A (Cell Biolabs,
Inc.). Cells (1.2×106) were transfected with AWPPH shRNA
or scrambled shControl (5′-UUUCCGAACGUGUCACGUdTdT-3′) for 48 h
prior to further experimentation.
MTT assay
The effect of AWPPH downregulation on MG63 and U2OS
cell proliferation was measured by MTT assay. Cells
(1×104/100 µl) were seeded in 96-well plates. MTT (50
µl) was added and the cells were incubated for 4 h. Subsequently,
150 µl DMSO was added to dissolve the purple formazan, and the
absorbance was read at 570 nm with a microplate reader (Thermo
Fisher Scientific, Inc.).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from OS tissues, the two OS cell lines
MG-63 and U2OS and the human osteoblast hFOB 1.19 cell line was
isolated with TRIzol® according to the manufacturer's
instructions. Total RNA concentration was determined with
photometric method using a NanoQuant plate (Tecan Group, Ltd.). RNA
(1 ng) was reverse transcribed using the Reverse EasyScript
One-Step gDNA Removal and cDNA Synthesis SuperMix (Trangene)
according to the manufacturer's instructions. RT-qPCR was performed
using SYBR-Green Master mix (Roche Life Science) in an ABI 7500
Real-time PCR instrument according to the following reactions:
Denaturation for 1 cycle at 95°C for 1 min, followed by 40 cycles
of 20 sec at 95°C and 40 sec at 58°C. GAPDH was used as an
endogenous control. The primers used were designed as follows:
AWPPH forward, 5′-CTGGATGGTCGCTGCTTTTTA-3′ and reverse,
5′-AGGGGGATGAGTCGTGATTT-3′; and GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′. The relative expression levels were
normalized to endogenous controls and assessed using the
2−ΔΔCq method (31).
Cell migration and invasion
assays
For the migration assay, 1×105 MG-63 and
U2OS cells were seeded in six-well plates and cultured at 37°C
overnight. A linear wound was carefully made with fine pipette-tips
in the middle of the well, cell debris was removed and the cells
were incubated with serum-free medium. The wounded monolayers were
photographed at 0 and 24 h after wounding using an inverted
microscope (magnification, 40×; Carl Zeiss AG).
For the invasion assay, 3×105 MG-63 and
U2OS cells were seeded in FBS-free DMEM in the upper chamber of a
24-well Matrigel-coated Transwell invasion insert (diameter, 6.4
mm; pore size, 8 µm; BD Biosciences). The lower chamber was filled
with DMEM supplemented with 20% FBS. Following 24 h of incubation,
the cells in the upper chamber were removed, and the cells that had
invaded the membrane were stained using crystal violet for 20 min
at room temperature. Five randomly selected fields were captured
with an upright optical microscope (magnification, ×10; Leica
Microsystems GmbH) and the migrated cells were counted. All
experiments were performed in triplicate at least three times.
Western blotting
MG-63 and U2OS cells were collected, and the total
protein was extracted using RIPA lysis buffer (cat. no. P0013K;
Beyotime Institute of Biotechnology) containing 1 mmol/l
phenylmethylsulfonyl fluoride protease inhibitor (cat. no. ST506;
Beyotime Institute of Biotechnology). Protein concentration of cell
lysates was determined using a Bradford Protein Assay Kit
(MultiSciences Biotech Co., Ltd.). Proteins (40 µg) were separated
by 12% SDS-PAGE and transferred to polyvinylidene difluoride
membranes. Membranes were incubated with antibodies against p-PI3K,
PI3K, p-Akt, Akt, Bcl-2, Bax, cleaved-caspase-3, caspase-3,
cleaved-caspase-9, caspase-9 and GAPDH at 4°C overnight. Membranes
were washed with PBST and incubated with secondary antibodies for 1
h at room temperature. Enhanced chemiluminescence reagent (Thermo
Fisher Scientific Inc.) was used to detect the signal on the
membrane. Relative expression level of proteins was normalized to
endogenous control GAPDH using Image-Pro Plus 6.0 (Media
Cybernetics Inc.).
Statistical analysis
P-values were calculated using Student's t-test or
one-way analysis of variance with SPSS software version 19.0 (IBM
Corp.). All data are expressed as the mean ± standard error of the
mean. P<0.05 was considered to indicate a statistically
significant difference.
Results
AWPPH is highly expressed in OS
tissues and cells
The expression levels of AWPPH in OS tissues, in the
two OS cell lines MG-63 and U2OS, and in the human osteoblast hFOB
1.19 cell line were detected by RT-qPCR. As presented in Fig. 1A, AWPPH expression levels were
significantly elevated in OS tissues compared with those in
non-cancerous matched bone tissues. In addition, the expression
levels of AWPPH were significantly increased in the MG-63 and U2OS
cell lines (Fig. 1B) compared with
those in hFOB1.19 cells. In order to investigate the biological
roles of AWPPH in OS cells, the MG-63 and U2OS cell lines were
stably depleted of AWPPH using AWPPH-specific shRNA. RT-qPCR
analysis reported that transfection with AWPPH shRNA effectively
decreased AWPPH mRNA expression in the MG-63 and U2OS cells,
compared with that in the control and shControl groups (Fig. 1C and D).
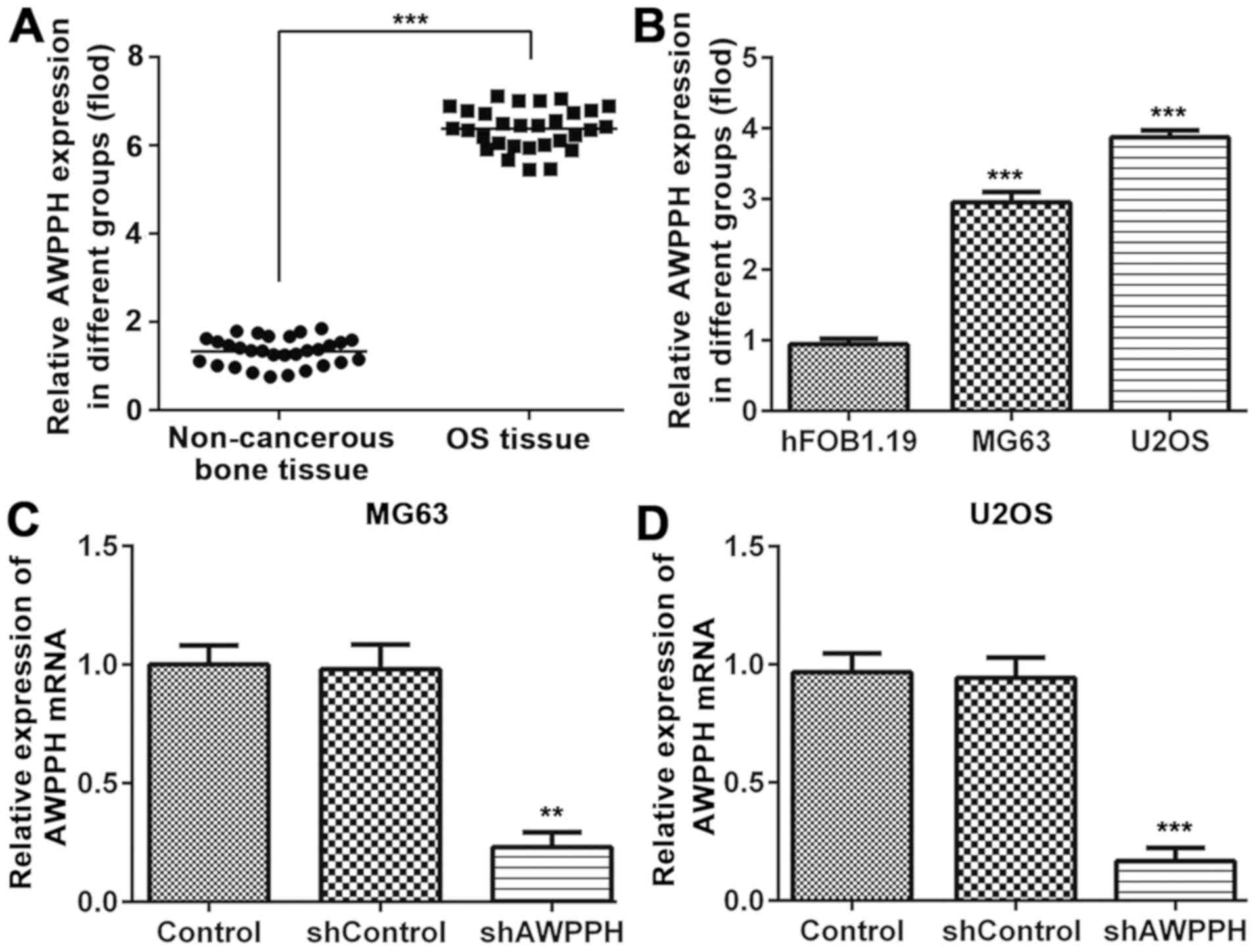 | Figure 1.AWPPH is highly expressed in OS
tissue and cells. The mRNA expression of AWPPH in (A) OS tissue and
(B) MG-63, U2OS and hFOB1.19 cells was measured by RT-qPCR. (C)
MG-63 and (D) U2OS cells were transfected with AWPPH shRNA and
blank vector, and mRNA expression of lncRNA AWPPH was measured by
RT-PCR. Data are expressed as the mean ± standard error of the mean
of 3 independent experiments in triplicate. ***P<0.001, compared
to the para-matched bone tissues. ***P<0.001, compared to the
hFOB1.19 cell, **P<0.01, ***P<0.001, compared to the control
group. Non-cancerous bone tissue, para-matched bone tissues; Sh,
short hairpin; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction. |
AWPPH downregulation inhibits OS cell
proliferation, migration and invasion
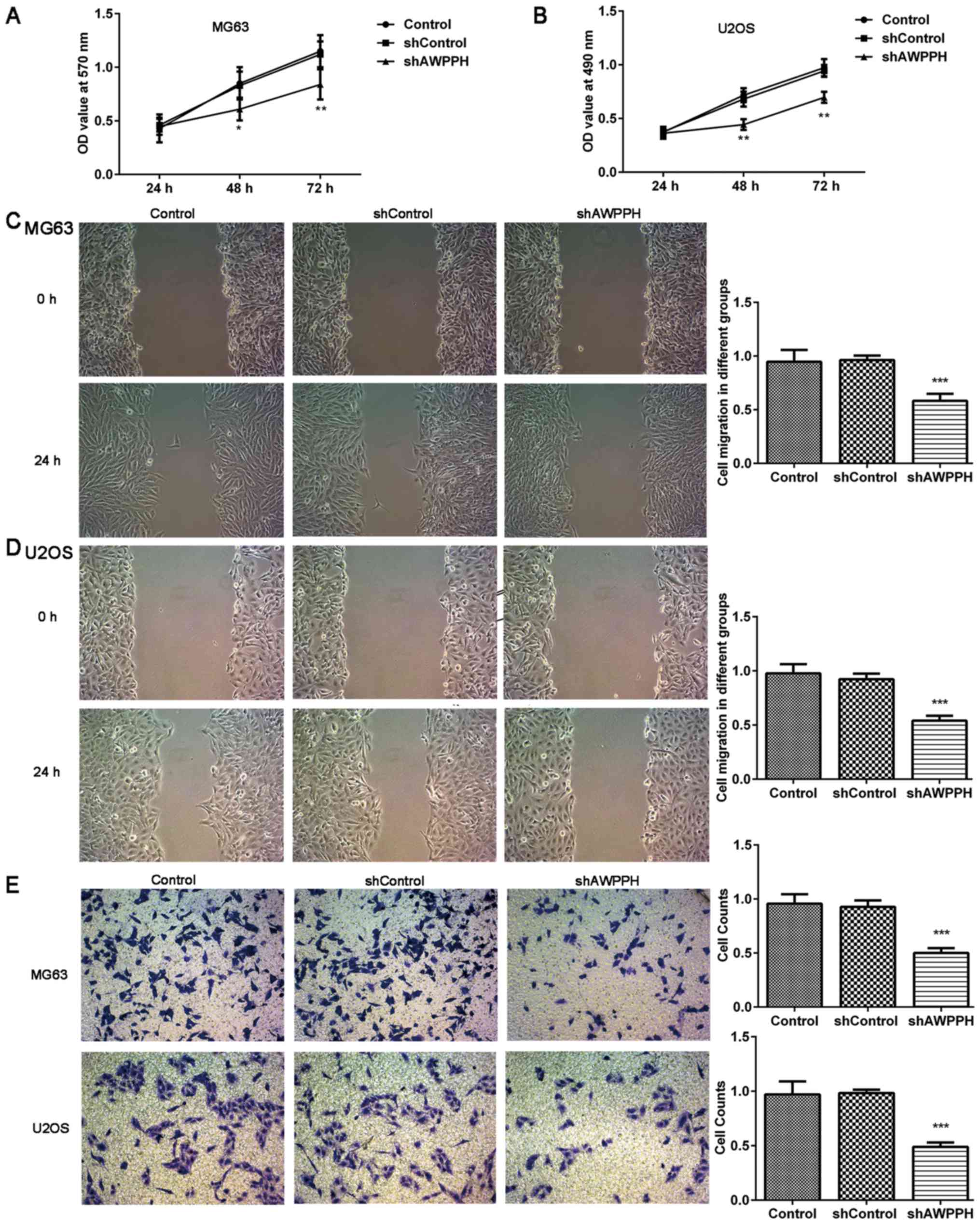
As presented in Fig. 2A
and B, MG63 and U2OS cell proliferation was significantly
decreased following 48 and 72 h of transfection with AWPPH shRNA,
compared with the negative control. The wound-healing assay
demonstrated that AWPPH depletion decreased the migration capacity
of the MG-63 (Fig. 2C) and U2OS
(Fig. 2D) cells. In addition, the
Transwell assay revealed that AWPPH depletion significantly
inhibited MG-63 and U2OS cell invasion (Fig. 2E). These data suggested that AWPPH
may serve a crucial role in OS cell proliferation, migration and
invasion.
AWPPH downregulation promotes OS cell
apoptosis-associated factors
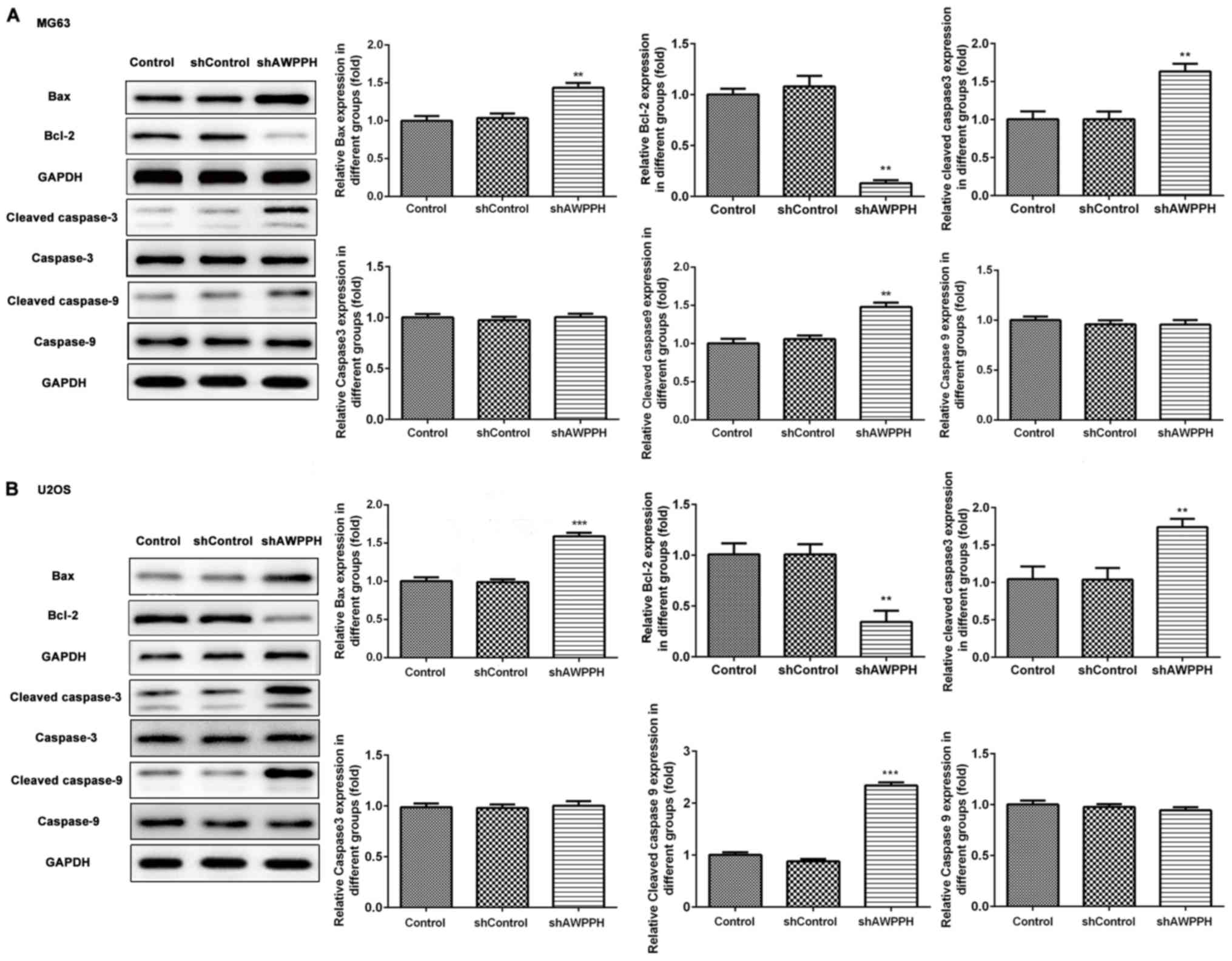
In order to investigate whether cell apoptosis
contributes to the decrease in cell viability, western blotting was
used to detect cell apoptosis-associated proteins. The results
demonstrated that cleaved-caspase-3, cleaved-caspase-9 and Bax
expressions were significantly increased, whereas Bcl-2 expression
was significantly decreased in the MG-63 and U2OS cells following
transfection with AWPPH shRNA (Fig. 3A
and B).
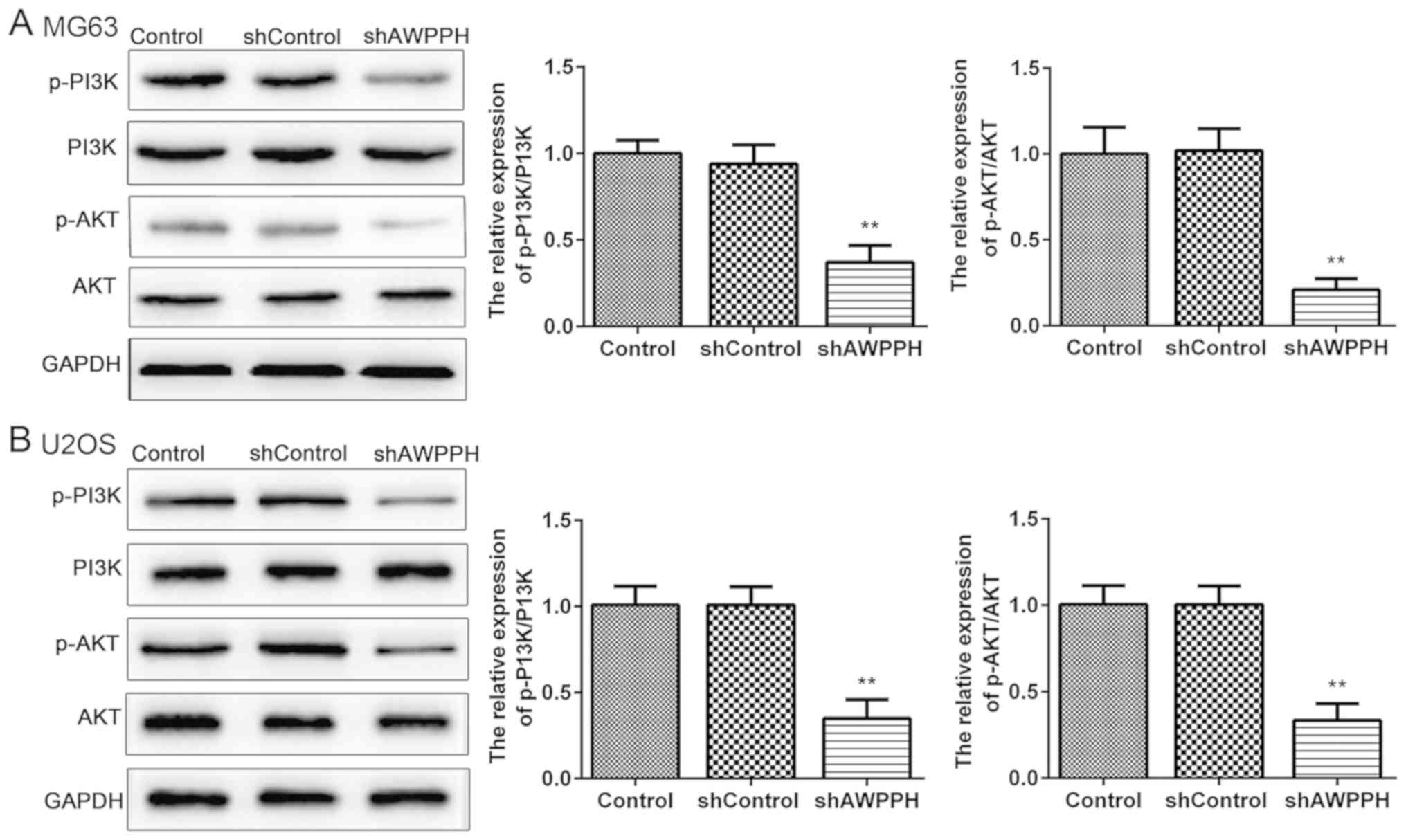
AWPPH downregulation inhibits the
PI3K/AKT pathway
As presented in Fig. 4A
and B, western blotting demonstrated that AWPPH depletion
significantly decreased the protein levels of p-PI3K and p-AKT in
the MG-63 and U2OS cells. These results suggested that AWPPH may
partly function by activating the PI3K/AKT pathway.
Discussion
OS is the most frequent type of primary bone cancer,
with an incidence of 0.2–3/100,000 per year (32,33). An
increasing number of studies have suggested that the aberrant
expression of lncRNAs is a potential driver event of tumorigenesis
and cancer progression (18,34,35). In
the present study, the AWPPH expression level was significantly
increased in OS tissues and cells compared with that in control
groups. Furthermore, AWPPH depletion decreased OS cell
proliferation, migration and invasion, promoted OS cell apoptosis
and inhibited the PI3K/AKT pathway. These results demonstrated that
AWPPH may be considered as a potential prognostic biomarker for
OS.
A recent study reported that AWPPH is highly
expressed in hepatocellular carcinoma (HCC) tissues, that it
promotes HCC cell proliferation and migration in vitro, and
that it promotes tumor growth and metastasis in vivo through
YBX1 (29). In addition, AWPPH is
highly expressed in bladder cancer (BC) and promotes cell
proliferation, autophagy and migration, and inhibits cell apoptosis
in BC by inhibiting SMAD family member 4 via enhancer of zeste 2
polycomb repressive complex 2 subunit (27). A previous study reported that
increased expression of AWPPH is correlated with incomplete
encapsulation, microvascular invasion, and advanced
Tumor-Node-Metastasis stage and Barcelona clinic liver cancer stage
in HCC (29). Additionally, survival
analysis and Cox proportional hazards regression analysis revealed
not only a correlation between AWPPH and poor recurrence-free
survival and overall survival, but the fact that it is an
independent prognostic factor of these measurements too (29). However, to the best of our knowledge,
there are currently no reagents that target AWPPH and no studies or
clinical tests on AWPHH expression in patients with OS, and the
expression and role of AWPPH in OS therefore remain unknown. The
present study demonstrated that AWPPH expression level was higher
in OS cells compared with that in the osteoblast hFOB1.19 cell
line. These results were consistent with those observed for AWPPH
expression in certain subtypes of HCC and BC (19,22). In
the present study, the role of AWPPH in OS carcinogenesis and the
one of the potential mechanisms underlying AWPPH function were
elucidated via AWPPH-knockdown. MTT assay confirmed that
AWPPH-knockdown decreased OS cell proliferation. In addition, the
results from the wound-healing and Transwell assays demonstrated
that AWPPH depletion suppressed OS cell migration and invasion.
Furthermore, western blotting analysis indicated that
AWPPH-knockdown induced cell apoptosis by regulating Bcl-2, Bax,
cleaved-caspase-3 and cleaved-caspase-9 protein expressions. These
results indicated that AWPPH may have an oncogenic role in OS.
Further investigation using a larger clinical sample size is
required to validate the expression of AWPPH in OS and to determine
its potential prognostic value.
Disease recurrence and metastasis occurrence
frequently result in the poor outcome of patients with OS. Numerous
studies have reported that the functional roles of lncRNAs in
cancer involve the carcinogenic or metastatic signaling pathways
(34,35). Investigating the association between
signaling pathways and lncRNAs is therefore critical in order to
develop novel strategies for the early diagnosis and treatment of
OS. A thorough investigation has been performed of the role of the
PI3K/AKT signaling pathway in cancer pathogenesis, and numerous
drugs targeting this pathway are currently being developed
(36). PI3K can change the protein
structure of AKT and activate it, when binding to growth factor
receptors, including EGFR, RAS and PTEN. PI3K can activate or
inhibit a series of downstream effectors, regulating therefore cell
proliferation, differentiation, apoptosis and migration. The
activation of Akt by PI3k leads to the phosphorylation of
apoptosis-related proteins, including Bax (37), Bad (38) and caspase-9 (39), inhibiting therefore their function of
apoptosis promoters. Furthermore, the structures of growth factor
receptors are all altered at some level in most solid tumors,
including colorectal cancer (36),
epithelial ovarian cancer (40) and
cervical cancer (41). The PI3K/AKT
signaling cascade and downstream effectors are therefore considered
as attractive pharmacological targets (42,43). In
addition, it has been reported that the downregulation of DEP
domain-containing mTOR-interacting protein inhibits the
proliferation, migration and survival of OS cells through the
PI3K/Akt/mTOR pathway (44). A
recent study reported that AWPPH activates the PI3K/AKT pathway in
HCC cells (29). In the present
study, AWPPH downregulation significantly decreased p-PI3K and
p-AKT expression levels, which indicated that the PI3K/AKT pathway
may participate in the oncogenic function of AWPPH.
In conclusion, the present study demonstrated that
AWPPH was highly expressed in OS cells, and that AWPPH-knockdown
suppressed OS cell proliferation, migration and invasion, and
promoted OS cell apoptosis, which may be mediated by PI3K/AKT
pathway activation. These data suggested that AWPPH may be
considered as a prognostic biomarker and a potential therapeutic
target in OS.
Acknowledgements
Not applicable.
Funding
This research was supported by the National Natural
Science Foundation of China (grants nos. 81272942, 81702666 and
81502328).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WD and DW wrote the manuscript, and collected and
interpreted the data. FJ and HZ designed the study and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Changhai Hospital. Written informed consent was
obtained from all patients.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rothermundt C, Seddon BM, Dileo P, Strauss
SJ, Coleman J, Briggs TW, Haile SR and Whelan JS: Follow-up
practices for high-grade extremity Osteosarcoma. BMC Cancer.
16:3012016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kager L, Zoubek A, Pötschger U, Kastner U,
Flege S, Kempf-Bielack B, Branscheid D, Kotz R, Salzer-Kuntschik M,
Winkelmann W, et al: Primary metastatic osteosarcoma: Presentation
and outcome of patients treated on neoadjuvant cooperative
osteosarcoma study group protocols. J Clin Oncol. 21:2011–2018.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anderson ME: Update on survival in
osteosarcoma. Orthop Clin North Am. 47:283–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Senerchia AA, Macedo CR, Ferman S,
Scopinaro M, Cacciavillano W, Boldrini E, Lins de Moraes VL, Rey G,
de Oliveira CT, Castillo L, et al: Results of a randomized,
prospective clinical trial evaluating metronomic chemotherapy in
nonmetastatic patients with high-grade, operable osteosarcomas of
the extremities: A report from the Latin American Group of
Osteosarcoma Treatment. Cancer. 123:1003–1010. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chung SW, Han I, Oh JH, Shin SH, Cho HS
and Kim HS: Prognostic effect of erroneous surgical procedures in
patients with osteosarcoma: Evaluation using propensity score
matching. J Bone Joint Surg Am. 96:e602014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng C, Chen ZQ and Shi XT: MicroRNA-320
inhibits osteosarcoma cells proliferation by directly targeting
fatty acid synthase. Tumour Biol. 35:4177–4183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li E, Zhang J, Yuan T and Ma B: MiR-145
inhibits osteosarcoma cells proliferation and invasion by targeting
ROCK1. Tumour Biol. 35:7645–7650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rinn JL and Chang HY: Genome regulation by
long noncoding RNAs. Annu Rev Biochem. 81:145–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ponjavic J, Ponting CP and Lunter G:
Functionality or transcriptional noise? Evidence for selection
within long noncoding RNAs. Genome Res. 17:556–565. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
ENCODE Project Consortium, ; Birney E,
Stamatoyannopoulos JA, Dutta A, Guigó R, Gingeras TR, Margulies EH,
Weng Z, Snyder M, Dermitzakis ET, et al: Identification and
analysis of functional elements in 1% of the human genome by the
ENCODE pilot project. Nature. 447:799–816. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirano T, Yoshikawa R, Harada H, Harada Y,
Ishida A and Yamazaki T: Long noncoding RNA, CCDC26, controls
myeloid leukemia cell growth through regulation of KIT expression.
Mol Cancer. 14:902015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen F, Mo J and Zhang L: Long noncoding
RNA BCAR4 promotes osteosarcoma progression through activating
GLI2-dependent gene transcription. Tumour Biol. 37:13403–13412.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raveh E, Matouk IJ, Gilon M and Hochberg
A: The H19 Long non-coding RNA in cancer initiation, progression
and metastasis-a proposed unifying theory. Mol Cancer. 14:1842015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Modali SD, Parekh VI, Kebebew E and
Agarwal SK: Epigenetic regulation of the lncRNA MEG3 and its target
c-MET in pancreatic neuroendocrine tumors. Mol Endocrinol.
29:224–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu XS, Wang F, Li HF, Hu YP, Jiang L,
Zhang F, Li ML, Wang XA, Jin YP, Zhang YJ, et al: LncRNA-PAGBC acts
as a microRNA sponge and promotes gallbladder tumorigenesis. EMBO
Rep. 18:1837–1853. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao J, Lv Y, Jin F, Liu Y, Ma Y, Xiong Y,
Liu L, Zhang S, Sun Y, Tipoe GL, et al: LncRNA HANR promotes
tumorigenesis and increase of chemoresistance in hepatocellular
carcinoma. Cell Physiol Biochem. 43:1926–1938. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu X, Feng Y, Zhang D, Zhao SD, Hu Z,
Greshock J, Zhang Y, Yang L, Zhong X, Wang LP, et al: A functional
genomic approach identifies FAL1 as an oncogenic long noncoding RNA
that associates with BMI1 and represses p21 expression in cancer.
Cancer Cell. 26:344–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng W, Si S, Zhang Q, Li C, Zhao F, Wang
F, Yu J and Ma R: Long non-coding RNA MEG3 functions as a competing
endogenous RNA to regulate gastric cancer progression. J Exp Clin
Cancer Res. 34:792015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun L, Yang C, Xu J, Feng Y, Wang L and
Cui T: Long noncoding RNA EWSAT1 promotes osteosarcoma cell growth
and metastasis through suppression of MEG3 expression. DNA Cell
Biol. 35:812–818. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Z, Yu X and Shen J: Long non-coding
RNAs: Emerging players in osteosarcoma. Tumour Biol. 37:2811–2816.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui X, Jing X, Long C, Tian J and Zhu J:
Long noncoding RNA MEG3, a potential novel biomarker to predict the
clinical outcome of cancer patients: A meta-analysis. Oncotarget.
8:19049–19056. 2017.PubMed/NCBI
|
|
23
|
Ying L, Huang Y, Chen H, Wang Y, Xia L,
Chen Y, Liu Y and Qiu F: Downregulated MEG3 activates autophagy and
increases cell proliferation in bladder cancer. Mol Biosyst.
9:407–411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin R, Chen Z, Ding Y, Hao J, Hu J and Guo
F: Long noncoding RNA MEG3 inhibits the proliferation of cervical
carcinoma cells through the induction of cell cycle arrest and
apoptosis. Neoplasma. 60:486–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang L, Wang G, Jia T, Zhang L, Li Y, Han
Y, Zhang K, Lin G, Zhang R, Li J and Wang L: Armored long
non-coding RNA MEG3 targeting EGFR based on recombinant MS2
bacteriophage virus-like particles against hepatocellular
carcinoma. Oncotarget. 7:23988–24004. 2016.PubMed/NCBI
|
|
26
|
Cao X, Zhuang S, Hu Y, Xi L, Deng L, Sheng
H and Shen W: Associations between polymorphisms of long noncoding
RNA MEG3 and risk of colorectal cancer in Chinese. Oncotarget.
7:19054–19059. 2016.PubMed/NCBI
|
|
27
|
Zhu F, Zhang X, Yu Q, Han G, Diao F, Wu C
and Zhang Y: LncRNA AWPPH inhibits SMAD4 via EZH2 to regulate
bladder cancer progression. J Cell Biochem. 119:4496–4505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu G, Wang W, Deng J and Dong S: LncRNA
AWPPH promotes the proliferation, migration and invasion of ovarian
carcinoma cells via activation of the Wnt/β-catenin signaling
pathway. Mol Med Rep. 19:3615–3621. 2019.PubMed/NCBI
|
|
29
|
Zhao X, Liu Y and Yu S: Long noncoding RNA
AWPPH promotes hepatocellular carcinoma progression through YBX1
and serves as a prognostic biomarker. Biochim Biophys Acta Mol
Basis Dis. 1863:1805–1816. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo D, Li Q, Lv Q, Wei Q, Cao S and Gu J:
MiR-27a targets sFRP1 in hFOB cells to regulate proliferation,
apoptosis and differentiation. PLoS One. 9:e913542014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bielack S, Carrle D and Casali PG; ESMO
Guidelines Working Group, : Osteosarcoma: ESMO clinical
recommendations for diagnosis, treatment and follow-up. Ann Oncol.
20 (Suppl 4):S137–S139. 2009. View Article : Google Scholar
|
|
33
|
Zhang J, Lan Q and Lin J: Identification
of key gene modules for human osteosarcoma by co-expression
analysis. World J Surg Oncol. 16:892018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bassett AR, Akhtar A, Barlow DP, Bird AP,
Brockdorff N, Duboule D, Ephrussi A, Ferguson-Smith AC, Gingeras
TR, Haerty W, et al: Considerations when investigating lncRNA
function in vivo. Elife. 3:e030582014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Danielsen SA, Eide PW, Nesbakken A, Guren
T, Leithe E and Lothe RA: Portrait of the PI3K/AKT pathway in
colorectal cancer. Biochim Biophys Acta. 1855:104–121.
2015.PubMed/NCBI
|
|
37
|
Gardai SJ, Hildeman DA, Frankel SK,
Whitlock BB, Frasch SC, Borregaard N, Marrack P, Bratton DL and
Henson PM: Phosphorylation of Bax Ser184 by Akt regulates its
activity and apoptosis in neutrophils. J Biol Chem.
279:21085–21095. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Quan JH, Cha GH, Zhou W, Chu JQ, Nishikawa
Y and Lee YH: Involvement of PI 3 kinase/Akt-dependent Bad
phosphorylation in Toxoplasma gondii-mediated inhibition of host
cell apoptosis. Exp Parasitol. 133:462–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cardone MH, Roy N, Stennicke HR, Salvesen
GS, Franke TF, Stanbridge E, Frisch S and Reed JC: Regulation of
cell death protease caspase-9 by phosphorylation. Science.
282:1318–1321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li C, Yu S, Wu S, Ni Y and Pan Z:
MicroRNA-936 targets FGF2 to inhibit epithelial ovarian cancer
aggressiveness by deactivating the PI3K/Akt pathway. Onco Targets
Ther. 12:5311–5322. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee MS, Jeong MH, Lee HW, Han HJ, Ko A,
Hewitt SM, Kim JH, Chun KH, Chung JY, Lee C, et al: PI3K/AKT
activation induces PTEN ubiquitination and destabilization
accelerating tumourigenesis. Nat Commun. 6:77692015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Porta C, Paglino C and Mosca A: Targeting
PI3K/Akt/mTOR signaling in cancer. Front Oncol. 4:642014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu B, Lv X, Gao F, Chen S, Wang S, Qing X,
Liu J, Wang B and Shao Z: Downregulation of DEPTOR inhibits the
proliferation, migration, and survival of osteosarcoma through
PI3K/Akt/mTOR pathway. Onco Targets Ther. 10:4379–4391. 2017.
View Article : Google Scholar : PubMed/NCBI
|